Abstract:
In a number of isolated blood vessel types, hypoxia causes an acute contraction that is dependent on the presence of nitric oxide and activation of soluble guanylyl cyclase. It is more pronounced when the preparations are constricted and is therefore termed hypoxic augmentation of vasoconstriction. This hypoxic response is accompanied by increases in the intracellular level of inosine 5′-triphosphate and in the synthesis of inosine 3′,5′-cyclic monophosphate (cIMP) by soluble guanylyl cyclase. The administration of exogenous cIMP or inosine 5′-triphosphate causes augmented vasoconstriction to hypoxia. Furthermore, the vasoconstriction evoked by hypoxia and cIMP is associated with increased activity of Rho kinase (ROCK), indicating that cIMP may mediate the hypoxic effect by sensitizing the myofilaments to Ca2+ through ROCK. Hypoxia is implicated in exaggerated vasoconstriction in the pathogenesis of coronary artery disease, myocardial infarction, hypertension, and stroke. The newly found role of cIMP may help to identify unique therapeutic targets for certain cardiovascular disorders.
Key Words: hypoxia, vasoconstriction, nitric oxide, soluble guanylyl cyclase, cIMP
(See editorial: Kuala Lumpur Emerging in Vascular Biology by Paul M. Vanhoutte. Journal of Cardiovascular Pharmacology, 2015 65:6;297–298)
INTRODUCTION
In the presence of the endothelium, hypoxia causes a rapid constriction in a number of blood vessel types.1–11 Such a response also occurs in arteries without endothelium treated with exogenous nitric oxide (NO).8,11 It is prevented by the inhibition of soluble guanylyl cyclase (sGC) but appears not to be related to guanosine 3′,5′-cyclic monophosphate (cGMP) signaling.8,11 Recent studies suggest that inosine 3′,5′-cyclic monophosphate (cIMP) synthesized by sGC may act as the mediator of such hypoxic “vasospasm.”11,12 This brief review discusses the characteristics of the endothelium-dependent hypoxic vasospasm, the synthesis of cIMP by sGC, and the role of this cyclic nucleotide in augmented vasoconstrictions in response to hypoxia.
CHARACTERISTICS OF HYPOXIC SPASM
The phenomenon that hypoxia can cause an acute contraction in systemic blood vessels was first reported in 1976 when describing experiments in isolated canine veins.1 This phenomenon has subsequently been confirmed in a number of blood vessel types including the canine femoral2,3 and coronary4–6 arteries, porcine coronary arteries,8,10,11 rat aortae and mesenteric arteries,9 and human and canine internal mammary arteries.6 The hypoxic constriction is more pronounced when the blood vessels are contracted. Therefore, it is more appropriate to be termed “hypoxic augmentation of vasoconstriction.”5 It occurs in arteries contracted with norepinephrine,2,3 phenylephrine,9 prostaglandin F2α,4,5,7 or the thromboxane A2 mimetic U46619.10,11 Thus, it is not related to a specific vasoconstrictor or restricted to a single blood vessel type.
The hypoxic augmentation of vasoconstriction occurs in arteries with endothelium but not in those without endothelium.4,5,8–11 It is not due to the release by the endothelial cells of a known endothelium-derived contracting factor, such as endothelin 1, constrictor prostanoids, or reactive oxygen species.8 The involvements of calcium-active potassium channels, inward rectifier potassium channels, L-type calcium channels, sodium–potassium ATPase, hyperpolarizing factors, gap junctions, adenylyl cyclase, and adenosine 3′,5′-cyclic monophosphate (cAMP)–dependent protein kinase (PKA) were excluded by studies with various pharmacological inhibitors.8
The endothelium-dependent hypoxic augmentation of vasoconstriction is abolished by the inhibition of endothelial NO synthase with nitro-l-arginine and by the inhibition of sGC with methylene blue, ODQ, or NS2028.5,8–11 It is restored in arteries without endothelium by exogenous NO donors or Bay 58-2667 (a heme-independent activator of sGC).5,8,11 These results indicate that activation of sGC is critically involved. Cyclic guanosine monophosphate is considered the only known functional molecule synthesized by sGC, but its action is to cause vasodilatation.13–15 Therefore, a suppressed formation of cGMP by sGC under hypoxia would be expected to promote vasoconstriction. However, the hypoxic response is not accompanied by significant changes in cGMP levels.8,11 It is also not affected by the inhibition of cGMP-dependent protein kinase (PKG, the primary downstream effector of the cyclic nucleotide).8,11 In arteries without endothelium, the absence of hypoxic augmentation of vasoconstriction is not restored by the cell-permeable cGMP analog 8-Br-cGMP or by the elevation of the intracellular level of cGMP with atrial natriuretic peptide.8 Hence, cGMP is unlikely to be involved. Since the hypoxic response is enhanced by inhibition of phosphodiesterase,5 the phenomenon cannot be mediated by a degradation product(s) of cGMP but rather is due to a product of sGC different from cGMP.
CYCLIC INOSINE 5′-MONOPHOSPHATE SYNTHESIZED BY sGC
sGC is a heterodimer composed of an α (α1 or α2) and a β (β1) subunit, with the α1β1 dimer being the dominant isoform in most tissues including blood vessels. The β subunit of sGC contains a haem moiety as a prosthetic group. The binding of NO to the Fe2+ center of the haem moiety leads to a conformational change and a several hundred-fold increase in the rate of cGMP synthesis.16 Seifert et al, using a highly sensitive and specific high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS), demonstrated that, in addition to cGMP, purified recombinant rat sGC can synthesize cIMP, cAMP, and xanthosine 3′,5′-cyclic monophosphate (cXMP) when Mg2+ is used as a cofactor. If Mg2+ is replaced with Mn2+, 2 more cyclic nucleotides [uridine 3′,5′-cyclic monophosphate (cUMP) and cytidine 3′,5′-cyclic monophosphate (cCMP)] can also be synthesized. Among these nucleoside 3′,5′-cyclic monophosphates, the maximal rate of cIMP formation is substantially greater than that of the others except cGMP (with Mg2+ as the cofactor: cIMP > cGMP >> cUMP >>> cXMP; with Mn2+ as the cofactor: cGMP > cIMP > cUMP>> cAMP > cCMP ≈ cXMP).17 Regarding the affinity of sGC for the substrate, the KM of inosine 5′-triphosphate [(ITP), the substrate for cIMP] is considerably greater than that of guanosine 5′-triphosphate [(GTP), the substrate for cGMP] if Mg2+ serves as the cofactor. However, when Mg2+ is replaced with Mn2+, the KM of ITP is lower than GTP.17 In intact HEK293 cells overexpressing sGC α1β1 and rat fetal lung fibroblast (RFL-6) cells endogenously expressing sGC, Mn2+ rather than Mg2+ may be the physiological cofactor for sGC.18
In isolated porcine coronary arteries, there is no difference in the basal levels of cIMP between arteries with or without endothelium whether treated or not with a sGC inhibitor, indicating a minor formation of the nucleotide by sGC under basal conditions.11 The cIMP content in cultured HEK293 cells overexpressing the isoform A of particulate guanylyl cyclases (pGC-A) is also reported to be below detection level.19 When exposed to hypoxia (25–30 mm Hg), porcine coronary arteries with endothelium exhibit an increase in cIMP level of the order of 311 pmole/mg protein.11 The levels of cIMP are also elevated in arteries without endothelium stimulated with exogenous NO or in arteries with endothelium treated with ITP, the substrate for cIMP formation. The elevation in cIMP under those conditions is further enhanced by hypoxia. These changes in cIMP are prevented by ODQ,11 indicating that cIMP is synthesized by sGC.11 In contrast to cIMP, in arteries with endothelium, the cGMP level is substantially greater in the absence than in the presence of ODQ, suggesting an active basal synthesis of cGMP by sGC.11 However, hypoxia has no significant effect on the cGMP levels.8,11
The stimulatory effect of hypoxia on the synthesis of cIMP by sGC in porcine coronary arteries is associated with an elevated intracellular level of ITP.11 Hence, an increased availability of the substrate is involved. ITP is primarily derived from ATP deamination.20–22 ATP is the most abundant nucleotide in cells, with an intracellular concentration in the low millimolar range.23 It has been estimated that approximately 10%–25% of the ATP pool can be deaminated to ITP.20 The intracellular level of cIMP is increased also by exogenous ATP.24 Under normal conditions, ITP is largely degraded by inosine triphosphatase and the intracellular level of ITP is very low.20 However, if hypoxia were to increase ATP deamination and/or inhibit inosine triphosphatase, the ITP level may increase,20–22 which would facilitate the increased synthesis of cIMP by sGC (Fig. 1).
FIGURE 1.
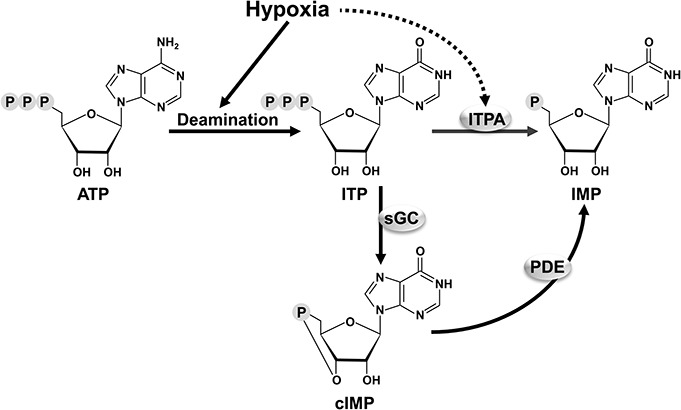
Possible mechanism for increased synthesis of cIMP by sGC in response to hypoxia. The intracellular ITP is primarily derived from the deamination of ATP, which is the most abundant nucleotide inside the cell. Under normal conditions, ITP is mostly converted to IMP by ITPA and the cytosolic level of ITP is negligible. Under hypoxia, ATP deamination is stimulated and/or ITPA is inhibited. This leads to elevated ITP level, increased formation of cIMP by sGC, and hence enhanced cIMP action. Cyclic IMP is degraded by phosphodiesterase (PDE). Full arrows indicate activation and dotted arrows indicate inhibition. IMP, inosine 5′-monophosphate; ITPA, inosine triphosphatase.
CYCLIC INOSINE 5′-MONOPHOSPHATE IN HYPOXIC VASOSPASM
In porcine coronary arteries with endothelium, the hypoxic augmentation of vasoconstriction is further enhanced by ITP in a manner sensitive to inhibition of sGC.11 In arteries without endothelium, the administration of cIMP restores the hypoxic augmentation.11 Therefore, it appears that cIMP derived from sGC is causally related to the response (Fig. 2).
FIGURE 2.
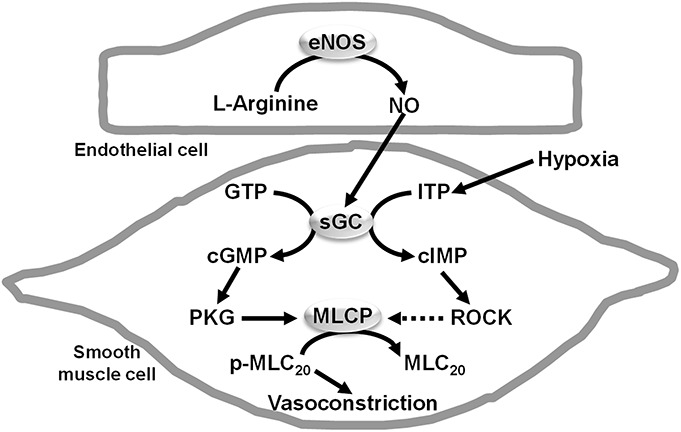
Proposed mechanism involving cIMP as a mediator in hypoxic augmentation of vasoconstriction. Hypoxia may stimulate the synthesis of cIMP by sGC, in part, by increasing the intracellular level of ITP. Cyclic IMP can activate Rho kinase (ROCK), resulting in reduced activity of MLCP, thus decreased dephosphorylation of phosphorylated myosin regulatory light chain (MLC20) and augmented vasoconstriction. Cyclic GMP may counteract the effect of ROCK by stimulating the activity of MLCP through cGMP-dependent protein kinase (PKG). Because hypoxia has no significant effect on cGMP formation, it appears not to play a significant role in hypoxic augmentation of vasoconstriction. Full arrows indicate activation and dotted arrows indicate inhibition (Modified from Gao and Vanhoutte12 by permission of Naunyn-Schmiedeberg's Archiv). IMP, inosine 5′-monophosphate; MLCP, myosin light chain phosphatase; GMP, guanosine monophosphate. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Cyclic inosine 5′-monophosphate or ITP has little effect on the basal tension of coronary arteries but markedly influences hypoxic facilitation of vasoconstriction.11 This observation suggests that cIMP ultimately may act by increasing the sensitivity of myofilaments of the vascular smooth muscle cells to Ca2+. In those cells, Ca2+ sensitization results from the activation of protein kinase C (PKC) or Rho kinase (ROCK), which results in reduced activity of myosin light-chain phosphatase, thus decreased dephosphorylation of phosphorylated myosin regulatory light chain (MLC20) and augmented vasoconstriction.25 The hypoxic augmentation of contraction in the porcine coronary artery is attenuated by inhibitors of ROCK (Y27632 and HA1077), suggesting the involvement of Rho kinase.8,11 ROCK causes Ca2+ sensitization primarily by inhibiting the activity of myosin light chain phosphatase through the phosphorylation of myosin phosphatase target subunit 1 (MYPT1) at Thr696 and Thr853 (human sequence).25,26 The phosphorylation of myosin phosphatase target subunit 1 at Thr853 is stimulated in arteries with endothelium by hypoxia and in arteries without endothelium treated with cIMP.11 In homogenates of porcine coronary arteries, cIMP activates ROCK at concentrations ranging from 10−7 M to 3 × 10−6 M,11 which is well within the physiological range for activation of protein kinases by cyclic nucleotides.27–29 It remains to be determined whether or not ROCK is activated directly by cIMP. This is the case for GTPase-RhoA, arachidonic acid, sphingosine phosphorylcholine, caspase 3, and granzyme B.30 In addition to ROCK, cIMP exhibits a very low affinity for PKG and PKA, classical targets for cyclic nucleotides, which makes these 2 types of protein kinase rather unlikely effectors for cIMP.31 Our previous experiments using various inhibitors have also excluded the possible involvement of PKG and PKA in mediating hypoxic augmentation of vasoconstriction.8
Cyclic guanosine monophosphate is so far considered the only molecule responsible for the actions of sGC.14,15 The findings that hypoxia stimulates the formation of cIMP in a manner sensitive to the inhibition of sGC and that this cyclic nucleotide promotes hypoxic vasoconstriction imply that cIMP derived from sGC may act as a novel signaling molecule. Cyclic inosine 5′-monophosphate is efficiently hydrolyzed by partially purified human recombinant phosphodiesterases 5A, 1B, 3A, and 3B expressed in Spodoptera frugiperda Sf9 insect cells.32,33 Hence, cIMP signaling can be not only turned on upon stimuli such as hypoxia but also can be effectively turned off.
Hypoxemia induces hyperconstriction of canine coronary arteries after reperfusion injury in vivo.7 Twelve weeks later, the arteries still exhibited potentiated endothelium-dependent hypoxic augmentation, sensitive to the inhibition of NO synthase.7 It is possible that cIMP is related to the abnormal responsiveness of these arteries. Repetitive hypoxia is involved in the pathogenesis of coronary artery disease, myocardial infarction, hypertension, and stroke in patients with obstructive sleep apnea.34–36 It is tempting to suggest that exaggerated vasoconstrictions due to increased cIMP formation by sGC during the sleep apnea episodes contribute to the development of these cardiovascular disorders. Clearly, challenges remain before accepting cIMP as a new signaling molecule and unraveling its role in physiology and pathophysiology. Those challenges include the definition of the stimuli and conditions that increase the availability of its precursor ITP, the elucidation of the enzymatic mechanisms underlying its synthesis and degradation, and the formal identification of its downstream targets.8,11,12,17,31 In particular, regarding the role of cIMP in hypoxic vasoconstriction, the development of specific inhibitors of the formation or action of cIMP may yield significant therapeutic benefits.
Footnotes
Supported in part by the National Natural Science Foundation of China Grant 81270341 and the Hong Kong Research Grant Council (University of Hong Kong—777507M and 17112914).
The authors report no conflicts of interest.
REFERENCES
- 1.Vanhoutte PM. Effects of anoxia and glucose depletion on isolated veins of the dog. Am J Physiol. 1976;230:1261–1268. [DOI] [PubMed] [Google Scholar]
- 2.De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall: importance of the endothelium. Circ Res. 1982;51:439–447. [DOI] [PubMed] [Google Scholar]
- 3.De Mey JG, Vanhoutte PM. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983;335:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubanyi GM, Vanhoutte PM. Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J Physiol. 1985;364:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graeser T, Vanhoutte PM. Hypoxic contraction of canine coronary arteries: role of endothelium and cGMP. Am J Physiol. 1991;261:H1769–H1777. [DOI] [PubMed] [Google Scholar]
- 6.Lin PJ, Pearson PJ, Schaff HV. Endothelium-dependent contraction and relaxation of the human and canine internal mammary artery: studies on bypass graft vasospasm. Surgery. 1991;110:127–134. [PubMed] [Google Scholar]
- 7.Pearson PJ, Lin PJ, Schaff HV, et al. Augmented endothelium-dependent constriction to hypoxia early and late following reperfusion of the canine coronary artery. Clin Exp Pharmacol Physiol. 1996;23:634–641. [DOI] [PubMed] [Google Scholar]
- 8.Chan CK, Mak J, Gao Y, et al. Endothelium-derived NO, but not cyclic GMP, is required for hypoxic augmentation in isolated porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2011;301:H2313–H2321. [DOI] [PubMed] [Google Scholar]
- 9.Nuñez C, Victor VM, Martí M, et al. Role of endothelial nitric oxide in pulmonary and systemic arteries during hypoxia. Nitric Oxide. 2013;37:17–27. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Chen Z, Liu J, et al. Endothelium-independent hypoxic contraction of porcine coronary arteries may be mediated by activation of phosphoinositide 3-kinase/Akt pathway. Vascul Pharmacol. 2014;61:56–62. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Zhang X, Ying L, et al. Cyclic IMP-synthesized by sGC as a mediator of hypoxic contraction of coronary arteries. Am J Physiol Heart Circ Physiol. 2014;307:H328–H336. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Vanhoutte PM. Tissues cIMPly do not lie. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:901–903. [DOI] [PubMed] [Google Scholar]
- 13.Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- 14.Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide. 2009;21:149–156. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y. The multiple actions of NO. Pflugers Arch. 2010;459:829–839. [DOI] [PubMed] [Google Scholar]
- 16.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–232. [DOI] [PubMed] [Google Scholar]
- 17.Beste KY, Burhenne H, Kaever V, et al. Nucleotidyl cyclase activity of soluble guanylyl cyclase α1β1. Biochemistry. 2012;51:194–204. [DOI] [PubMed] [Google Scholar]
- 18.Bähre H, Danker KY, Stasch JP, et al. Nucleotidyl cyclase activity of soluble guanylyl cyclase in intact cells. Biochem Biophys Res Commun. 2014;443:1195–1199. [DOI] [PubMed] [Google Scholar]
- 19.Beste KY, Spangler CM, Burhenne H, et al. Nucleotidyl cyclase activity of particulate guanylyl cyclase A: comparison with particulate guanylyl cyclases E and F, soluble guanylyl cyclase and bacterial adenylyl cyclases CyaA and edema factor. PLoS One. 2013;8:e70223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behmanesh M, Sakumi K, Abolhassani N, et al. ITPase-deficient mice show growth retardation and die before weaning. Cell Death Differ. 2009;16:1315–1322. [DOI] [PubMed] [Google Scholar]
- 21.Sakumi K, Abolhassani N, Behmanesh M, et al. ITPA protein, an enzyme that eliminates deaminated purine nucleoside triphosphates in cells. Mutat Res. 2010;703:43–50. [DOI] [PubMed] [Google Scholar]
- 22.Simone PD, Pavlov YI, Borgstahl GE. ITPA (inosine triphosphate pyrophosphatase): from surveillance of nucleotide pools to human disease and pharmacogenetics. Mutat Res. 2013;753:131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gribble FM, Loussouarn G, Tucker SJ, et al. A novel method for measurement of submembrane ATP concentration. J Biol Chem. 2000;275:30046–30049. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson DR, Price RH. The production of cIMP by toad bladder, and its effects on transport of water and salt. FEBS Lett. 1973;34:207–212. [DOI] [PubMed] [Google Scholar]
- 25.Somlyo AP, Somlyo AV. Ca2+sensitivity of smooth muscle and nonmusclemyosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Portugal AD, Negash S, et al. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L678–L684. [DOI] [PubMed] [Google Scholar]
- 27.Dhanakoti S, Gao Y, Nguyen MQ, et al. Involvement of cGMP-dependent protein kinase in the relaxation of ovine pulmonary arteries to cGMP and cAMP. J Appl Physiol (1985). 2000;88:1637–1642. [DOI] [PubMed] [Google Scholar]
- 28.Qin X, Zheng X, Qi H, et al. cGMP-dependent protein kinase in regulation of basal tone and in nitroglycerin and nitric oxide induced relaxation in porcine coronary artery. Pflugers Arch. 2007;454:913–923. [DOI] [PubMed] [Google Scholar]
- 29.Wolter S, Golombek M, Seifert R. Differential activation of cAMP- and cGMP-dependent protein kinases by cyclic purine and pyrimidine nucleotides. Biochem Biophys Res Commun. 2011;415:563–566. [DOI] [PubMed] [Google Scholar]
- 30.Duong-Quy S, Bei Y, Liu Z, et al. Role of Rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol Ther. 2013;137:352–364. [DOI] [PubMed] [Google Scholar]
- 31.Seifert R. Is cIMP a second messenger with functions opposite to those of cGMP? Naunyn Schmiedebergs Arch Pharmacol. 2014;387:897–899. [DOI] [PubMed] [Google Scholar]
- 32.Reinecke D, Burhenne H, Sandner P, et al. Human cyclic nucleotide phosphodiesterases possess a much broader substrate-specificity than previously appreciated. FEBS Lett. 2011;585:3259–3262. [DOI] [PubMed] [Google Scholar]
- 33.Reinecke D, Schwede F, Genieser HG, et al. Analysis of substrate specificity and kinetics of cyclic nucleotide phosphodiesterases with N'-methylanthraniloyl-substituted purine and pyrimidine 3',5'-cyclic nucleotides by fluorescence spectrometry. PLoS One. 2013;8:e54158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fava C, Montagnana M, Favaloro EJ, et al. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost. 2011;37:280–297. [DOI] [PubMed] [Google Scholar]
- 35.Khayat R, Patt B, Hayes D., Jr Obstructive sleep apnea: the new cardiovascular disease. Part I: obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev. 2009;14:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanhoutte PM, Shimokawa H, Tang EH, et al. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf). 2009;196:193–222. [DOI] [PubMed] [Google Scholar]


