The crystal structure of the M. tuberculosis MarR family protein Rv0880 is described.
Keywords: MarR, Mycobacterium tuberculosis, transcription factor, MarR family protein
Abstract
Rv0880 from the pathogen Mycobacterium tuberculosis is classified as a MarR family protein in the Pfam database. It consists of 143 amino acids and has an isoelectric point of 10.9. Crystals of Rv0880 belonged to space group P1, with unit-cell parameters a = 54.97, b = 69.60, c = 70.32 Å, α = 103.71, β = 111.06, γ = 105.83°. The structure of the MarR family transcription regulator Rv0880 was solved at a resolution of 2.0 Å with an R cryst and R free of 21.2 and 24.9%, respectively. The dimeric structure resembles that of other MarR proteins, with each subunit comprising a winged helix–turn–helix domain connected to an α-helical dimerization domain.
1. Introduction
All living organisms have molecular systems that enable them to resist a variety of toxic substances and environmental stresses. Proteins belonging to the multiple antibiotic-resistance regulator (MarR) family have been reported to regulate the expression of proteins conferring resistance to multiple antibiotics and pathogenic factors (Perera & Grove, 2010 ▶; Grove, 2013 ▶). In Escherichia coli, resistance to numerous antimicrobial agents is believed to be determined primarily via efflux pumps, the expression of which is controlled by the binding of MarR repressor proteins to their cognate DNA, preventing initiation of gene transcription (Lomovskaya et al., 1995 ▶).
Knowledge of the structures of MarR family regulators has contributed to the understanding of their mechanism of action. To date, the structures of more than 20 MarR family proteins have been solved and deposited in the Protein Data Bank. These proteins are homodimers comprising a largely helical dimerization domain linked to a DNA-binding domain that contains a winged helix–turn–helix (wHTH) motif. MarR family proteins repress the activity of their target genes by binding as dimers to upstream pseudopalindromic sequences in the promoters of regulated proteins (Perera & Grove, 2010 ▶). MarR proteins typically function as repressors via one of two mechanisms: either by disrupting disulfide-bridge formation when MarR cysteines are oxidized or by the binding of a co-inducer such as salicylate. For example, the oxidation of cysteines in the MarR protein OhrR in Bacillus subtilis results in structural changes such that it is no longer able to bind to DNA (Soonsanga et al., 2008 ▶; Eiamphungporn et al., 2009 ▶), and the binding of salicylate to ST170 in Sulfolobus tokodaii strain 7 (Yu et al., 2009 ▶) leads to a major rearrangement in the dimerization region of this MarR protein such that its DNA-binding domain is significantly altered, preventing DNA recognition.
Nine million new cases of tuberculosis, caused by the pathogen Mycobacterium tuberculosis (Mtb), are reported annually (World Health Organization, 2014 ▶), and multidrug-resistant (MDR), extensively drug-resistant (XDR) and most recently totally drug-resistant (TDR) strains of Mtb have emerged and are spreading globally. Five MarR family proteins are encoded in the Mtb genome; the potential role of these regulatory proteins in drug resistance is unclear and is worthy of investigation. Given the potential importance of the MarR family of proteins in Mtb, we have performed structural studies on Rv0880, a previously uninvestigated protein which has been annotated as a MarR protein, and here describe its structure at 2.0 Å resolution.
2. Materials and methods
2.1. Macromolecule production
The Rv0880 gene was amplified from M. tuberculosis strain H37Rv genomic DNA by PCR and ligated into the vector pET-28a(+) (Novagen) using NdeI and HindIII restriction sites, generating a plasmid encoding recombinant Rv0880 protein with a 6×His tag at its N-terminus (pET-28aΩRv0880) which was then transformed into E. coli BL21(DE3) cells. Large-scale cultures were grown to an OD600 of between 0.6 and 0.8; Rv0880 expression was then induced by incubation with IPTG (final concentration of 0.5 mM) for 16 h at 16°C.
Bacterial cells were collected, suspended in ice-cold lysis buffer consisting of 20 mM Tris pH 7.4, 10 mM imidazole, 500 mM NaCl and subjected to high-pressure homogenization. Cell debris was removed by centrifugation at 30 000g for 40 min at 4°C. The crude lysate was loaded onto an Ni2+-chelating column pre-equilibrated with 20 mM Tris pH 7.4, 10 mM imidazole, 500 mM NaCl, which was then washed with six column volumes of buffer consisting of 80 mM imidazole, 500 mM NaCl, 20 mM Tris pH 7.4. The Rv0880 protein was then eluted with two column volumes of buffer consisting of 300 mM imidazole, 500 mM NaCl, 20 mM Tris pH 7.4. The eluted protein was concentrated to 0.5 ml using an Amicon Ultra-4 10K centrifugal filter device (Millipore) and loaded onto a Superdex 200 (GE Healthcare) size-exclusion column pre-equilibrated with 20 mM Tris pH 7.4, 150 mM NaCl, 5% glycerol at a flow rate of 0.5 ml min−1. Fractions containing the pure protein were pooled and concentrated to 12 mg ml−1. After checking the purity by SDS–PAGE analysis, purified Rv0880 was flash-frozen in liquid nitrogen and stored at −80°C. Macromolecule-production information is summarized in Table 1 ▶.
Table 1. Macromolecule-production information.
| Source organism | M. tuberculosis H37Rv |
| DNA source | M. tuberculosis H37Rv genome |
| Forward primer | GGAATTCCATATGGTGCTTGACAGCGATGCGCG |
| Reverse primer | CCCAAGCTTTCACGGGCTTTCGTCGACCAG |
| Cloning vector | pET-28a |
| Expression vector | pET-28a |
| Expression host | E. coli BL21(DE3) |
| Complete amino-acid sequence of the construct produced | MGSSHHHHHHSSGLVPRGSHMVLDSDARLASDLSLAVMRLSRQLRFRNPSSPVSLSQLSALTTLANEGAMTPGALAIRERVRPPSMTRVIASLADMGFVDRAPHPIDGRQVLVSVSESGAELVKAARRARQEWLAERLATLNRSERDILRSAADLMLALVDESP |
2.2. Crystallization
Purified Rv0880 protein at a concentration of 12 mg ml−1 in 20 mM Tris pH 7.4, 150 mM NaCl, 5% glycerol was used in all crystallization experiments. Crystallization experiments were performed at 16°C using the hanging-drop vapour-diffusion method. Crystallization screening kits from Hampton Research (Index, Crystal Screen and Crystal Screen 2) were used to determine the initial crystallization conditions for the Rv0880 protein. Typically, 1 µl drops of protein solution were mixed with an equal volume of screening solution and equilibrated over a reservoir containing 0.2 ml reservoir solution. Crystallization information is summarized in Table 2 ▶.
Table 2. Crystallization.
| Method | Hanging-drop vapour diffusion |
| Plate type | 16-well hanging-drop plate |
| Temperature (K) | 289 |
| Protein concentration (mgml1) | 12 |
| Buffer composition of protein solution | 20 mM Tris pH 7.4, 150mM NaCl, 5% glycerol |
| Composition of reservoir solution | 0.1M HEPES, 5%(v/v) ()-2-methyl-2,4-pentanediol, 12%(w/v) PEG 6000 |
| Volume and ratio of drop | 1.0l; 1:1 |
| Volume of reservoir (ml) | 0.2 |
2.3. Data collection and processing
Native Rv0880 crystals were soaked for 30 s in reservoir solution supplemented with 15% glycerol and flash-cooled in a stream of cold nitrogen at 100 K. X-ray diffraction data were collected at an in-house data-collection facility using a Rigaku R-AXIS IV++ image plate and a Rigaku MicroMax-007 copper rotating-anode generator operating at 60 mA and 45 kV. A total of 360 frames were collected with an oscillation step of 1° and an exposure of 3 min per frame. The crystal-to-detector distance was maintained at 150 mm. Diffraction data were processed with iMosflm (Battye et al., 2011 ▶; Powell et al., 2013 ▶) and scaled with SCALA (Winn et al., 2011 ▶; Cowtan et al., 2011 ▶).
2.4. Structure solution and refinement
The structure of Rv0880 was solved by molecular replacement with Rosetta (Terwilliger et al., 2012 ▶) from the PHENIX package (Adams et al., 2002 ▶, 2010 ▶) using the structure of a putative transcriptional regulator protein from Pseudomonas aeruginosa PA01 (PDB entry 2hr3; Midwest Center for Structural Genomics, unpublished work) as a search model. Model building and structural refinement were performed with Coot (Emsley & Cowtan, 2004 ▶) and PHENIX, respectively. Data-collection and refinement statistics are summarized in Tables 3 ▶ and 4 ▶. All structural figures were rendered in PyMOL (http://www.pymol.org).
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | Rigaku MicroMax-007 |
| Wavelength () | 1.5418 |
| Temperature (K) | 100 |
| Detector | R-AXIS IV++ image plate |
| Crystal-to-detector distance (mm) | 150 |
| Rotation range per image () | 1 |
| Total rotation range () | 360 |
| Exposure time per image (s) | 180 |
| Space group | P1 |
| a, b, c () | 54.97, 69.60, 70.32 |
| , , () | 103.71, 111.06, 105.83 |
| Mosaicity () | 0.5 |
| Resolution range () | 41.152.00 (2.112.00) |
| Total No. of reflections | 218999 (30572) |
| No. of unique reflections | 55124 (7737) |
| Completeness (%) | 94.3 (90.3) |
| Multiplicity | 4.0 (4.0) |
| I/(I) | 12.3 (2.4) |
| R r.i.m. | 0.071 (0.543) |
| Overall B factor from Wilson plot (2) | 29.7 |
Table 4. Structure solution and refinement.
Values in parentheses are for the outer shell.
| Resolution range () | 37.1082.002 |
| Completeness (%) | 94.01 |
| Cutoff | 1.96 |
| No. of reflections, working set | 52178 |
| No. of reflections, test set | 2793 |
| Final R cryst | 0.212 |
| Final R free | 0.249 |
| No. of non-H atoms | |
| Protein | 6181 |
| Ligand | 0 |
| Water | 529 |
| Total | 6710 |
| R.m.s. deviations | |
| Bonds () | 0.006 |
| Angles () | 1.222 |
| Average B factors (2) | 41.7 |
| Protein | 41.7 |
| Water | 41.0 |
| Ramachandran plot | |
| Most favoured (%) | 98.0 |
| Allowed (%) | 1.0 |
| Disallowed (%) | 1.0 |
3. Results and discussion
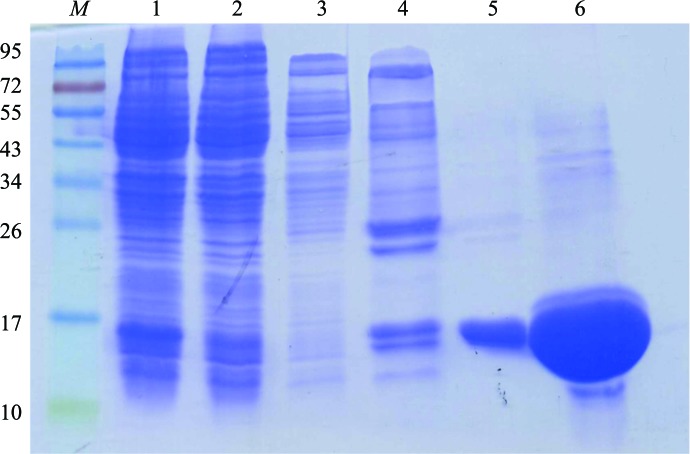
The Rv0880 gene is 432 bp in length and codes for 143 amino-acid residues. The isoelectric point of the protein is calculated to be 10.9. Purified Rv0880 gave a band of approximately 16 kDa on SDS–PAGE (Fig. 1 ▶), which is in good agreement with the calculated molecular weight.
Figure 1.
Purification of Rv0880. Monitoring protein purification using SDS–PAGE. Lane M, molecular-weight markers (labelled in kDa); lane 1, whole cell lysate after IPTG induction of protein expression; lane 2, flowthrough after the cell lysate was loaded onto an Ni2+-chelating column; lanes 3, 4, 5 and 6, flowthrough after elution with buffer containing 40, 80, 120 or 300 mM imidazole, respectively.
Crystals grew after 7 d at 16°C in Crystal Screen 2 solution No. 30 [0.1 M HEPES pH 7.5, 5%(v/v) (±)-2-methyl-2,4-pentanediol, 10%(w/v) PEG 6000]. To optimize the crystal, a systematic grid refinement was employed by varying the concentration of the precipitant (PEG 6000) from 5 to 15% in 1% steps and the pH value of the reservoir solution from 7.0 to 8.0 in steps of 0.1. The best crystals were obtained in a final optimized reservoir solution consisiting of 0.1 M HEPES pH 7.2, 5%(v/v) (±)-2-methyl-2,4-pentanediol, 12%(w/v) PEG 6000.
X-ray diffraction data were indexed and integrated using iMosflm (Battye et al., 2011 ▶), giving a data set to 2.0 Å resolution that had a completeness of 94.3% and an overall R merge of 7.1%. The crystals belonged to space group P1, as determined using phenix.xtriage (Terwilliger et al., 2012 ▶), and six molecules were present in the unit cell. The solvent content of the Rv0880 crystal was 44.5%, and the calculated Matthews value was 2.2 Å3 Da−1.
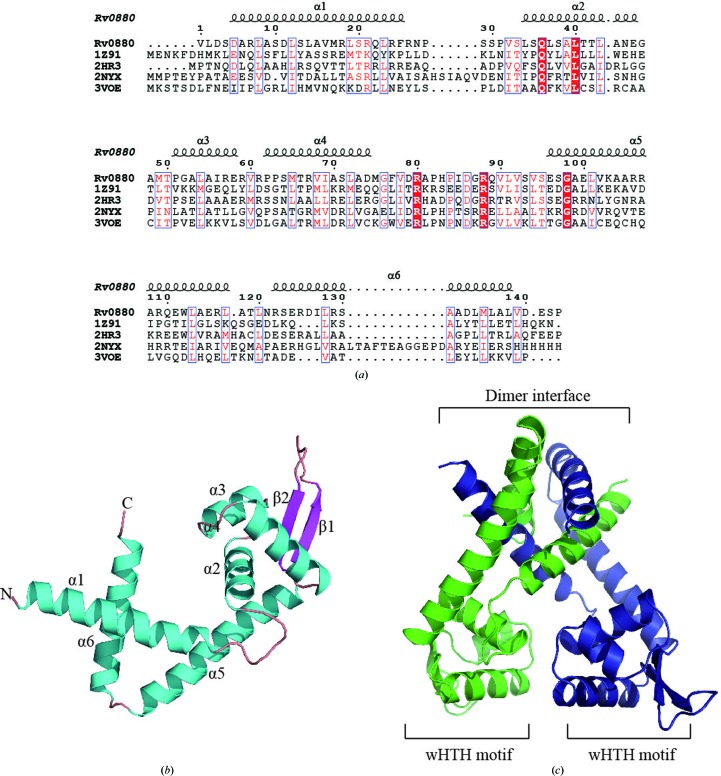
Like other MarR proteins, Rv0880 is present as a dimer and belongs to the α/β family of proteins. It consists of six α-helices and two β-strands arranged in the order α1–α2–α3–α4–β1–β2–α5–α6 in the primary structure. Each subunit is composed of two functional domains: the dimerization domain, which includes helices α1, α5 and α6, and the DNA-binding domain, which includes α2, α3, α4, β1 and β2. The N- and C-termini of each monomer, located in the α1 and α6 helices, respectively, are closely intertwined and form the dimer interface, which is stabilized by hydrophobic and hydrogen-bond interactions between residues located within these regions. Residues located within the α2–α3–α4–β1–β2 structure form a wHTH DNA-binding motif (Fig. 2 ▶ c) which is distal to the dimer interface.
Figure 2.
Structure of Rv0880. (a) Primary sequence alignment of Rv0880 with other structurally characterized MarR family members from B. subtilis (PDB entry 1z91; Hong et al., 2005 ▶), P. aeruginosa (PDB entry 2hr3; Midwest Center for Structural Genomics, unpublished work), M. tuberculosis (Rv1404; PDB entry 2nyx; TB Structural Genomics Consortium/Integrated Center for Structure and Function Innovation, unpublished work) and E. coli K-12 (PDB entry 3voe; H. Lou, R. Zhu & Z. Hao, unpublished work). The multiple sequence alignment was made with ClustalW (Thompson et al., 2002 ▶) and displayed with secondary structures using ESPript (Gouet et al., 1999 ▶). (b) Ribbon diagram of the Rv0880 subunit. Secondary-structural elements are labelled and the N- and C-termini are labelled N and C, respectively. (c) Ribbon diagram of the Rv0880 dimer.
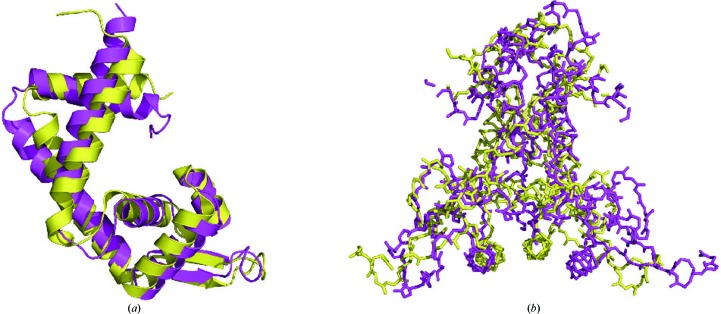
Although Rv0880 shares less than 20% sequence identity with other MarR family proteins (Fig. 2 ▶ a), it possesses the same core fold as other members of this family of regulators. While the structure of these proteins is quite similar, their dimerization is quite different. For example, when the structure of Rv0880 is superimposed onto the MarR family protein from E. coli (PDB entry 3voe; H. Lou, R. Zhu & Z. Hao, unpublished work; Fig. 3 ▶), it matches it closely with an r.m.s.d. of 2.5 Å. However, the orientation of the monomers in these two dimers is different. Analysis of the dimer interface using PISA (Krissinel & Henrick, 2007 ▶) showed that in PDB entry 3voe the interface, which contains eight hydrogen bonds and four salt bridges, buries a total area of 3819 Å2 and encompasses 46 residues from monomer A and 49 residues from monomer B. In Rv0880, the total area of the interface, which contains 22 hydrogen bonds and 15 salt bridges, is 5477 Å2 and encompasses 62 residues from monomer A and 63 residues from monomer B.
Figure 3.
Structural comparison of Rv0880 (magenta) with the MarR family protein from E. coli (PDB entry 3voe; yellow). (a) Superposition of the Rv0880 monomer with PDB entry 3voe. (b) Superposition of the Rv0880 dimer with PDB entry 3voe.
The structural information on Rv0880 that we have obtained supports its annotation as a MarR family protein. It will be interesting to investigate the functional mechanism of this protein and to determine whether it plays a role in drug resistance in M. tuberculosis.
Supplementary Material
PDB reference: Rv0880, 4yif
Acknowledgments
We would like to gratefully acknowledge the help of the staff of the Protein Science Core Facility Center at the Institute of Biophysics, Chinese Academy of Science. Financial support for this project was provided by the Chinese Ministry of Science and Technology 973 program (2011CB910302).
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Adams, P. D., Grosse-Kunstleve, R. W., Hung, L.-W., Ioerger, T. R., McCoy, A. J., Moriarty, N. W., Read, R. J., Sacchettini, J. C., Sauter, N. K. & Terwilliger, T. C. (2002). Acta Cryst. D58, 1948–1954. [DOI] [PubMed]
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Cowtan, K., Emsley, P. & Wilson, K. S. (2011). Acta Cryst. D67, 233–234. [DOI] [PMC free article] [PubMed]
- Eiamphungporn, W., Soonsanga, S., Lee, J. W. & Helmann, J. D. (2009). Nucleic Acids Res. 37, 1174–1181. [DOI] [PMC free article] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Gouet, P., Courcelle, E., Stuart, D. I. & Métoz, F. (1999). Bioinformatics, 15, 305–308. [DOI] [PubMed]
- Grove, A. (2013). Curr. Biol. 23, R142–R143. [DOI] [PubMed]
- Hong, M., Fuangthong, M., Helmann, J. D. & Brennan, R. G. (2005). Mol. Cell, 20, 131–141. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Lomovskaya, O., Lewis, K. & Matin, A. (1995). J. Bacteriol. 177, 2328–2334. [DOI] [PMC free article] [PubMed]
- Perera, I. C. & Grove, A. (2010). J. Mol. Cell Biol. 2, 243–254. [DOI] [PubMed]
- Powell, H. R., Johnson, O. & Leslie, A. G. W. (2013). Acta Cryst. D69, 1195–1203. [DOI] [PMC free article] [PubMed]
- Soonsanga, S., Lee, J. W. & Helmann, J. D. (2008). Mol. Microbiol. 68, 978–986. [DOI] [PubMed]
- Terwilliger, T. C., DiMaio, F., Read, R. J., Baker, D., Bunkóczi, G., Adams, P. D., Grosse-Kunstleve, R. W., Afonine, P. V. & Echols, N. (2012). J. Struct. Funct. Genomics, 13, 81–90. [DOI] [PMC free article] [PubMed]
- Thompson, J. D., Gibson, T. J. & Higgins, D. G. (2002). Curr. Protoc. Bioinformatics, Unit 2.3. 10.1002/0471250953.bi0203s00. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- World Health Organization (2014). Global Tuberculosis Report 2014. Geneva: World Health Organization. http://www.who.int/tb/publications/global_report/en/.
- Yu, L., Fang, J. & Wei, Y. (2009). Biochemistry, 48, 2099–2108. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: Rv0880, 4yif