Abstract
Chronic inflammation is a key pathogenic process in age-related macular degeneration (AMD). Amyloid-beta (Aβ) is a constituent of AMD drusen and promotes the activation of NLRP3 inflammasome which facilitates the production of cytokines. We investigated the role of transcription factor NF-κB in the activation of inflammasome in the RPE and the effect of vinpocetine, a dietary supplement with inhibitory effect on NF-κB. ARPE19/NF-κB-luciferase reporter cells treated with Aβ demonstrated enhanced NF-κB activation that was significantly suppressed by vinpocetine. Intraperitoneal injection of vinpocetine (15 mg/kg) inhibited NF-κB nuclear translocation and reduced the expression and activation of NLRP3, caspase-1, IL-1β, IL-18, and TNF-α in the RPE of adult rats that received intraocular Aβ, as measured by retinal immunohistochemistry and Western blot. Cytokine level in the vitreous was assayed using multiplex suspension arrays and revealed significantly lower concentration of MIP-3α, IL-6, IL-1α, IL-1β, IL-18, and TNF-α in vinpocetine treated animals. These results suggest that the NF-κB pathway is activated by Aβ in the RPE and signals the priming of NLRP3 inflammasome and the expression of pro-inflammatory cytokines including the inflammasome substrates IL-1β and IL-18. NF-κB inhibition may be an effective approach to stem the chronic inflammatory milieu that underlies the development of AMD. Vinpocetine is a potentially useful anti-inflammatory agent that is well-tolerated in long term use.
Keywords: AMD, amyloid-β, NF-κB, vinpocetine, RPE, inflammasome, cytokine
1. Introduction
Age-related macular degeneration (AMD) is a leading cause of visual impairment among the elderly in developed countries (The Eye Diseases Prevalence Research Group, 2004). Advanced AMD accounts for the majority of vision loss and manifests as choroidal neovascularization (wet form) or geographic atrophy (dry form). Chronic, subclinical inflammation in the outer retina is thought to promote AMD pathogenesis long before symptom onset (Anderson et al., 2002; Hageman et al., 2001). Extracellular deposits beneath the retinal pigment epithelium (RPE), known as drusen, are hallmarks of AMD and contain protein and lipid components capable of stimulating inflammation (Hageman et al., 2001; Mullins et al., 2000; Jiang et al., 2012). Amyloid-beta (Aβ), a peptide found in senile plaques in the brain in Alzheimer’s disease (AD), is a constituent of drusen and is believed to contribute to the inflammatory state in the outer retina (Anderson et al., 2004; Johnson et al., 2002).
Aβ is a physiologic peptide that exists in two forms: the 1-42 form is associated with AD plaques while the 1-40 form is more prevalent in the eye and drusen (Isas et al., 2010; Prakasam et al., 2010). Aβ 1-40 activates inflammatory/immune response pathways but not acute toxicity in RPE cells, in keeping with the insidious onset of AMD (Kurji et al., 2010). More recently, the pro-inflammatory effect of Aβ 1-40 was verified in vivo using an intravitreal injection model that demonstrated upregulation of NLR family, pyrin domain containing 3 (NLRP3) inflammasome associated products (interleukin 1 beta (IL-1β), IL-18), cytokines (IL-6, tumor necrosis factor alpha (TNF-α)), and increased microglia activation (Liu et al., 2013). The NLRP3 inflammasome is an intracellular multi-protein complex that recruits and cleaves caspase-1 when activated; this inflammasome complex with activated caspase-1 in turn cleaves IL-1β and IL-18 pro-peptides into their mature forms (Halle et al., 2008; Martinon et al., 2002; Tarallo et al., 2012). A host of diverse molecules have been identified as activation signals, including pathogen-associated molecular patterns (PAMPs) such as pore-forming toxins, environmental irritants, danger-associated molecular patterns (DAMPs) such as ATP, crystals, and proteins such as Aβ (Di Virgilio. 2013; Masters and O’Neill. 2011; Tschopp and Schroder. 2010). Aβ is being investigated as a potential target for dry AMD (Ding et al., 2011), however, concomitant suppression of inflammation, specifically the inflammasome, may be a novel approach in attenuating the stimulus that underlies the early development of AMD and preventing its progression to vision-threatening late stages.
Vinpocetine is a modified vinca alkaloid extracted from the periwinkle plant, widely used as dietary supplement in Europe and Asia for cognitive impairment and cerebrovascular diseases (Szatmari and Whitehouse. 2003; Tamaki and Matsumoto. 1985). Recent evidence demonstrates that it possesses an anti-inflammatory property via the suppression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation in a variety of cell types (Jeon et al., 2010). Compared to traditional steroids and non-steroidal anti-inflammatory drugs (NSAIDs), vinpocetine has no known significant side effects, thus making it an attractive alternate anti-inflammatory agent for long term use. The role of NF-κB in ocular tissue has not been studied in the context of AMD, but it is believed that NF-κB pathways supply the necessary signals to prime the NLRP3 inflammasome complex for activation as well as the production of pro-peptides that are substrates for the inflammasome (Bauernfeind et al., 2009; Segovia et al., 2012). As a potent pro-inflammatory transcription factor, NF-κB is classically activated in response to cellular insult by TNF-α (Bouwmeester et al., 2004) via intermediate kinases and in turn, regulates the expression of cytokines including TNF-α (Blackwell and Christman. 1997). TNF-α is upregulated by the RPE and retina in the presence of Aβ (Liu et al., 2013) and is capable of causing damage to photoreceptor cells (Nakazawa et al., 2011). Therefore, further investigation into the effect of NF-κB inhibition on the expression of inflammatory mediators is essential towards a better understanding of AMD pathogenesis and therapy.
We aimed to characterize the response of NF-κB to Aβ stimulation in the eye and examine the effect of vinpocetine on NLRP3 inflammasome activation and pro-inflammatory cytokine expression. We focused on the RPE cells in particular because they are the key homeostatic regulator in the outer retina and their dysfunction is hypothesized to be one of the first steps in the development of AMD. Targeting the NF-κB pathways may suppress early events associated with inflammasome activation, and provide an avenue for future treatment strategies for chronic inflammatory retinal diseases such as AMD.
2. Methods
2.1. Peptide and vinpocetine preparation
Aβ 1-40 oligomeric peptide (American Peptide, Sunnyvale CA) was prepared according to previously described methods (Kurji et al., 2010) and stored in 1.4 μg/μL aliquots. Vinpocetine (67358-1G) was purchased from AK Scientific Inc (Union City CA). Vinpocetine was dissolved in dimethyl sulfoxide (DMSO) as vehicle at a stock concentration of 5 mg/ml and diluted to the desired concentration based on published figures for in vitro (Pereira et al., 2000) and in vivo activity (Jeon et al., 2010).
2.2. Establishment of ARPE19/NF-κB-luciferase reporter cell line
To measure NF-κB activation, an NF-κB specific reporter expression plasmid was constructed: the NF-κB specific promoter was composed of six tandem NF-κB consensus binding sites and a minimal CMV promoter fragment. Following the NF-κB specific promoter is a luciferase reporter gene. The entire NF-κB reporter unit was cloned into an eukaryotic expression vector pcDNA3.1(+). ARPE19 cells (ATCC® CRL-2302™) at passage 4 were seeded in a 6-well plate and cultured for 24 h. At the time of transfection, cell confluence was approximately 80%. The ARPE19 cells were transfected by 2 μg of NF-κB-luciferase expression plasmid using 4 μL of FuGene HD (Roche Applied Science, Indianapolis IN). The transfected cells were subcultured in diluted cell concentration and selected by G418 (1 mg/mL) in culture medium for four weeks. The monoclonal stable reporter cells resistant to G418 selection were expanded and tested for the response to rhTNF-α stimulation. The monoclonal stable cells with the best response to rhTNF-α stimulation were used for subsequent experiment.
2.3. In vitro evaluation of NF-κB activation and the effect of vinpocetine
To verify the inhibitory effect of vinpocetine on NF-κB in ocular cell type, we compared vinpocetine to BAY11-7082, a classic inhibitor of NF-κB (Lee et al., 2012). ARPE19/NF-κB reporter cells were seeded in a 24-well plate and cultured overnight. The reporter cells were washed twice with PBS and pretreated with vinpocetine (50 μM) or Bay11-7082 (10 μM) in DME/F12 medium without serum at 37 °C in 5% CO2 for 1 h, followed by addition of the known NF-κB stimulus TNF-α (2.5 ng/mL) into the medium above and cultured for 8 h.
To assess the effect of vinpocetine on RPE’s response to Aβ, three treatments were prepared: Aβ 1-40 (1 μM) containing 2.5% DMSO, vinpocetine (50 μM) containing 2.5% DMSO, and 1 μM Aβ 1-40 combined with 50 μM vinpocetine containing 2.5% DMSO. 2.5% DMSO served as vehicle control. All treatments were prepared in DME/F12 medium without serum. Reporter cells were incubated with each treatment at 37 °C in 5% CO2 for 8 h. The transcriptional level of the luciferase gene that is downstream of the NF-κB responsive element is a sensitive surrogate indicator of NF-κB activation state (Lemon and Tjian. 2000; Napetschnig and Wu. 2013), therefore NF-κB activation was quantified by luciferase mRNA level via q-PCR. Total RNA was isolated from tissue using RNAqueous-4PCR kit (Ambion, Austin TX) and reverse transcribed into cDNA using the High Capacity RNA-to-cDNA Master Mix (Invitrogen, Carlsbad CA). 130 ng total RNA from each treatment group was used with the following luciferase primers: forward: TCACAGAATCGTCGTATGC; reverse: CGTGATGGAATGGAACAAC. q-PCR was carried out on the 7500Fast SDS (Applied Biosystems, Carlsbad CA) with the following cycling conditions: 95 °C for 30 s, 50 °C for 30 s, 72 °C for 45 s, 40 cycles. Melting curve analysis was automatically performed immediately after amplification. Each treatment group was compared to vehicle control and the results expressed in mRNA fold change normalized to housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCT method.
2.4. Animal treatment studies
Animal procedures were carried out according to the protocol reviewed and approved by the University of British Columbia Animal Care Committee and conformed to the Canadian Council on Animal Care guidelines. All animal studies were performed in accordance with the Resolution on the Use of Animals in Research of the Association of Research in Vision and Ophthalmology. Five and half month-old female Long-Evans rats (Charles River Laboratory, Wilmington MA) weighing an average of 450 g were used. Animals were raised on standard rodent diet and housed with environmental enrichment in 12 h-light/dark cycle. Anesthesia was induced with 5% halothane in 70/30 mixture of N2O and oxygen, and maintained with 1.5% halothane throughout surgical procedures. On Day 0, a single intraocular injection of Aβ 1-40 (7 μg) was performed on all animals according to described procedures (Liu et al., 2013). Treatment group received vinpocetine (n = 13) at a dosage of 15 mg/kg body weight, and control group received converted volume of DMSO (n = 13), as intraperitoneal (IP) injection 1 h prior to the intraocular injections, and then repeated daily for three subsequent days. Animals were sacrificed on Day 4 and eyes were immediately enucleated and frozen (for Western blot and vitreous analysis) or fixed in 4% paraformaldehyde in Dulbecco’s phosphate buffered saline (Invitrogen, Carlsbad CA) for 48–72 h prior to tissue processing for immunohistochemistry.
2.5. Immunohistochemistry
Fixed eye tissues were embedded in paraffin, oriented in the sagittal plane and cut at 4 μm thickness through the axis defined by the pupil and the optic nerve per established protocol (Ning et al., 2008). Antibodies against NF-κB p65 subunit, IL-1β, IL-18, TNF-α, NLRP3, and caspase-1 were used to stain the paraffin sections. Antibody dilution and specifications are listed in Table 1. Sections were deparaffinized and rehydrated by standard procedures. All sections underwent antigen retrieval in proteinase K solution (20 μg/ml, pH 8.0; Sigma Aldrich, St. Louis MO) for 10 min at room temperature except for NF-κB. NF-κB sections underwent antigen retrieval in 10 mM sodium citrate buffer and microwave heating for 20 min at high power (800 W). Sections were then washed 3 times in phosphate buffered saline (PBS, pH 7.4), treated with 0.3% H2O2 for 15 min, and blocked for 20 min with 3% normal serum diluted in 0.3% Triton-X (TX)-100-PBS solution to minimize nonspecific staining. Tissue sections were then incubated in specific primary antibodies diluted in serum and PBS with 0.3% TX-100 for 1 h at room temperature and then overnight at 4 °C. Negative controls were obtained by treating the sections with an irrelevant IgG isotype at the same concentration in place of the primary antibody incubation. After incubation in the primary antibodies, sections were thoroughly washed and incubated in secondary antibodies for 30 min at room temperature and rinsed 3 times in PBS, followed by incubation in avidin biotin peroxidase complex (ABC Standard Elite kit; Vector Laboratories, Burlingame CA) according to the manufacturer’s protocol. Next, sections were developed in Vector VIP peroxidase substrate (Vector Laboratories, Burlingame CA) or 3 amino-9-ethylcarbazole peroxidase substrate (AEC; Vector Laboratories, Burlingame CA) chromogenic reactions for 2–5 min. Sections developed in VIP (purple labeling) were counterstained with Methyl Green (Vector Laboratories, Burlingame CA) for 1 min at room temperature then rinsed, dehydrated and coverslipped with Permount (Thermo Fisher Scientific, Waltham MA) for light microscopy. Sections developed in AEC (red labeling) were counterstained with Mayer’s Hematoxylin (Sigma Aldrich, St. Louis MO) for 1 min at room temperature and then rinsed and coverslipped with aqueous mounting medium VectaMount (Vector Laboratories, Burlingame CA).
Table 1.
List of primary antibodies.
| Antigen | Type | Dilution | Specificity | Source and catalog no. |
|---|---|---|---|---|
| Phosphorylated NF-κB p65 | Rabbit Polyclonal | 1:75 | Detects Ser 276 residue in phosphorylated NF-κB p65 of mouse, rat and human origin. | Santa Cruz Biotechnology, Dallas, TX; sc-101749 |
| Interleukin-1 beta (IL-1β) | Goat Polyclonal | 1:400 | Detects rat IL-1 β/IL-1F2 in cultured cells or tissue sections | R&D Systems, Minneapolis, MN; AF-501-NA |
| Interleukin-18 (IL-18) | Rabbit Polyclonal | 1:100 | Detects IL-18 of mouse, rat and human origin. | Santa Cruz Biotechnology, Dallas, TX; sc-7954 |
| NLR family, pyrin domain containing 3 (NLRP3) | Goat Polyclonal | 1:1000 | Recognizes the C-terminus of human NLRP3. Cross-reacts with rat. | AbD Serotec (Bio-Rad), Hercules, CA; AHP1774 |
| Caspase-1 | Rabbit Monoclonal | 1:300 | Recognizes human caspase-1 amino acid 250 to C-terminus (both pro-peptide and cleaved peptide). Cross-reacts with rat and mouse. | Abcam, Cambridge, UK; ab108362 |
| Tumor necrosis factor-alpha (TNF-α) | Rabbit Polyclonal | 1:1000 | Detects TNF-α existing in forms of Type II membrane protein and extracellular soluble protein in human, mouse and rat. | Abcam, Cambridge, UK; ab66579 |
| Caspase-1 (WB) | Mouse Monoclonal | 1:1000 | Recognizes P20 subunit of human caspase-1. Cross-reacts with rat and mouse. | R&D Systems, Minneapolis, MN; MAB6215 |
| GAPDH (WB) | Mouse Monoclonal | 1:10,000 | Recognizes 36 kD monomeric subunit of rabbit GAPDH. Cross-reacts with rat. | EMD Millipore, Billerica, MA; CB1001 |
Sections from each animal in the two groups (Aβ + vehicle and Aβ + vinpocetine, n = 5 per group) were processed at the same time to allow direct comparisons of chromogenic intensity. Four sections were examined per animal for each antibody in a masked fashion. Within each section, an intact, 1500 μm linear strip of retina centered on the optic nerve was evaluated with a 40× objective lens (Eclipse 80i; Nikon, Tokyo, Japan). As NF-κB translocates to the nucleus when the NF-κB pathway is activated, we counted immunoreactive NF-κB nuclei in the RPE layer. Both positively labeled nuclei and non-labeled nuclei were counted in each 1500 μm field, and a mean percentage of labeled nuclei (compared to total nuclei) was obtained.
Semi-quantitative analysis for caspase-1, NLRP3, IL-18, IL-1β and TNF-α was carried out in a masked manner. Within each 1500 μm field, the RPE, Bruch’s membrane (BM), and choroid (CH) were analyzed. Immunostaining intensity was scored from 0 to 3. A score of 0 indicates no detectable staining above background as determined by comparison with the negative control sections. The most intense immunoreactivity was classified as a score of 3. For intermediate intensity levels, a score of 1 was given to samples with the weakest immunolabeling, while a score of 2 represented intermediate immunolabeling. Examples of semi-quantitative scoring are shown on Figs. 2 and 3. The mean semi-quantitative score of Aβ + vinpocetine was normalized to that obtained from Aβ + vehicle, which was set at 100% (Figs. 2 and 3).
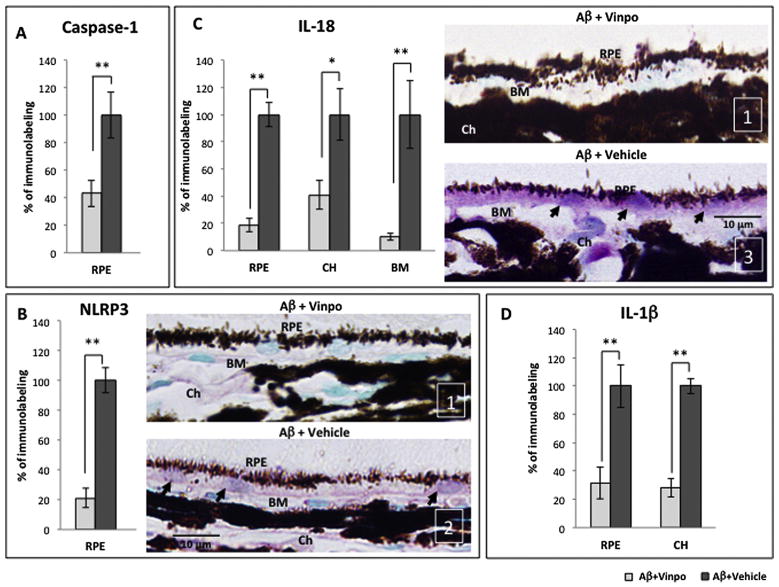
Fig. 2.
Vinpocetine reduced the expression of NLRP3-related components and pro-inflammatory cytokine products in vivo. (A) Immunoreactivity of total caspase-1 (pro-peptide and cleaved forms) is reduced by vinpocetine in the RPE of Aβ-treated animals (Aβ + vinpo), compared to the vehicle group (Aβ + vehicle). The semi-quantitative immunoreactivity score in Aβ + vinpo rats was normalized to that obtained from the Aβ + vehicle group which was set at 100% in all graphs. (B) Vinpocetine suppressed the amount of NLRP3 protein in the RPE of Aβ-treated animals compared to the vehicle group. Representative pictures from Aβ + vinpo rats and Aβ + vehicle rats are shown (right). Positive immunoreactivity of RPE (arrows) is purple due to VIP chromogen. Nuclei appear green due to counterstaining with Methyl Green. Representative semi-quantitative scoring is shown in the lower right corner of the micrograph (white) and indicate immunoreactivity intensity, from 1 (weakest) to 3 (most intense). (C) Vinpocetine caused a 60–80% decrease in IL-18 level in the outer retina (RPE, Bruch’s membrane (BM) and choroid (CH)) compared to the vehicle group. Representative micrographs reveal prominent IL-18 immunoreactivity within the RPE (purple, arrows) in Aβ + vehicle animals. In contrast, the cross section from Aβ + vinpo animals exhibited minimal IL-18 immunoreactivity. (D) The immunoreactivity of IL-1β in the RPE and choroid is suppressed by vinpocetine in Aβ-injected eyes. Scale bar: 10 μm. Immunoreactivity was scored in 4 retinal cross sections per animal (n = 5 per group). Histogram represents mean and SEM. Statistical analysis was performed using a Mann–Whitney U test, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
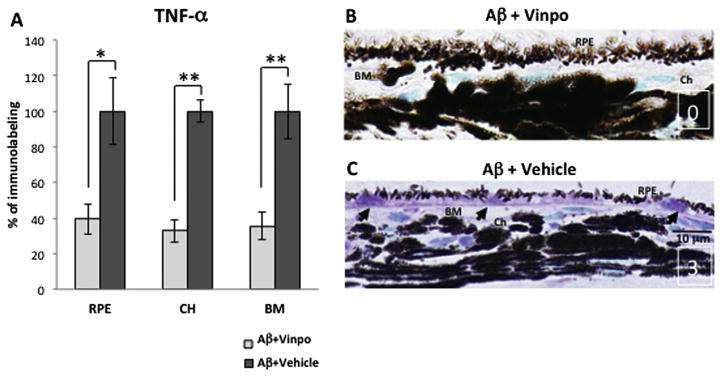
Vinpocetine decreased non-inflammasome-related cytokine TNF-α expression. (A) Vinpocetine significantly reduced the immunoreactivity of TNF-α in the RPE, choroid (CH) and Bruch’s membrane (BM) by approximately 60%. (B, C) Representative micrographs from Aβ + vinpo rats and Aβ + vehicle rats are displayed. The positive immunoreactivity is shown in purple due to the VIP chromogen. The nuclei appear green due to the counterstaining with Methyl Green. Representative semi-quantitative scoring is shown in the lower right corner of the micrographs (white) and indicates immunoreactivity intensity, with “0” indicating background levels (observed in control cross sections in which the primary antibody was replace with a non-specific IgG), and “3” indicating the highest immunoreactivity intensity level. Intense TNF-α labeling was seen in the RPE cytoplasm, the cytoplasm of choroidal cells and in the extracellular matrix including BM from Aβ + vehicle animals (C), while the immunoreactivity in Aβ + vinpo eye demonstrated significantly less TNF-α immunoreactivity in the outer retinal layers (B). Scale bar: 10 μm. Immunoreactivity was scored in 4 retinal cross sections per animal (n = 5 per group). Histogram represents the mean and SEM. Statistical analysis was performed using a Mann–Whitney U test, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.6. Western blot
Western blot was employed to further distinguish the proportion of active and inactive caspase-1 in the treatment groups. Vehicle and vinpocetine treated rat retina/RPE/choroid tissues were isolated microsurgically and immersed in 200 μL of ice-cold RIPA buffer (Thermo Scientific, Rockford IL) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis IN). The tissues were completely homogenized using rotor-stator homogenizer and supernatant was obtained by centrifugation at 14,800 g for 10 min at 4 °C. Total protein concentration was quantitated using BCA Protein Assay kit (Thermo Scientific, Rockford IL). Protein samples were treated in reducing conditions (2×SDS loading buffer with 2% β-mercaptoethanol) and boiled at 100 °C for 5 min. The treated samples were directly subjected to 5–12.5% SDS-PAGE. 50 μg of total protein was loaded in each lane. N = 5 for SDS-PAGE from each group. The gel was transferred to a PVDF membrane and the membrane was blocked in blocking buffer (PBS containing 5% skim milk and 0.2% Tween-20) at room temperature for 1 h. The membrane was then incubated in blocking buffer containing 1:1000 diluted primary mouse antibody against caspase-1 (R&D Systems, Minneapolis MN) at 4 °C for overnight. Finally, the membrane was incubated in blocking buffer containing 1:1000 diluted secondary anti-mouse antibody conjugated with HRP (R&D systems, Minneapolis MN) at room temperature for 2 h. Standard enhanced chemiluminescence (ECL) method (Thermo Scientific, Rockford IL) was used to detect the intensity of full length (45 kD) and cleaved (20 kD) caspase-1 protein bands from vehicle and vinpocetine treated samples. For the purpose of GAPDH detection as loading control, the same membrane from the caspase-1 study was stripped in stripping buffer (TrisHCL (pH: 6.8): 1 mM, SDS: 1%, β-mercaptoethanol: 1%) at room temperature for overnight. The above blotting method was repeated with 1:10,000 diluted primary mouse antibody against GAPDH (EMD Millipore, Billerica MA). The intensity of cleaved caspase-1 (20 kD) and GAPDH (36 kD) protein bands was measured using software ImageJ (NIH, Bethesda MD). The final relative intensity of cleaved caspase-1 (P20) was normalized with respect to the corresponding GAPDH intensity.
2.7. Suspension array assay
An ELISA-based cytokine assay was carried out by the Bio-Plex 200 System (Bio-Rad Laboratories, Hercules CA). As part of the in vivo experiment, rat vitreous was collected during dissection and aliquoted (n = 8). Samples, standards and blanks were prepared according to manufacturer’s instructions. Signals were generated using biotinylated detection antibody and streptavidinphycoerythrin (SAPE). Raw median fluorescent intensity (MFI) data was captured and analyzed using Bio-Plex Manager software 4.1 (Bio-Rad Laboratories, Hercules CA), under a standard high photomultiplier tube setting. Vitreous cytokine concentrations were determined in pg/mL. Normalized fold change was calculated as a ratio of cytokine concentrations in the Aβ + vinpocetine group over Aβ + vehicle group. The negative reciprocal of this result is plotted, ie. fold change less than −1 indicates higher cytokine concentration in the vehicle group than vinpocetine group.
2.8. Statistical analysis
For the in vitro NF-κB activation study, comparisons were made between vehicle control and the vinpocetine treatment groups, respectively. In addition, the Aβ only group was compared to Aβ + vinpocetine group. Similarly, the TNF-α only group was compared to TNF-α + BAY and TNF-α + vinpocetine groups. Statistical analysis of results of q-PCR studies was performed using one-tailed Student’s t-test (GraphPad Software, Inc., La Jolla CA) and significance level at p ≤ 0.05.
Statistical analysis for semi-quantitative data from IL-1β, IL-18, TNF-α, NLRP3, and caspase-1 immunohistochemistry in the in vivo studies was undertaken using the non-parametric Mann–Whitney U test, with significance set at p < 0.05 (GraphPad Software, Inc., La Jolla CA). The statistical analysis for NF-κB p65 subunit was performed on the cell counts of positively labeled relative to unlabeled nuclei in the RPE layer. The Mann–Whitney U test was used to determine the significance which was set at p < 0.05.
Western blot data of full length and cleaved forms of caspase-1 were analyzed by first normalizing the 20 kD band luminescence to that of GAPDH band, and then comparisons between Aβ + vehicle and Aβ + vinpocetine groups were undertaken. Mann–Whitney test was used to determine statistical significance which was set at p < 0.05.
Suspension array data was obtained via the Bio-Plex Manager software 4.1 (Bio-Rad Laboratories). The cytokine concentration in the Aβ + vinpocetine group was compared to that of Aβ + vehicle group and a Mann–Whitney test was used to determine statistical significance, with p < 0.05 considered significant.
3. Results
3.1. NF-κB activation is induced by Aβ and suppressed by vinpocetine in vitro
The mechanism of NF-κB inhibition by vinpocetine has been examined in blood and epithelial cell types (Jeon et al., 2010) but not in an ocular cell. To investigate NF-κB activation, we transfected ARPE19 with an NF-κB-luciferase plasmid, selected and established a reporter cell line. When challenged by TNF-α, a known stimulator of NF-κB, ARPE19/NF-κB reporter cells demonstrated a robust NF-κB response (Fig. 1A), consistent with the nature of RPE as an active cell with pro-inflammatory capabilities. Vinpocetine markedly suppressed this response, as did BAY11-7082, a classic NF-κB inhibitor (Fig. 1A), suggesting that ARPE19 is susceptible to vinpocetine, and vinpocetine is as effective as BAY11-7082.
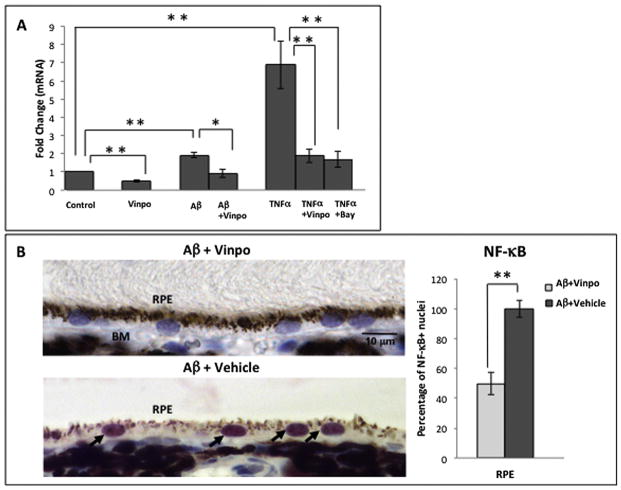
Fig. 1.
NF-κB activation is stimulated by Aβ and inhibited by vinpocetine in the RPE. (A) ARPE19/NF-κB-luciferase reporter cells were first challenged with TNF-α to determine NF-κB activation in this cell type, and if this response is susceptible to inhibition. Data are presented as mRNA fold change normalized to control conditions. TNF-α (2.5 ng/mL) stimulation resulted in a 7-fold increase in NF-κB activation in ARPE19 reporter cells. An established NF-κB inhibitor, BAY11-7082 (10 μM), suppressed NF-κB activation by more than 3 fold. Vinpocetine (50 μM) also inhibited TNF-α-induced NF-κB activation by more than 3 fold. Stimulation with Aβ (1 μM) resulted in a nearly-2 fold increase in NF-κB activation and co-treatment with vinpocetine (50 μM) diminished this NF-κB activation. Treatment with vinpocetine alone reduced NF-κB activation level to less than that of control. Data are represented as the mean and error bars denote standard deviation (SD). Statistical analysis was performed using a one-tailed Student’s t-test, *p < 0.05, **p < 0.01. (B) Adult rats received an intravitreal injection of 5 μL of 1.4 μg/μL Aβ 1-40 (oligomeric form) and an intraperitoneal injection of either vinpocetine (15 mg/kg) or vehicle (2.5% DMSO). Immunohistochemistry was used to highlight NF-κB labeling intensity and nuclear translocation of NF-κB in the RPE. The Aβ + vehicle group demonstrated intense red AEC labeling of NF-κB concentrated in the RPE nucleus (arrows), while Aβ + vinpo group displays minimal NF-κB immunoreactivity. Tissue sections were counterstained with hematoxylin resulting in blue nuclei, and the combination of blue counterstain with red AEC chromogen results in purple nuclei signifying activated NF-κB in the Aβ + vehicle retina. Note that Aβ + vinpo retina illustrated blue (not purple) nuclei, identifying lack of nuclear translocation of NF-κB. Scale bar: 10 μm. Total number of RPE nuclei and NF-κB immunopositive nuclei were counted in 4 retinal cross sections per animal (n = 5 per group). Histogram represents the mean and error bars denote standard error of mean (SEM). Statistical analysis was performed using Mann–Whitney U test, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Given that NF-κB is readily activated in ARPE19 cells, we next investigated whether NF-κB is involved in Aβ-mediated inflammatory pathway. ARPE19/NF-κB reporter cells treated with 1 μM Aβ showed a 1.92 ± 0.25 fold increase (mean ± SD, p < 0.01) in NF-κB activation (Fig. 1A) compared to DMSO control group. In contrast, the group treated with both Aβ and vinpocetine showed significantly reduced NF-κB activation relative to the Aβ-alone group (0.91 ± 0.21 fold, p < 0.05), to a level comparable to that of the control group. Treatment of RPE cells with 50 μM vinpocetine without Aβ decreased the level of NF-κB activation below control (0.49 ± 0.05 fold, p < 0.01) (Fig. 1A). This likely reflects inhibition of constitutive NF-κB activation that is present in ARPE19/NF-κB reporter cells.
3.2. NF-κB activation is induced by Aβ and suppressed by vinpocetine in vivo
Next, we followed up the cell culture study by assessing the activation of NF-κB in vivo using rats that received intraocular Aβ and IP injection of either vinpocetine or vehicle. Intraocular injection has been shown to effectively deliver Aβ peptide to the outer retina and RPE in earlier studies without the confounding effect of mechanical disturbances to the RPE layer due to subretinal delivery (Liu et al., 2013). Parenteral route for vinpocetine administration was chosen due to its good blood–brain (and hence blood-ocular) penetration, and poor oral bioavailability (Polgar et al., 1985). Using a primary antibody against the phosphorylated p65 subunit of NF-κB, we probed the RPE layer in retinal cross section. NF-κB is sequestered in the cytoplasm in the inactive state and translocates into the nucleus when activated by phosphorylation. The nuclear presence of the p65 subunit therefore is an indicator of NF-κB activation. The control group receiving intravitreal Aβ and a vehicle IP injection (Aβ + vehicle) demonstrated intense NF-κB labeling within the RPE nucleus (arrows, Fig. 1B, left bottom). In contrast, the treatment group that received intravitreal Aβ and a vinpocetine IP injection (Aβ + vinpo) showed a predominance of nuclei without intranuclear NF-κB immunolabeling (Fig. 1B, left top). The absence of nuclear translocation indicates a lack of NF-κB activation in the RPE in the vinpocetine treated animals. Quantitative analysis revealed that vinpocetine treatment reduced the number of NF-κB positive RPE nuclei by 50% (Fig. 1B, right). Similar effects of vinpocetine treatment have been noted in microglia (Zhao et al., 2011), vascular smooth muscle cells, umbilical vein endothelial cells and lung epithelial cells (Jeon et al., 2010).
3.3. Vinpocetine reduced the expression of NLRP3-related genes and pro-inflammatory cytokines in vivo
The NLRP3 inflammasome is a critical cellular apparatus for the detection of noxious stimuli, including Aβ, and the subsequent production of mature pro-inflammatory cytokines to defend against cellular insults (Halle et al., 2008; Masters and O’Neill, 2011). NF-κB has been proposed as a prerequisite priming mechanism for inflammasome activation (Bauernfeind et al., 2009; Segovia et al., 2012). Based on the premise that NLRP3 inflammasome activation relies on NF-κB, we evaluated whether vinpocetine can inhibit inflammasome and its ability to produce cytokines.
We first assessed for the expression of cytosolic protein caspase-1 in retinal tissues. Caspase-1 is synthesized as a pro-peptide and must be cleaved in the inflammasome complex to become the active species. Analysis of retinal sections in the Aβ+vinpo group revealed that vinpocetine reduced total caspase-1 immunoreactivity (both cleaved and full length caspase-1) by more than 50% in the RPE (Fig. 2A). We next examined NLRP3 protein within RPE cytoplasm. Following vinpocetine treatment, there was a dramatic decrease of 80% in NLRP3 immunolabeling intensity compared to vehicle treatment (Fig. 2B, left). Inspection of the RPE layer revealed relatively weak immunoreactivity in the Aβ + vinpo group compared to the conspicuous cytoplasmic labeling of NLRP3 in the Aβ + vehicle group (Fig. 2B, right).
IL-18 and IL-1β are end products of NLRP3 inflammasome activation and are secreted by the RPE. Bruch’s membrane and the choroid were included in the analysis of secreted cytokines given their close proximity to the RPE and role in progressive AMD changes. IL-18 immunoreactivity in the Aβ + vinpo group was greatly reduced in the RPE, choroid, and Bruch’s membrane (Fig. 2C, left). Immunohistological sections of the Aβ + vehicle group showed prominent IL-18 labeling intracellularly in the RPE and in the choroidal cells and in BM (Fig. 2C). The immunolabeling pattern of IL-1β closely mirrored that of IL-18; vinpocetine treatment significantly decreased immunohistochemical levels of IL-1β in the RPE and choroid (Fig. 2D). Lower level of these cytokines within the cellular cytoplasm and in the extracellular matrix suggests suppression of both the pro-peptides and the secreted mature protein products in animals treated with vinpocetine.
3.4. Vinpocetine decreased TNF-α expression
The NLRP3 inflammasome is one of many pro-inflammatory pathways regulated by NF-κB. In vivo stimulation of RPE with Aβ also provoked increased expression of TNF-α, a powerful cytokine associated with NF-κB signaling (Blackwell and Christman. 1997) but not dependent on inflammasome processes. TNF-α has important ocular effects, thus we assessed the effect of vinpocetine on Aβ-induced TNF-α expression. Immunohistochemistry results demonstrated that vinpocetine significantly inhibited the production of TNF-α to less than 50% of the vehicle control level in the RPE, choroid and BM (Fig. 3A). Immunohistochemistry demonstrated intense TNF-α labeling in the RPE cytoplasm, the cytoplasm of choroidal cells and in the extracellular matrix including BM (Fig. 3C). Sections from the vinpocetine treatment group demonstrated significantly less TNF-α immunoreactivity in the outer retinal layers (Fig. 3B). This observation supports the inhibitory effect of vinpocetine on non-inflammasome associated NF-κB pathways, in keeping with its putative mechanism of action.
3.5. Western blot verifies suppression of caspase-1 activation
Quantitative analysis of NF-κB translocation to the RPE nucleus and the semi-quantitative analysis of cytokines in immunohistochemical studies point to a strong inhibitory effect of vinpocetine on NF-κB and its downstream pathways in the outer retina. However, because retinal immunohistochemistry could not distinguish between the pro- and cleaved forms of caspase-1, it was not clear if inflammasome activation was suppressed by vinpocetine treatment. To further study this, Western blot was performed on cell lysates from retinal tissues obtained from Aβ + vinpo and Aβ + vehicle treated rats. Cleaved caspase-1 bands (20 kD) were significantly reduced in the vinpocetine treatment group, indicating suppression of inflammasome activation (Fig. 4A). Normalizing the 20 kD band luminescence to the housekeeping gene GADPH eliminates potential difference in amount of total protein loaded. The cleaved caspase-1 protein is dramatically diminished by 86% compared to the vehicle treated group (p < 0.05, Fig. 4B).
Fig. 4.
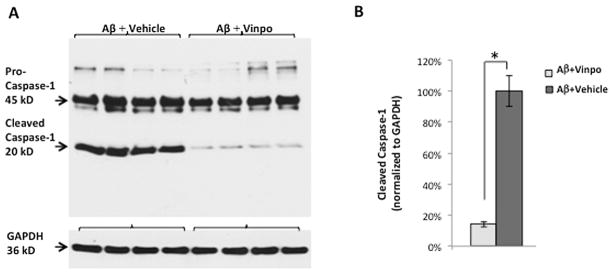
Western blot of caspase-1 shows a significant reduction in the cleaved portion of the peptide in the vinpocetine-treated group (n = 5 per group). (A) Protein lysate (including neuroretina, RPE and choroid) from Aβ + vehicle group shows intense bands at 45 kD and 20 kD, corresponding to the uncleaved (pro-peptide) and cleaved (active) forms of caspase-1, respectively. Vinpocetine markedly reduced the 20 kD band intensity consistent with a decrease in caspsase-1 activation. GAPDH (36 kD) was used to visualize protein loading levels. (B) Standard ECL method was used to quantify the 20 kD band luminescence normalized to that of GAPDH. The cleaved (active) caspase-1 peptide is reduced by greater than 80% in the Aβ + vinpo group compared to the Aβ + vehicle group. Histogram represents the mean and SEM. Statistical analysis was performed using a Mann Whitney U test, *p < 0.05.
3.6. Vinpocetine led to decreased intravitreal cytokine concentration
The over-expression of pro-inflammatory cytokines generated by Aβ is not only present locally in the outer retina but also evident in the vitreous (Liu et al., 2013). Here we measured the effect of vinpocetine on secreted cytokines in the vitreous. The levels of cytokine in the vitreous from animals that received Aβ and vinpocetine was compared with those that received Aβ and vehicle and the results normalized as fold change (Fig. 5). IL-1β (−3.00 ± 0.15 fold) and Il-18 (−2.54 ± 0.35 fold), mature secreted products associated with NLRP3 inflammasome activation, were all statistically significantly lowered in vitreous from the Aβ + vinpo group, consistent with immunohistochemical results demonstrating a lowering of these cytokines by vinpocetine treatment. Four other cytokines and chemokines demonstrated statistically significant and greater than 2.0 fold reduction by vinpocetine: MIP-3α (−5.39 ± 0.23 fold), IL-6 (−4.74 ± 0.58 fold), IL-1α (−4.19 ± 0.41 fold), and TNF-α (−2.12 ± 0.19 fold). These cytokines represent NF-κB activation products and chemokines that are produced by RPE, and immune cells such as lymphocytes and microglia, and function in different arms of the immune response pathway (Chang and Dong. 2011; Chen et al., 2011b; Dinarello. 2009).
Fig. 5.
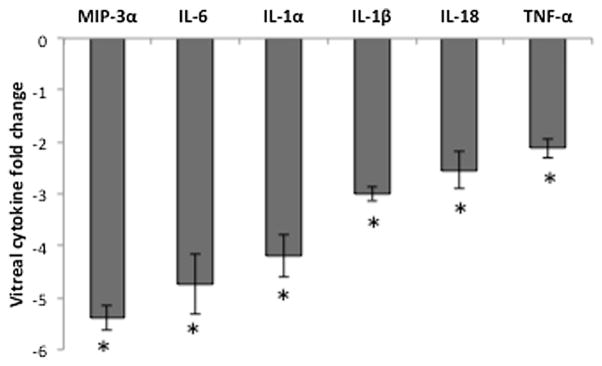
Vinpocetine reduces secreted cytokines in the vitreous after Aβ injection. Vitreous from vinpocetine and vehicle-treated animals (n = 8 per group) were pooled, and assayed using an ELISA based suspension array. Fold change was obtained by dividing the concentration of cytokine (in pg/mL) in the vehicle group by that of the vinpocetine group; negative sign denotes a downregulation due to vinpocetine treatment. Concentrations of secreted MIP-3α, IL-6, IL-1α, IL-1β, IL-18, and TNF-α were reduced by at least 2-fold in the Aβ + vinpo group. Histogram represents the mean and SEM comparisons of the Aβ + vinpo and Aβ + vehicle. Statistical analysis was performed using a Mann Whitney U test, *p < 0.05.
4. Discussion
New evidence is emerging to support the role of specific inflammatory mechanisms in response to drusen components and how these pathways may contribute to the development of AMD. Although the majority of disease burden from vision loss derives from the advanced forms of AMD, it is known that drusen accumulation starts decades earlier and may promote a chronic state of parainflammation in the outer retina (Hageman et al., 2001; Lin et al., 2013; Xu et al., 2009). Therefore, effective early AMD intervention requires the understanding of these inflammatory events and the identification of valid cellular targets. The present study focuses on a novel therapeutic agent, vinpocetine, in an animal model of the subclinical stages of AMD, and expands on our earlier findings by further exploring the pro-inflammatory effect of a drusen component, Aβ, on the RPE. In this model, a single intravitreal injection of a drusen component, Aβ, results in a pro-inflammatory milieu and inflammasome activation in the outer retina that emulates features that are hypothesized to exist in early AMD, without inducing features of late AMD such as cell death (geographic atrophy) or angiogenesis (choroidal neovascularization) (Liu et al., 2013). By using an intravitreal, rather than a subretinal delivery, unnecessary complications due to mechanical disruption of the RPE layer are avoided in this model.
NF-κB is an important transcription factor that facilitates a wide range of cellular processes pertaining to inflammation, immune response, cell survival and proliferation. Given that inflammation is considered a driving force behind the development of AMD, it is logical to consider NF-κB as a potential key mediator of pathogenesis. Indeed, our results support that Aβ, a physiologic peptide in drusen, stimulates NF-κB activation in the RPE (Fig. 1). NF-κB is constitutively expressed by the RPE and is activated by diverse signals such as monocytes co-culture (Bian et al., 2003), TNF-α, IL-17A (Chen et al., 2011a), and Alu-RNA (Kerur et al., 2013). Vinpocetine was highly effective at suppressing Aβ-induced NF-κB activation in the RPE both in vitro and in vivo. Vinpocetine (trade name Cavinton®) is a dietary supplement widely used in Europe and Asia for prevention against stroke and memory loss (Medina. 2010) due to its vasoactive and neuroprotective properties (Nivison-Smith et al., 2014). More recently it was shown to inhibit NF-κB-associated inflammatory pathway by inactivating IKK (Jeon et al., 2010). IKK normally phosphorylates the inhibitory IκB molecule, which then releases NF-κB to enter the nucleus. Thus, by inactivating IKK, vinpocetine in effect would keep NF-κB sequestered in the cytoplasm. Our results demonstrated that vinpocetine inhibited NF-κB activation and nuclear translocation in vitro and in vivo, in keeping with the aforementioned mechanism (Fig. 1). These results suggest that NF-κB plays an active role in the pro-inflammatory state induced by drusen components in the outer retina and is suppressed by vinpocetine.
NLRP3 inflammasome is implicated in a growing list of conditions including sepsis, gout, diabetes mellitus and atherosclerosis (Strowig et al., 2012). Numerous studies have established a role for NLRP3 inflammasome in promoting inflammation and RPE damage in response to factors associated with macular degeneration such as lysosomal destabilization (Tseng et al., 2013), oxidative stress (Kauppinen et al., 2012), and Alu RNA (Tarallo et al., 2012). The present study highlights drusen component Aβ as an important stimulus for inflammasome activation in the RPE, in keeping with similar observation of Aβ’s effect in microglia (Halle et al., 2008).
Although vinpocetine is not known to directly interfere with NLRP3 inflammasome assembly or activation, it effectually suppressed inflammasome activation and the production of cytokines in our study (Fig. 2), presumably in a NF-κB-dependent manner. The NLRP3 inflammasome is tightly regulated and its activation requires two signals (Masters and O’Neill, 2011). NF-κB is an essential priming signal that promotes the expression of inflammasome components as well as that of the substrates IL-1β and IL-18 (Bauernfeind et al., 2009). Aβ oligomers and other misfolded proteins can serve as the second, activating signal that triggers inflammasome assembly (Masters and O’Neill, 2011). By preventing NF-κB activation, vinpocetine inhibits the expression of downstream pathways. Our data demonstrated a decrease in the synthesis of NLRP3 protein, which logically should lead to less inflammasome complex assembly and the recruitment of caspase-1. Without being incorporated into the NLRP3 complex, pro-caspase-1 cannot be cleaved and remains in its full-length form (Fig. 4). Consequently, NLRP3 inflammasome overall is inactive and there is less IL-1β and IL-18 being secreted locally (Fig. 2C, D).
In addition to blocking inflammasome activation, vinpocetine has a suppressive effect on a multitude of cytokines due to the central position of NF-κB in the immune-response cascade. We determined that vinpocetine decreases the intracellular labeling (Fig. 2) of IL-18 and IL-1β in the RPE and secreted in the vitreous (Fig. 5), indicating a reduction in the pro-peptides and further disabling of the NLRP3 inflammasome pathway. NF-κB inhibition also impaired the secretion of non-inflammasome related cytokines in the vitreous, including TNF-α (Fig. 3), IL-6 IL-1α, and MIP-3α (Fig. 5). TNF-α propagates NF-κB activation and its presence in the outer retina has been shown to facilitate photoreceptor death (Nakazawa et al., 2011). IL-6 is a powerful cytokine that mediates inflammatory response in a variety of disease states and is implicated in the progression of AMD (Seddon et al., 2005). IL-1α is a homolog of IL-1β but is cleaved by the protease calpain instead of caspase-1 (Kavita and Mizel. 1995), although more recent evidence suggests there is a partial association between IL-1α and NLRP3 inflammasome (Yazdi et al., 2010). MIP-3α exerts chemotaxic effect on lymphocytes and was highly elevated in the vitreous post-intravitreal Aβ injections in rats (Liu et al., 2013). Taken together, these affected cytokines may act on different aspects of the inflammatory pathway in all cells of the retina.
Among the limitations of the present study, the time course of this study did not allow us to delineate the effect of a chronic inflammatory cytokine exposure on RPE physiology or any potential benefit of vinpocetine long term (ie. greater than 4 days). As such, further studies with an expanded time frame are needed to assess the utility of vinpocetine in early AMD. While this study demonstrated that vinpocetine inhibited Aβ-induced NF-κB activation, additional questions remain. The pathway by which Aβ activate NF-κB remains under investigation; recent evidence suggests that receptor for advanced glycation endproduct (RAGE) and Toll-like receptor 4 (TLR4) are involved in Aβ recognition and subsequent NF-κB signaling (Kim et al., 2013; Meneghini et al., 2013). NF-κB is a non-specific transcription factor that is not only involved in chronic inflammation related to degenerative changes but also important in acute inflammatory response to cellular insults such as infection or injury, as well as in wound healing. Complete inhibition of this crucial transcription factor may affect other cellular processes with unforeseen consequences. However, given vinpocetine is presently used as a dietary supplement in Europe and Asia (Medina, 2010), a clinical evaluation of these populations may provide the answer to the long term sequelae of an NF-κB inhibitor.
5. Conclusion
In our earlier study, we found that the transcription factor NF-κB is implicated in RPE’s response to Aβ by providing the priming signal for NLRP3 inflammasome activation and the synthesis of pro-inflammatory cytokines. Systemic vinpocetine, a well-tolerated anti-inflammatory agent, inhibited NF-kB activation in the RPE as well as NF-κB-dependent processes including inflammasome activation and cytokine production. NF-κB is a key upstream regulator of the inflammatory pathways that is not only activated by Aβ but is likely involved in the RPE’s response to other pathological, age-related stimuli and drusen components such as carboxyethylpyrrole (CEP) and advanced glycation endproduct (AGE) (Lin et al., 2013). NF-κB inhibition may be a useful approach to control the chronic inflammation that is believed to drive the degenerative processes in early AMD, but more studies are needed to ascertain other cellular changes that may be affected by long-term inhibition of this important transcription factor.
Acknowledgments
The authors would like to acknowledge Meysam Abbasi for technical assistance, and support by Vancouver Hospital + UBC Foundation, Faculty of Medicine (UBC). This study was funded by Canadian Institutes of Health Research Grant (CIHR MOP-97806) to JAM.
References
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Elner VM. Human RPE-monocyte co-culture induces chemokine gene expression through activation of MAPK and NIK cascade. Exp Eye Res. 2003;76:573–583. doi: 10.1016/s0014-4835(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, Angrand P, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon A, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin A, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G. A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kijlstra A, Chen Y, Yang P. IL-17A stimulates the production of inflammatory mediators via Erk1/2, p38 MAPK, PI3K/Akt, and NF-B pathways in ARPE-19 cells. Mol Vis. 2011a;17:3072–3077. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011b;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev. 2013;65:872–905. doi: 10.1124/pr.112.006171. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Ding J, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Rickman CB. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2011;108:E279–E287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-[beta] Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, Langen R, Chen J. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010;51:1304–1310. doi: 10.1167/iovs.09-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Li JD, Yan C. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci USA. 2010;107:9795–9800. doi: 10.1073/pnas.0914414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, To E, Cui J, Cao S, Gao J, Matsubara J. Drusen and pro-inflammatory mediators in the post-mortem human eye. J Clin Exp Ophthalmol. 2012;3:208. doi: 10.4172/2155-9570.1000208. http://dx.doi.org/10.4172/2155-9570.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells – implications for age-related macular degeneration (AMD) Immunol Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Kavita U, Mizel SB. Differential sensitivity of interleukin-1 alpha and -beta precursor proteins to cleavage by calpain, a calcium-dependent protease. J Biol Chem. 1995;270:27758–27765. doi: 10.1074/jbc.270.46.27758. [DOI] [PubMed] [Google Scholar]
- Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos-Carvalho A, Yasuma T, Yasuma R, Kim Y, Hinton DR, Kirschning CJ, Gelfand BD, Ambati J. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54:7395–7401. doi: 10.1167/iovs.13-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Ahn JW, Kim H, Ha HJ, Lee SW, Kim HK, Lee S, Hong HS, Kim YH, Choi CY. Two beta-strands of RAGE participate in the recognition and transport of amyloid-beta peptide across the blood brain barrier. Biochem Biophys Res Commun. 2013;439:252–257. doi: 10.1016/j.bbrc.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Kurji KH, Cui JZ, Lin T, Harriman D, Prasad SS, Kojic L, Matsubara JA. Microarray analysis identifies changes in inflammatory gene expression in response to amyloid-β stimulation of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:1151–1163. doi: 10.1167/iovs.09-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Rhee MH, Kim E, Cho JY. BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediat Inflamm. 2012;2012:416036. doi: 10.1155/2012/416036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- Lin T, Walker GB, Kurji K, Fang E, Law G, Prasad SS, Kojic L, Cao S, White V, Cui JZ, Matsubara JA. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62:369–381. doi: 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Gao J, Cao S, Sandhu N, Cui JZ, Chou CL, Fang E, Matsubara JA. Inflammatory mediators induced by amyloid-beta in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:2225–2237. doi: 10.1167/iovs.12-10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Masters SL, O’Neill LAJ. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med. 2011;17:276–282. doi: 10.1016/j.molmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Medina AE. Vinpocetine as a potent antiinflammatory agent. Proc Natl Acad Sci USA. 2010;107:9921–9922. doi: 10.1073/pnas.1005138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini V, Bortolotto V, Francese MT, Dellarole A, Carraro L, Terzieva S, Grilli M. High-mobility group box-1 protein and beta-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-B axis: relevance for Alzheimer’s disease. J Neurosci. 2013;33:6047–6059. doi: 10.1523/JNEUROSCI.2052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- Nakazawa T, Kayama M, Ryu M, Kunikata H, Watanabe R, Yasuda M, Kinugawa J, Vavvas D, Miller JW. Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:1384–1391. doi: 10.1167/iovs.10-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-β deposits lead to retinal degeneration in a mouse model of Alzheimer Disease. Invest Ophthalmol Vis Sci. 2008;49:5136–5143. doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivison-Smith L, Acosta ML, Misra S, O’Brien BJ, Kalloniatis M. Vinpocetine regulates cation channel permeability of inner retinal neurons in the ischaemic retina. Neurochem Int. 2014;66C:1–14. doi: 10.1016/j.neuint.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Pereira C, Agostinho P, Oliveira CR. Vinpocetine attenuates the metabolic dysfunction induced by amyloid beta-peptides in PC12 cells. Free Radic Res. 2000;33:497–506. doi: 10.1080/10715760000301041. [DOI] [PubMed] [Google Scholar]
- Polgar M, Vereczkey L, Nyary I. Pharmacokinetics of vinpocetine and its metabolite, apovincaminic acid, in plasma and cerebrospinal fluid after intravenous infusion. J Pharm Biomed Anal. 1985;3:131–139. doi: 10.1016/0731-7085(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Prakasam A, Muthuswamy A, Ablonczy Z, Greig NH, Fauq A, Rao KJ, Pappolla MA, Sambamurti K. Differential accumulation of secreted AbetaPP metabolites in ocular fluids. J Alzheimer’s Dis. 2010;20:1243–1253. doi: 10.3233/JAD-2010-100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. TLR2/MyD88/NF-kB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Szatmari SZ, Whitehouse PJ. Vinpocetine for cognitive impairment and dementia. Cochrane Database Syst Rev. 2003;1:CD003119. doi: 10.1002/14651858.CD003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki N, Matsumoto S. Agents to improve cerebrovascular circulation and cerebral metabolism – vinpocetine. Nihon Rinsho. 1985;43:376–378. [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand B, Dridi S, Kerur N, Kim Y, Cho W, Kaneko H, Fowler B, Bogdanovich S, Albuquerque RC, Hauswirth W, Chiodo V, Kugel J, Goodrich J, Ponicsan S, Chaudhuri G, Murphy M, Dunaief J, Ambati B, Ogura Y, Yoo J, Lee D, Provost P, Hinton D, Núñez G, Baffi J, Kleinman M, Ambati J. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D’Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, Tschopp J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci USA. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Yu JZ, Li QY, Ma CG, Lu CZ, Xiao BG. TSPO-specific ligand Vinpocetine exerts a neuroprotective effect by suppressing microglial inflammation. Neuron Glia Biol. 2011;7:187–197. doi: 10.1017/S1740925X12000129. [DOI] [PubMed] [Google Scholar]