Abstract
Background
Immunoassay urine drug screening cups that detect use for two or more days are commonly used in addiction treatment settings. Until recently, there has been no comparable immunoassay test for alcohol use in these settings.
Objectives
The aim of this study was to assess the agreement of a commercially available ethyl glucuronide immunoassay (EtG-I) test conducted at an outpatient addiction clinic and lab-based EtG mass spectrometry (EtG-MS) conducted at a drug testing laboratory at three cut-off levels. High agreement between these two measures would support the usefulness of EtG-I as a clinical tool for monitoring alcohol use.
Methods
Forty adults with co-occurring alcohol dependence and serious mental illnesses submitted 1068 urine samples over a 16-week alcohol treatment study. All samples were tested using EtG-I on a benchtop analyzer and 149 were randomly selected for EtG-MS analysis at a local laboratory. Agreement was defined as the number of samples where EtG-I and EtG-MS were both above or below a specific cut-off level. Agreement was calculated at low cut-off levels (100 and 250 ng/ml), as well as at a higher cut-off level (500 ng/ml) recommended by most by commercial drug testing laboratories.
Results
Agreement between EtG-I and EtG-MS was high across all cut-off levels (90.6% at 100 ng/ml, and 96.6% at 250 and 500 ng/ml).
Conclusions
EtG immunoassays conducted at low cut-off levels in point-of-care testing settings have high agreement with lab-based EtG-MS. EtG-I can be considered a useful clinical monitoring tool for alcohol use in community-based addiction treatment settings.
Keywords: Alcohol biomarkers, alcohol use disorders, ethyl glucuronide, outpatient addiction treatment, urine drug testing
Introduction
Immunoassays are used to assess illicit drug use in outpatient addiction clinics because they provide rapid results and can detect substance use in urine for two or more days depending on the drug of abuse (1,2). Rapid results and a long detection window allow clinicians to monitor patient outcomes in a clinical setting where patients are not seen daily. Despite alcohol use being the most common reason for referral to specialty substance abuse treatment, there is no comparable alcohol biomarker that can be used in clinical settings and detect alcohol use for two or more days (3,4).
Currently available alcohol biomarkers have limitations for use in clinical settings.
Measures of blood alcohol content (i.e. breath tests) return results immediately but only detect alcohol use in the previous several hours. This method is primarily intended to assess current intoxication, rather than ongoing abstinence (5). Transdermal alcohol monitors allow for continuous monitoring of drinking, but are relatively expensive, and concerns exist regarding their feasibility and convenience (6). High levels of enzymes such as gamma-glutamyl-tansferase (GGT) can be detected in alcohol-dependent people, but have limited utility in detecting low levels of drinking, or infrequent, non-chronic binge drinking (7,8). Phosphatidylethanol (PEth) has shown potential as a sensitive biomarker for detecting drinking in the past 1–7 days (9–11), but its use is not feasible in many outpatient addiction clinics as it requires blood collection (9). Ethyl sulfate (EtS) has performed well as a biomarker for recent (up to 36 hours) drinking (12,13). However, to our knowledge there is no commercially available EtS immunoassay; samples must be sent to an MS reference laboratory with a delay of several days for receipt of results.
Ethyl glucuronide (EtG) is a metabolite of alcohol with the potential to overcome the shortcomings of aforementioned metabolites as a clinical tool for alcohol detection in outpatient addiction treatment settings. EtG has reported detection times in urine ranging from 2–5 days (14). EtG can be collected from a variety of bodily tissues (e.g. hair, toenails), including urine, the most feasible collection method at an outpatient addiction clinic (15). Thermo Scientific/ Microgenics has developed a commercially available EtG immunoassay – DRI (Diagnostic Reagents Incorporated) EtG – that can be easily conducted in a clinical setting using a relatively small benchtop analyzer (Indiko Clinical and Specialty Chemistry System, Thermo Scientific, Fremont, CA, USA). EtG-I test results are obtained within 20 min, and the immunoassay provides a semi-quantitative assessment of drinking, allowing for use of multiple cut-off levels. Overall, the rapid return of results and ease-of-use when combined with a simple benchtop analyzer make EtG-I a promising tool for clinical researchers and clinicians seeking an alcohol biomarker test with improved utility.
Prior research with EtG has suggested the use of a higher cut-off level (i.e. 500 ng/ml) due to concerns about positive EtG tests from incidental alcohol exposure; e.g. using alcohol-based hand sanitizer (16,17). However, higher cut-off levels may result in under-detection of drinking (18,19) and few studies have examined EtG at low cut-off levels (i.e. 100 and 250 ng/ml). EtG analysis via mass spectrometry (EtG-MS) has been previously used to detect low to high levels of alcohol use (19,20). Previous studies comparing EtG-I to EtG-MS observed moderate to high levels of agreement (14,16,21,22). While some of these studies investigated the low cut-off level of 100 ng/ml, they differ from the current study in that samples were collected from non-alcohol-dependent populations (16,22). Alcohol-dependent populations consume alcohol more frequently and in greater quantities than the general population and are thus more likely to produce EtG frequently, and sustain a higher level of the metabolite in their bodies. Other studies used a higher cut-off level (500 ng/ml), but also investigated non-alcohol-dependent populations (21) or participants currently undergoing alcohol detox (14). EtG-I analyses in these studies were conducted by laboratory technicians on the advanced Hitachi 902 Automatic (22) and Olympus AU640 (14,16,21) analyzers.
The aim of the current study was to examine agreement of EtG-I tests conducted at an outpatient addiction clinic as part of an experimental research study and EtG-MS tests conducted at a SAMHSA-certified drug testing laboratory with alcohol-dependent adults with serious mental illnesses. Agreement was defined as the number of samples where EtG-I and EtG-MS results fell below or above a specific cut-off level. Agreement was compared across low (100 and 250 ng/ml) and higher, more commonly used (500 ng/ml) cut-off levels. We hypothesized that the level of agreement between EtG-I and EtG-MS would be high across cut-off levels. To our knowledge, the present study is the first to evaluate the agreement between EtG-I and EtG-MS tests at low cut-off levels (below 500 ng/ml) when collected from individuals with alcohol dependence receiving outpatient treatment using instrumentation (Thermo Scientific Indiko) operated by staff with brief training from the immunoassay manufacturer. High agreement between EtG-I and EtG-MS in the present study would suggest that EtG-I is a valuable clinical tool for addiction treatment providers.
Materials and methods
Participants
Participants were 40 adults with DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) diagnoses of alcohol dependence and serious mental illness (i.e. schizophrenia, bipolar, or re-occurring major depression) receiving services at a certified outpatient addiction clinic located in a community mental health center in Seattle, Washington. All participants were enrolled in a randomized controlled trial of contingency management for alcohol use (clinicaltrials.gov identifier: NCT01567943). Participants were randomly assigned to one of two treatment groups, including contingency management (CM) and a non-contingent control group (NC). Participants in the CM group received prize draws only upon submission of alcohol-negative urine and breath samples. The CM group also received gift cards based on outpatient addiction treatment group attendance. The NC group was semi-yoked, receiving a number of prize draws calculated based on the prize draws earned by the CM group in the previous week. The NC group also received gift cards based on the amount of gift cards earned by the CM group for attending outpatient addiction treatment in the previous week. The average age of participants was 46.1 years (SD=13.2), and the majority of participants were male (67.5%) and white (55%). Other races included Black (30%), Hispanic (5%) Multiracial (5%), American Indian or Alaska Native (2.5%), and Other (2.5%). Primary psychiatric diagnoses were 68.4% psychotic disorder and 31.6% mood disorder.
Study procedures
Urine samples were collected three times per week for up to 16 weeks. Participants were asked to refrain from using alcohol-based hand sanitizer, mouthwash, and other products containing ethanol. EtG-I testing was conducted on the day of sample collection onsite at the outpatient addiction clinic by clinical research staff using spectrophotometry on a Thermo Scientific Indiko. DRI EtG semi-quantitative enzyme immunoassay tests were conducted using EtG 100, 500, 1000 and 2000 ng/ml, and Negative calibrators and EtG 100 and 375 ng/ml controls. Antibody/Substrate and Enzyme Conjugate reagents were used. When controls deviated more than 25% from given concentrations, the analyzer was recalibrated and controls were rerun before immunoassays were conducted. To prevent bacterial hydrolysis, all samples, calibrators, controls, and reagents were refrigerated at 4 °C until analyses were conducted. The analyzer was calibrated once per week and samples were collected and analyzed on Mondays, Wednesdays and Fridays. Eight drops of urine from each sample were required for analysis. A cut-off level of 100 ng/ml was used for the contingency management intervention. EtG-I is linear up to 2000 ng/ml, with an analytical range of 0–2000 ng/ml. Dilution was conducted according to manufacturer’s guidelines when sample results displayed an error message indicating high absorbance and was only required for 10 samples out of the total 1068 samples. Samples requiring dilution were rerun using four drops Negative calibrator as the diluent and four drops of the urine sample. Creatinine was not analyzed. Each analysis and calibration procedure took 10–20 min. One to five urine samples from each participant were randomly selected for confirmatory analysis based on a random number list generated at study startup, rather than all EtG-I positive samples. Samples were refrigerated at 4 °C for less than 48 h before being sent via overnight courier to a local drug testing laboratory (Sterling Reference Laboratories) for EtG-MS and EtS-MS analysis on an AB Sciex triple quadrupole tandem mass spectrometer (Framingham, MA, USA). For EtG-MS, the limit of detection (LOD) was 50 ng/ml, the lower limit of quantification (LLOQ) was 100 ng/ml, and the calibrators used were 250, 1000 and 2500 ng/ml. For EtG-I, the LOD was 0 ng/ml, the LLOQ was <100 ng/ml, and the calibrators used were Negative, 100, 500, 1000 and 2000 ng/ml. As demonstrated in Table 1, confirmatory analyses were conducted on roughly equal (according to the 100 ng/ml cut-off level) numbers of positive and negative samples. Study procedures were approved by the University of Washington Institutional Review Board.
Table 1.
Agreement between EtG immunoassay (EtG-I) and EtG mass spectrometry (EtG-MS) across multiple cut-offs in 149 urine samples (n=40).
| EtG-MS − | EtG-MS + | Agreement | Kappa | |
|---|---|---|---|---|
| 100 ng/ml | ||||
| EtG-I − | 73 | 1 | 90.6% | 0.83* |
| EtG-I + | 13 | 62 | ||
| 250 ng/ml | ||||
| EtG-I − | 87 | 3 | 96.6% | 0.92* |
| EtG-I + | 2 | 57 | ||
| 500 ng/ml | ||||
| EtG-I − | 94 | 3 | 96.6% | 0.91* |
| EtG-I + | 2 | 50 | ||
Kappa criteria was statistically significant at p<0.05. Agreement is indicated by the number of positive and negative EtG-I and EtG-MS tests that were both above or below each cut-off level.
Data analysis
Percentage agreement between EtG-I and EtG-MS analyses was calculated at 100, 250 and 500 ng/ml cut-off levels. Interrater agreement was calculated using Kappa coefficients, and interpreted using Cicchetti (23) standards. Kappa criteria for significance was set at p<0.05. The median EtG values for EtG-I and EtG-MS were also calculated. Results of both variables were recoded, forcing a similar distribution of 100– 2000 ng/ml for both variables. The overall association between EtG-I and EtG-MS was calculated using the non-parametric Kendall’s tau-b correlation coefficient. For the correlational analysis, both variables were again recoded to a distribution of 100–2000. Values that fell below 100 or above 2000 ng/ml were recoded to 100 and 2000 ng/ml, respectively. Statistical analyses were conducted with the gmodels package (24) in R-3.0.1 (25).
Results
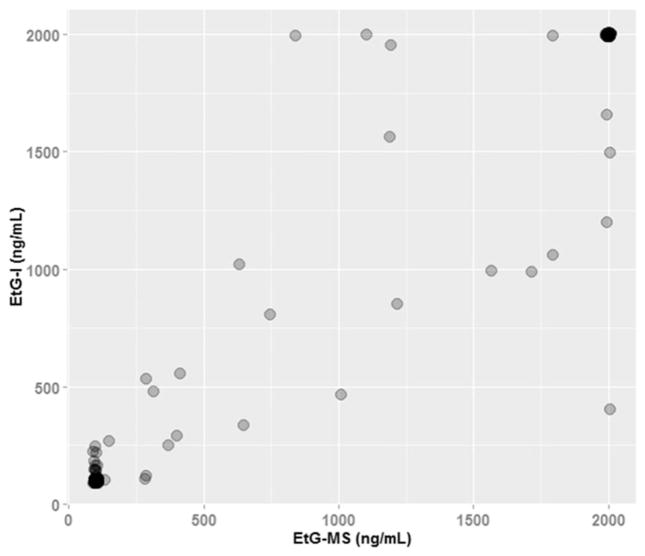
Across all data analyzed for this study, the median value of EtG-I was 385.2 ng/ml and the median value of EtG-MS was 305.5 ng/ml. Figure 1 describes the association between EtG-I and EtG-MS for all samples that fell between 100 and 2000 ng/ml. At 100 ng/ml, 73 observations had perfect agreement; at 2000 ng/ml, 36 observations had perfect agreement. The overall correlation between EtG-I and EtG-MS was strong, rτ=0.91, p<0.001. Agreement between clinic-based EtG-I and lab-based EtG-MS was high across all three cut-off levels. At the 100 ng/ml cut-off level, 90.6% of EtG-I and EtG-MS results were either both above or below the cut-off level, indicating agreement. This agreement was 96.6% at the 250 and 500 ng/ml cut-off levels (see Table 1). In terms of disagreement, EtG-I was on average 54.7 ng/ml (SD=60.4) above or below the cut-off level at 100 ng/ml, 53.5 ng/ml (SD=72.7) at 250 ng/ml, and 41.3 ng/ml (SD=80.1) at 500 ng/ml. Disagreements did not appear to be associated with gender, treatment group, or body mass index (a number calculated from a person’s weight and height that is a reliable indicator for assessing obesity for most people). Six of the 24 total disagreements were produced by a sole participant. This participant’s samples were unaffected by bacterial hydrolysis or chloral hydrate, two possible explanations for EtG false positives. To our knowledge, individual characteristics of this participant (BMI=26.6, male, white, aged 64 years) would not cause false positive EtG results. The remaining disagreements were produced by unique participants.
Figure 1.
Scatterplot of EtG-mass spectrometry and EtG-immunoassay results of 149 urine samples (n=40). Note. At 100 ng/ml, 73 observations had perfect agreement; at 2000 ng/ml, 36 observations had perfect agreement. The overall correlation between EtG-I and EtG-MS was rτ=0.91, p<0.001. Upon exclusion of the samples with perfect agreement at ≤100 ng/ml and ≥2000 ng/ml, correlation between the remaining 40 samples was rτ=0.64, p<0.001.
Discussion
Results of this study indicate high levels of agreement between EtG-I tests conducted at an outpatient addiction clinic and EtG-MS tests conducted at a certified drug-testing laboratory. Agreement was high at both the commonly used 500 ng/ml cut-off level and low cut-off levels of 100 and 250 ng/ml. This is consistent with agreement between onsite immunoassay drug tests used in clinical care and MS tests (18). When disagreements occurred they were small in magnitude (M=49.8 ng/ml, SD=84.77). Therefore, EtG-I appears to be a reliable objective measure of alcohol use that can be used by addiction clinics to monitor alcohol use.
This study has several limitations: First, tests did not include regular dilution or creatinine analysis. We forwent these procedures to emulate the way tests may be conducted by actual clinicians in an outpatient addiction clinic setting. Second, as participants were diagnosed with serious mental illnesses and alcohol dependence, it is possible these results may not be generalizable to other populations. To our knowledge, there are no documented interactions between psychiatric medications prescribed to some participants (most commonly quetiapine, trazodone, and hydroxizine) and EtG. The only medication related to EtG false positives is chloral hydrate, which was not reported by participants. Third, temperature stability and microbial activity in samples are potential issues in immunoassay testing, but were minimized via refrigeration of samples (26). Fourth, in order to attain accreditation to conduct onsite EtG-I analyses and bill insurance providers if desired, addiction treatment agencies would be required to attain status as a moderate complexity laboratory, requiring specialized lab documentation and dedicated laboratory personnel.
Despite these limitations, results of the present study suggest that EtG-I tests, which can assess alcohol use up to five days prior, can be conducted in an outpatient addiction clinic. Importantly, low cut-off levels (100 and 250 ng/ml) may be more effective at identifying low levels of drinking than the higher and more commonly used 500 ng/ml cut-off level (16,27). However, results at the 100 ng/ml cut-off level should be interpreted with caution and used in conjunction with self-reported drinking data, as there is a higher rate of false positives at this level. This cut-off level may be most useful in abstinence-based programs interested in detecting any alcohol use without serious consequences for positive tests, rather than forensic settings. The low cut-off level of 250 ng/ml may be optimal for use in clinical or forensic settings as its agreement with EtG-MS is equal to that of the 500 ng/ml cut-off level that is typically used by reference laboratories. It is recommended that clinicians and researchers interested in using EtG-I use this information to select the appropriate cut-off level for their purposes. Based on our findings and its ease-of-use, EtG-I appears to be well suited to fulfill the role of a rapid and non-invasive alcohol biomarker that can be used in clinical research or outpatient addiction treatment settings.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper. The current study was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism, (R01 AA020248, PI: McDonell). NIAAA had no input into the data analysis, interpretation or conclusions of this study.
References
- 1.Chermack ST, Roll J, Reilly M, Davis L, Kilaru U, Grabowski J. Comparison of patient self-reports and urinalysis results obtained under naturalistic methadone treatment conditions. Drug Alcohol Depend. 2000;59:43–49. doi: 10.1016/s0376-8716(99)00106-4. [DOI] [PubMed] [Google Scholar]
- 2.Ries RK, Dyck DG, Short R, Srebnik D, Snowden M, Comtois KA. Use of case manager ratings and weekly urine toxicology tests among outpatients with dual diagnoses. Psychiatr Serv. 2002;53:764–766. doi: 10.1176/appi.ps.53.6.764. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration (SAMHSA) Treatment episode dataset 1999–2009: National admissions to substance abuse treatment services. 2011 [Google Scholar]
- 5.Zuba D. Accuracy and reliability of breath alcohol testing by hand-held electrochemical analysers. Forensic Sci Int. 2008;178:29–33. doi: 10.1016/j.forsciint.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Gurvich EM, Kenna GA, Leggio L. Use of novel technology-based techniques to improve alcohol-related outcomes in clinical trials. Alcohol Alcoholism. 2013;48:712–719. doi: 10.1093/alcalc/agt134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg DM. Structural, functional, and clinical aspects of gamma-glutamyltransferase. CRC Crit Rev Clin Lab Sci. 1980;12:1–5. doi: 10.3109/10408368009108725. [DOI] [PubMed] [Google Scholar]
- 8.Rosalki SB. Gamma-glutamyl transpeptidase. Adv Clin Chem. 1975;17:53–107. doi: 10.1016/s0065-2423(08)60248-6. [DOI] [PubMed] [Google Scholar]
- 9.Helander A, Peter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcoholism. 2012;47:552–557. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- 10.Varga A, Hansson P, Johnson G, Alling C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clin Chim Acta. 2000;299:141–150. doi: 10.1016/s0009-8981(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 11.Wurst F, Thon N, Aradottir S, Hartmann S, Wiesbeck G, Lesch O, Skala K, et al. Phosphatidylethanol: normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addict Biol. 2010;15:88–95. doi: 10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 12.Helander A, Beck O. Ethyl sulfate: a metabolite of ethanol in humans and a potential biomarker of acute alcohol intake. J Anal Toxicol. 2005;29:270–274. doi: 10.1093/jat/29.5.270. [DOI] [PubMed] [Google Scholar]
- 13.Wurst F, Dresen S, Allen JP, Wiesbeck G, Graf M, Weinmann W. Ethyl sulphate: a direct ethanol metabolite reflecting recent alcohol consumption. Addiction. 2006;101:204–211. doi: 10.1111/j.1360-0443.2005.01245.x. [DOI] [PubMed] [Google Scholar]
- 14.Helander A, Böttcher M, Fehr C, Dahmen N, Beck O. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcoholism. 2008;44:55–61. doi: 10.1093/alcalc/agn084. [DOI] [PubMed] [Google Scholar]
- 15.Crunelle CL, Yegles M, van Nuijs AL, Covaci A, De Doncker M, Maudens KE, Sabbe B, et al. Hair ethyl glucuronide levels as a marker for alcohol use and abuse: a review of the current state of the art. Drug Alcohol Depend. 2014;134:1–11. doi: 10.1016/j.drugalcdep.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Böttcher M, Beck O, Helander A. Evaluation of a new immunoassay for urinary ethyl glucuronide testing. Alcohol Alcoholism. 2008;43:46–48. doi: 10.1093/alcalc/agm153. [DOI] [PubMed] [Google Scholar]
- 17.Substance Abuse and Mental Health Services Administration (SAMHSA) The role of biomarkers in the treatment of alcohol use disorders. SAMHSA Advisory. 2012;11 [Google Scholar]
- 18.McDonell MG, Srebnik D, Angelo F, Sugar AM, Howell D, Rainey C, Roll J, et al. Evaluation of ethyl glucuronide immunoassay urinalysis in five alcohol dependent outpatients. Am J Addiction. 2011;20:482–484. doi: 10.1111/j.1521-0391.2011.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosano TG, Lin J. Ethyl glucuronide excretion in humans following oral administration of and dermal exposure to ethanol. J Anal Toxicol. 2008;32:594–600. doi: 10.1093/jat/32.8.594. [DOI] [PubMed] [Google Scholar]
- 20.Albermann ME, Musshoff F, Madea B. A high-performance liquid chromatographic-tandem mass spectrometric method for the determination of ethyl glucuronide and ethyl sulfate in urine validated according to forensic guidelines. J Chromatogr Sci. 2012;50:51–56. doi: 10.1093/chromsci/bmr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crews B, Latyshev S, Mikel C, Almazan P, West R, Pesce A, West C. Improved detection of ethyl glucuronide and ethyl sulfate in a pain management population using high-throughput LC-MS/ MS. J Opioid Manag. 2010;6:415–421. doi: 10.5055/jom.2010.0039. [DOI] [PubMed] [Google Scholar]
- 22.Turfus SC, Vo T, Niehaus N, Gerostamoulos D, Beyer J. An evaluation of the DRI-ETG EIA method for the determination of ethyl glucuronide concentrations in clinical and post-mortem urine. Drug Test Anal. 2013;5:439–445. doi: 10.1002/dta.414. [DOI] [PubMed] [Google Scholar]
- 23.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. [Google Scholar]
- 24.Warnes GR. [last accessed 1 April 2014];gmodels: various R programming tools for model fitting. Computer software manual. Available from: http://CRAN.R-project.org/package=gmodels (R package version 2.15.4.1)
- 25.R Core Team. R: a language and environment for statistical computing [Computer software manual] Vienna, Austria: 2013. [last accessed 1April 2014]. Available from: http://www.R-project.org/ [Google Scholar]
- 26.Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728–1730. doi: 10.1373/clinchem.2005.051565. [DOI] [PubMed] [Google Scholar]
- 27.Jatlow P, O’Malley S. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968–975. doi: 10.1111/j.1530-0277.2010.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]