Abstract
As treatment of ischemic stroke remains a challenge with respect to the failure of numerous neuroprotective attempts, there is an ongoing need for better understanding of pathophysiological mechanisms causing tissue damage. Although ischemic outcomes have been studied extensively at the cellular and molecular level using histological and biochemical methods, properties of ischemia-affected brain tissue with respect to mechanical integrity have not been addressed so far. As a novel approach, this study used fluorescence-based detection of regions affected by experimental thromboembolic stroke in combination with scanning force microscopy to examine mechanical alterations in selected rat brain areas. Twenty-five hours after onset of ischemia, a decreased elastic strength in the striatum as the region primarily affected by ischemia was found compared with the contralateral nonaffected hemisphere. Additional intrahemispheric analyses showed decreased elastic strength in the ischemic border zone compared with the more severely affected striatum. In conclusion, these data strongly indicate a critical alteration in mechanical tissue integrity caused by focal cerebral ischemia. Further, on the basis of data that have been obtained in relation to the ischemic border zone, a shell-like pattern of mechanical tissue damage was found in good accordance with the penumbra concept. These findings might enable the development of specific therapeutic interventions to protect affected areas from critical loss of mechanical integrity.
Keywords: mechanical tissue characterization, penumbra concept, stroke, thromboembolic model
Introduction
Ischemic stroke still represents one of the leading causes of death worldwide 1. Despite enormous efforts to reduce mortality, symptoms, and personal burden related to acute ischemic stroke 2, treatment is limited to intravenous recombinant tissue plasminogen activator (rtPA) as the only approved drug-related therapy that has shown beneficial effects for patient’s outcome 3. Although this therapy has increasingly been used during the last few years 4, only a minority of patients appear to be eligible because of limitations such as the narrow time window of 4.5 h from the onset of symptoms. Further, treatment with rtPA involves the risk of hemorrhagic transformation or even intracerebral hemorrhage in ischemia-affected brain areas, which is associated with poor clinical outcome related to exacerbation of tissue damage.
With the aim of improving stroke treatment, preclinical research has recently been intensified, resulting in a more detailed understanding of pathophysiological mechanisms responsible for tissue damage including cellular loss 2,5. As perhaps the most popular and consistent model during the last few decades, the concept of ischemic penumbra distinguishes a core with critical decrease in oxygen supply and subsequent tissue damage from the surrounding penumbra, whereas the latter shows – at least in part – preserved cellular integrity 6. As the initial description classifies areas strictly according to the amount of impaired local blood flow, the penumbra concept was adapted to modern techniques of stroke visualization such as multiparameter MRI or computed tomography 7. In terms of the mechanical properties of brain tissue affected by focal ischemia, the assumption emerged that areas in the center of focal ischemia – growing naturally over time 5 – show a more decreased integrity than the border zone as well as adjacent nonaffected regions, although specific verifications are lacking. However, this hypothesis should be considered with caution in terms of therapeutic interventions as a progression of the area attributed to significantly decreased tissue integrity is considered to be more sensitive to complications such as rtPA-related hemorrhagic transformation or even intracerebral hemorrhage. Consequently, stroke patients were traditionally not treated with rtPA if cerebral imaging indicated a relevant proportion of the ischemic core related to the affected vascular territory 3,8.
As the theoretical concept of different degrees of stroke-induced tissue damage has not been confirmed by methods providing details on mechanical properties, this study, for the first time, focused on a direct mechanical tissue characterization in areas affected by experimental focal cerebral ischemia. In addition to a verification approach of the widely accepted penumbra concept, these data appear to be essential for the development of advanced therapeutic strategies.
Methods
Study setup
Male Wistar rats (n=10) with a mean body weight of 308±26 g, provided by Charles River (Sulzfeld, Germany), were subjected to thromboembolic middle cerebral artery occlusion as described below. To ensure sufficient cerebral ischemia, rats were screened for neurobehavioral deficits at 4 and 24 h after the onset of ischemia using the Menzies score (ranging from 0, no deficit, to 4, spontaneous contralateral circling 9); animals included in the study had to present a score of at least 2. To enable detection of brain regions showing ischemia-associated alterations indicated by diminished blood–brain barrier integrity, fluorescein isothiocyanate-conjugated albumin (FITC-albumin, 20 mg dissolved in 1 ml saline; Sigma, Taufkirchen, Germany 10), usually not entering the blood–brain barrier 11, was administered intravenously at 24 h through a femoral catheter. After a circulation period of 1 h, rats were sacrificed using CO2 inhalation and perfused with ∼200 ml of 0.9% saline, immediately followed by careful removal of the brain from the skull.
Experiments involving animals were conducted according to the European Communities Council Directive (86/609/EEC) and were approved by the local authorities (reference number TVV-34/11; Landesdirektion Sachsen, Leipzig, Germany). Generally, efforts were made to minimize the number and suffering of animals, which were housed in a temperature-controlled and humidity-controlled room with 12 h of a light/dark cycle and with free access to food and water.
Experimental focal cerebral ischemia
Thromboembolic middle cerebral artery occlusion was performed according to the model originally described by Zhang et al. 12, with some minor modifications. Briefly, after preparation of right-sided cervical arteries, a polyethylene tube with a tapered end was inserted into the external carotid and moved forward through the internal carotid artery up to the origin of the middle cerebral artery. Here, a blood clot with a medium length of about 4 cm was injected, originating from rat blood that was collected on the previous day and allowed to clot in a polyethylene tube on a warming pad at 37°C for 2 h, followed by overnight storage at 4°C. Afterwards, the catheter was removed, and both the carotid stump and the wound were closed. Generally, surgical interventions were performed in anesthetized rats using 2.0 to 2.5% isoflurane (Isofluran Baxter; Baxter, Unterschleißheim, Germany; mixture: 70% N2O/30% O2), applied by a commercial vaporizator (VIP 3000; Matrix, New York, New York, USA). To avoid anesthesia-related cooling, a thermostatically controlled warming pad (Fine Science Tools, Heidelberg, Germany) was used to adjust the body temperature to about 37.0°C.
Tissue preparation and semiquantification of elastic strength
Perfused rat brains were cut coronally using a Leica VT 1200 s vibrating microtome (Leica Microsystems, Nussloch, Germany) producing slices of 350 μm thickness, which were stored temporarily in Hank’s balanced salt solution upon transportation. Shortly after the cutting procedure (<30 min), slices were glued with commercially available histoacryl surgical glue (B. Braun Surgical, Rubi, Spain) to microscope slides immediately before the measurements and submerged in Hank’s balanced salt solution throughout the measurement. The ischemia-affected brain areas were identified by extravasation of the fluorescently detectable FITC-albumin 10 using an inverted fluorescence microscope (Leica DM ILM; Leica Microsystems, Wetzlar, Germany).
The scanning force microscope (SFM) NanoWizard I (JPK Instruments, Berlin, Germany) was used for measurement of the elastic strength (Young’s modulus) in selected brain areas, starting in the ischemia-affected striatum as identified by the fluorescence signal of FITC-albumin (designated to the region of ischemia). Viscous contributions were ignored in these initial measurements as measurements with an oscillating SFM cantilever would have been too complex 13. Three positions were usually selected for each of the targeted brain areas (ischemia, control, and border zone), whereas in the approach addressing potential bordering effects, six positions were analyzed in each region. At each position, usually 15 force–distance curves (minimum 4, maximum 26) were recorded with a spacing of 10 μm between individual curves, and unreliable values were excluded from further processing. The slices were then manually moved about 500 μm to the next position. Images were taken at each position to mark the positions on the slice. For control tissues, further brain regions were analyzed located either on the contralateral hemisphere (control) or within the ischemia-associated bordering zone on the ipsilateral hemisphere (border zone). Thus, the applied model of tissue characterization was based on ∼180 to 360 single measurements per brain slice.
To obtain well-defined tip geometry with a large contact area, cantilevers were modified by gluing 6 μm diameter polystyrene beads (Polysciences, Warrington, Pennsylvania, USA) to the tip of soft (10–100 mN/m) cantilevers for contact mode (Pointprobe; NanoWorld, Neuchâtel, Switzerland). Elastic constants, that is, the Young’s modulus, were obtained by fitting a Hertz model to the force–indentation curves, where F is the force, R is the radius of the bead, E is the Young’s modulus, υ=0.5 is the Poisson ratio, and δ is the indentation (see subsequent equation).
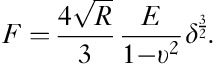 |
Data calculations
Data were processed by calculating rounded means of Young’s modulus measured, first grouped by the position obtained and then by the brain region, resulting in a single mean value for each of targeted brain regions (ischemia, control, and border zone) in each animal. Calculations were generally carried out using the statistical software package SPSS, version 20.0 (IBM Corp., New York, USA).
Results
The applied model of thromboembolic stroke resulted in sufficient cerebral ischemia as indicated by significant and consistent neurobehavioral deficits at two observational time points (Menzies score at 4 h, 2.9±0.9; at 24 h, 3.0±0.7), which allowed the recruitment of all 10 rats for the study. However, brains of three rats were used for the establishment of a measurement routine (i.e. scanning parameters of the SFM) to determine the elastic strength in selected brain slices. Three further animals had to be excluded because of severe tissue damage and/or insufficient detection of FITC-albumin extravasation, impeding correct measurements. Overall, the presented data originated from four rat brains significantly affected by focal cerebral ischemia. Thereby, ischemia-affected slices of two animals each were used for interhemispheric (ischemia vs. control) and intrahemispheric (ischemia vs. border zone) comparisons, respectively.
Twenty-five hours after the onset of ischemia, interhemispheric comparison of mechanical properties between ischemic and nonischemic areas (Fig. 1a, a′) indicated decreased elastic strength in the striatum as the brain region that is primarily affected by the thromboembolic stroke model used (Fig. 1a″, animals 1 and 2). Although the difference between both hemispheres appears to be relatively small, the trend toward decreased values of elastic strength proved robust in the two animals analyzed.
Fig. 1.
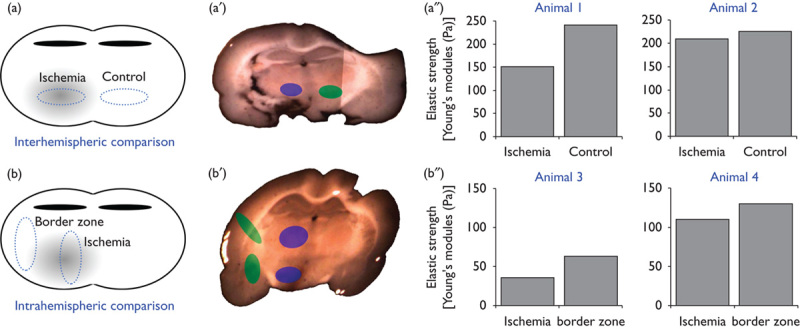
Mechanical characterization of the rat brain subjected to thromboembolic focal cerebral ischemia. Using an interhemispheric (a, a′) and intrahemispheric (b, b′) setup of measurements on coronal brain slices, mechanical properties of different brain areas depending on the amount of ischemic affection were assessed by scanning force microscopy. The analyses (a″, b″) indicated decreased elastic strength in areas primarily affected by experimental focal cerebral ischemia, consistently found in both the interhemispheric and the intrahemispheric approach. The latter is focused on potential bordering effects and apparently shows a shell-like pattern of impaired tissue integrity with respect to the striatum as the area of ischemic origin. Bars represent means per animal.
To further address detectable differences in the mechanical properties between the most ischemia-affected area and associated border zones (Fig. 1b, b′), extravasation of FITC-albumin was used to identify these regions with reference to the intensity of the fluorescence signal. Thereby, slightly decreased values for elastic strength were found in the ischemic border zone compared with the more severely affected striatum, which showed even more decreased values (Fig. 1b″, animals 3 and 4). As a situation comparable to the interhemispheric approach, the differences between both regions on the same hemisphere were relatively small, but the trend toward decreased values was found consistently in the two animals analyzed.
Discussion
The present study was focused on mechanical properties of the rat brain as affected by focal cerebral ischemia to reinforce or even attenuate the hypothesis that ischemic stroke not only results in numerous morphological changes (i.e. on the cellular level) but also leads to mechanical alterations – an issue that has been neglected until now. For this purpose, an SFM was used, which has been applied previously to explore mechanical characteristics in the unaffected rodent brain in terms of a demarcation between white and gray matter 14, as well as physical properties of isolated cell types 15–19. As the translational relevance of earlier pathophysiological studies was often limited by the poor comparability of the stroke models applied with respect to the human condition 20, efforts have been made to mimic focal cerebral ischemia close to the nature of human stroke pathophysiology. Therefore, this study was based on a thromboembolic rat model of ischemic stroke, which has been reported to provide excellent comparability 21 because embolism represents a major cause of ischemic events in humans 22. Furthermore, the model applied has previously been shown to result in significant ischemia-associated cellular alterations as assessed by immunofluorescence labeling of diverse cell types 10 and breakdown of the functionally relevant blood–brain barrier 11, qualifying this model to close the gap to the lack of mechanical characterization of related brain areas. To consider existing theories of ischemia-related tissue damage, that is, the penumbra concept 5,6, the present study included both an interhemispheric as well as an intrahemispheric comparison of mechanical properties to capture bordering effects.
The data obtained strongly indicate that one day after embolic occlusion of the middle cerebral artery, the striatum, as the primarily affected area, provides the most impaired tissue integrity as indicated by decreased elastic strength compared with the contralateral as well as ipsilateral bordering zone. With respect to the well-established concept of the ischemic penumbra 5,6, these data support the perspective of a shell-like pattern of tissue affection because of focal cerebral ischemia. In addition to earlier histological and biochemical reports focusing on graduated ischemic damage in the rodent 23 and the human brain 24, this study provides novel evidence for altered mechanical properties of brain areas critically affected by focal cerebral ischemia. Moreover, neuronal cells are known to be highly mechanosensitive and alter their degree of differentiation in response to mechanical alterations of their microenvironment 25. This feedback loop might represent an additional explanation for extended tissue damage caused by ischemic stroke as the altered mechanical properties of the tissue that is primarily affected by ischemia could critically influence neighboring areas.
However, because of its novel aspect, the present study is not devoid of limitations. First, despite the efforts made toward measurement of tissue’s elastic strength as reflected by the high number of single measurements per brain slice, the final sample size is limited to four animals (two in each group investigated), impeding analyses on statistical significance beyond the descriptive level. Second, some animals had to be excluded as clear identification of the FITC-albumin signal as a prerequisite for identification of ischemic tissue was lacking. Third, observations were limited to a single time point (25 h after the onset of ischemia), although mechanical tissue alterations are expected to provide a temporal profile analogous to data emerging from histological studies (e.g. 23). Fourth, characterization of mechanical properties is generally influenced by the surface quality of the targeted object, which might be modified by the technique of tissue extraction applied. These issues need to be addressed by future studies involving further improved methods of tissue preparation and refined scanning protocols. Despite the given limitations, the data presented strongly support the view of a gradually impaired mechanical integrity in brain areas subjected to focal ischemia, and, therefore, represents valuable ancillary information that complements previous reports using multiparameter characterization that did not take this perspective into consideration.
Conclusion
In this study, SFM indicated that experimental focal cerebral ischemia critically affects mechanical properties in affected areas, which appear to be pronounced in the area of ischemic origin, supporting the view of a shell-like pattern of tissue damage. These findings and the suggestion to include mechanical analyses in preclinical models of ischemic stroke may help to adapt already existing clinical treatments (i.e. rtPA) or to develop therapeutic interventions with the objective of protecting critically affected tissue from serious complications such as hemorrhagic transformation. Consequently, future research should focus on strategies leading to an improved cellular stabilization or at least an attenuation of impaired cellular integrity during ischemic stroke. From this perspective, stabilizing elements in both the extracellular space (e.g. the extracellular matrix) and the intracellular compartment (for instance microtubule-associated proteins) appear as relevant targets for pharmacological modifications during the early phase of stroke.
Acknowledgements
The authors are grateful to Professor Andreas Reichenbach (Paul Flechsig Institute for Brain Research, University of Leipzig, Leipzig, Germany) for critically reading an earlier manuscript version. This study has been carried out as an investigator-initiated approach, emerging from a local interdisciplinary stroke research group without external funding. Therefore, the authors would like to thank all the contributing departments for providing internal support to this project.
Author’s contributions: D.M.: designed the study setup, carried out regulatory affairs on animal experiments, conducted animal experiments and calculations, and wrote the manuscript; W.H.: codesigned the study setup and revised the manuscript critically; M.K.: performed tissue preparation and carried out critical revisions to the manuscript; C.H.: enabled animal experiments and provided comments on the manuscript; J.A.K.: carried out senior supervision of the biomechanical experiments and revisions to the manuscript; and T.F.: performed mechanical tissue characterization and revised the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015; 131:434–441. [DOI] [PubMed] [Google Scholar]
- 2.Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis 2008; 25:268–278. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 4.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008; 39:924–928. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22:391–397. [DOI] [PubMed] [Google Scholar]
- 6.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke 1981; 12:723–725. [DOI] [PubMed] [Google Scholar]
- 7.Kidwell CS, Wintermark M, de Silva DA, Schaewe TJ, Jahan R, Starkman S, et al. Multiparametric MRI and CT models of infarct core and favorable penumbral imaging patterns in acute ischemic stroke. Stroke 2013; 44:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomalla G, Schwark C, Sobesky J, Bluhmki E, Fiebach JB, Fiehler J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke 2006; 37:852–858. [DOI] [PubMed] [Google Scholar]
- 9.Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery 1992; 31:100–106. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 10.Michalski D, Grosche J, Pelz J, Schneider D, Weise C, Bauer U, et al. A novel quantification of blood–brain barrier damage and histochemical typing after embolic stroke in rats. Brain Res 2010; 1359:186–200. [DOI] [PubMed] [Google Scholar]
- 11.Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D. Blood–brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One 2013; 8:e56419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res 1997; 766:83–92. [DOI] [PubMed] [Google Scholar]
- 13.Mahaffy RE, Shih CK, MacKintosh FC, Käs J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys Rev Lett 2000; 85:880–883. [DOI] [PubMed] [Google Scholar]
- 14.Christ AF, Franze K, Gautier H, Moshayedi P, Fawcett J, Franklin RJ, et al. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J Biomech 2010; 43:2986–2992. [DOI] [PubMed] [Google Scholar]
- 15.Lu YB, Franze K, Seifert G, Steinhäuser C, Kirchhoff F, Wolburg H, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA 2006; 103:17759–17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu YB, Iandiev I, Hollborn M, Körber N, Ulbricht E, Hirrlinger PG, et al. Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J 2011; 25:624–631. [DOI] [PubMed] [Google Scholar]
- 17.Knorr M, Koch D, Fuhs T, Behn U, Käs JA. Stochastic actin dynamics in lamellipodia reveal parameter space for cell type classification. Soft Matter 2011; 7:3192. [Google Scholar]
- 18.Franze K, Gerdelmann J, Weick M, Betz T, Pawlizak S, Lakadamyali M, et al. Neurite branch retraction is caused by a threshold-dependent mechanical impact. Biophys J 2009; 97:1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuhs T, Goegler M, Brunner CA, Wolgemuth CW, Kaes JA. Causes of retrograde flow in fish keratocytes. Cytoskeleton (Hoboken) 2014; 71:24–35. [DOI] [PubMed] [Google Scholar]
- 20.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40:2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007; 87:179–197. [DOI] [PubMed] [Google Scholar]
- 22.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001; 32:2735–2740. [DOI] [PubMed] [Google Scholar]
- 23.Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab 2000; 20:1011–1032. [DOI] [PubMed] [Google Scholar]
- 24.Sairanen T, Szepesi R, Karjalainen-Lindsberg ML, Saksi J, Paetau A, Lindsberg PJ. Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in ischemic human stroke. Acta Neuropathol 2009; 118:541–552. [DOI] [PubMed] [Google Scholar]
- 25.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310:1139–1143. [DOI] [PubMed] [Google Scholar]


