Abstract:
Emerging evidence has shown that aldosterone blockers reduced the incidence of ventricular arrhythmias in patients with myocardial infarction (MI). However, the mechanism remains unknown. In this study, we investigated the mechanism by which spironolactone, a classic aldosterone blocker, regulates hyperpolarization-activated cyclic nucleotide-gated channel (HCN) protein expression in ischemic rat myocardium after MI. Eighteen rats surviving 24 hours after MI were randomly assigned into 3 groups: MI, spironolactone, and spironolactone + antagomir-1. Six sham-operated rats had a suture loosely tied around the left coronary artery, without ligation. The border zone of the myocardial infarct was collected from each rat at 1 week after MI. HCN2 and HCN4 protein and messenger RNA (mRNA) level were measured in addition to miRNA-1 levels. Spironolactone significantly increased miRNA-1 levels and downregulated HCN2 and HCN4 protein and mRNA levels. miRNA-1 suppression with antagomir-1 increased HCN2 and HCN4 protein levels; however, HCN2 and HCN4 mRNA levels were not affected. These results suggested that spironolactone could increase miRNA-1 expression in ischemic rat myocardium after MI and that the upregulation of miRNA-1 expression partially contributed to the posttranscriptional repression of HCN protein expression, which may contribute to the effect of spironolactone to reduce the incidence of MI-associated ventricular arrhythmias.
Key Words: myocardial infarction, HCN2, HCN4, spironolactone, miRNA-1
INTRODUCTION
The benefits of aldosterone blockers (ABs) in patients after myocardial infarction (MI) with heart failure have been well demonstrated.1 The Eplerenone Post-acute Myocardial Infarction Heart failure Efficacy and Survival Study1 reported that low doses of AB decreased the incidence of cardiovascular mortality among patients with moderate to severe heart failure after MI, with a 21% reduction rate of sudden death from cardiac causes. The value of ABs in patients after acute myocardial infarction (AMI) without heart failure is also currently determined in a recent clinical trial.2 This trial found that early AB in patients presenting for primary percutaneous coronary intervention for acute ST-elevation MI is clearly associated with a reduction in the incidence of life-threatening ventricular arrhythmia. The Aldosterone Blockade Early After Acute Myocardial Infarction3 study, a multicenter, open-labeled, randomized trial designed to assess the effects of an early AB in patients after AMI, has been completed in July 2014, and the results of which are eagerly awaited because they may lead to changes in ST-elevation MI management. A recent study4 showed that ABs prevented ventricular arrhythmias induced by aldosterone during ischemia–reperfusion by multiple antiarrhythmic properties, including the reduction of sinoatrial node (SA) beating rate and classes I and III antiarrhythmic effects.
The surviving ventricular myocytes surrounding an infarct play a key role in the occurrence of ventricular arrhythmias after AMI.5 Changes in the ion channel activities in ventricular myocytes after AMI constitute cardiac electrical remodeling, which may be an important mechanism of ventricular arrhythmias.6 The hyperpolarization-activated current (If) is a mixed Na+ and K+ inward current that is activated by membrane hyperpolarization and encoded by the hyperpolarization-activated cyclic nucleotide-gated channel (HCN) genes, which encode 4 isoforms (HCN1-4).7–9 Abnormal expression of HCN channels in cardiac regions might lead to electrical instability in ventricular myocytes and induce ventricular arrhythmias.10 A recent study11 found that ABs reduced ventricular arrhythmias due to decreased HCN protein expression; this may play an important role in reducing mortality after MI. miRNA-1 is specifically expressed in skeletal muscle and cardiac myocytes12,13 and might regulate the expression of some ion channel proteins by posttranscriptional mechanisms. It is unclear whether the reduction of HCN protein expression by AB treatment after MI is related to posttranscriptional repression by miRNA-1. In this study, we examined the effect of spironolactone, a classic AB, on HCN protein expression. Antagomir-1, which can efficiently and stably knock down miRNA-1,14 was used to determine whether the observed reduction in HCN protein expression was due to the posttranscriptional repression by miRNA-1.
MATERIALS AND METHODS
Experimental Model
The rat experiments were conducted in accordance with the Experimental Animal Regulations of Chongqing Medical University. Male Sprague Dawley rats weighing 200–250 g were used in the study. Rat with MI was established by ligation of the left anterior descending coronary artery.15 Sprague Dawley rats were anaesthetized with an intraperitoneal injection of 10% chloral hydrate sodium (0.3 mL/100 g). The tracheae of the rats were cannulated, and the animals were mechanically ventilated using a rodent ventilator. The chest of each rat was opened by a left thoracotomy, and the heart was quickly removed from the thoracic cavity. The left anterior descending coronary artery was ligated between the left atrial appendage and the pulmonary artery outflow tract by a 6-0 suture. The heart was repositioned into the chest, and the chest was then closed. All surgical procedures were performed under sterile conditions. MI was assessed using visual cyanosis and electrocardiography (segment elevation continued for 30 minutes). Eighteen rats surviving 24 hours after the surgery were randomly assigned into 3 groups: MI rats receiving 2 mL saline by intragastric administration for 7 days and daily injection of 0.2 mL saline through the tail vein from the fourth day to the seventh day after MI (MI, n = 6), MI rats receiving spironolactone intragastrically administered at a dosage of 80 mg·kg−1·d−1 for 7 days and daily injection of 0.2 mL saline through the tail vein from the fourth day to the seventh day after MI (spironolactone, n = 6), and MI rats receiving spironolactone intragastrically administered at a dosage of 80 mg·kg−1·d−1 for 7 days and daily injection of antagomir-1 through the tail vein at a dosage of 80 mg·kg−1·d−1 in 0.2 mL saline from the fourth day to the seventh day after MI (spironolactone + antagomir-1, n = 6). In the sham-operated rats, a suture was loosely tied around the left coronary artery without ligation (sham, n = 6).
Tissue Collection
One week after MI, the rats were placed under anesthesia, and the hearts were removed from the chests. The infarcted areas were visually identified by a pale and thin appearance. The area of the myocardium that spread about 1.0 mm from the infarct scar represented the border zone of the MI.
Synthesis of Antagomir-1
Antagomirs have been used previously to inhibit miRNA expression.14 Antagomir-1 was synthesized by RiboBio Co. (Guangzhou, China). Antagomir-1 is a single-stranded RNA analog complementary to the sequence of mature miRNA-1 (5′-UGGAAUGUAAAGAAGUGUGUAU-3′) that is chemically modified and cholesterol-conjugated from a hydroxyprolinol-linked cholesterol solid support and 2′-OMe phosphoramidites.
RNA Extraction and SYBR Green Quantitative PCR Analysis
Total RNA was isolated from 50 to 100 mg frozen tissue samples using the RNAiso Plus reagent (TaKaRa, China) following the manufacturer's instructions. The expression of miRNA-1 was determined by quantitative real-time polymerase chain reaction (qRT-PCR) using an All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia Inc.). In brief, the total RNA isolated from tissue samples was reverse transcribed with an oligo-dT adapter. qRT-PCR was performed using SYBR Green detection with All-in-One miRNA quantitative polymerase chain reaction (qPCR) primers and the universal adapter PCR primer. The miRNA-1 qPCR primers were purchased from GeneCopoeia, Inc. The catalog numbers of the All-in-One miRNA qPCR primers were as follows: rno-miR-1∼RmiRQP1188 and snRNA U6∼RmiRQP9003 (the company did not provide sequence information for these primers). The PCR for miRNA-1 amplification was conducted with the CFX96TM Real-Time PCR Detection System (Bio-Rad). miRNA-1 levels were normalized to snRNA U6. The relative level was calculated using the 2−ΔΔCt method.
To quantitatively detect HCN2 and HCN4 messenger RNA (mRNA) expression, first-strand complementary DNA was synthesized from l μg of total RNA with a PrimeScript RT Reagent Kit (TaKaRa, China). The complementary DNA was used for qRT-PCR with a ShineSybr Real-time qPCR MasterMix Kit (ShineGene, China) on a CFX96 Real-Time PCR Detection System (Bio-Rad). The following primers were used: HCN2 forward: 5′-CAAGGGCAACAAGGAGATGA-3′, HCN2 reverse: 5′-GTGAGTAGAGGCGACAGTAGGTG-3′; HCN4 forward: 5′-CGCATCCACGACTACTACGAAC-3′, HCN4 reverse: 5′-GGTCTGCATTGGCGAACAG-3′; and β-actin forward: 5′-CCCATCTATGAGGGTTACGC-3′, β-actin reverse: 5′-TTTAATGTCACGCACGATTTC-3′. HCN2 and HCN4 mRNA expression was normalized to β-actin. The relative levels were calculated using the 2−ΔΔCt method. All qRT-PCR reactions were performed in triplicate.
Western Blot Analysis
Total protein was extracted from the samples. The protein extracts were separated by 6% SDS-PAGE gels for HCN4 and 8% gels for HCN2 and then electroblotted onto polyvinylidene difluoride membranes (Millipore). After blocking for 2 hours with 5% nonfat dry milk in TBS at room temperature, the membranes were incubated with primary antibodies overnight at 4°C and subsequently incubated with secondary antibody for 1 hour at 37°C. The primary antibodies used were the rabbit polyclonal antibodies anti-HCN2 (1:500, ab65704; Abcam), anti-HCN4 (1:300, ab65703; Abcam), and anti-GAPDH (1:1000, ab37168; Abcam), whereas the secondary antibody was Goat Anti-Rabbit IgG H&L (1:10,000, ab97051; Abcam). The protein bands from the Western blots were visualized using enhanced chemiluminescence detection reagents (Applygen Technologies Inc., Beijing, China). All bands were analyzed using ImageJ software to verify the relative levels of HCN2 and HCN4 normalized to GAPDH.
Statistical Analysis
SPSS version 17.0 was used for the statistical analyses. All data are expressed as the mean ± SD. Statistical comparisons were performed using one-way analysis of variance and two-sided SNK tests. P < 0.05 was considered statistically significant.
RESULTS
Comparison of HCN2 and HCN4 mRNA Expression in Ischemic Left Ventricular Myocardium Between Groups
The HCN2 mRNA expression significantly increased by 1.29- and 0.59-fold in the MI and spironolactone groups, respectively as compared with that in the sham group (P < 0.05). Compared with the MI group, spironolactone significantly decreased the HCN2 mRNA expression in ischemic left ventricular myocardium by 30% (P < 0.05). Simultaneously, the HCN4 mRNA expression markedly increased by 1.07- and 0.73-fold in the MI and spironolactone groups, respectively, as compared with that in the sham group (P < 0.05). Compared with the MI group, spironolactone significantly decreased the HCN4 mRNA expression in ischemic left ventricular myocardium by 16% (P < 0.05). However, no significant changes were observed in the HCN2 and HCN4 mRNA levels between the spironolactone + antagomir-1 group and the spironolactone group (P > 0.05) (Table 1 and Fig. 1).
TABLE 1.
HCN2 and HCN4 mRNA Expression in Ischemic Left Ventricular Myocardium
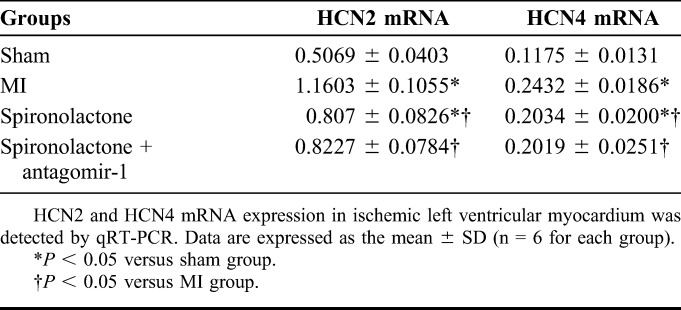
FIGURE 1.
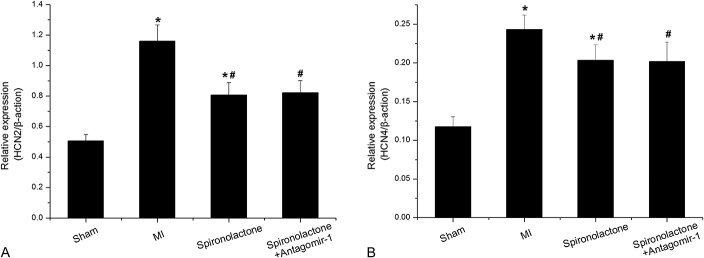
HCN2 and HCN4 mRNA expression in ischemic left ventricular myocardium was detected by qRT-PCR. Data are expressed as the mean ± SD (n = 6 for each group). *P < 0.05 versus sham group; #P < 0.05 versus MI group.
Comparison of HCN2 and HCN4 Protein Expression in Ischemic Left Ventricular Myocardium Between Groups
The HCN2 protein expression significantly increased by 1.35- and 0.17-fold, and the HCN4 protein expression significantly increased by 1.75- and 0.32-fold in the MI and the spironolactone groups, respectively, as compared with that in the sham group (all P < 0.05). Compared with the MI group, spironolactone significantly downregulated the HCN2 and HCN4 protein expression in ischemic left ventricular myocardium by 50% and 52%, respectively (P < 0.05). When miRNA-1 suppressed with antagomir-1, there was a significant increase of HCN2 and HCN4 protein expression in the spironolactone + antagomir-1 group compared with that in the spironolactone group (P < 0.05) (Figs. 2, 3).
FIGURE 2.
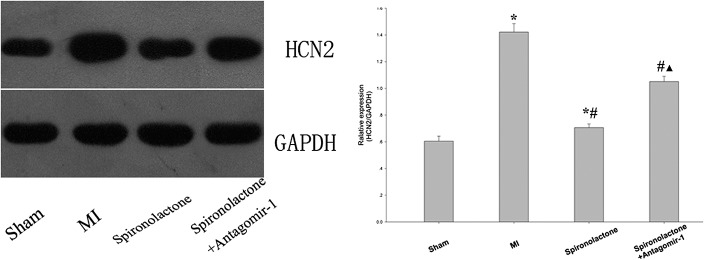
HCN2 protein expression in ischemic left ventricular myocardium was detected by Western blotting. Data are expressed as the mean ± SD (n = 6 for each group). *P < 0.05 versus sham group; #P < 0.05 versus MI group; ▲P < 0.05 versus spironolactone group.
FIGURE 3.
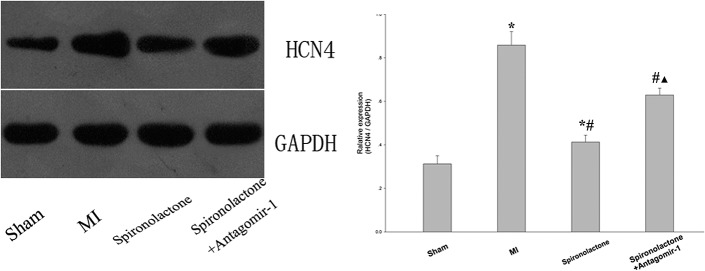
HCN4 protein expression in ischemic left ventricular myocardium was detected by Western blotting. Data are expressed as the mean ± SD (n = 6 for each group). *P < 0.05 versus sham group; #P < 0.05 versus MI group; ▲P < 0.05 versus spironolactone group.
Comparison of Micro-RNA-1 Expression in Ischemic Left Ventricular Myocardium Between Groups
miRNA-1 expression was significantly downregulated by 75% and 42% in the MI and spironolactone groups, respectively, as compared with that in the sham group (P < 0.05). However, miRNA-1 expression significantly increased by 1.34-fold in the spironolactone group compared with the MI group (P < 0.05). When miRNA-1 suppressed with antagomir-1, the miRNA-1 expression significantly decreased by 45% in the spironolactone + antagomir-1 group compared with that in the spironolactone group (P < 0.05) (Table 2 and Fig. 4).
TABLE 2.
Micro-RNA-1 Expression in Ischemic Left Ventricular Myocardium
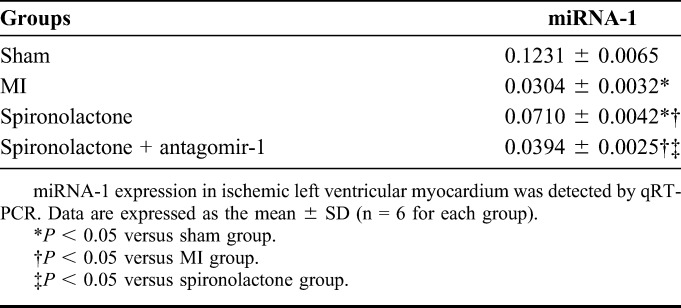
FIGURE 4.
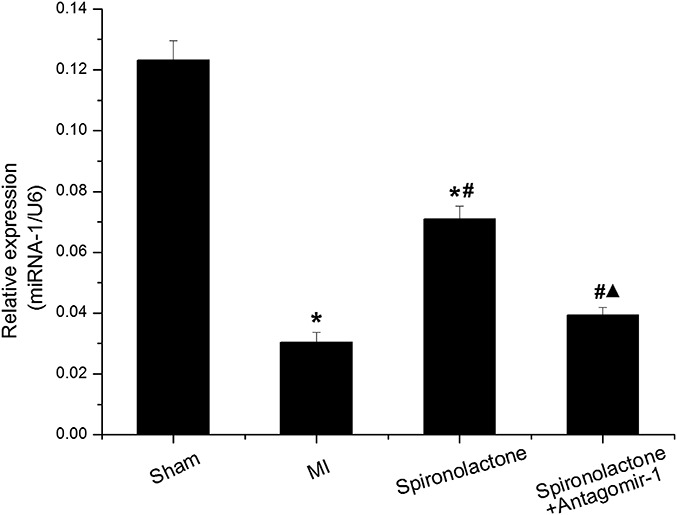
miRNA-1 expression in ischemic left ventricular myocardium was detected by qRT-PCR. Data are expressed as the mean ± SD (n = 6 for each group). *P < 0.05 versus sham group; #P < 0.05 versus MI group; ▲P < 0.05 versus spironolactone group.
DISCUSSION
AMI is a leading cause of death in the industrialized world,16 and ventricular arrhythmias are the main cause of death in patients with AMI.17 Studies on the mechanism of ventricular arrhythmia have contributed to the development of therapeutic strategies for patients with MI and have societal significance. However, this mechanism is complex and remains unclear. Previous studies have suggested that sympathetic remodeling and myocardial tissue remodeling may be involved in the occurrence of ventricular arrhythmias after MI.18,19 However, recent studies have found that changes in the activity of ion channels in ventricular myocytes constitute cardiac electrical remodeling, which may play an important role in the pathogenesis of ventricular arrhythmias.6 Therefore, an understanding of the regulatory mechanism of electrical remodeling after MI has important clinical significance.
The hyperpolarization-activated current (If) is a mixed Na+/K+ inward current that is activated by hyperpolarization and is encoded by HCN genes.7 Recently, 4 isoforms (HCN1-4) have been identified in mammals by molecular cloning; these isoforms exhibit different expression patterns in heart and brain cells.8,9 Three isoforms (HCN1, HCN2, and HCN4) have been identified in cardiac tissues. HCN2 is predominant in ventricular myocardium, and HCN4 is predominant in SA cells. A previous study found that If could produce automatic activity not only in SA node cells but also in the atria, atrioventricular nodes, Purkinje fibers, and ventricles.20 When the SA node is impaired, latent pacemaker cells play a complementary role in pacemaking. However, overactivation of the If in cardiac regions can result in improved automaticity from the ectopic focus, thereby leading to atrial and ventricular arrhythmias.10 Previous studies have found that HCN2 and/or HCN4 gene expression is increased in pathological ventricular myocardium, for example, in heart failure, ventricular hypertrophy, and atrial fibrillation.21–23 Our previous study found that HCN2 and HCN4 gene expression was upregulated and exhibited dynamic changes in ischemic rat myocardium at 4 weeks post-AMI; the highest levels were observed at 1 week post-AMI. Consistent with the changes in HCN2 and HCN4 gene expression, ventricular arrhythmia occurred post-AMI and peaked at 1 week post-AMI.15 Milliez et al24 found that the selective If inhibitor ivabradine reduced ventricular premature complexes by 89% in severe chronic heart failure after MI. Furthermore, a recent study has also found that ivabradine reduced ischemia–reperfusion–induced ventricular arrhythmias predominantly because of its selective heart rate lowering effects in rat model of acute ischemia–reperfusion.25 In summary, ventricular arrhythmias after MI are closely related to abnormal HCN gene expression in the ventricular myocardium.
Currently, the mechanism by which HCN gene expression increases after MI is unclear. Previous studies have shown that the mechanism might be associated with β2-adrenergic activity, MiRP1, and endothelin-1.26–28 A recent study found that the renin–angiotensin–aldosterone system might play an important role in the regulation of HCN gene expression. Muto et al29 found that aldosterone promoted HCN2 and HCN4 mRNA and protein expression in cultured neonatal rat ventricular myocytes by binding to the mineralocorticoid receptor and that ABs (spironolactone and eplerenone) counteracted this effect. In this study, we found that spironolactone significantly reduced HCN4 protein expression in rat ischemic left ventricular myocardium after MI, in agreement with Song et al.11 However, we also found that spironolactone could reduce HCN2 protein expression, whereas Song et al found that spironolactone had no significant effect on HCN2 protein expression; this discrepancy could potentially be due to differences in the tissue samples collected or other factors. Further study is required to confirm our results.
miRNAs are small conserved RNAs (approximately 22 nucleotides in length) that can negatively regulate gene expression in plants and animals, primarily through base pairing to the 3′ untranslated region of target mRNAs; this action results in mRNA cleavage and/or translational repression.30 miRNA-1 is specifically expressed in skeletal muscle and cardiac myocytes12,13 and is broadly involved in the development of cardiovascular diseases. Several studies suggested that miRNA-1 plays a role in cardiac injury by affecting the expression of insulin growth factor-1,31 Bcl-2,32 heat shock protein 60.33 Other studies showed that miRNA-1 promotes cardiac arrhythmogenesis by inhibiting the expression of connexin 43, Kir2.1, and the PP2A regulatory subunit B56alpha.13,34 Sayed et al35 demonstrated that miRNA-1 regulates cardiac hypertrophy by targeting cyclin-dependent kinase 9 (Cdk9), fibronectin, Ras homolog enriched in brain (Rheb), and Ras GTPase-activating protein (RasGAP). Luo et al36 found that the HCN2 and HCN4 genes are targets for posttranscriptional repression by miRNA-1 in hypertrophic myocytes. Our results showed that miRNA-1 levels were significantly decreased in rat ischemic left ventricular myocardium after MI, in agreement with the results of D'Alessandra et al.37 Spironolactone significantly increased miRNA-1 expression; this increase was accompanied by the downregulation of HCN2 and HCN4. Furthermore, miRNA-1 suppression with antagomir-1 increased HCN2 and HCN4 protein levels; however, HCN2 and HCN4 mRNA levels were not affected, indicating that spironolactone stimulates miRNA-1 expression in the rat ischemic left ventricular myocardium after MI and that the upregulation of miRNA-1 expression partly contributes to the posttranscriptional repression of HCN protein expression.
CONCLUSIONS
In summary, our results demonstrate for the first time that spironolactone can increase miRNA-1 expression in rat ischemic left ventricular myocardium after MI and that the upregulation of miRNA-1 expression partially contributes to the posttranscriptional repression of HCN protein expression, which may contribute to the effect of spironolactone to reduce the incidence of MI-associated ventricular arrhythmias.
ACKNOWLEDGMENTS
The authors thank the National Natural Science Foundation of China (NSFC: 81270211 and 81100088), Specialized Research Fund for the Doctoral Program of Higher Education (20125503110009), and Science and Technology Research Project of Chongqing Education Commission (KJ130324) for foundation support.
Footnotes
Supported by the National Natural Science Foundation of China (NSFC: 81270211 and 81100088), Specialized Research Fund for the Doctoral Program of Higher Education (20125503110009), and Science and Technology Research Project of Chongqing Education Commission (KJ130324).
The authors report no conflicts of interest.
REFERENCES
- 1.Pitt B, Remme W, Zannad F, et al. ; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 2.Beygui F, Labbé JP, Cayla G, et al. Early mineralocorticoid receptor blockade in primary percutaneous coronary intervention for ST-elevation myocardial infarction is associated with a reduction of life-threatening ventricular arrhythmia. Int J Cardiol. 2013;167:73–79. [DOI] [PubMed] [Google Scholar]
- 3.Beygui F, Vicaut E, Ecollan P, et al. Rationale for an early aldosterone blockade in acute myocardial infarction and design of the ALBATROSS trial. Am Heart J. 2010;160:642–648. [DOI] [PubMed] [Google Scholar]
- 4.Alexandre J, Beygui F, Puddu PE, et al. Electrophysiological and antiarrhythmic properties of Potassium Canrenoate during myocardial ischemia-reperfusion. J Cardiovasc Pharmacol Ther. 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Breithardt G, Borggrefe M, Martinez-Rubio A, et al. Pathophysiological mechanisms of ventricular tachyarrhythmias. Eur Heart J. 1989;10(suppl E):9–18. [DOI] [PubMed] [Google Scholar]
- 6.Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation. 2005;112:2517–2529. [DOI] [PubMed] [Google Scholar]
- 7.Barbuti A, Baruscotti M, Difrancesco D. The pacemaker current: from basis to the clinics. J Cardiovasc Electrophysiol. 2007;18:342–347. [DOI] [PubMed] [Google Scholar]
- 8.Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci. 1999;868:741–764. [DOI] [PubMed] [Google Scholar]
- 9.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. [DOI] [PubMed] [Google Scholar]
- 10.Stieber J, Hofmann F, Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc Med. 2004;14:23–28. [DOI] [PubMed] [Google Scholar]
- 11.Song T, Yang J, Yao Y, et al. Spironolactone diminishes spontaneous ventricular premature beats by reducing HCN4 protein expression in rats with myocardial infarction. Mol Med Rep. 2011;4:569–573. [DOI] [PubMed] [Google Scholar]
- 12.Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang B, Lin H, Xiao J, et al. The muscle-specific microRNA-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. [DOI] [PubMed] [Google Scholar]
- 14.Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with “antagomirs”. Nature. 2005;438:685–689. [DOI] [PubMed] [Google Scholar]
- 15.Xia S, Wang Y, Zhang Y, et al. Dynamic changes in HCN2, HCN4, KCNE1, and KCNE2 expression in ventricular cells from acute myocardial infarction rat hearts. Biochem Biophys Res Commun. 2010;395:330–335. [DOI] [PubMed] [Google Scholar]
- 16.Fiedler J, Thum T. MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol. 2013;33:201–205. [DOI] [PubMed] [Google Scholar]
- 17.Benito B, Josephson ME. Ventricular tachycardia in coronary artery disease. Rev Esp Cardiol (Engl Ed). 2012;65:939–955. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Xuan Y-L, Hu H-S, et al. Risk of ventricular arrhythmias after myocardial infarction with diabetes associated with sympathetic neural remodeling in rabbits. Cardiology. 2012;121:1–9. [DOI] [PubMed] [Google Scholar]
- 19.St John Sutton M, Lee D, Rouleau JL, et al. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation. 2003;107:2577–2582. [DOI] [PubMed] [Google Scholar]
- 20.Barbuti A, Difrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–223. [DOI] [PubMed] [Google Scholar]
- 21.Stillitano F, Lonardo G, Zicha S, et al. Molecular basis of funny current(If) in normal and failing human heart. J Mol Cell Cardiol. 2008;45:289–299. [DOI] [PubMed] [Google Scholar]
- 22.Cerbai E, Barbieri M, Mugelli A. Occurrence and properties of the hyperpolarization-activated current if in ventricular myocytes from normotensive and hypertensive rats during aging. Circulation. 1996;94:1674–1681. [DOI] [PubMed] [Google Scholar]
- 23.Lai LP, Su MJ, Lin JL, et al. Measurement of funny current (I(f)) channel mRNA in human atrial tissue: correlation with left atrial filling pressure and atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:947–953. [DOI] [PubMed] [Google Scholar]
- 24.Milliez P, Messaoudi S, Nehme J, et al. Beneficial effects of delayed ivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. Am J Physiol Heart Circ Physiol. 2009;296:H435–H441. [DOI] [PubMed] [Google Scholar]
- 25.Ng FS, Shadi IT, Peters NS, et al. Selective heart rate reduction with ivabradine slows ischaemia-induced electrophysiological changes and reduces ischaemia-reperfusion-induced ventricular arrhythmias. J Mol Cell Cardiol. 2013;59:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grf EM, Heubach JF, Ravens U. The hyperpolarization-activated current if in ventricular myocytes of nontransgenic and beta2-adrenoceptor overexpressing mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:131–139. [DOI] [PubMed] [Google Scholar]
- 27.Qu J, Kryukova Y, Potapova IA, et al. MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J Biol Chem. 2004;279:43497–43502. [DOI] [PubMed] [Google Scholar]
- 28.Stillitano F, Sartiani L, DePaoli P, et al. Expression of the hyperpolarization-activated current, I(f), in cultured adult rat ventricular cardiomyocytes and its modulation by hypertrophic factors. Pharmacol Res. 2008;57:100–109. [DOI] [PubMed] [Google Scholar]
- 29.Muto T, Ueda N, Opthof T, et al. Aldosterone modulates if current through gene expression in cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H2710–H2718. [DOI] [PubMed] [Google Scholar]
- 30.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 31.Yu XY, Song YH, Geng YJ, et al. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376:548–552. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Zheng J, Sun Y, et al. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. [DOI] [PubMed] [Google Scholar]
- 33.Shan ZX, Lin QX, Deng CY, et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–3600. [DOI] [PubMed] [Google Scholar]
- 34.Terentyev D, Belevych AE, Terentyeva R, et al. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res. 2009;104:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayed D, Hong C, Chen IY, et al. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. [DOI] [PubMed] [Google Scholar]
- 36.Luo X, Lin H, Pan Z, et al. Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol Chem. 2008;283:20045–20052. [DOI] [PubMed] [Google Scholar]
- 37.D'Alessandra Y, Devanna P, Limana F, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]


