Abstract
Objective:
The aim was to investigate the efficacy and safety of erythropoietin (EPO) to prevent acute kidney injury (AKI) in patients with critical illness or perioperative care.
Methods:
Randomized controlled trials comparing EPO with placebo for AKI prevention in adult patients with critical illness or perioperative care were searched in MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, the Web of Science, and Clinical Trials.gov until October 2014. The outcomes of interest included the incidence of AKI, dialysis requirement, mortality, and adverse event. Fixed effect model was used to calculate the pooled risk ratio (RR) and 95% confidence interval (CI) for eligible studies.
Results:
Ten randomized controlled trials involving 2759 participants were identified and included in the analysis. Compared with placebo, EPO administration did not reduce the incidence of AKI (RR, 0.97; 95% CI, 0.79–1.19; P = 0.782), dialysis requirement (RR, 0.72; 95% CI, 0.31–1.70; P = 0.457), or mortality (RR, 0.96; 95% CI, 0.78–1.18; P = 0.705). Moreover, EPO had no effect on the risk of adverse events, but estimations of RR were difficult due to their relatively infrequent occurrence.
Conclusions:
This meta-analysis suggests that prophylactic administration of EPO in patients with critical illness or perioperative care does not prevent AKI, dialysis requirement, or mortality.
Key Words: critical illness, perioperative care, erythropoietin, acute kidney injury, meta-analysis
INTRODUCTION
Acute kidney injury (AKI), defined as an abrupt drop of renal function within a short period, is a frequent and serious complication in the intensive care unit (ICU) or after surgery, with an incidence of 7.7%–42% in patients with previous normal renal function.1,2 AKI or even a minor increase in serum creatinine level from baseline was independently associated with increased length of hospitalization, health care costs, cardiovascular events, and mortality.3,4 Although there have been many clinical studies on the protection of kidney function in patients with critical illness or perioperative care, such as the administration of N-acetylcysteine, atrial natriuretic peptide, and fenoldopam, the results of such interventions are somewhat contradictory or unproven.5 Consequently, it is paramount that more attention should be paid to explore effective preventative and therapeutic strategies for the management of AKI.
Erythropoietin (EPO), a 30-kDa glycoprotein hormone, is produced by the kidney to regulate the hematopoiesis in bone marrow, and recombinant human EPO has been widely used in the treatment of anemia, especially in end-stage renal disease and certain hematologic diseases.6 Interestingly, there is considerable evidence indicating that EPO acts as a novel renoprotective agent against ischemic, toxic, and septic AKI in animal experiments by reducing apoptosis, stimulating cell proliferation and eliciting its antioxidative and anti-inflammatory functions.7,8 Recently, a few randomized controlled trials (RCTs) analyzed the role of EPO to prevent AKI in patients within the ICU or after surgery who were at high risk of AKI.9–18 However, the results from these trials were inconsistent, partly because they involved single-site studies with small-scale samples.
Therefore, we conducted a systematic review and meta-analysis of RCTs to determine whether the use of EPO in patients with critical illness or perioperative care could ameliorate the incidence of AKI and assess its adverse event.
METHODS
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement recommendations.19
Search Strategy
A comprehensive search was conducted in MEDLINE (through OVID), EMBASE (through OVID), the Cochrane Central Registry of Controlled Trials, and the Web of Science from inception to October 2014. Medical subject headings, entry terms, and text word searches included the following terms: “critical illness,” “critical care,” “intensive care,” “ICU,” “severely ill,” “perioperative care,” “perioperative period,” “erythropoietin,” “epoetin,” “EPO,” “erythropoiesis stimulating protein,” “ darbepoetin,” “acute kidney injury,” “acute renal injury,” “acute renal insufficiency,” “acute kidney insufficiency,” “acute renal failure,” “acute kidney failure,” “acute tubular necrosis,” “AKI,” “ARF,” and “ATN.” There was no restriction on language or publication date. In addition, other potentially relevant studies were searched from the Clinical Trials database (http://clinicaltrials.gov/) for completed trials and the references cited in the retrieved articles and pertinent reviews.
Study Selection Criteria
All titles, abstracts, and full articles were independently searched and evaluated using predesigned inclusion and exclusion criteria by 2 investigators (C.Z. and Z.C.L.). Any discrepancies were resolved by consensus with a third investigator (Q.M.L.) if necessary, which was infrequent.
Studies were included when the following inclusion criteria were met: (1) RCTs, (2) adult patients (age ≥ 18 years) with critical illness or perioperative care, (3) use of EPO for prevention at least in 1 treatment group, (4) control group receiving placebo or usual treatment, (5) reported incidences of AKI in both groups, and (6) for more than 1 publication on the same trial, data from the most recent or complete report were used. Exclusion criteria were as follows: (1) nonrandomized or pseudo-randomized design, retrospective study, case report, and case series; (2) enrolled participants undergoing chronic dialysis therapy, nephrectomy, or transplant surgery (heart, liver, or kidney) due to their complex situations; (3) lack of a control group; (4) studies not addressing the target outcome as mentioned above; and (5) use of EPO as a treatment agent after the occurrence of AKI.
Data Extraction and Risk of Bias Assessment
Two investigators (Q.M.L. and X.X.) independently extracted data from all eligible trials and assessed the risk of bias. Disagreements were resolved through consensus with a third investigator (C.Z.). Data included the first author, publication year, nation of origin, study participants, sample size, whether EPO was used previously, mean age, proportions of male patients, hypertension, and diabetes mellitus, mean baseline hemoglobin and serum creatinine levels, mean blood transfusion volume, treatment regimens of the EPO-based intervention and control groups, definition of AKI, and outcomes measured. The primary outcome was the incidence of AKI, the secondary outcomes included dialysis requirement, 30-day mortality, and the adverse events. When the study had several dosage intervention arms,14 all EPO intervention arms were combined as one arm by weighted means. In the case of missing or incomplete data, the corresponding author of the original trial was contacted by e-mail for additional information.
The risk of bias in the eligible trials was assessed according to the Cochrane Collaboration tool (version 5.1.0),20 which included 7 items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. In each item, an answer of “low risk of bias” suggested sufficient and correct information, “high risk of bias” indicated that the item was reported incorrectly, and “unclear risk of bias” meant insufficient or unmentioned details for judgment.
Statistical Analysis
For dichotomous outcomes (the incidence of AKI, dialysis requirement, and mortality), data were pooled as risk ratio (RR) with 95% confidence interval (CI). The between-study heterogeneity was quantified by Cochran's Q-statistic and the inconsistency index (I2). If there was significant heterogeneity among studies (PQ-statistic < 0.10 and I2 > 50%), the random effect model (DerSimonian and Laird method) was adopted to pool the results; otherwise, the fixed effect model (Mantel-Haenszel method) was used. Subgroup analysis was performed based on (1) entirely perioperative care, (2) no use of EPO previously, (3) at least 2 doses of EPO, and (4) patients at high risk for AKI according to each study. Sensitivity analysis was conducted to assess the influence of individual studies on the pooled estimate of effects by withdrawing 1 study at a time. Publication bias was explored by the funnel plot and Egger's test. An asymmetric funnel plot and PEgger's test less than 0.05 indicated a significant publication bias. The assessment for the risk of bias was performed with Review Manager software (version 5.3 from http://tech.cochrane.org/revman). All statistical analyses were conducted using the Stata/SE 12.0 software (Stata Corporation, College Station, TX). A P value less than 0.05 by a 2-sided test was considered to be statistically significant.
RESULTS
A flow diagram of the study selection is demonstrated in Figure 1. The initial search yielded 615 citations, of which 72 were duplicate studies that were excluded. After reviewing the titles and abstracts of 543 citations, 516 articles were removed because of irrelevant content. Upon review of the full text of 27 articles, ten eligible RCTs with 2759 participants were identified for this meta-analysis.9–18
FIGURE 1.
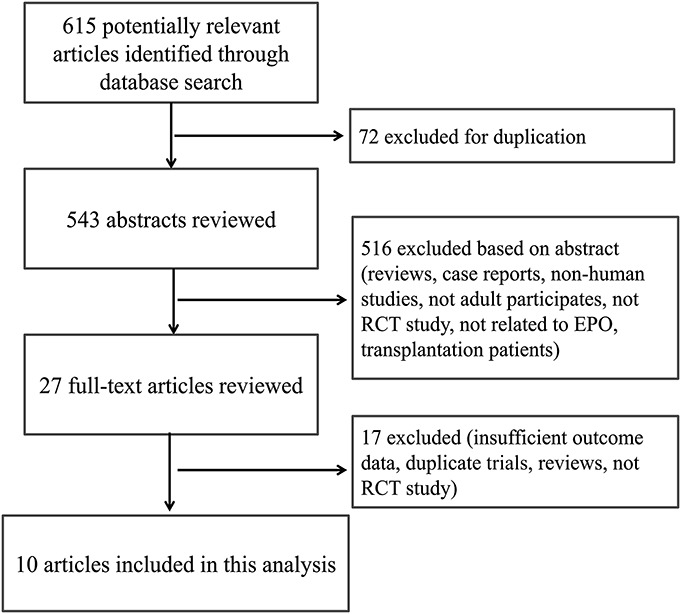
Flow diagram of article selection approach.
Study and Patient Characteristics
The detailed characteristics are summarized in Table 1. The included studies spanned from 2002 to 2014, and with the exception of 2 articles,9,10 the sample size of all other studies was relatively small (63–108 participants). Four trials were conducted in Korea,12,13,16,17 2 in the United States,9,10 and the others in New Zealand,11 Switzerland,14 Thailand,15 and Sweden.18 Napolitano et al10 reanalyzed the data from critically ill trauma patients in EPO-2 trial,9 which had been included in this meta-analysis, so only the part of EPO-3 trial was included in this analysis. The intention to treat of participants in 1 study included AKI patients on randomization,11 and for the objective of this meta-analysis, the data of the subgroup not AKI on randomization were used in this analysis. One study13 was the follow-up of a previous trial21; therefore, the incidence of AKI was extracted from the former, and the baseline data were extracted from the latter. All studies enrolled participants from single-site center except 3.9–11 With respect to the participants setting, 8 studies were performed in perioperative period, 6 of which were conducted in patients with elective cardiac surgery,12–16,18 one assessed patients undergoing thoracic aorta surgery with hypothermic cardiac arrest,17 and the rest one partly included patients with scheduled cardiothoracic surgery.11
TABLE 1.
Baseline Clinical Characteristics of Included Studies
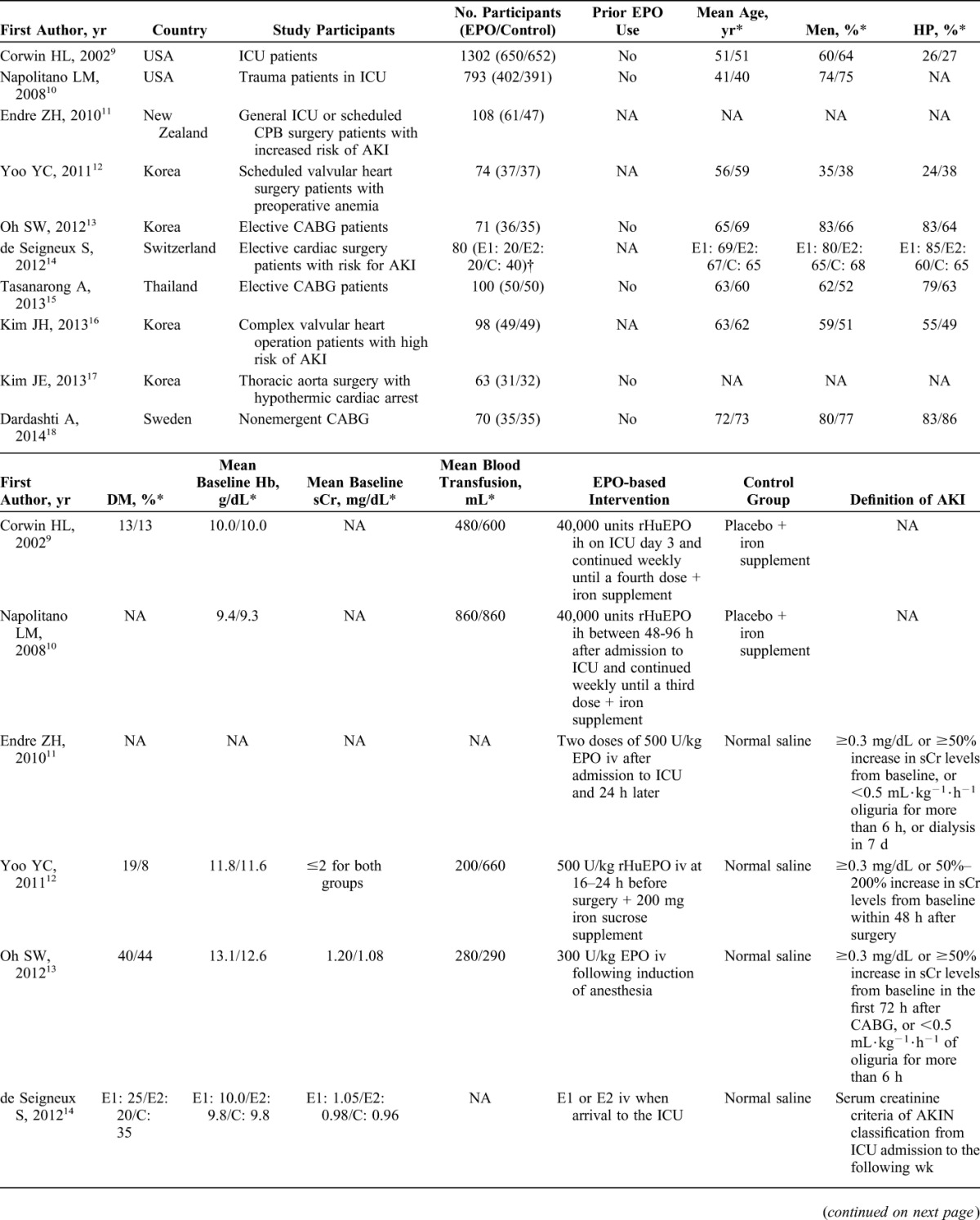
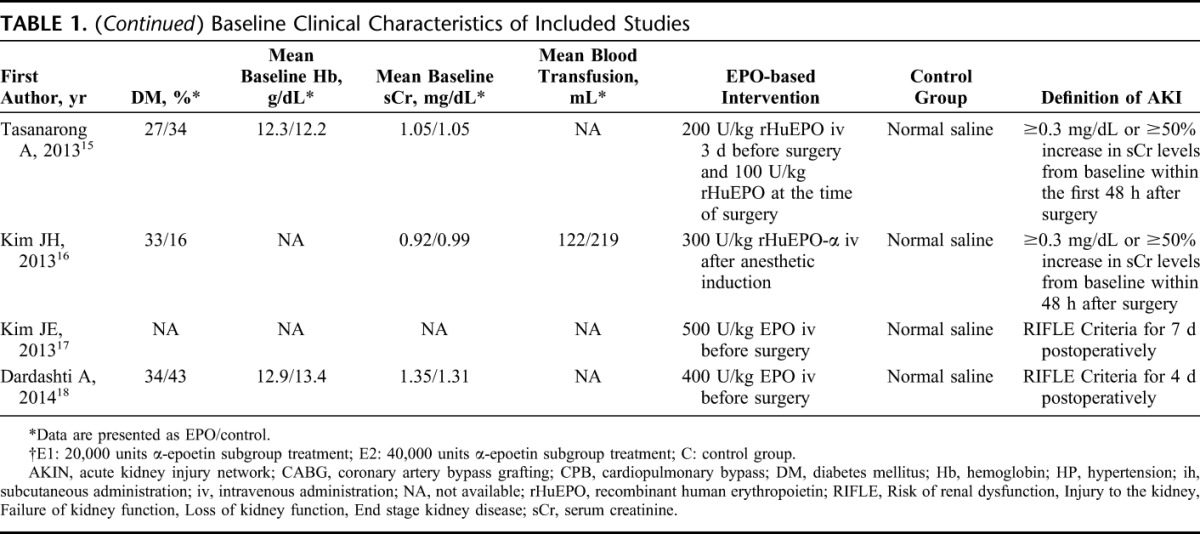
Six studies excluded patients who used EPO previously,9,10,13,15,17,18 whereas other studies did not specify this issue. In 3 of the 10 studies, a high risk for developing AKI, the definitions of which were not the same, was included in the enrollment criteria.11,14,16 In 8 studies, baseline end-stage renal disease or dialysis was set as a compulsory exclusion criterion.9–13,15,16,18 When reported, mean baseline hemoglobin levels ranged from 9.3 to 13.1 g/dL,9,10,12–15,18 and mean baseline serum creatinine levels varied from 0.92 to 1.35 mg/dL.13–16,18 The type of erythropoiesis-stimulating agent used in all of the intervention groups was EPO-alpha or -beta, but not darbepoietin. The one-time EPO dosage was either fixed as 20,000 units or 40,000 units in 3 studies9,10,14 or was based on participant weight ranging from 100 units/kg to 500 units/kg in the other 7 studies. The participants in 6 studies received only a single administration of EPO12–14,16–18; however, participants received at least 2 doses in the other studies. Two studies provided supplementary iron therapy to both the intervention and control groups,9,10 and 1 study provided an iron sucrose supplement only to the intervention group.12 There was considerable variation in the definition of AKI across these studies, which was not clear in 2 studies.9,10
Risk of Bias Assessment
The quality of the included studies is presented in Figure 2. All ten studies reported randomization, but 7 studies described the methods of randomization,9–12,14,16,18 and only 4 studies showed the details of concealed allocation.11,13,15,18 All studies reported double blinding and no or few missing outcome data with the reason. Eight studies provided the clinical trial registration number online with a low risk of bias in selective outcome reporting.10,11,13–18 Because of the unbalanced use of iron supplementation in 2 groups, 1 study exhibited a high risk of bias in the item “other biases.”12
FIGURE 2.
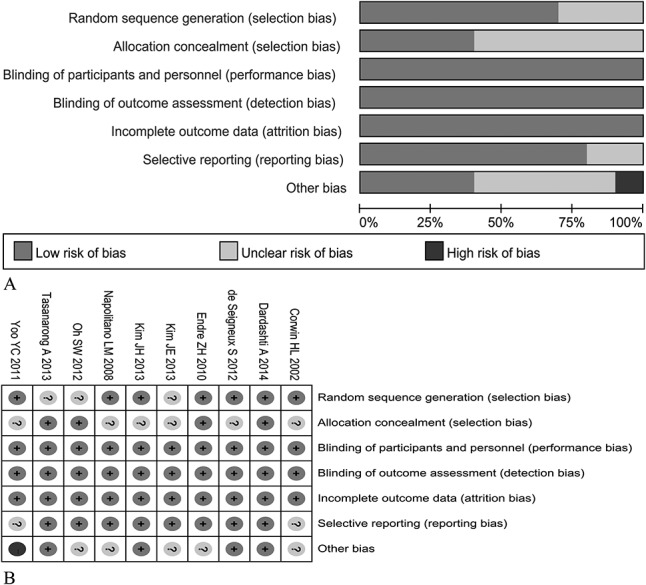
Risk of bias in included studies. A, Risk of bias graph demonstrates the percentages of included studies for each item in the tool; (B) risk of bias summary illustrates the review author's judgments with a cross-tabulation for each eligible study.
Effect of EPO on AKI Prevention
All ten eligible RCTs reported that the overall incidence of AKI was 10.57% (147 of 1391 participants) in the EPO-based intervention group and 10.38% (142 of the 1368 participants) in the control group. Pooled analysis using a fixed effect model showed that there was no significant difference for preventing the development of AKI between the EPO and control groups (RR, 0.97; 95% CI, 0.79–1.19; P = 0.782), as shown in Figure 3A. Meanwhile, the test for between-study heterogeneity among the included trials was not significant (I2 = 20.2% and P = 0.257).
FIGURE 3.
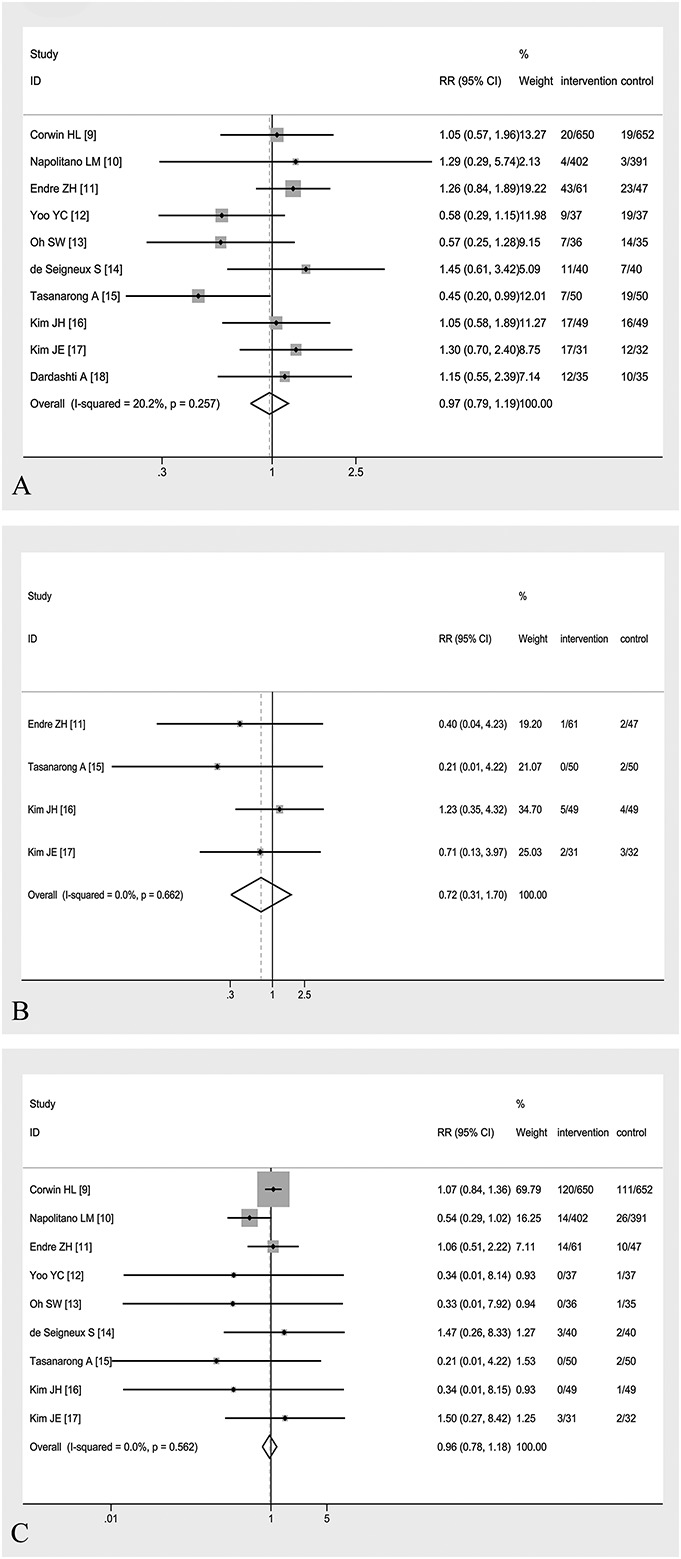
Forest plots of RR estimates with the corresponding 95% CI for (A) the incidence of AKI, (B) dialysis requirement, and (C) mortality in patients receiving EPO therapy versus control. The columns of intervention and control were presented as number of events/number of participants.
Stratified analyses of studies with entirely perioperative care demonstrated no significant difference between the EPO and control groups using a fixed effect model (RR, 0.86; 95% CI, 0.66–1.12; P = 0.260; I2 = 33.9%; P = 0.169).12–18 Among studies that excluded patients with prior EPO use, similar result of RR was obtained (RR, 0.89; 95% CI, 0.66–1.21; P = 0.467; I2 = 23.3%, P = 0.259).9,10,13,15,17,18 Among patients who received at least 2 doses of EPO9–11,15 or were at high risk of AKI according to each study,11,14,16 the prevention of AKI remained nonsignificant (RR, 0.99; 95% CI, 0.73–1.35; P = 0.962; I2 = 44.3%, P = 0.145 and RR, 1.22; 95% CI, 0.89–1.67; P = 0.215; I2 = 0%, P = 0.805, respectively). The sensitivity analysis after exclusion of 1 study at a time demonstrated that the above pooled result was robust and did not change much depending on any single study (Fig. 4). It should be noted that after removing the study,12 which gave iron supplement only to EPO intervention group, the overall RR was 1.02 (95% CI, 0.82–1.27; P = 0.824) without statistical significance.
FIGURE 4.
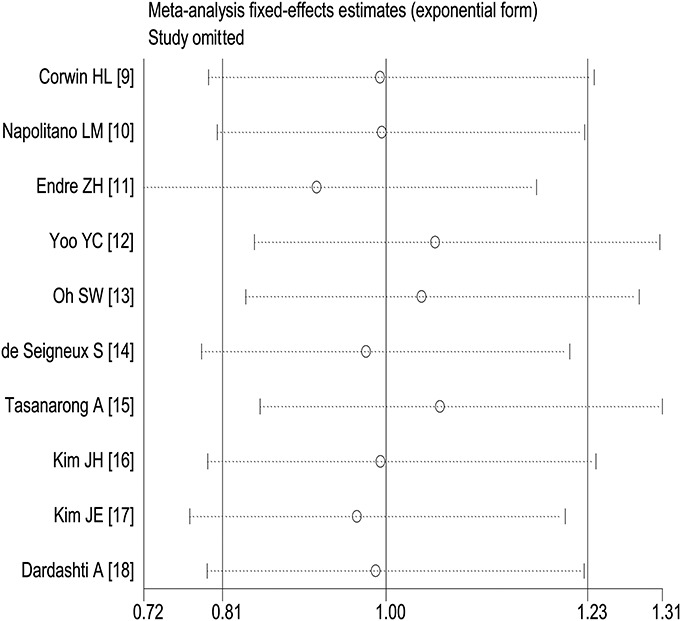
The sensitivity analysis by removing each individual study at a time is shown on the pooled effect size of the incidence of AKI.
The funnel plot and Egger's test analysis indicated that there was no evidence of publication bias for the AKI outcome (Fig. 5A for funnel plot; P = 0.346 for Egger's test).
FIGURE 5.
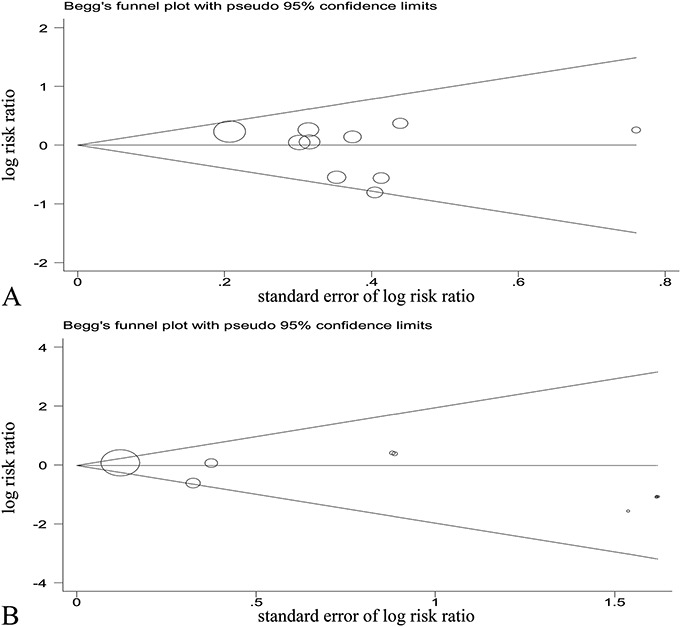
The funnel plots for publication bias tests of studies assessing the effect of EPO on (A) the incidence of AKI and (B) mortality.
Effect of EPO on Dialysis Requirement
The dialysis requirement was reported in 7 studies11,13–18 totaling 590 analyzable patients, and in 3 of these studies, no patient was treated with dialysis in either group.13,14,18 The overall incidence of dialysis was 2.65% (8 of the 302 participants) in the EPO-based intervention group and 3.82% (11 of the 288 participants) in the control group. By meta-analysis for 4 studies using a fixed effect model,11,15–17 there was no significant difference of mortality between the EPO and control groups (RR, 0.72; 95% CI, 0.31–1.70; P = 0.457) without significant between-study heterogeneity (I2 = 0%, P = 0.662), as shown in Figure 3B. Because there were only 4 articles in the literature with the effective data, the publication bias was not investigated.
Effect of EPO on Mortality
The ten included studies demonstrated that overall mortality was 11.07% (154 of the 1391 participants) in the EPO-based intervention group and 11.40% (156 of the 1368 participants) in the control group. Mortality was ascertained in-hospital in 3 studies,13,15,16 at 30 days in 5 studies,9–12,14 and unclear in the remaining study.17,18 Pooled analysis using a fixed effect model showed that there was no significant difference of mortality between the EPO and control groups (RR, 0.96; 95% CI, 0.78–1.18; P = 0.705) without significant between-study heterogeneity (I2 = 0%, P = 0.562), as shown in Figure 3C. Meanwhile, there was no evidence of publication bias for the mortality outcome (Fig. 5B for funnel plot; P = 0.162 for Egger's test).
Adverse Events
Six studies demonstrated that none of the patients who received the EPO intervention suffered from adverse events, which were associated with the use of drug, throughout the study period, such as hypertension, symptomatic thrombosis, myocardial infarction, stroke, headache, and seizures.12–16,18 One study did not report adverse events,17 and 1 study reported adverse events for the entire participants without specifically describing the specific group.11 Corwin et al9 showed that the incidence of deep thrombophlebitis was 2.15% (14/650) among the EPO intervention group and 2.30% (15/652) in the control group without significant difference. Napolitano et al10 reported that the incidence of clinically relevant thrombovascular events was 16.42% (66/402) in the EPO intervention group and 12.53% (49/391) in the control group with an RR of 1.31 (95% CI, 0.93–1.85).
DISCUSSION
In this present meta-analysis of 10 RCTs with 2759 participants, we found that compared with placebo, prophylactic EPO therapy for patients who are critically ill or under perioperative care was not associated with a significant reduction in the incidence of AKI, dialysis requirement, or mortality. Meanwhile, the effect of EPO on AKI prevention was consistent with the above result for the stratified analyses on patients with entirely perioperative care, no previous EPO use, or more than 1 dose of EPO. In addition, EPO therapy was not associated with adverse events in these studies, which appeared to be safe in this kind of patients.
AKI is a frequent and serious complication occurring in the critically ill or surgical patients and is associated with increased length of hospital stay, occurrence of end-stage renal disease, and mortality.22 Ischemia and reperfusion injury, inflammation, oxidative stress, apoptosis, etc. have all been demonstrated as crucial factors in the development of AKI.23 Recently, results from several animal studies indicated that EPO exerts a renoprotective effect against AKI induced by cardiopulmonary bypass or sepsis through mechanisms of reducing ischemia/reperfusion injury, inhibiting apoptosis, and alleviating inflammatory responses.8,24,25 However, the benefits of EPO in these animal studies were not reproducible in clinical trials or in this meta-analysis. There are several plausible reasons for this discrepancy. First, many patients included in these trials suffered from some comorbidities, such as hypertension, diabetes, dyslipidemia, or ischemic heart disease and had complicated pathophysiological conditions, which might reduce the effects of EPO.26,27 Second, compared with animal experiments, the dosage of EPO in clinical studies is relatively lower, which might be unable to obtain therapeutic effects. Third, the variable time of EPO treatment in clinical studies is not the same as animal experiments, and the optimal time has not been established.
This meta-analysis has several strengths as following. Only 1 EPO intervention study was enrolled in a previous meta-analysis without estimating the risk of AKI,28 and 9 additional RCTs with over 2600 participants were included into our meta-analysis. The majority of trials in this analysis had good or moderate methodological quality, which indicated that the results were not susceptible to be influenced by the biases of original researches. Meanwhile, most trials investigated the effect of EPO on clinically hard end points such as dialysis requirement and short-term mortality. Both the between-study heterogeneity and publication bias were relatively minimal without statistical significance, which made the pooled effect size less likely to be affected. In addition, the appropriate stratified analyses and sensitivity analyses, which removed one study at a time, were performed with consistent results. Therefore, the results of our meta-analysis are reliable with cautiously speaking.
There are several limitations of this study that should be taken into account. First, several inclusion trials might not have sufficient sample size with designed only at the single-center level.12–17 In the future, more large multicenter RCTs with adequately participants will be needed to elucidate the prevention role of EPO. Second, there were differences in patient selection, treatment regimen, and the definition of AKI across these trials. An accepted uniform EPO study protocol would reduce the degree of variation among the studies. Finally, our meta-analysis was based on aggregated data, not on individual patient-level data. As a consequence, the results might be less accurate and could not be adjusted for certain confounding factors, such as age, comorbidities, and laboratory parameters, which might influence the true therapeutic effect of EPO.
CONCLUSIONS
In summary, this meta-analysis, based on currently available RCT evidence, suggests that prophylactic EPO treatment of patients with critical illness or under perioperative care does not reduce the incidence of AKI, dialysis requirement, or death. Considering the limitations of this study, larger scale, well-designed multicenter RCT studies using optimal doses and administration times are needed to investigate the role of EPO in AKI prevention.
Footnotes
Supported by grants from the National Key Basic Research Program of China (Grant No. 2011CB504005), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2011BAI10B05), the National Natural Science Foundation of China (Grant No. 81170765), and the Guangdong Natural Science Foundation (Grant No. S2011020002359).
The authors report no conflicts of interest.
C. Zhao and Z. Lin have contributed equally to this study.
REFERENCES
- 1.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008;36:S146–S151. [DOI] [PubMed] [Google Scholar]
- 2.Herrera-Gutiérrez ME, Seller-Pérez G, Sánchez-Izquierdo-Riera JA, et al. Prevalence of acute kidney injury in intensive care units: the “COrte de prevalencia de disFunción RenAl y DEpuración en críticos” point-prevalence multicenter study. J Crit Care. 2013;28:687–694. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liotta M, Olsson D, Sartipy U, et al. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol. 2014;113:70–75. [DOI] [PubMed] [Google Scholar]
- 5.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 6.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care. 2008;14:621–626. [DOI] [PubMed] [Google Scholar]
- 8.Coldewey SM, Khan AI, Kapoor A, et al. Erythropoietin attenuates acute kidney dysfunction in murine experimental sepsis by activation of the β-common receptor. Kidney Int. 2013;84:482–490. [DOI] [PubMed] [Google Scholar]
- 9.Corwin HL, Gettinger A, Pearl RG, et al. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288:2827–2835. [DOI] [PubMed] [Google Scholar]
- 10.Napolitano LM, Fabian TC, Kelly KM, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma. 2008;65:285–297. [DOI] [PubMed] [Google Scholar]
- 11.Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int. 2010;77:1020–1030. [DOI] [PubMed] [Google Scholar]
- 12.Yoo YC, Shim JK, Kim JC, et al. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115:929–937. [DOI] [PubMed] [Google Scholar]
- 13.Oh SW, Chin HJ, Chae DW, et al. Erythropoietin improves long-term outcomes in patients with acute kidney injury after coronary artery bypass grafting. J Korean Med Sci. 2012;27:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Seigneux S, Ponte B, Weiss L, et al. Epoetin administrated after cardiac surgery: effects on renal function and inflammation in a randomized controlled study. BMC Nephrol. 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasanarong A, Duangchana S, Sumransurp S, et al. Prophylaxis with erythropoietin versus placebo reduces acute kidney injury and Neutrophil Gelatinase-associated Lipocalin in patients undergoing cardiac surgery: a randomized, double-blind controlled trial. BMC Nephrol. 2013;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Shim JK, Song JW, et al. Effect of erythropoietin on the incidence of acute kidney injury following complex valvular heart surgery: a double blind, randomized clinical trial of efficacy and safety. Crit Care. 2013;17:R254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JE, Ham SY, Shim YH. Prevention of acute kidney injury by erythropoietin in patients undergoing thoracic aorta surgery with hypothermic circulatory arrest. Eur J Anaesthesiol. 2013;30(suppl 51):63–64. [Google Scholar]
- 18.Dardashti A, Ederoth P, Algotsson L, et al. Erythropoietin and protection of renal function in cardiac surgery (the EPRICS Trial). Anesthesiology. 2014;121:582–590. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011). Available at: http://handbook.cochrane.org. Accessed October 1, 2014.
- 21.Song YR, Lee T, You SJ, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. 2009;30:253–260. [DOI] [PubMed] [Google Scholar]
- 22.Clec'h C, Gonzalez F, Lautrette A, et al. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: a competing risks analysis. Crit Care. 2011;15:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–825. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Huang H, Wu H, et al. Erythropoietin attenuates cardiopulmonary bypass-induced renal inflammatory injury by inhibiting nuclear factor-κB p65 expression. Eur J Pharmacol. 2012;689:154–159. [DOI] [PubMed] [Google Scholar]
- 25.Stoyanoff TR, Todaro JS, Aguirre MV, et al. Amelioration of lipopolysaccharide-induced acute kidney injury by erythropoietin: involvement of mitochondria-regulated apoptosis. Toxicology. 2014;318:13–21. [DOI] [PubMed] [Google Scholar]
- 26.Ghaboura N, Tamareille S, Ducluzeau PH, et al. Diabetes mellitus abrogates erythropoietin-induced cardioprotection against ischemic-reperfusion injury by alteration of the RISK/GSK-3β signaling. Basic Res Cardiol. 2011;106:147–162. [DOI] [PubMed] [Google Scholar]
- 27.Matějková Š, Scheuerle A, Wagner F, et al. Carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine kidney ischemia/reperfusion injury. Intensive Care Med. 2013;39:497–510. [DOI] [PubMed] [Google Scholar]
- 28.Zacharias M, Mugawar M, Herbison GP, et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2013;9:CD003590. [DOI] [PMC free article] [PubMed] [Google Scholar]


