Abstract
Background
Aging is highly associated with neurodegeneration and atrophy of the brain. Evidence suggests that personality variables are risk factors for reduced brain volume. We examine whether falls-related self-efficacy is independently associated with brain volume.
Method
A cross-sectional analysis of whether falls-related self-efficacy is independently associated with brain volumes (total, grey, and white matter). Three multivariate regression models were constructed. Covariates included in the models were age, global cognition, systolic blood pressure, functional comorbidity index, and current physical activity level. MRI scans were acquired from 79 community-dwelling senior women aged 65 to 75 years old. Falls-related self-efficacy was assessed by the Activities Specific Balance Confidence (ABC) Scale.
Results
After accounting for covariates, falls-related self-efficacy was independently associated with both total brain volume and total grey matter volume. The final model for total brain volume accounted for 17% of the variance, with the ABC score accounting for 8%. For total grey matter volume, the final model accounted for 24% of the variance, with the ABC score accounting for 10%.
Conclusion
We provide novel evidence that falls-related self-efficacy, a modifiable risk factor for healthy aging, is positively associated with total brain volume and total grey matter volume.
Trial Registration
ClinicalTrials.gov Identifier: NCT00426881.
Keywords: self-efficacy, brain volume, older women
Cognitive decline and dementia is a significant health care and societal issue. Currently worldwide, 24.3 million people have dementia; the 4.6 million new cases of dementia that occur annually represent one new case every 7 seconds [1]. The number of people affected is projected to double every 20 years to over 80 million by 2040 [1].
The risk of cognitive impairment and dementia increases with age. Age is believed to increase risk for dementia because it is independently associated with brain atrophy [2]. Between the ages 30 and 90 years, roughly 15% of the cerebral cortex and 25% of white matter in humans are lost [3]. Given that by 2030, 14% of the world’s population will be over age 65, there is an urgent need to identify modifiable risk factors for age-related neurodegeneration [4].
Well-known modifiable risk factors for reduced brain volume in older adults include physical inactivity, high body mass index, and chronic conditions such as hypertension [5]. Evidence also suggests that personality variables such as low self-esteem are risk factors. Specifically, Pruessner and colleagues [6] found that high self-esteem moderated age-related patterns in cognitive decline, cortisol regulation, and global brain volume decline in older adults aged 60 to 84 years old. Most recently, Jackson and colleagues [7] demonstrated the effect of personality on age-related reduction in brain volume. Compared with individuals who displayed higher conscientiousness (i.e., their ability to complete tasks they committed to), those with higher neuroticism demonstrated significantly reduced brain volume in prefrontal and medial temporal regions [7].
A personality variable that has not been previously examined in the context of age-related reduction in brain volume is self-efficacy. Self-efficacy is distinct from self-esteem. Self-efficacy is defined as confidence in one’s ability to achieve a desired outcome, whereas self-esteem is a general feeling about one’s self-worth. According to Bandura’s Social Cognitive Theory [8], an individual’s perceived capability to perform an activity is a better predictor of activity in a particular domain than an individual’s actual physical ability to complete the activity.
Previous studies highlight the importance of self-efficacy in healthy aging[9, 10]. For example, a large population-based study demonstrated that older men’s instrumental efficacy beliefs at baseline were positively associated with change in verbal memory over a 2.5 year follow-up [10]. Falls-related self–efficacy is also independently associated with mobility performance in older adults [9]. In turn, both cognitive function and mobility performance are related to brain health [11]. Thus, we hypothesize that falls-related self-efficacy may be of particular importance to brain volume and an essential component of healthy aging. In this study, we examined whether falls related self-efficacy independently associated with brain volume in community-dwelling older women after accounting for global cognitive function and known covariates.
METHOD
Participants
The total sample for this cross-sectional analysis consisted of 79 women who consented and completed a randomized controlled trial of exercise (NCT00426881) that aimed to examine the effect of once weekly and twice-weekly resistance training on cognitive performance of executive functions. The design and the primary results of the Brain Power study have been reported elsewhere [12]. Briefly, participants enrolled in Brain Power were: aged 65 to 75 years, community-dwelling, and had a Mini-Mental State Examination (MMSE) score ≥ 24. Participants were enrolled and randomized by the Research Coordinator to one of three groups: once-weekly resistance training (1x RT), twice-weekly resistance training (2x RT), or twice-weekly balance and tone (BAT).
This study was approved by the relevant university and hospital ethics boards. All participants gave written informed consent prior to participants in the study.
Dependent Variable: Brain Volume – MRI
Brain volume was measured via high-resolution, T1-weighted structural MRI images obtained using a Philips Achieva 3T scanner (TR = 8 ms, TE = 3.7 ms, bandwidth = 2.26 kHz, voxel size = 1 × 1 × 1 mm). Brain tissue volume, normalized for subject head size, was estimated with SIENAX [13], part of FSL (FMRIB’s Software Library, Version 4.1.4) [14]. SIENAX starts by extracting brain and skull images from the single whole-head T1 image [15]. The brain image was then affine-registered to Montreal Neurological Institute (MNI) 152 space. Next, tissue-type segmentation with partial volume estimation was carried out in order to calculate total volume of brain tissue, total white matter volume, and total grey matter volume.
Independent Variables
Comorbidity
Functional comorbidity index (FCI) was calculated to estimate the degree of comorbidity associated with physical functioning [16]. This scale’s score is the total number of comorbidities.
Global Cognition
We assessed global cognition using the MMSE. The MMSE is a widely used and well-known questionnaire used to screen for cognitive impairment (i.e., MMSE <24) [17]. It is scored on a 30-point scale with a median score of 28 for healthy community dwelling octogenarians with more than 12 years of education [17]. The MMSE may underestimate cognitive impairment for frontal system disorders because it has no items specifically addressing cognitive function [17].
Blood Pressure
Resting systolic blood pressure and diastolic blood pressure were recorded in duplicate, using an voscillometric sphygmomanometer, the Omron HEM-775 Values were presented as an average of two recordings that were taken in minute apart.
Physical Activity
Current physical activity level was assessed by the valid and reliable Physical Activities Scale for the Elderly (PASE) questionnaire. This 12-item scale measures the average number of hours per day spent participating in leisure, household, and occupational physical activities over the previous seven-day period.
Falls-Related Self-Efficacy
The 16-item Activities-Specific Balance Confidence (ABC) Scale [18] assesses falls-related self-efficacy with each item rated from 0% (no confidence) to 100% (complete confidence). The ABC Scale score is correlated with other measures of self-efficacy, distinguishes between individuals of low and high mobility, and corresponds with balance performance measures [18].
Data Analysis
We analyzed all data using SPSS version 13.0. We report descriptive data for all variables of interest. This cross-sectional analysis of the Brain Power study is at baseline prior to commencement of any exercise intervention. For data that are normally distributed we report mean and standard deviation and frequencies depending on the measure. For data that were significantly skewed, we report median and interquartile range. We used the Pearson product moment correlation coefficient to determine the level of association between brain volume and age, FCI, global cognition, systolic blood pressure, physical activity, and falls-related self-efficacy. Total brain volume, total white matter volume, and total grey matter volume (all normalized by head size) were used as our dependent variables; separate correlation coefficients and multiple linear regression models were run with each dependent variable. In our multiple linear regression model, age, FCI, global cognition, systolic blood pressure, and physical activity, were statistically controlled by forcing these six variables into the regression model first. These independent variables were determined based on the results of the Pearson product moment coefficient analyses (i.e., alpha level < 0.05) and assumed biological relevance. Falls-related self-efficacy was then entered into the model after adjusting for known covariates. We assessed the assumptions of normality of the residuals and heteroscedasticity.
Results
Of the 155 participants who consented and were randomized at baseline, 135 completed the 12-month trial. Seventy-nine of the 135 participants consented and completed baseline MRI scanning.
Participants
Table 1 reports descriptive statistics for our variables of interest for this cohort. Participants included in our MRI sub-study were similar on demographic characteristics. At baseline, this cohort of community-dwelling senior women has a mean total brain volume of 14.0E5 ± 0.06 mm3, a mean total grey matter region of 7.33E5 ± 0.03 mm3 and a mean total white matter region of 6.70E5 ± 0.03 mm3. Further, the mean ABC score was 89 ± 12 (max 100).
Table 1.
Characteristics of the Brain Power cohort at baseline
| Variable at Baseline | (N=79) | |
|---|---|---|
| Mean | Standard Deviation | |
| Total Brain Volume (mm3) | 14.0E5 | 0.06 |
| Total Grey Matter Volume (mm3) | 7.3E5 | 0.03 |
| Total White Matter Volume (mm3) | 6.7E5 | 0.03 |
| Age (years) | 69 | 3 |
| Function Comorbidity Index | 1.8 | 1.5 |
| MMSE (max 30 pts) | 28 | 1 |
| Systolic Blood Pressure | 139 | 21 |
| Physical Activity Scale for the Elderly | 129 | 61 |
| Activities Specific Balance Confidence (%) | 89 | 12 |
Correlation Coefficients
Table 2 reports the correlation coefficients between independent variables of interest and brain volume. Systolic blood pressure and physical activity levels were significantly associated with total brain volume (p < 0.05). Age (p < 0.01) and systolic blood pressure (p < 0.05) were significantly associated with total grey matter volume. The ABC score was significantly associated with both total brain volume (Figure 1a) and total grey matter volume (Figure 1b) (p < 0.01).
Table 2.
Correlation coefficient matrix for brain volume (N=79)
| Variable at Baseline | Total Brain Volume (mm3) | Grey Matter Volume (mm3) | White Matter Volume (mm3) |
|---|---|---|---|
| Age | −0.165 | −0.288** | −0.108 |
| Function Comorbidity Index | 0.024 | 0.091 | −0.038 |
| Mini-Mental State Examination (max 30) | −0.007 | 0.033 | −0.111 |
| Systolic Blood Pressure | −0.166* | −0.242* | −0.073 |
| Physical Activities Scale for the Elderly | −0.201* | 0.154 | 0.093 |
| Activities Specific Balance Confidence Scale | 0.352** | 0.401** | 0.224* |
p < 0.05
p < 0.01
Figure 1.
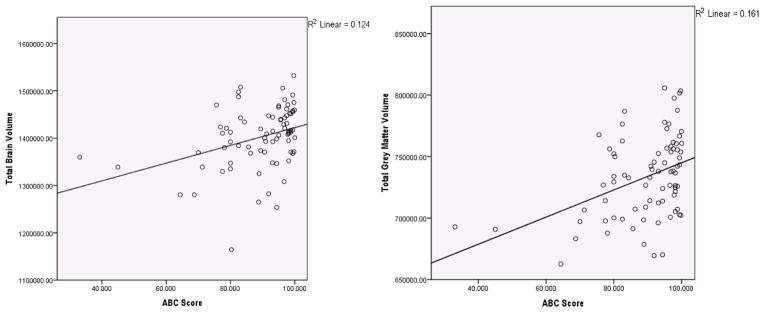
(a) Total brain volume (mm3) versus falls-related self-efficacy (ABC Score) (b) Total grey matter brain volume (mm3) versus falls-related self-efficacy (ABC Score)
Multivariate Linear Regression
The ABC score was significantly associated with total brain volume and total grey matter volume after adjusting for known covariate (p < 0.05). The total variance accounted for by the final model for total brain volume was 17% and for total grey matter volume was 24% (Table 3). The ABC score accounted for an additional 8% of the total variance in the final model for total brain volume. The ABC Scale accounted for an additional 10% of the total variance in the final model for total grey matter volume.
Table 3.
Multiple Linear Regression Summary for Brain Volume versus Falls-Related Self-Efficacy (N=79)
| Total Brain Volume (mm3) | Total Grey Volume (mm3) | |||
|---|---|---|---|---|
| Independent Variables | Unstandardized β (Standard Error) | P-value | Unstandardized β (Standard Error) | P-value |
| Model 1 | R2 0.090 | R2 0.144 | ||
| Age | −2962 (2842) | 0.300 | −2914 (1423) | 0.044* |
| FCI | 383 (4878) | 0.938 | 2133 (2443) | 0.385 |
| MMSE | −3057 (6500) | 0.640 | −511 (3255) | 0.876 |
| Systolic Blood Pressure | −426 (378) | 0.264 | −245 (189) | 0.200 |
| PASE | 236 (119) | 0.052 | 96 (60) | 0.113 |
| Model 2 | R2 0.172 | R2 0.244 | ||
| Age | −1011 (2824) | 0.721 | −1801 (1393) | 0.200 |
| FCI | 2261 (4735) | 0.635 | 3202 (2337) | 0.175 |
| MMSE | −4365 (6260) | 0.488 | −1256 (3089) | 0.685 |
| Systolic Blood Pressure | −370 (363) | 0.311 | −213 (179) | 0.238 |
| PASE | 172 (117) | 0.147 | 59 (58) | 0.308 |
| ABC Scale | 1677 (626) | 0.009** | 955 (309) | 0.003** |
p < 0.05
p < 0.001
FCI = Functional Comorbidity Index
MMSE = Mini Mental State Examination
PASE = Physical Activity Scale for the Elderly
ABC = Activities of Balance Confidence Scale
Discussion
This study provides novel evidence that falls-related self-efficacy is positively and independently associated with brain volume (both total and grey matter) among high functioning community-dwelling senior women. To our knowledge, our study is the first to demonstrate the independent contribution of falls-related self-efficacy to brain volume after accounting for key covariates (i.e., age, FCI, global cognition, systolic blood pressure and physical activity). Importantly, falls-related self-efficacy was independently associated with grey matter – brain tissue that is particularly sensitive to aging effects and pathological processes (e.g., Alzheimer’s disease) [19]. In addition, Taki and colleagues [20] recently showed that total grey matter volume was significantly associated with cognitive performance in healthy older adults. Hence, our findings extend previous investigations that highlight the importance of falls-related self-efficacy for healthy aging [9].
Falls-related self-efficacy does not exist only among older adults with a history of falls; reduced falls-related self-efficacy is reported by 30% or more of older adults who have no history of falling [21]. Fear of falling may lead to self-imposed activity restriction that is not due to actual physical impairments [22]. Further, fear of falling is independently contributes to functional decline and the loss of independence among older adults [23]. Taking the results of our study with those of previous studies, self-efficacy appears to be an essential psychosocial characteristic of healthy aging [7]. Studies have highlighted the importance of self-efficacy for the maintenance of mobility, balance, functional status, social function and cognitive function among older adults [7, 9]. More recently, studies have demonstrated an association between personality and differences in regional brain volume associated with healthy aging [7]. We also recently demonstrated that improved falls-related self-efficacy is positively and independently associated with increased usual gait speed among community-dwelling older women [12]; improved gait speed is associated with substantial reduction in mortality [24]. Previous research has also demonstrated the association between brain atrophy and poor gait speed [25]. Further, spatial characteristics of gait are associated with distinct brain networks in older adults [26]. Hence, targeting falls-related self-efficacy may also have indirect benefits on mobility in older adults. In summary, it would appear that healthy aging promotion strategies should target self-efficacy. Further, given the establish relationship between falls and cognition [12], it seems only appropriate to target falls-related self-efficacy in the context of promoting healthy aging in seniors.
This study generates numerous research questions as to the mechanisms that underpin the independent association between falls-related self-efficacy and measures of brain volume. An underlying mechanism may relate to allostatic load, defined by McEwen and Gianaros [27] as the wear-and-tear on the body and brain that results from chronic dysregulation (i.e., overactivity or underactivity) of mediators of allostasis. Allostasis is defined as the active process of responding to a challenge to the body (e.g., psychological stress) by triggering chemical mediators of adaptations that operate in a nonlinear network.
Low self-efficacy is a form of psychological stress. When faced with a challenging everyday cognitive-behavioural task, healthy older adults with low self-efficacy experience greater stress, as demonstrated by an exaggerated response of the hypothalamic-pituitary-adrenal (HPA) axis and increased production of glucocorticoids (GCs), compared with those without low self-efficacy [28].
Without intervention, low self-efficacy also is a persistent psychological state. According to Schulkin and colleagues [29], chronic psychological stress results in allostatic load. Specifically, chronic stress can lead to poor habituation of the HPA axis and dysregulation of the GCs release. Documented central effects of chronic psychological stress include shortening of dendrites, loss of spine synapses, suppression of neurogenesis, and reduced brain volume – especially in the hippocampus [30].
Finally, lifestyle behaviours of older adults with low falls-related falls efficacy may increase the negative effect of allostatic load. For example, older adults with low falls-related falls efficacy often restrict activity to reduce the risk of falling. However, this often leads to social isolation and a sedentary lifestyle. Yet, both social integration and physical activity are identified by McEwen and Gianaros [27] as two of the most important interventions approaches for allostatic load.
Taking the results of our study with those of previous studies, self-efficacy appears to be an essential personality variable for healthy aging [7]. Studies have highlighted the importance of self-efficacy for the maintenance of mobility, balance, functional status, cognitive function, and social function among older adults [7, 9, 12]. Hence, it would appear that healthy aging promotion strategies should target self-efficacy.
We recognize that our cross-sectional study design did not allow us to ascertain the temporal relationship between falls-related self-efficacy and brain volume. Also, we did not assess the association between falls-related self-efficacy and the specific regions that are primarily responsible for the allodynamic processes, such as the hippocampus, amygdale, and the prefrontal cortex [27]. Further, we highlighted that previous studies demonstrated personality-related factors such as self-esteem [6] and personality [7] to directly affect age-related brain volume. Although our study highlighted an additional and unique personality variable – self-efficacy independently contributes to brain volume, a limitation of our study is that we cannot compare directly the contribution of self-efficacy with other personality related factors because we did not measure such factors. Last, we note that our study sample included only high functioning community dwelling older women without cognitive impairments. The relationship between falls-related self-efficacy and brain volume may differ in lower functioning older adults, cognitively impaired older adults, or older men. Thus, future prospective population-based studies are needed to determine whether our present findings can be generalized to more heterogeneous populations.
Conclusions
Our study, conducted among older community dwelling women, for the first time highlights that falls-related self-efficacy is positively associated with brain volume (both total and grey matter) after accounting for age, FCI, global cognition, systolic blood pressure and physical activity.
Key Points.
No previous studies have examined the independent contribution of falls-related self-efficacy to brain volume
Falls-related self-efficacy was independently associated with brain volume – total and grey matter
Promotion of self-efficacy may be an essential component of healthy aging
Acknowledgments
We thank the Brain Power study participants.
Funding
The Vancouver Foundation (BCM06-0035, Operating Grant to TLA), Michael Smith Foundation for Health Research (MSFHR, Establishment Grant (CI-SCH-063(05-1)CLIN) to TLA), and Canadian Institute for Health Research (CIHR, MOB-93373 to TLA) provided funding for this study. LSN is a MSFHR Senior Graduate trainee and a Natural Sciences and Engineering Research Council of Canada Doctoral trainee. TLA is funded by a MSFHR Scholar Award and CIHR New Investigator. JCD is funded by Vancouver Coastal Health and a CIHR and MSFHR Postdoctoral Fellowship. These funding agencies did not play a role in study design. We obtained approval for the Brain Power study from UBC Clinical Ethics Review Board.
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
Author’s Contributions
TLA was principal investigator for the Brain Power study. TLA and JCD were responsible for study concept and design, acquisition of data, data analysis and interpretation, writing and reviewing of the manuscript. JCD, TLA, LSN, CLH, and LB drafted and revised the manuscript. JCD, LSN, CLH and TLA acquired and analyzed the data.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 3.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiology of aging. 1991;12(4):336–338. doi: 10.1016/0197-4580(91)90013-a. discussion 352-335. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella K, He W. An Aging World: 2008 International Population Reports. 2009 [Google Scholar]
- 5.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol. 2007;254(6):713–721. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 6.Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuro Image. 2005;28(4):815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Ambrose T, Khan KM, Donaldson MG, Eng JJ, Lord SR, McKay HA. Falls-related self-efficacy is independently associated with balance and mobility in older women with low bone mass. J Gerontol A Biol Sci Med Sci. 2006;61(8):832–838. doi: 10.1093/gerona/61.8.832. [DOI] [PubMed] [Google Scholar]
- 10.Seeman T, McAvay G, Merrill S, Albert M, Rodin J. Self-efficacy beliefs and change in cognitive performance: MacArthur Studies of Successful Aging. Psychology and aging. 1996;11(3):538–551. doi: 10.1037//0882-7974.11.3.538. [DOI] [PubMed] [Google Scholar]
- 11.Shenkin SD, Rivers CS, Deary IJ, Starr JM, Wardlaw JM. Maximum (prior) brain size, not atrophy, correlates with cognition in community-dwelling older people: a cross-sectional neuroimaging study. BMC Geriatr. 2009;9:12. doi: 10.1186/1471-2318-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, De Stefano N, Jenkinson M, Matthews PM. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr. 2001;25(3):466–475. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. Journal of clinical epidemiology. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Myers A, Powell L, Maki B, Holliday P, Brawley L, Sherk W. Psychological indicators of balance confidence: Relationship to actual and perceived abilities. Journal of gerontology. 1996;51A:M37–M43. doi: 10.1093/gerona/51a.1.m37. [DOI] [PubMed] [Google Scholar]
- 19.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 20.Taki Y, Kinomura S, Sato K, Goto R, Wu K, Kawashima R, Fukuda H. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 75(2):170–176. doi: 10.1016/j.bandc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. Journal of gerontology. 1994;49(3):M140–147. doi: 10.1093/geronj/49.3.m140. [DOI] [PubMed] [Google Scholar]
- 22.Howland J, Lachman ME, Peterson EW, Cote J, Kasten L, Jette A. Covariates of fear of falling and associated activity curtailment. Gerontologist. 1998;38(5):549–555. doi: 10.1093/geront/38.5.549. [DOI] [PubMed] [Google Scholar]
- 23.Cumming RG, Salkeld G, Thomas M, Szonyi G. Prospective study of the impact of fear of falling on activities of daily living, SF-36 scores, and nursing home admission. J Gerontol A Biol Sci Med Sci. 2000;55(5):M299–305. doi: 10.1093/gerona/55.5.m299. [DOI] [PubMed] [Google Scholar]
- 24.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 25.Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. Journal of the American Geriatrics Society. 1999;47(3):330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special Article: Gait Measures Indicate Underlying Focal Gray Matter Atrophy in the Brain of Older Adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen BS, Gianaros PJ. Stress- and Allostasis-Induced Brain Plasticity. Annual Review of Medicine. 62(1):431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeman TE, Berkman LF, Gulanski BI, Robbins RJ, Greenspan SL, Charpentier PA, Rowe JW. Self-esteem and neuroendocrine response to challenge: MacArthur studies of successful aging. J Psychosom Res. 1995;39(1):69–84. doi: 10.1016/0022-3999(94)00076-h. [DOI] [PubMed] [Google Scholar]
- 29.Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neuroscience and biobehavioral reviews. 1994;18(3):385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]


