Abstract:
Although mesenchymal stem cells (MSCs) have a therapeutic potential for the repair of tissue injuries, their poor viability in damaged tissue limits their effectiveness. Statins can induce an increased production of heme oxygenase-1 (HO-1), which may prevent this detrimental effect in MSCs. We investigated the protective effect of statin-induced overexpression of HO-1 by examining changes in gene expression and function in MSCs after pitavastatin treatment. The relative expression of the HO-1 and endothelial nitric oxide synthase genes in MSCs was significantly increased after treatment with pitavastatin (PitaMSCs). Immunocytological analysis showed that PitaMSCs also stained with phospho-Akt. After exposure to oxidative stress, PitaMSCs showed increased resistance to induced cell death compared with control MSCs. Under serum starvation conditions, MSCs treated with 1 μM pitavastatin showed enhanced cell proliferation and a marked increase in vascular endothelial growth factor production compared with control MSCs. Interestingly, PitaMSCs showed enhanced tube formation under both normoxia and hypoxia. These results demonstrate that pitavastatin can enhance endogenous HO-1 expression in MSCs, which may protect the cells into the environment of oxidative stress with partial activation of endothelial nitric oxide synthase and Akt phosphorylation.
Key Words: heme oxygenase-1, statins, mesenchymal stem cells, cytoprotection
INTRODUCTION
Mesenchymal stem cells (MSCs) have been shown to have considerable potential in stem cell therapies particularly in ischemic heart disease. However, the relatively poor viability of MSCs in injured tissues, probably as a result of oxidative stress, limits their repair capabilities. Heme oxygenase-1 (HO-1), the rate-limiting enzyme for heme degradation, catalyzes the stepwise degradation of heme to produce equimolar quantities of biliverdin, iron, and carbon monoxide.1 HO-1 has been identified as a protein with antioxidant, anti-inflammatory, and cytoprotective functions through the effects of its metabolites.2 We previously reported that transient overexpression of the human HO-1 gene in MSCs, achieved using a plasmid vector, improved cardiac function in a myocardial infarction model.3 Therefore, MSCs that overexpress HO-1 may have the potential to act as high-functioning stem cells for clinical applications of stem cell therapy.
Upregulation of HO-1 expression is induced by many different factors, such as cytokines (interleukin-10, tumor necrosis factor), endotoxins, angiotensin II, hypoxia, and various drugs.4,5 Thus, HO-1 augmentation may indicate an endogenous defensive system in cells or organs.6,7 Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, such as statins, have been demonstrated to influence production of inflammatory cytokines and other mediators to moderate pleiotropic effects8 and to markedly upregulate HO-1 gene expression in a cell- and species-specific manner.9 However, few data exist regarding the impact of endogenous overexpression of HO-1 in MSCs on the survival and functional capabilities of the cells. In this study, we assessed the effect of pitavastatin-induced endogenous expression of HO-1 in cultures of MSCs.
MATERIALS AND METHODS
Preparation of Bone Marrow–derived MSCs
All of the animal experiments were performed in accordance with the internationally accepted guidelines from the Animal Care Committee of Kanazawa University, which approved our experimental protocol.
MSC expansion and isolation were performed according to previously described methods.10 Briefly, male Lewis rats (200–300 g; Japan SLC, Hamamatsu, Japan) were anesthetized by injection of pentobarbital sodium (30 mg/kg body weight intraperitoneally) with buprenorphine (0.03 mg/kg subcutaneously) for pain control. Immediately after killed, bone marrow was harvested by flushing the femoral and tibial cavities with phosphate–buffered saline. The cells were then cultured in standard medium [Gibco's minimum essential medium alpha (MEM alpha)] with 10% fetal bovine serum (FBS; ICN Biomedicals, Inc, Irvine, CA), 100 U/mL penicillin, and 100 μg/mL streptomycin. Nonadherent cells were removed, and the medium was replaced. Cell culture experiments were performed at 37°C in 5% humidified CO2. In all experiments, adherent and spindle-shaped MSCs were used at passages 3–5. Phenotypic analysis of adhered cultured cells from the three-fifth passage showed strongly expressed CD29, CD73, CD90, and SSEA-1 using standard flow cytometry techniques (JSAN Biosciences, La Jolla, CA).10 In contrast, these cells were negative for CD14, CD34, CD45, and SSEA-4. Cultured MSCs (1 × 105 cells per well) were incubated in control media or treated with pitavastatin (0, 0.1, 0.5, and 1 μM; Kowa Co, Ltd, Nagoya, Japan) for the specified times. Pitavastatin-treated cells are referred to in this article as PitaMSCs.
Polymerase Chain Reaction Analysis of Changes in Expression of Endothelial Nitric Oxide Synthase, HO-1, and Hypoxia-inducible Factor-1α Genes
The levels of endothelial nitric oxide synthase (eNOS) and HO-1 mRNAs were determined in MSCs treated with pitavastatin for 6 or 24 hours.11 The level of HIF-1α messenger RNA (mRNA) in MSCs treated for 24 hours with pitavastatin (1 μM) was assessed in the hypoxic state (anaerobic bench, 5% O2). mRNA levels for rat HO-1, eNOS, HIF-1α, and GAPDH were quantified using the SYBR Green 2-step real-time reverse transcriptase-polymerase chain reaction (RT-PCR) protocol. Briefly, total RNA was extracted from cells with guanidine isothiocyanate (RNeasy Mini Kits; QIAGEN, Valencia, CA). Total RNA (500 ng) was used to synthesize first-strand complementary DNA (cDNA) using the First-Strand Synthesis System for RT-PCR (Invitrogen). The cDNA product was amplified by PCR. The PCR reaction mixture was prepared using the SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA). Each sample was analyzed in duplicate using the conditions recommended by the manufacturer. The following primers were used: rat HO-1 sense primer, 5′-AGCTCTATCGTGCTCGC-3′, and anti-sense primer, 5′-GTGTTCCTCTGTCAGCAGT-3′; rat eNOS sense primer, 5′-GGACCCAAGTTTCCTCGAGTAA-3′, and anti-sense primer, 5′-GGATCCCAAGCAGCGTCTT-3′; HIF-1α sense primer, 5′-ACAAGTCACCACAGGACAG-3′, and anti-sense primer, 5′-AGGGAGAAAATCAAGTCG-3′; GAPDH sense primer, 5′-ATGGCACAGTCAAGGGTGAGA-3′, and anti-sense primer, 5′-CGCTCCTGGAAGATGGTGAT-3′. After initial denaturation at 95°C for 60 seconds, a 2-cycle procedure was used (denaturation at 95°C for 15 seconds, annealing and extension at 60°C for 1 minute) for 40 cycles in an ABI PRISM 7700 Sequence Detector System (PE Applied Biosystems). Using the manufacturer's software, real-time PCR data were plotted as the ΔRn fluorescence signal versus the cycle number. The cycle threshold was defined as the cycle number at which the ΔRn crossed this threshold. The expression of each gene was normalized against GAPDH mRNA and calculated relative to the control using comparative cycle threshold methods.
Immunofluorescent Staining of HO-1 and Phospho-Akt
MSCs that had been treated with pitavastatin (1 μM) for 24 hours were immunostained for HO-1. We set the concentration of pitavastatin from the result of real-time RT-PCR. MSCs treated with the same concentrations of pitavastatin (1 μM) under serum-free conditions for 6 hours11 were immunostained for phospho-Akt. The MSCs were fixed with 4% paraformaldehyde for 20 minutes and permeabilized with 70% ethyl alcohol. After blocking with 1% bovine serum albumin (Wako Pure Chemical Industries, Osaka, Japan) for 30 minutes, the fixed cells were incubated with a primary antibody [anti-HO1, 1:100; StressGen Biotechnologies, Victoria, BC, and anti-Phospho-Akt (Ser473), 1:200; Cell Signaling Tech, Beverly, MA] overnight at 4 °C. After washing with phosphate–buffered saline, the cells were incubated for 1 hour with a fluorochrome-conjugated secondary antibody (1:50; Dako A/S, Glostrup, Denmark). Nuclei were stained with Hoechst 33342 (Wako Pure Chemical Industries). Stained cells were viewed and photographed using a BZ-9000 fluorescence microscope (Keyence Corporation, Osaka, Japan).
Western Blot Analysis
Western blot analysis was performed with duplicate fashion. Fifty micrograms of cell lysate was resolved in 10% sodium dodecyl sulfate-polyacrylamide gels. After electrophoresis, the proteins were electrotransferred to polyvinylidene difluoride membranes, blocked with 10 mM Tris-HCl, 2% SDS, 20% sucrase, 0.06% bromophenol blue, and 100 mM DTT and incubated with the appropriate antibodies: rabbit anti–HO-1 (1:200, ADI-SPA-895; Enzo Life Science, Tokyo, Japan), rabbit anti-Akt (pan) (1:2000, #4691; Cell Signaling), and rabbit anti–phospho-Akt (1:2000, #4060; Cell Signaling). The blots were then incubated with anti-rabbit HRP-conjugated secondary antibody (1:1000, sc-2054; Santa Cruz). The protein bands were measured with Luminescent Image Analyzer (LAS-4000 mini; Fujifilm Co Ltd, Tokyo, Japan).
Analysis of Cell Viability Under Conditions of Oxidative Stress and Secretion of Vascular Endothelial Growth Factor
For oxidative stress experiments, MSCs or PitaMSCs (0.1, 0.5, and 1 µM) were seeded in 96-well microculture plates (1 × 104 cells per well, passage 4) for 24 hours. The culture medium was replaced with fresh medium containing 400 μM H2O2 (Mitsubishi Gas Chemical Company, Tokyo, Japan) for 2 hours12 or under hypoxic conditions for 24 hours on an anaerobic bench. After this incubation period, the medium was replaced with fresh medium, and cell viability and functional capacity were assessed using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI). Twenty microliters of MTS solution was added to each well, and the microculture plates were incubated for 1–4 hours at 37°C in a humidified 5% CO2 atmosphere. The quantity of formazan product was measured by absorbance at 490 nm with a microtiter plate reader (Multiskan JX Version 1.1; Thermo Fisher Scientific, Waltham, MA).
We also analyzed vascular endothelial growth factor (VEGF) levels in paracrine media (10 cm culture dishes) from MSCs and PitaMSCs (1 µM) after a 24-hour culture under normoxic or hypoxic conditions. VEGF levels from paracrine media were measured using enzyme-linked immunosorbent assay Kits (R&D systems Diagnostic, Salem, NH).
Assays for Proliferation and Tube Formation
MSCs (5 × 104 cells per well, passage 3) were seeded on 96-well plates in growth medium, then serum-starved overnight as standard. After incubation with pitavastatin (0.1, 0.5, and 1 µM) for 24 hours, 10 µM BrdU was added for 2 hours. Cell proliferation rates were measured by a BrdU incorporation assay (Cell proliferation ELISA; Roche Diagnostics GmbH, Mannheim, Germany) according to the vendor's protocol; cell numbers in harvested plates were quantified using a plate reader (Thermo Labsystems, Franklin, MA).
The in vitro tube formation assay examines the potential for endothelial-like cells to form capillary-like tubules when planted on a reconstituted basement membrane of Matrigel. Chamber slides were coated with undiluted Matrigel solution (In Vitro Angiogenesis Assay kit; Trevigen, Gaithersburg, MD) and allowed to solidify overnight in a humidified 37 °C CO2 incubator. MSCs from primary cultures were plated at a density of at least 5 × 104 cells per test well and serum-starved overnight. Serum-free medium containing pitavastatin was added, and the slides were further incubated under normoxic or hypoxic conditions. Tube formation was quantified by counting the capillary branches in randomly selected high-power (×40) microscopic fields.
Statistical Analysis
All data are presented as mean ± SD. The data from different treatments were compared by one-way analyses of variance for repeated measures (Stat-View version 5.0; SAS, Cary, NC). Further analyses were performed using unpaired Student's t tests or post hoc Bonferroni/Dunn tests. Differences were considered significant at P < 0.05.
RESULTS
Pitavastatin-induced Expression of HO-1 and eNOS
MSCs incubated with pitavastatin for 24 hours were found to adhere to the tissue culture dish and did not show any morphological changes. The relative expression of HO-1 increased with pitavastatin concentration from 1.01 ± 0.04 in the control to 1.48 ± 0.13 (P < 0.05) in cells treated with 1 µM (Figure 1A). Likewise, eNOS mRNA levels increased from 0.82 ± 0.19 in the control to 1.41 ± 0.12 (P < 0.05) in cells treated with 1 µM (Figure 1B).
FIGURE 1.
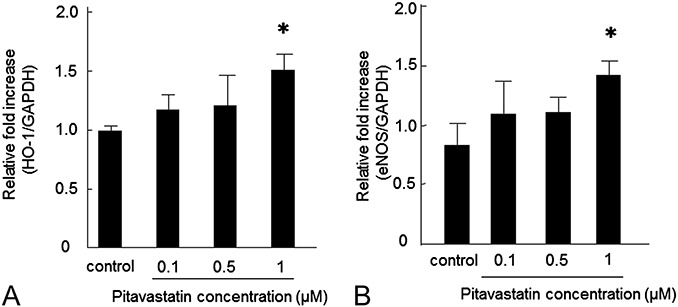
Effect of pitavastatin treatment on HO-1 and eNOS mRNA levels in MSCs. HO-1 (A) and eNOS (B) mRNA levels increased in a dose-dependent manner after pitavastatin treatment. Data are shown as mean ± SD (n = 6). ∗P < 0.05 versus control.
Immunocytological analysis showed enhanced HO-1 staining in MSCs cultured for 24 hours with pitavastatin (1 µM) by comparison without pitavastatin (Figure 2A). As other reactions, pitavastatin (1 µM) treatment for 6 hours also enhanced Akt phosphorylation in the MSCs (Figure 2B).
FIGURE 2.
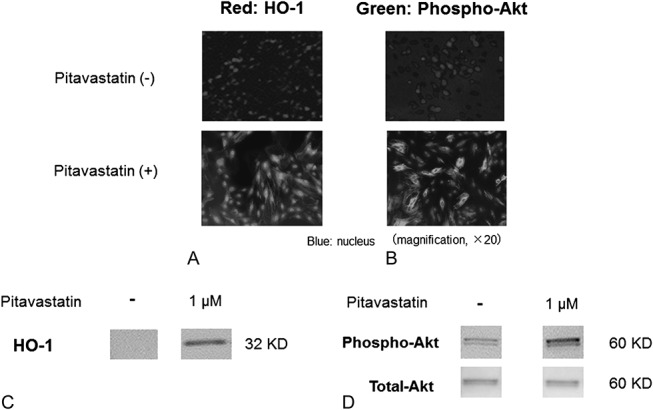
Expression of HO-1 (A) and phospho-Akt (B) in MSCs (PitaMSCs) with or without pitavastatin treatment. Immunostaining (A and B) and Western blotting (C and D) analyses with duplicate fashion showed that both HO-1 and Akt phosphorylation were enhanced by pitavastatin treatment. The reason why we detected at least 2 bands of phospho-Akt by Western blotting using anti-phospho-Akt (Ser473) antibody might be that Akt was phosphorylated at several sites including Thr308, Tyr315, and Tyr326.
Western bolt analysis with duplicate fashion showed the enhanced protein expression of HO-1 (Figure 2C) and phospho-Akt in whole cell lysate from PitaMSCs (1 μM) without the change of total-Akt level (Figure 2D).
Protective Effect of Pitavastatin Against Oxidative Stress and Serum Starvation
A high concentration (400 μM) of H2O2 caused a dramatic decrease in cell viability, which was represented by the ratio of surviving cells under the oxidative stress to surviving cells under the non-oxidative stress in both MSCs and PitaMSCs (0.1 μM). However, the same concentration of H2O2 had a significantly lower effect on cell viability in PitaMSCs treated with 0.5 (40% ± 8%) and 1 μM (43% ± 6%) pitavastatin in comparison with the control (18% ± 4%, P < 0.05, Figure 3). This suggests that the induced overexpression of HO-1 and eNOS genes by pitavastatin provided a protective effect against the oxidative stress imposed by a high concentration of H2O2.
FIGURE 3.
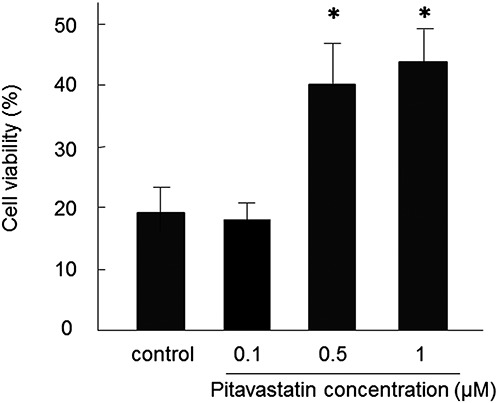
Effects of pitavastatin-induced increase in HO-1 expression on cell viability during oxidative stress. Cell viability (vertical axis) was evaluated in cells exposed to H2O2 (400 µM) for 2 hours. Data are mean ± SD (n = 8). ∗P < 0.05 versus control.
With regard to the effects of serum starvation, MSCs treated with 1 μM pitavastatin showed a significantly higher proliferation rate compared with untreated cells in the absence of FBS (0.33 ± 0.04 vs. 0.24 ± 0.02, P < 0.05; Figure 4). Moreover, the proliferation rate of the PitaMSCs was comparable with that of MSCs cultured in the presence of FBS.
FIGURE 4.
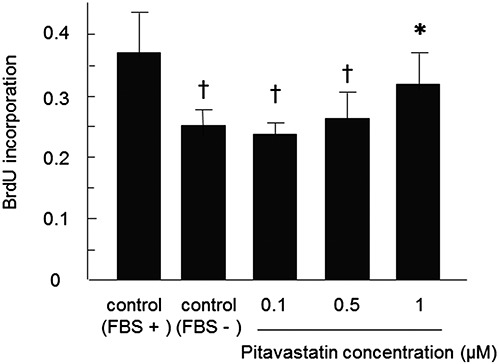
Effect of pitavastatin on MSC proliferation. Cultured MSCs were treated with a range of pitavastatin concentrations (0, 0.1, 0.5, and 1 µM) in serum-free medium or medium containing 10% FBS. Data are mean ± SD (n = 6). ∗P < 0.05 versus control (FBS−), †P < 0.05 versus control (FBS+).
Secretion of VEGF and Hypoxia-induced Expression of HIF-1α
In the hypoxic state, VEGF secretion was significantly enhanced by pitavastatin (1 µM) [706 ± 38 (pitava−) vs. 796 ± 60, P < 0.05; Figure 5A]; however, VEGF secretion was essentially unchanged under normoxic conditions. HIF-1α expression with pitavastatin (1 µM) was elevated only in cells cultured under hypoxic conditions [1.7 ± 0.1 (pitava−) vs. 2.1 ± 0.1, P < 0.05; Figure 5B], suggesting that pitavastatin-induced VEGF secretion under hypoxic conditions might be related to HIF-1α expression.
FIGURE 5.
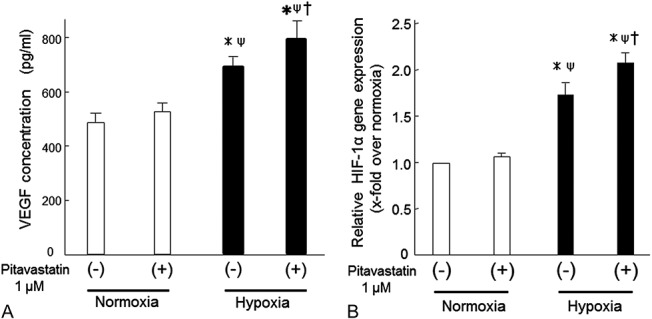
A, VEGF production and (B) expression of the hypoxia-inducible factor (HIF)-1 gene in MSCs before and after pitavastatin treatment. Changes in VEGF concentrations (A, vertical axis) in culture media from MSCs and PitaMSCs under normoxia or hypoxia. Real-time RT-PCR analysis of HIF-1α mRNA levels (B) in MSCs or PitaMSCs that were cultured for 24 hours under normoxia or hypoxia. Data are mean ± SD (n = 6). ∗P < 0.05 versus normoxia (pitava−), ΨP < 0.05 versus normoxia (pitava+), †P < 0.05 versus hypoxia (pitava−).
Tube Formation in Pitavastatin-treated MSCs
To evaluate the functional capability of PitaMSCs, we examined tube formation in vitro. The rate of tube formation was low in the absence of FBS in both normoxic and hypoxic conditions; however, clear tube formation was evident in cultures with FBS. In the absence of FBS, pitavastatin treatment caused a clear increase in the numbers of tubes formed after 24 hours in normoxic [182 ± 21 branches per well (pitava+) vs. 130 ± 13 branches per well (FBS+), P < 0.05] and hypoxic [142 ± 18 branches per well (pitava+) vs. 95 ± 10 branches per well (FBS+), P < 0.05; Figure 6A–B] cultures.
FIGURE 6.
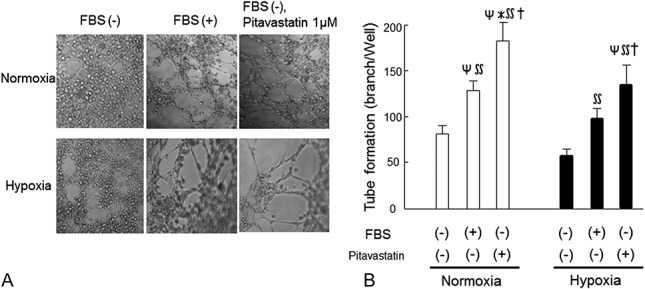
Effect of pitavastatin treatment on capillary tube formation in cultured MSCs. A, Representative photographs of MSCs cultured on Matrigel after incubation under normoxia (upper) or hypoxia (lower). Culture of MSCs was performed without serum (left), with serum (center), and with serum-free plus pitavastatin (1 µM, right) (n = 4 for each group). B, Quantitative analysis of tube formation. Data are mean ± SD (n = 4) for each group. ΨP < 0.05 versus normoxia (FBS−, pitava−), ∗P < 0.05 versus normoxia (FBS+, pitava−), ∬P < 0.05 versus hypoxia (FBS−, pitava−), †P < 0.05 versus hypoxia (FBS+, pitava−).
DISCUSSION
Some clinical studies of stem cell–based therapies for ischemic heart disease have reported only modest beneficial effects.13–15 One possible reason for this relative lack of effect may be the poor viability of transplanted cells, which may lack resistance to oxidative stress in the ischemic heart.16,17 We previously reported that plasmid-mediated overexpression of HO-1 in MSCs resulted in the cells showing improved antioxidative and antiapoptotic properties, which enabled a higher rate of survival after transplantation compared with unmodified MSCs.18 Grosser et al19 demonstrated the induction of HO-1 expression by lovastatin and simvastatin in endothelial cells. As their report suggested that statins might induce HO-1 expression in other cell types, such as MSCs, we investigated whether pitavastatin could increase expression of antioxidant genes in stem cells for use in clinical applications.
Our analyses showed that expression of HO-1, eNOS, and phosphorylated-Akt was enhanced by pitavastatin20 and provided protection for the MSCs from oxidative stress. Statins rapidly promote the activation of Akt in endothelial cells, leading to eNOS activity.11 The activation of Akt is also suggested to be responsible for endothelial cell proliferation and survival.21 Enhancement of eNOS activity was suggested to play a significant role in the cell protective, cardioprotective, and antiapoptotic effects of statins.22,23
Weis et al described a biphasic effect of statins on the proliferation of human endothelial cells.24 In this study, we observed that MSC proliferation increased after treatment with 1 µM pravastatin under serum deprivation conditions. This suggests a fundamental codependence among HO-1, eNOS, and Akt in mediating cytoprotection against oxidative stress probably with cell apoptosis or death.
HIF-1α in MSCs is related to the hypoxic status of the cells and increases expression of VEGF, hepatic growth factor, and insulin-like growth factor-1 under hypoxic conditions.25 HIF-1α and VEGF expression may be regulated by NO pathway associated with hematopoietic stem cell and neural stem cell proliferative and survival under the hypoxic condition.26 HO-1 is a stress-inducible enzyme that regulates angiogenesis through the induction of VEGF.12 We found here that the combined effects of increased HO-1 and HIF-1α expression induced by pitavastatin in hypoxic cells, which might occur in an ischemic environment, were associated with increased secretion of VEGF. Furthermore, we found that pitavastatin could enhance the rate of tube formation by MSCs under both normoxia and hypoxia, thus resulting in an improvement to the angiogenic properties of the MSCs. These results suggest that pitavastatin produces improvement in both the quality and function of MSCs.
MSC transplantation may provide a cardiac protection effect in a paracrine fashion by abundant cytokine secretion in ischemic heart injury.18,27,28 Strong statins, such as pitavastatin, can reduce cardiac events in the early phases of ischemic heart injury,29 and it also has been reported that a combination of statin and MCS transplantation improves revascularization in ischemic limbs.30 Although we have not yet assessed the effect of PitaMSC transplantation in a vivo study using an infarction model, our results here would indicate that the cells might enhance myocardial protection against ischemic injury.
CONCLUSIONS
In conclusion, this study demonstrates that pitavastatin might improve the survival of MSCs by enhancing endogenous HO-1 with partial activation of eNOS and Akt phosphorylation and might result in improvements to MSC functions, such as proliferation and tube formation.
ACKNOWLEDGMENTS
The authors thank Mayumi Yoshida for excellent technical assistance. In addition, they thank Kowa Co, Ltd (Nagoya, Japan) for the gift of pitavastatin.
Footnotes
Supported by grants from the Ministry of Education, Science and Culture of Japan, and from the Ministry of Health, Welfare and Labor of Japan.
Presented in part at the American College of Cardiology 61st Annual Scientific Session, March 2012, Chicago, IL.
Dr. Masakazu Yamagishi received lecture fees from Kowa Pharma Inc. The other authors report no conflicts of interest.
M. Kawashiri, C. Nakanishi, and T. Tsubokawa have contributed equally.
REFERENCES
- 1.Shibahara S, Yoshizawa M, Suzuki H, et al. Functional analysis of cDNA for two types of human hemeoxygenase and evidence for their separate regulation. J Biochem. 1993;113:214–218. [DOI] [PubMed] [Google Scholar]
- 2.Otterbein LE, Choi AM. Hemeoxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. [DOI] [PubMed] [Google Scholar]
- 3.Tsubokawa T, Yagi K, Nakanishi C, et al. Impact of anti-apoptotic and ant-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:H1320–H1329. [DOI] [PubMed] [Google Scholar]
- 4.Geraldes P, Yagi K, Ohshiro Y, et al. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J Biol Chem. 2008;283:34327–34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otterbein LE, Soares MP, Yamashita K, et al. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. [DOI] [PubMed] [Google Scholar]
- 6.Choi AM. Heme oxygenase-1 protects the heart. Circ Res. 2001;89:105–107. [PubMed] [Google Scholar]
- 7.Choi BM, Kim BR. Upregulation of heme oxygenase-1 by brazilin via the phosphatidylinositol 3-kinase/Akt and ERK pathways and its protective effect against oxidative injury. Eur J Parmacol. 2008;580:12–18. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita K, Ollinger R, McDaid J, et al. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J. 2006;20:776–778. [DOI] [PubMed] [Google Scholar]
- 9.Lee TS, Chang CC, Zhu Y, et al. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. [DOI] [PubMed] [Google Scholar]
- 10.Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Xu Z, Kiajima I, et al. Effect of different statins on endothelial nitric oxide synthase and AKT phosphorylation in endothelial cells. Int J Cardiol. 2008;127:33–39. [DOI] [PubMed] [Google Scholar]
- 12.Lakkisto P, Kytö V, Forsten H, et al. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635:156–164. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Avilés F, San Román JA, García-Frade J, et al. Experimental and clinical regeneration capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. [DOI] [PubMed] [Google Scholar]
- 14.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. [DOI] [PubMed] [Google Scholar]
- 16.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. [DOI] [PubMed] [Google Scholar]
- 17.Song SW, Chang W, Song BW, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27:1358–1365. [DOI] [PubMed] [Google Scholar]
- 18.Jo J, Nagaya N, Miyahara Y, et al. Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial infarction: benefit of a novel nonviral vector, cationized dextran. Tissue Eng. 2007;13:313–322. [DOI] [PubMed] [Google Scholar]
- 19.Grosser N, Hemmerle A, Berndt G, et al. The antioxidant defense protein heme oxygenase 1 is a novel target for statins in endothelial cells. Free Radic Biol Med. 2004;37:2064–2071. [DOI] [PubMed] [Google Scholar]
- 20.Tsubokawa T, Nakanishi C, Tagawa S, et al. Impact of pitavastatin pretreatment on survival and functional activities of mesenchymal stem cell: possible implication for cell transplantation therapy. J Am Coll Cardiol. 2012;59(suppl):E1039. [Google Scholar]
- 21.Jiang BH, Zheng JZ, Aoki M, et al. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2002;97:1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi N, Takeshima H, Fukushima H, et al. Cardioprotective effects of pitavastatin on cardiac performance and remodeling in failing rat hearts. Am J Hypertens. 2008;22:176–182. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui AJ, Gustafsson T, Fischer H, et al. Simvastatin enhances myocardial angiogenesis induced by vascular endothelial growth factor gene transfer. J Mol Cell Cardiol. 2004;37:1235–1244. [DOI] [PubMed] [Google Scholar]
- 24.Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. [DOI] [PubMed] [Google Scholar]
- 25.Tamama K, Kawasaki H, Kerpedjieva SS, et al. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem. 2011;112:804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madri JA.Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(suppl 4):95–104. [PubMed] [Google Scholar]
- 27.Dai W, Hale SL, Kloner RA. Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regen Med. 2007;2:63–68. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi C, Nagaya N, Ohnishi S, et al. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ J. 2011;75:2260–2268. [DOI] [PubMed] [Google Scholar]
- 29.Hibi K, Kimura T, Kimura K, et al. ; JAPAN-ACS Investigators. Clinically evident polyvascular disease and regression of coronary atherosclerosis after intensive statin therapy in patients with acute coronary syndrome: serial intravascular ultrasound from the Japanese assessment of pitavastatin and atorvastatin in acute coronary syndrome (JAPAN-ACS) trial. Atherosclerosis. 2001;219:743–749. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhang R, Li Y, et al. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Mol Biol Rep. 2012;39:285–293. [DOI] [PubMed] [Google Scholar]


