Abstract
Colonization by Lactobacillus in the female genital tract is thought to be critical for maintaining genital health. However, little is known about how genital microbiota influence host immune function and modulate disease susceptibility. We studied a cohort of asymptomatic young South African women and found that the majority of participants had genital communities with low Lactobacillus abundance and high ecological diversity. High diversity communities strongly correlated with genital pro-inflammatory cytokine concentrations in both cross-sectional and longitudinal analyses. Transcriptional profiling suggested that genital antigen presenting cells sense gram-negative bacterial products in situ via Toll-like receptor 4 signaling, contributing to genital inflammation through activation of the NF-κB signaling pathway and recruitment of lymphocytes by chemokine production. Our study proposes a mechanism by which cervicovaginal microbiota impact genital inflammation and thereby may affect a woman's reproductive health, including her risk of acquiring HIV.
Introduction
The female genital tract (FGT) maintains a finely-tuned immune response that balances reproductive tolerance with protection against genital infections. While inflammatory responses are beneficial and required to effectively eliminate several sexually transmitted infections (STIs), the presence of elevated genital inflammation in women prior to HIV exposure paradoxically increases the risk of disease acquisition (Lajoie et al., 2012; Morrison et al., 2014). Studies of the microbial causes of this inflammation have primarily focused on established pathogens such as Chlamydia trachomatis, Neisseria gonorrhoeae, HSV-2, and Trichomonas vaginalis. The potential role of commensal cervicovaginal bacteria, which number ~108 per gram of vaginal fluid (Delaney and Onderdonk, 2001), in modulating immune responses in the FGT is largely unknown.
The bacterial microbiome of the healthy FGT is thought to be exceedingly simple, predominated by a single Lactobacillus species in the majority of white premenopausal women (Chaban et al., 2014; Drell et al., 2013; Huttenhower, 2012; Ravel et al., 2011; Zhou et al., 2007). These Lactobacilli benefit the host by inhibiting the growth of pathogenic bacteria and fungi through the production of bacteriocins, lactic acid, and hydrogen peroxide (Aroutcheva et al., 2001; Ghartey et al., 2014). Bacterial vaginosis (BV) is an alteration of microbial communities in this ecological niche in which Lactobacilli are replaced by Gardnerella and Mobiluncus species. BV is associated with a 1.5–2-fold increased risk of acquiring C. trachomatis, N. gonorrhoeae, T. vaginalis, and HIV (Atashili et al., 2008; Brotman et al., 2010), which raises the question of whether specific genital microbial communities in asymptomatic women may more broadly modulate disease susceptibility.
To determine the contribution of cervicovaginal microbiota to genital inflammation, we evaluated a cohort of asymptomatic HIV-negative young South African women. Only a minority of study participants had Lactobacillus-dominant cervicovaginal communities, despite a lack of clinical symptoms, redefining what is considered to be normal in this region. We found a strong in vivo relationship between high-diversity bacterial communities lacking Lactobacillus dominance and genital pro-inflammatory cytokine levels. We identified specific bacterial species within the high-diversity communities that elicit pro-inflammatory cytokines and provide evidence that endocervical antigen presenting cells (APCs) sense microbial lipopolysaccharide (LPS) and produce a myriad of pro-inflammatory cytokines and T cell chemoattractants. Our data provide important insight into the mechanism by which bacterial microbiota impact host immunity and suggest potential interventions to reduce disease susceptibility in women in sub-Saharan Africa.
Results
A minority of South African women in FRESH have Lactobacillus dominant genital communities
We began by assessing the baseline bacterial microbiome in participants from the FRESH (Females Rising through Education, Support and Health) study, a cohort enrolling HIV-negative, 18- to 23-year-old, black South African women. Following isolation of nucleic acid from cervical swabs, we sequenced variable region 4 (V4) of the bacterial 16S rRNA gene to assess bacterial abundances (Caporaso et al., 2012) (Figure 1A). We clustered the observed bacterial communities into four distinct community types based on the dominant bacterial species, herein referred to as “cervicotypes” (CTs). CT1 was primarily composed of non-iners Lactobacillus (i.e. higher percentage of sequencing reads from non-iners Lactobacillus than L. iners, Gardnerella, or Prevotella); CT2 was Lactobacillus iners dominant; CT3 had Gardnerella dominance; and CT4 lacked a consistent dominant species but communities all included Prevotella (Figure 1A and S1). Visualization of the same samples using a principal coordinates plot, which represents the phylogenetic distance between samples, supported the dominance-based clustering, though CT3 and CT4 are a continuum (Figure 1B).
Figure 1.
16S rRNA sequencing analysis of cervical swabs reveals low Lactobacillus abundance and four distinct bacterial community structures. (A) Heatmap of bacterial taxa identified by 16S V4 sequencing of cervical swabs collected from 94 women. Cervicotypes (CTs) were determined based on the dominant species: Lactobacillus crispatus (CT1), L. iners (CT2), Gardnerella (CT3), and mixed microflora containing Prevotella (CT4). Nugent scores and bacterial alpha diversity are also shown. (B) Principal coordinates analysis using the weighted UniFrac distance metric on the same 94 samples, colored by CT. See also Figure S1 and Table S1.
We found that only 37% of participants had Lactobacillus dominant cervicovaginal communities. This is in contrast to published reports of white and black women in developed countries in which 90% and 62% of women respectively demonstrated Lactobacillus dominance (Ravel et al., 2011; Zhou et al., 2007). Of those women with Lactobacillus dominance in our study, 77% primarily had Lactobacillus iners (CT2). L. iners is biologically distinct from other Lactobacilli due to its unique adaptation to survive with diverse community members (Macklaim et al., 2013) and greater pathogenic potential (Doerflinger et al., 2014; Rampersaud et al., 2011).
Of the 63% of women in our cohort who did not have Lactobacillus dominance, 45% had Gardnerella dominant communities (CT3). The remaining 55% of women did not have a consistent predominant bacterial taxon, though each community was found to have at least 10% Prevotella abundance (CT4). Additionally, only half of the women in CT4 had BV, as measured by the Nugent criteria, which assesses a Gram stained vaginal wet prep based on bacterial morphology; none of these women reported symptoms. Overall, the bacterial community structures seen in these asymptomatic young South African women were characterized by a predominance of communities with high ecological diversity and low abundance of non-iners Lactobacillus.
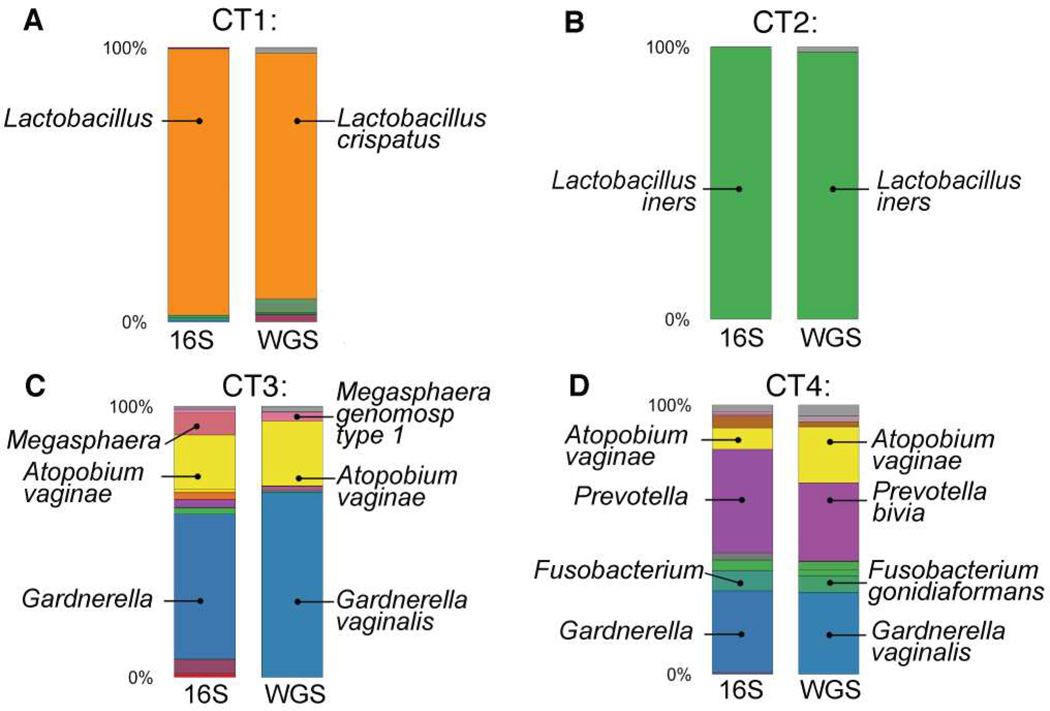
We next confirmed the 16S rRNA sequencing results and resolved specific community members at a species level by performing whole-genome shotgun (WGS) sequencing on cervical bacterial DNA from a subset of 12 women representing all four CTs (Figure S2A). WGS has proven useful for species-level taxonomic resolution when different bacterial species share nearly identical 16S rRNA variable regions (Jaspers and Overmann, 2004; Segata et al., 2012). The taxonomic classification by WGS had good agreement with 16S-based classification and STI PCR results (Figure 2 and S2B). The additional species-level resolution provided by WGS also revealed that both of the women with CT1 analyzed by WGS had Lactobacillus crispatus dominant microflora (Figure 2A and S2B). We extended the analysis to every woman within CT1 using taxonomic identification by oligotyping, a method which detects patterns in subtle nucleotide variations within the 16S gene (Eren et al., 2011), and found that nearly all women had identical sequences that aligned to L. crispatus (Figure S2C), further validating that CT1 was mostly comprised of L. crispatus. We therefore demonstrated close agreement between taxonomic classifications obtained using 16S and WGS, and further used WGS to provide species level resolution of specific bacterial community members.
Figure 2.
16S V4 sequencing and whole-genome shotgun sequencing (WGS) identify very similar bacterial abundances for all CTs, with higher resolution taxonomic identification provided by WGS. Participant #6 represents CT1 (A), #1 represents CT2 (B), #7 represents CT3 (C), #11 represents CT4 (D). See also Figure S2.
Cervicovaginal bacterial communities are not associated with sexually transmitted infections (STIs), hormonal contraceptive usage, or sexual behavior
Women with BV have been shown to have a higher incidence and prevalence of STIs (Brotman et al., 2010; Wiesenfeld et al., 2003), be less likely to use hormonal contraceptives (Bradshaw et al., 2013a), and be more likely to engage in high-risk sexual behavior (Bradshaw et al., 2013b). However, we did not find a difference in the prevalence of an active STI or STI symptoms between cervicotypes, though the association with N. gonorrhoeae was difficult to ascertain due to its low prevalence in this group (Table S1). Additionally, we saw no difference in hormonal contraceptive method, condom use, or reported sexual activity (Table S1). Thus, there was no evidence that the high ecological diversity in CT4 was due to behavioral, infectious, or exogenous hormonal etiologies.
Large variation in baseline genital immune activation is not explained by STIs
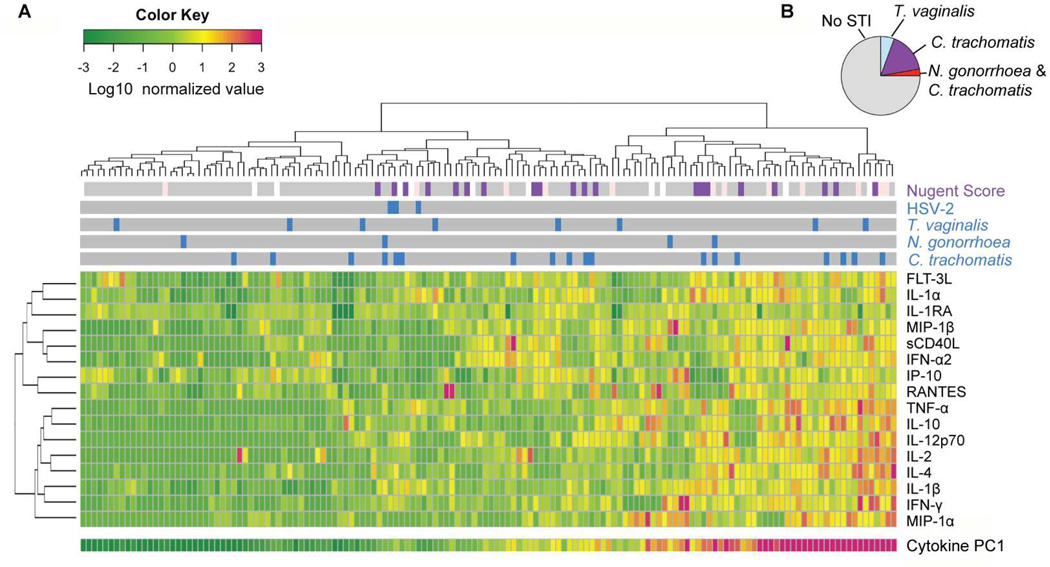
Genital immune activation has been described as an important risk factor for disease of the FGT, such as obstetric complications (Inglis et al., 1994; Lockwood et al., 1994) and HIV acquisition (Lajoie et al., 2012; Morrison et al., 2014). We therefore assessed the cohort’s baseline genital immune activation by measuring the concentrations of 17 soluble cytokines in cervicovaginal lavage (CVL) fluid from all participants who had completed at least one mucosal sampling. The cytokine panel included canonical pro-inflammatory cytokines (IL-1α, IL-1β, TNF-α), interferons (IFN-γ, IFN-α2), chemokines (MIP-1α, MIP-1β, RANTES, IP-10, IL-8), regulators of inflammation (IL-1 receptor antagonist (IL-1RA), IL-10), differentiation markers (IL-4, IL-12p70), and activation and proliferation markers (soluble CD40L, FLT-3L, IL-2). There was a wide range in CVL pro-inflammatory cytokine levels, with over a 1,000-fold difference in some cytokines (e.g. IL-8 and IL-1α) among participants. We performed unsupervised clustering to visualize inflammatory patterns (Figure 3A) and found strong positive correlations (Spearman’s ρ > 0.4) between 45% of cytokine pairs (Figure S3A).
Figure 3.
Asymptomatic women display a broad range of baseline genital inflammation that is not explained by STIs. (A) Heatmap of cervicovaginal lavage cytokines from 146 women, each represented by a column. Nugent scores and active STIs (blue: C. trachomatis, N. gonorrhoea, T. vaginalis, and/or HSV-2 positive; gray: negative for that STI) are also displayed. Principal component (PC) analysis was performed on the normalized cytokine concentrations and the first PC (PC1) explained 41% of variation. (B) Pie chart of the STI prevalence in women in the highest quartile of inflammation (n=35). See also Figure S3 and Table S2.
To reduce the dimensionality of the cytokine dataset to a smaller number of covarying components, we performed principal component analysis (PCA) on the normalized cytokine concentrations. We found that the first principal component (PC1) explained 41% of the variation in cytokines; PC1 represented the general presence of inflammatory cytokines, with high and nearly equal loading by all cytokines except for IL-1RA and IP-10, which had a negligible contribution (Figure S3B).
We next asked whether women with the highest levels of cytokine inflammation had active STIs. Chlamydia trachomatis was the most prevalent STI in this cohort, with 13% of women testing positive at the first visit. We found that women with C. trachomatis had higher inflammation than women without an STI (p=0.0226, Mann-Whitney test) but saw no trend with N. gonorrhoeae (Figure S3C–E), consistent with previous studies (Hedges et al., 1998; Rasmussen et al., 1997). Of the 35 women in the top quartile of inflammation, 75% of them did not have a detectable STI (Figure 3B). Cytokine levels also did not correlate with sexual frequency, condom use, hormonal contraception use, or self-reported STI symptoms (Fisher’s exact test with p=0.05 significance level, Table S2). Thus, the cause of elevated genital inflammation in the majority of women remained unexplained.
High-diversity bacterial communities are strongly associated with pro-inflammatory genital cytokines
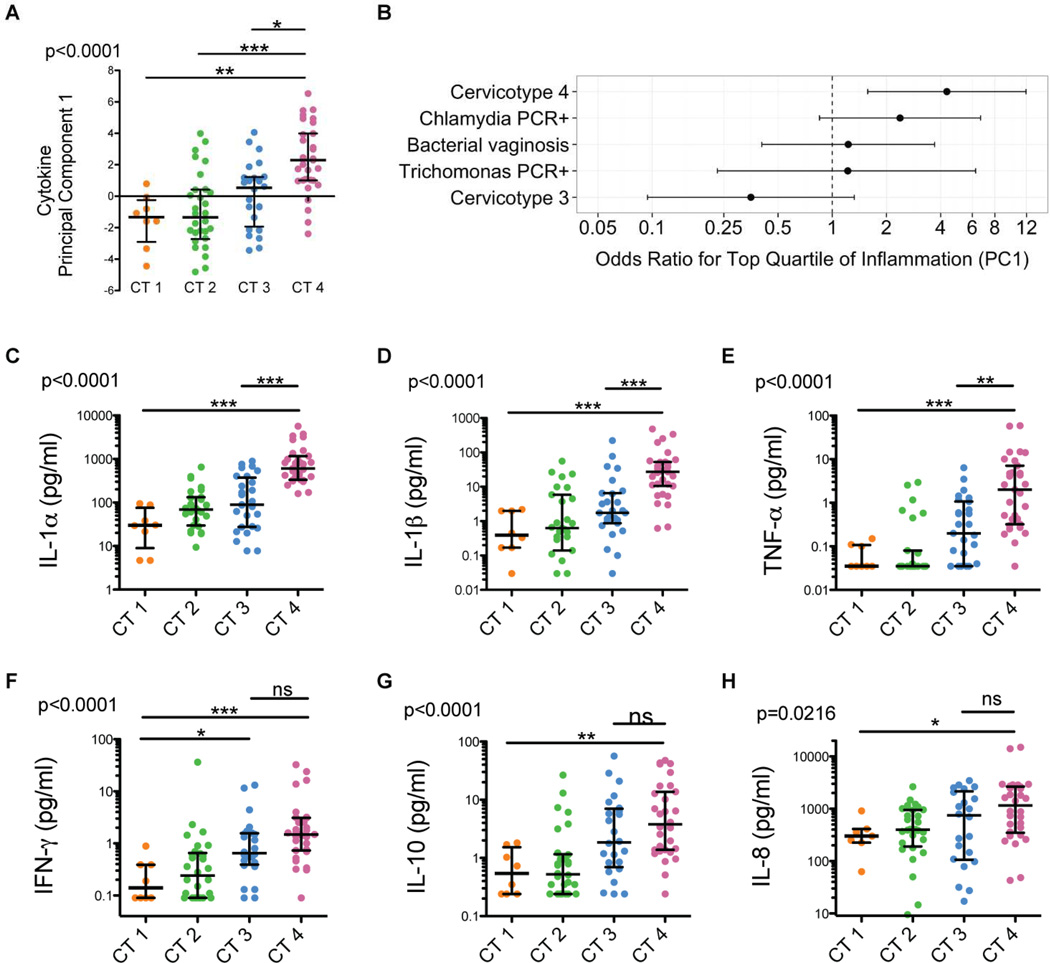
To assess whether microbial communities might account for non-STI associated genital inflammation, we compared genital pro-inflammatory cytokine levels among CTs, with the hypothesis that Lactobacillus crispatus dominant CT1 would have the lowest inflammation due to the beneficial effects attributed to L. crispatus (Ghartey et al., 2014). To determine aggregate differences in cytokine concentrations, we first examined the value of cytokine PC1 in each CT. There was a highly significant difference across the cervicotypes (p<0.0001); between CT1 and CT4 (p<0.01); CT2 and CT4 (p<0.001), and between CT3 and CT4 (p<0.05) (Kruskal-Wallis with post-test, Figure 4A), even after excluding women with any STIs (Figure S4A)
Figure 4.
Genital pro-inflammatory cytokine levels vary significantly with microbial community structure and most strongly correlate with CT4 bacterial communities.
(A) Cytokine PC1 values from women with bacterial communities CT1–4.
(B) Odds ratios and 95% confidence intervals representing the likelihood that a woman with cervicotype 4, an active chlamydia infection, bacterial vaginosis (Nugent score >7), an active Trichomonas infection, or cervicotype 3 is within the top quartile of proinflammatory cytokine levels (as determined by cytokine PC1) versus below the 75th percentile.
(C–H) Cervicovaginal lavage IL-1α, IL-1β, TNF-α, IFN-γ, IL-10, and IL-8 concentrations in women with bacterial communities CT1–4. Median and IQR shown. Significance levels were determined by a Kruskal-Wallis test and asterisks denote post-test significance level (* p < 0.05; ** p < 0.01; *** p < 0.001; n=94). See also Figure S4.
To determine how strongly the presence of CT4, STIs, or BV associated with inflammation (defined by highest quartile of cytokine PC1), we calculated the odds ratios for each and found that only CT4 was significantly associated with cytokine PC1 (OR 4.33, 95% CI: 1.575 –11.92; p=0.0046), though there was a trend for PCR-positive chlamydia (OR 2.380, 95% CI: 0.8497 – 6.666; p=0.1329) (Figure 4B). This indicated that women with CT4 were over four times more likely to have elevated genital pro-inflammatory cytokines than those with CT1–3, and that CT4 was a better predictor of inflammation than STIs or BV.
We next determined which of the cytokines that comprise PC1 were different between CTs. We observed significant increases in IL-1α, IL-1β, TNF-α, IFN-γ (p<0.001, Figure 4C–F), IL-10 (p<0.01, Figure 4G), and IL-8 (p<0.05, Figure 4H), as well as IL-12p70, IL-4, and FLT-3L (p<0.01, Figure S4B–D) in CT4 relative to CT1. Even after excluding women with any STIs, individuals with CT4 still had significantly higher levels of each of these cytokines relative to those with CT1 (Figure S4E–M). We observed a less pronounced increase in cytokines in CT3, with significantly higher IFN-γ than CT1, and a trend towards higher TNF-α, IL-8, and IL-10. Thus, the highly diverse bacterial communities in CT4, and to a lesser extent CT3, were strongly associated with the presence of multiple pro-inflammatory cytokines, suggesting specific genital bacteria could induce a robust local immune response.
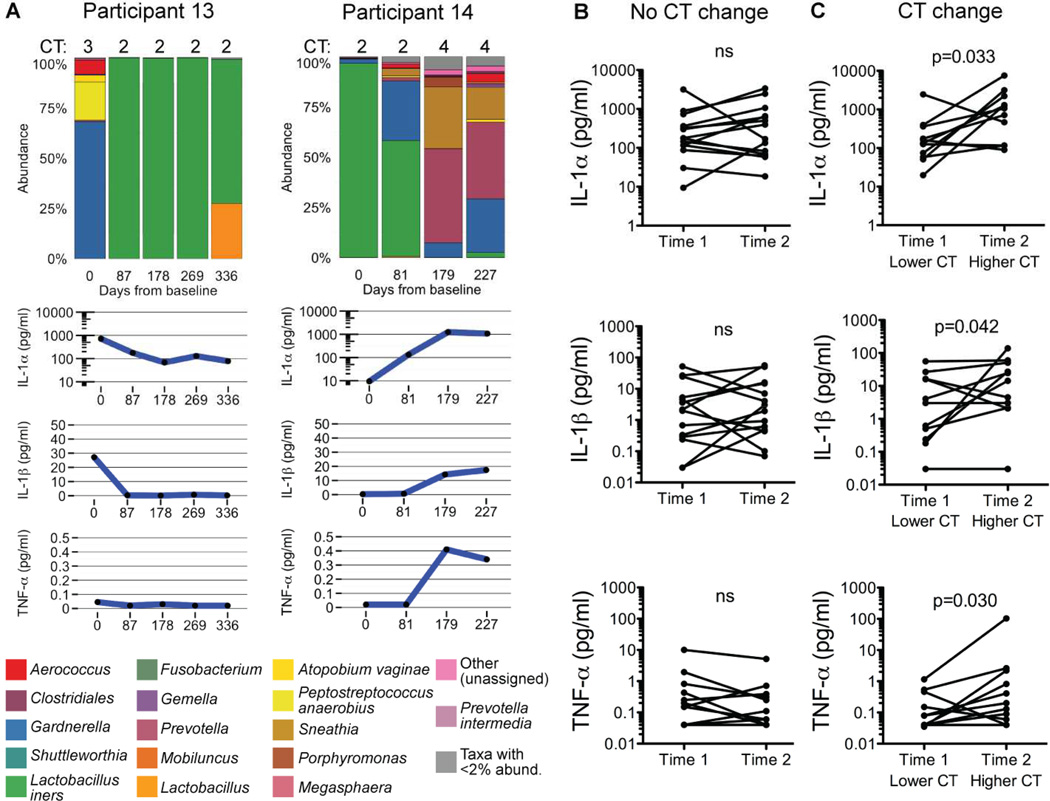
Longitudinal changes in genital microbial communities correlate with pro-inflammatory cytokines
Because of the close association between genital bacterial communities and inflammation in the cross sectional analysis, we assessed the stability of these factors over time. We performed 16S sequencing and measured soluble cytokines on matched longitudinal cervical swabs and CVLs from all women who had been followed for 6–12 months at the time of analysis. In 58% of sequential time points, women remained in the same CT (Figure S5A). Of those who had changes in microbial communities, there was a close association between CTs and IL-1α, IL-1β, and TNF-α, which we focused on because they had the strongest associations with CTs in the cross-sectional analysis. Two representative individuals who experienced CT changes are shown in Figure 5A (also see Figure S5, and associated metadata in Table S3). When we assessed sequential time points for which there was not a CT change, we did not find a significant difference in the canonical pro-inflammatory cytokines (p>0.05, one-tailed Wilcoxon matched pairs test; Figure 5B). However, there were significant increases in IL-1α (p=0.033), IL-1β (p=0.042), and TNF-α (p=0.030) in adjacent time points with transitions between a lower and higher CT (one-tailed Wilcoxon matched pairs test; Figure 5C). Thus, intraindividual changes in genital bacterial communities were tightly linked to inflammatory changes and further support the reciprocal relationship between bacterial communities and genital inflammation.
Figure 5.
Intraindividual longitudinal genital microbiome changes correlate with pro-inflammatory cytokine levels.
(A) Cervicovaginal IL-1α, IL-1β, and TNF-α concentrations with bacterial taxa identified by 16S sequencing from matched longitudinal cervical swabs and CVLs collected from two representative participants, #13 and #14.
(B–C) Cervicovaginal IL-1α, IL-1β, and TNF-α concentrations from serial time points with either no change in CT (B) or a change (C) (one-tailed Wilcoxon matched pairs test). The order of time points was standardized such that the lower cervicotype was entered as the first time point and the higher cervicotype was entered as the second time point, regardless of the actual chronological order. Any transition, e.g. from CT1 to CT2 and from CT3 to CT4, was considered to be a CT change. See also Figure S5 and Table S3.
Identification of bacterial species that cause pro-inflammatory cytokine secretion
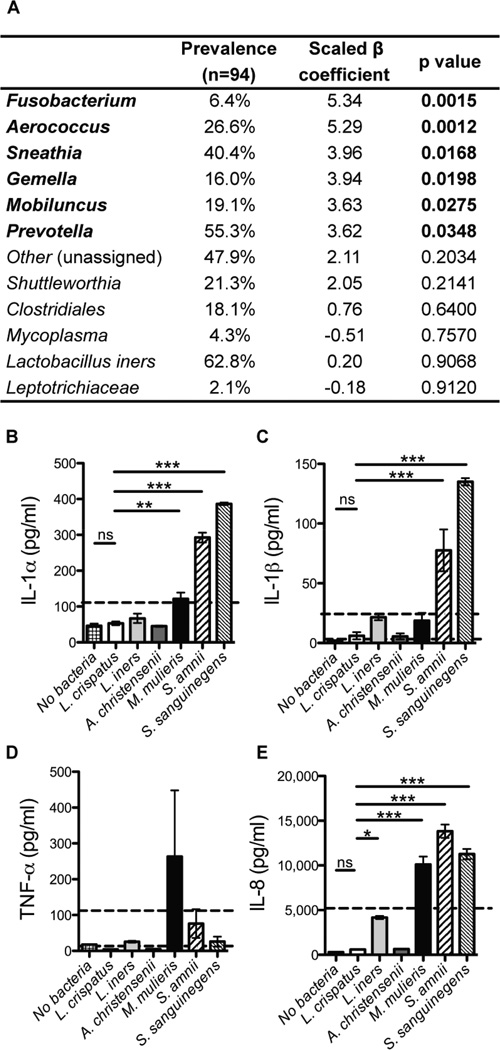
We next asked which bacteria in CT4 were most highly correlated with inflammation. To select bacterial taxa to use in a multivariate model, we performed univariate Spearman correlations between cytokine PC1 and each taxon found with over 0.1% abundance (Figure S6A). The eleven taxa with highly significant correlations (p≤ 0.005) were included as predictor variables in the multivariate analysis. Since many taxa were highly correlated (Figure S6B), the ridge method was used to correct for multicollinearity and revealed six genera significantly correlated with cytokine PC1: Fusobacterium, Aerococcus, Sneathia, Gemella, Mobiluncus, and Prevotella (Figure 6A). Species-level identification from whole-genome sequencing (Figure S2A) provided a set of candidate bacteria to test in vitro.
Figure 6.
Bacterial species highly correlated with pro-inflammatory cytokines in vivo also stimulate vaginal epithelial cells to produce the same cytokines in vitro.
(A) Ridge regression analysis with cytokine PC1 as the response variable and bacterial abundances as the predictor variables. The bacterial prevalence amongst the 94 subjects is also shown; taxa must have an abundance of at least 1% within a sample to be considered present.
(B–E) IL-1α, IL-1β, TNF-α, and IL-8 secretion by vaginal epithelial cells after in vitro application of 15 log10 CFU/cm2 of each bacterial species. MALP-2 (a TLR 2/4 agonist; 25nM) treatment was used as a positive control (value denoted by dashed line). P values were determined by a Dunnett test, compared to Lactobacillus crispatus. Data shown as mean and SEM, with two biological replicates. See also Figure S6 and Table S4.
We used these candidates to test the causative relationship between individual bacterial species and genital inflammation with an in vitro epithelial cell co-culture assay, since epithelial cells play critical roles as anatomic barriers and in the innate immune response to bacteria in the FGT (Fichorova et al., 2011; Wira et al., 2005). We co-cultured immortalized human vaginal epithelial cells with bacterial isolates and measured secreted pro-inflammatory cytokines in the supernatant. Consistent with our in vivo findings, Prevotella amnii, Mobiluncus mulieris, Sneathia amnii, and Sneathia sanguinegens induced higher levels of IL-1α, IL-1β, and IL-8 than Lactobacillus crispatus (Figure 6B–E, Table S4). Prevotella, the genus consistently present in CT4, was able to induce significant cytokine secretion even at an input colony forming unit (CFU) as low as 2 log10 (Table S4). Additionally, L. iners induced moderate IL-8 secretion, consistent with reports that it can have moderate pro-inflammatory activity, while L. crispatus and Aerococcus christensenii did not elicit any cytokine secretion relative to the absence of bacteria. Low levels of TNF-α were detected in response to these bacteria. These data suggest that epithelial cells sense cervicovaginal bacteria in a species specific manner and contribute to the observed pro-inflammatory cytokines found in women in CT4.
Genital antigen presenting cells (APCs) from women with high diversity genital communities are activated by microbial products in vivo
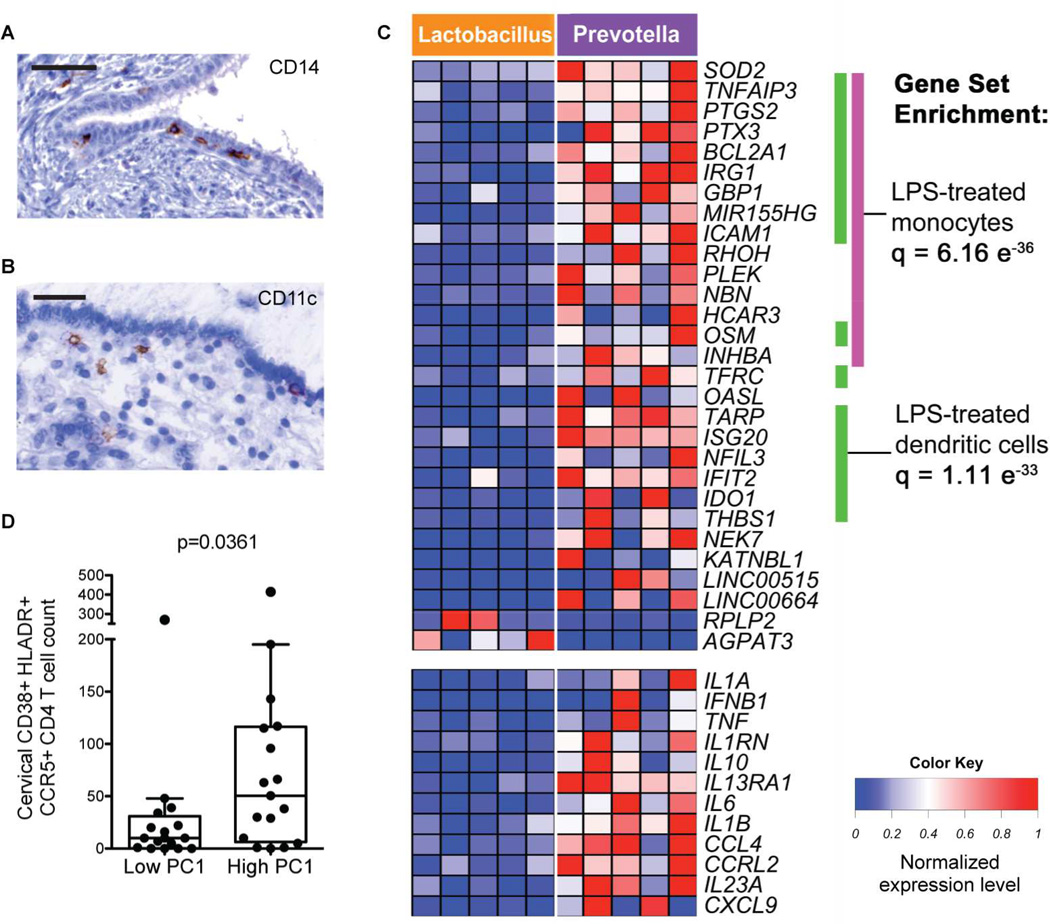
APCs in the cervix are closely associated with the epithelium (Figure 7A and B) and sense microbes in conjunction with epithelial cells (Hickey et al., 2011). Because the context in which APCs are stimulated can have important effects on their phenotype and ability to prime adaptive immune cells (Littman and Pamer, 2011; Ochiel et al., 2010), we performed phenotypic and transcriptional analyses on sorted APCs obtained by cervical cytobrush to understand bacterial sensing in situ (Figure S7A). We compared APCs from women in CT1 or CT2, who had gram-positive Lactobacillus dominance, to those in CT4, additionally excluding anyone with an active STI. Using flow cytometry, we saw no significant difference in the frequency of cervical APC subsets (CD11c+ dendritic cells or CD14+ monocytes or macrophages) (Figure S7B). However, there were pronounced transcriptional differences amongst the cervical APCs; 35 genes were significantly upregulated and 2 were downregulated in women with Prevotella dominant communities relative to those with Lactobacillus dominance (false discovery rate [FDR] <0.05; Figures 7C and S7C). The genes upregulated in CT4 were enriched in NF-κB, Toll-like receptor (TLR), NOD-like receptor, and TNF-α signaling pathways. Gene set enrichment analysis revealed that 20 of the 35 genes upregulated in CT4 APCs were shared with monocytes treated with a low dose of LPS (a TLR4 agonist; q=6.16e-36) and 19 genes were shared with dendritic cells treated with LPS (q=1.11e-33). Ingenuity Pathway Analysis also identified LPS as the most likely upstream regulator to be activated in CT4 APCs (p=9.47e-26). These results are consistent with our bacterial pathway analysis using the microbial DNA shotgun sequences, which demonstrated that LPS biosynthesis was the second most enriched pathway in the high inflammation samples compared to those with low inflammation (Table S5).
Figure 7.
Transcriptional profiling suggests that CT4 bacterial products are sensed by cervical antigen presenting cells (APCs), which contribute to genital inflammation by producing a myriad of pro-inflammatory cytokines and critical T cell chemoattractants.
(A, B) CD14 (A) and CD11c (B) staining of endocervical tissue. Scale bar: 50 µm.
(C) Heatmap of genes differentially expressed in cervical APCs from women with Lactobacillus dominant communities (>95% abundance) (from left to right: 2 in CT1, 3 in CT2) versus those with high Prevotella abundance (>25%) (n=5 in CT4). Women with STIs were excluded. Only genes with a false discovery rate < 0.05 are shown. Gene set enrichment analysis was used to determine statistically significant similarities with annotated gene sets.
(D) Number of live endocervical CD38+ HLADR+ CCR5+ CD4 T cells from women in the highest and lowest quintile of cytokine PC1 (Mann-Whitney, n=16 per group). See also Figure S7 and Table S5.
In addition to LPS, Ingenuity Pathway Analysis also predicted IFN-γ (p=6.31e-24), IL-1-β (p=1.51e-23), and CSF2 (colony stimulating factor 2, p=6.74e-22) to be upstream regulators of the CT4 APCs, which was consistent with higher levels of IFN-γ and IL-1β in the CVLs of women with CT4. Also consistent with the CVL cytokine measurements, CT4 APCs had significantly higher expression of many pro-inflammatory cytokine genes, such as IL1A (5.8-fold), IL1B (4.3-fold), TNF (6.5-fold), IL10 (10.6-fold), IFNB1 (23.4-fold), IL23A (7.3-fold), and IL6 (8.9-fold), compared to CT1 and CT2 APCs (Figure 7C). Of relevance to cellular recruitment, CT4 APCs also upregulated the chemokines CCL4 (MIP-1β, a CCR5 ligand, 5.7-fold), CXCL9 (MIG, a CXCR3 ligand, 9-fold), and CCL8 (MCP-2, a CCR1 and CCR5 ligand, 6.3-fold). These transcriptional differences were not observed when comparing APCs from peripheral blood between CTs (Figure S7C). Thus, the transcriptional profile of cervical APCs indicated that they were responding to a CT4 bacterial product such as LPS and subsequently initiating acute inflammation and lymphocyte recruitment, and that these differences were not a reflection of systemic inflammatory activation.
Women with elevated pro-inflammatory genital cytokines have increased activated cervical HIV target cells
Given the close linkage between CT4 bacteria and genital inflammation, we sought to determine whether pro-inflammatory cytokines would correlate with the presence of activated HIV target cells in the genital tract. Activated CCR5+ CD4+ T cells at the cervical mucosal surface are likely the first susceptible cells to encounter HIV upon sex with an infected partner (Haase, 2010) and support greater HIV replication upon infection than resting cells (Meditz et al., 2011). We found a significantly higher number of activated (CD38+ HLA-DR+) CCR5+ CD4+ T cells in the endocervical canal of women in the top quintile of cytokine PC1 relative to those in the bottom quintile (p=0.0361, Figure 7D), but no significant difference in activated CCR5+ CD4+ T cells in the peripheral blood (Figure S7D). Thus, the observed increase in genital HIV target cells supports the link between high diversity microbial communities and HIV acquisition risk.
Discussion
By integrating metagenomic, immunologic, transcriptional, and behavioral datasets from a population of young South African women, we demonstrated a strong link between high-diversity cervicovaginal bacterial community structures and genital inflammation in vivo in both cross sectional and longitudinal analyses. Women with CT4 were over four times more likely to have high pro-inflammatory genital cytokine levels than those with other CTs and on average had higher levels of genital inflammation than women with BV or STIs. We provide evidence that these high diversity communities in the FGT are sensed by both epithelial cells and APCs. APCs specifically appear to respond to LPS via Toll-like receptor 4 signaling, contributing to genital inflammation through NF-κB activation and recruitment of lymphocytes by chemokine production. This was consistent with the bacterial pathway analysis that demonstrated that LPS biosynthesis was highly enriched in bacterial communities from women with high genital inflammation. This genital inflammation was accompanied by increased numbers of activated HIV infectable CD4 T cells in the cervix, providing a potential cellular link to increased HIV acquisition risk. Our study therefore identified a mechanism by which the predominant cervicovaginal communities found in our cohort of South African women impact genital inflammation and thereby likely modulate HIV acquisition risk.
Only 37% of participants had CT1 or CT2 bacterial communities, clearly indicating that Lactobacillus dominance was not typical in this population. This is in contrast to studies in the U.S., in which 90% of white women were found to have Lactobacillus dominance (Ravel et al., 2011), but consistent with previous studies that use Gram stain analyses to demonstrate a high prevalence of BV in sub-Saharan African women (Jespers et al., 2014; Myer et al., 2005). We reported a common occurrence of low Lactobacillus, high-diversity communities even in the absence of BV and provided a comprehensive characterization of bacterial taxa using high-throughput sequencing.
The etiology of the low Lactobacillus abundance in our cohort remains unexplained. It was not associated with sexual behavior, contraceptive usage or demographic characteristics and we speculate that host genetic factors may play a role. Previous studies demonstrate a lower frequency of L. crispatus and L. jensenii and a higher frequency of Prevotella in black and Hispanic women compared to white and Asian women in the U.S. (Ravel et al., 2011; Zhou et al., 2007). The high diversity vaginal communities seen in black American women show general similarities to those seen in black South African women, specifically with high levels of Prevotella, but the prevalence of these high-diversity communities was approximately half of that observed in South Africans. Studies of African cohorts have also demonstrated a higher intestinal Prevotella abundance than in American and European cohorts (De Filippo et al., 2010; Yatsunenko et al., 2012) suggesting that vaginal seeding from the bacteria-rich rectum (El Aila et al., 2009) may also influence the observed differences. Overall, our findings support the growing understanding that Lactobacillus dominance is not tantamount to a “healthy” female genital tract microbiome in all women (Ravel et al., 2011), and they highlight the value of bacterial characterization in broader geographic regions.
Within CT4, we found that Sneathia sanguinigens, Sneathia amnii, Mobiluncus mulieris, and Prevotella amnii were capable of inducing the secretion of IL-1α, IL-1β, and IL-8 from human vaginal epithelial cells in vitro. Aerococcus was highly associated with inflammation in vivo but did not induce cytokine secretion in vitro, possibly due to the particular bacterial isolate that we tested or because it is merely well-adapted to surviving in inflamed tissue but not a primary driver of inflammation. We did not test Fusobacterium, but Fusobacterium nucleatum has been shown to be pro-inflammatory and tumorigenic in the gut (Kostic et al., 2013). Overall, several CT4 bacteria were capable of directly inducing pro-inflammatory cytokine production from epithelial cells in vitro. The strong correlation between bacterial communities and genital inflammation that we demonstrated may help explain the increased genital immune activation seen in other sub-Saharan cohorts compared to those in the U.S. (Cohen et al., 2010). While the proportion of American women with high diversity communities may be lower, preliminary results demonstrated that they were also associated with elevated pro-inflammatory genital cytokines (data not shown).
To begin to understand how CT4 bacteria are sensed by the host immune system, we performed transcriptional analyses on antigen presenting cells (APCs) isolated from cervical cytobrushes. APCs from women with high Prevotella abundance demonstrated a marked response to LPS, as well as to IFN-γ and IL-1β. This likely reflects both the stimulation by gram-negative bacteria and effects of the cytokine milieu, as women with CT4 had higher levels of both IFN-γ and IL-1β in the CVL. The CT4 APCs also were more activated and mature, with higher expression of CD80, ICAM-1, and MHC II, and were likely contributing to both T cell priming and effector function control (Iijima et al., 2011). Additionally, miR-155 was strongly upregulated in CT4 APCs and has been shown previously to be critical for APCs to effectively activate T cells (Rodriguez et al., 2007).
Based upon transcriptional data and in vitro assays, we propose that cervicovaginal bacteria are sensed through epithelial cells and APCs, activating TLR and NF-κB pathways that cause secretion of chemokines that recruit activated CCR5+ CD4+ T cell targets to the genital tract. Additionally, cytokines such as TNF-α and IFN-γ may directly damage the columnar epithelial barrier of the endocervix by disrupting tight junctions, which enables both bacterial and viral translocation (Madara and Stafford, 1989; McGee et al., 1992). Thus, bacteria-induced genital inflammation may increase HIV acquisition through multiple local mechanisms in the FGT. This is supported by prior observations that pro-inflammatory cytokines in CVLs are associated with an over 3-fold increased risk of HIV acquisition in women (Karim, 2012) and that HIV-1 exposed women who remain seronegative have lower levels of genital IFN-γ, IL-1α, and IP-10 (Lajoie et al., 2012).
Our work supports the role of the bacterial microbiome in modulating HIV risk and suggests that interventions targeting genital microbiota, such as narrow-spectrum antibiotics or probiotics, may reduce HIV acquisition in women. In addition, our results indicate that the genital microbiome can significantly alter local host inflammation and may more broadly impact efforts to create effective microbicides, induce mucosal vaccine responses, curtail cervical cancer progression, and improve reproductive health in women.
Experimental Procedures
Study cohort
Participants were recruited through the Females Rising through Education, Support, and Health (FRESH) study, a prospective, 12-month observational study of women 18–23 years old conducted outside of Durban, South Africa. At the time of data analysis, 146 participants had completed at least one mucosal sampling. The study protocol was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (Durban, South Africa) and the Massachusetts General Hospital Institutional Review Board (2012P001812/MGH; Boston, MA). Informed consent was obtained after the nature and possible consequences of the study were explained.
Sample Collection
Twice weekly volunteers had a finger prick blood draw for HIV RNA testing and every three months had a pelvic exam, peripheral blood draw, and completed an HIV risk questionnaire administered by a counselor. The mucosal sample collection involved the collection of ectocervical and midvaginal swabs (Catch-All, Epicentre), a cervicovaginal lavage, and an endocervical cytobrush, as described in the Supplemental Experiment Procedures.
BV and sexually transmitted infection (STI) detection
Vaginal smear gram staining, Nugent scoring for BV, and STI testing were performed by Global Labs (Durban, South Africa).
Bacterial 16S rRNA gene sequencing
Nucleic acid was extracted from cervical swabs by bead beating in phenol:chloroform:isoamyl alcohol (25:24:1, pH 7.9, Ambion), followed by an isopropanol precipitation, as described in the Supplemental Experimental Procedures. Extracted DNA was amplified using the 16S V4 primer constructs (Caporaso et al., 2012) 515F and Golay-barcoded 806R for PCR amplification and Illumina sequencing. The cleaned amplicon pool was sequenced on a single 1×300 bp run on an Illumina MiSeq machine. 16S sequencing reads were processed using the QIIME (Caporaso et al., 2010) version 1.8.0 analysis pipeline. Samples from the full data set were then assigned to the four cervicotypes based on a simple assessment of dominance (i.e. abundance). Details can be found in Supplemental Experimental Procedures.
Metagenomic shotgun DNA sequencing and taxonomic classification
Shotgun DNA libraries were made from total nucleic acid isolated from 12 cervical swab samples; the same total nucleic acid was used for 16S amplification. DNA was isolated from the total nucleic acid using an AllPrep DNA/RNA Micro Kit (Qiagen) and sheared (S2 sonicator, Covaris). DNA libraries were prepared using the NEBNext DNA Library Construction kit (New England Biolabs) and sequenced on a single paired-end 250 bp Illumina MiSeq run. Bacterial species were identified using the MetaPhlAn2 package v.2.0.0.b (Segata et al., 2012).
Bacterial functional pathways analysis
Reads aligning to the human genome or PhiX were removed from quality controlled and adapter trimmed shotgun DNA sequences. The remaining reads were assigned to KEGG genes using a translated BLAST search, and HUMAnN (was used to identify the differentially abundant microbial pathways (Abubucker et al., 2012).
Cytokine measurements in CVLs
Cytokines were measured using a human cytokine/chemokine multiplexed bead assay (Millipore). Cervicovaginal lavages (CVL) were analyzed within six months of collection and underwent a single freeze-thaw cycle. Additional information can be found in the Supplemental Experimental Procedures.
Bacterial strains and colonization assays
Isolates were obtained from the Culture Collection University of Göteborg (Sweden), the American Type Culture Collection (ATCC, Manassas, VA), and collaborators. See Supplemental Experimental Procedures for further information.
Immunophenotyping of cervical cytobrush samples
Cervical and peripheral blood cells were stained with a violet viability dye (Life Technologies) and labeled with a cocktail of antibodies, described in detail in the Supplemental Experimental Procedures. Cells were sorted on a FACS Aria III (BD Biosciences) directly into TRIzol (Life Technologies), vortexed vigorously, and stored at −80°C. Data were processed using FlowJo software (Treestar). See Figure S7A for gating strategy.
Transcriptional analysis of sorted cells
RNA was extracted from cells sorted into TRIzol using a chloroform-based method. RNA quality and quantity were assessed using the Bioanalyzer (Agilent) and RINs were consistently above 9. The RNA-seq libraries were prepared according to the Single Cell RNA Barcoding and Sequencing method originally developed for single cell RNA-seq (SCRB-seq (Soumillon et al., 2014)) and adapted to extracted total RNA. Digital gene expression values were analyzed using the edgeR package (Robinson et al., 2010).
Statistics
Mann-Whitney Wilcoxon test was used to compare continuous data between two groups. For comparisons between more than two groups, Kruskal-Wallis test with post hoc analyses was used. Wilcoxon signed rank was used to compare continuous data between two time-points. Fisher’s exact test was used for comparing categorical data among two or more groups. Spearman’s correlation coefficients were used to examine associations between variables; only correlations with a P value lower than the specified cut-off are displayed. Principal component and cluster analyses were used to obtain summary measures for cytokine/bacteria variables and to identify study subjects with similar cytokine/bacteria profiles. Multivariate analyses using ridge regression (Cule and De Iorio, 2013) were used to identify the correlates of higher cytokine values. P-values are two-sided and are not adjusted for multiple testing. The analyses were performed using R Studio and Prism (GraphPad).
Supplementary Material
Highlights.
Most African women studied had genital communities with low Lactobacillus abundance
Unlike the gut, high diversity cervicovaginal communities are pro-inflammatory
Specific bacteria induce cytokine production from genital APCs and epithelial cells
Acknowledgements
We would like to thank the FRESH participants; T. Cele for performing the pelvic exams; T. Sikhakhane, S. Ngcobo, and S. Zungu for clinical support; H. Shen for flow cytometric assistance; A. Lisanti for performing tissue staining; B. Corleis, C. Gosmann, and G. Olson for critical feedback and assistance; S. Karim, L. Liebenberg, N. Samsunder, and N. Garrett for helpful discussions regarding mucosal sampling; C. Monaco and S. Handley for discussions about sample preparation and sequencing; and L. Hoisington-Lopez for sequencing support. This work was supported the Collaboration for AIDS Vaccine Discovery of the Bill and Melinda Gates Foundation, the International AIDS Vaccine Initiative (IAVI) (UKZNRSA1001) and the NIAID (1R01AI111918). D.S.K. received additional support from the Burroughs Wellcome Fund. M.N.A. was supported by award number T32GM007753 from the NIGMS, and the Paul and Daisy Soros Fellowship. R.N.F. and H.S.Y were supported by the NIAID (5R01AI079085). T.N. received additional support from the South African Research Chairs Initiative and an International Early Career Scientist Award from the Howard Hughes Medical Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.N.A., E.H.B., and B.A.B. performed the nucleic acid extraction and bacterial sequencing; K.E.D., N.P., M.N.A., and D.S.K. performed flow cytometry; M.N.A. performed the CVL cytokine measurements and data analyses; H.S.Y. and R.N.F. designed and performed the in vitro bacterial-epithelial cell co-culture experiments and cytokine measurements; R.N.F. and M.E.S. provided expertise in vaginal mucosal immunity and genital sampling; K.E.D. and A.M. collected behavioral data; N.K. processed and managed clinical samples; M.S.G. was involved with statistical analyses; M.S. and C.N. performed the digital gene expression analysis; C.H. and H.H.V. were involved with experimental design and data analysis; T.N., K.L.D., B.D.W., and D.S.K. designed and managed the clinical study; M.N.A. and D.S.K. prepared the manuscript. All authors discussed the results and commented on the manuscripts. The authors declare no competing financial interests.
References
- Abubucker S, Segata N, Goll J, Schubert AM, Izard J, Cantarel BL, Rodriguez-Mueller B, Zucker J, Thiagarajan M, Henrissat B, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS computational biology. 2012;8:e1002358. doi: 10.1371/journal.pcbi.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. Defense factors of vaginal lactobacilli. American journal of obstetrics and gynecology. 2001;185:375–379. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. Aids. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, De Guingand D, Morton AN, Fairley CK. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013a;56:777–786. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- Bradshaw CS, Walker J, Fairley CK, Chen MY, Tabrizi SN, Donovan B, Kaldor JM, McNamee K, Urban E, Walker S, et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PloS one. 2013b;8:e57688. doi: 10.1371/journal.pone.0057688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, Schwebke JR. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. The Journal of infectious diseases. 2010;202:1907–1915. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME journal. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban B, Links MG, Jayaprakash TP, Wagner EC, Bourque DK, Lohn Z, Albert AY, van Schalkwyk J, Reid G, Hemmingsen SM, et al. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome. 2014;2:23. doi: 10.1186/2049-2618-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CR, Moscicki AB, Scott ME, Ma Y, Shiboski S, Bukusi E, Daud I, Rebbapragada A, Brown J, Kaul R. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. Aids. 2010;24:2069–2074. doi: 10.1097/QAD.0b013e32833c323b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cule E, De Iorio M. Ridge regression in prediction problems: automatic choice of the ridge parameter. Genetic epidemiology. 2013;37:704–714. doi: 10.1002/gepi.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney ML, Onderdonk AB. Nugent score related to vaginal culture in pregnant women. Obstetrics and gynecology. 2001;98:79–84. doi: 10.1016/s0029-7844(01)01402-8. [DOI] [PubMed] [Google Scholar]
- Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. The Journal of infectious diseases. 2014;209:1989–1999. doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspollu A, Vain E, Saarma I, Salumets A, Donders GG, Metsis M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PloS one. 2013;8:e54379. doi: 10.1371/journal.pone.0054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aila NA, Tency I, Claeys G, Verstraelen H, Saerens B, Santiago GL, De Backer E, Cools P, Temmerman M, Verhelst R, Vaneechoutte M. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC infectious diseases. 2009;9:167. doi: 10.1186/1471-2334-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren AM, Zozaya M, Taylor CM, Dowd SE, Martin DH, Ferris MJ. Exploring the diversity of Gardnerella vaginalis in the genitourinary tract microbiota of monogamous couples through subtle nucleotide variation. PloS one. 2011;6:e26732. doi: 10.1371/journal.pone.0026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. mBio. 2011;2:e00168-00111. doi: 10.1128/mBio.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghartey JP, Smith BC, Chen Z, Buckley N, Lo Y, Ratner AJ, Herold BC, Burk RD. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PloS one. 2014;9:e96659. doi: 10.1371/journal.pone.0096659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Hedges SR, Sibley DA, Mayo MS, Hook EW, 3rd, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. The Journal of infectious diseases. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. Journal of reproductive immunology. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers E, Overmann J. Ecological significance of microdiversity: identical 16S rRNA gene sequences can be found in bacteria with highly divergent genomes and ecophysiologies. Applied and environmental microbiology. 2004;70:4831–4839. doi: 10.1128/AEM.70.8.4831-4839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespers V, Crucitti T, Menten J, Verhelst R, Mwaura M, Mandaliya K, Ndayisaba GF, Delany-Moretlwe S, Verstraelen H, Hardy L, et al. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PloS one. 2014;9:e109670. doi: 10.1371/journal.pone.0109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim SS. CAPRISA: Partnering for Scientific Innovation in HIV Prevention and Treatment. Conference on Retroviruses and Opportunistic Infections. 2012 [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell host & microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie J, Juno J, Burgener A, Mogk K, Wachihi C, Mwanjewe J, Plummer FA, Kimani J, Ball TB, Fowke KR. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunology. 2012;5:277–287. doi: 10.1038/mi.2012.7. [DOI] [PubMed] [Google Scholar]
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell host & microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome. 2013;1:12. doi: 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. The Journal of clinical investigation. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee ZA, Clemens CM, Jensen RL, Klein JJ, Barley LR, Gorby GL. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microbial pathogenesis. 1992;12:333–341. doi: 10.1016/0882-4010(92)90096-7. [DOI] [PubMed] [Google Scholar]
- Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, McCarter M, Mawhinney S, Campbell TB, Lie Y, Coakley E, et al. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. Journal of virology. 2011;85:10189–10200. doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, Salata R, Doncel GF. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. Journal of acquired immune deficiency syndromes. 2014;66:109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. The Journal of infectious diseases. 2005;192:1372–1380. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- Ochiel DO, Ghosh M, Fahey JV, Guyre PM, Wira CR. Human uterine epithelial cell secretions regulate dendritic cell differentiation and responses to TLR ligands. Journal of leukocyte biology. 2010;88:435–444. doi: 10.1189/jlb.1009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud R, Planet PJ, Randis TM, Kulkarni R, Aguilar JL, Lehrer RI, Ratner AJ. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. Journal of bacteriology. 2011;193:1034–1041. doi: 10.1128/JB.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. The Journal of clinical investigation. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nature methods. 2012;9:811–814. doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumillon M, Cacchiaelli D, Semrau S, van Oudenaarden A, Mikkelsen T. Characterization of directed differentiation by high-throughput single-cell RNA-Seq. bioRxiv. 2014 [Google Scholar]
- Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;36:663–668. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. American journal of reproductive immunology. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA, Forney LJ. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. The ISME journal. 2007;1:121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.