Abstract:
Calorie restriction (CR) is one of the most effective nonpharmacological interventions protecting against cardiovascular disease, such as hypertension in the systemic circulation. However, whether CR could attenuate pulmonary arterial hypertension (PAH) is largely unknown. The PAH model was developed by subjecting the rats to a single subcutaneous injection of monocrotaline. CR lowered mean pulmonary arterial pressure (mPAP) and reduced vascular remodeling and right ventricular hypertrophy in PAH rats. Meanwhile, CR attenuated endothelial dysfunction as evidenced by increased relaxation in response to acetylcholine. The beneficial effects of CR were associated with restored sirtuin-1 (SIRT1) expression and endothelial nitric oxide synthase (eNOS) phosphorylation and reduced eNOS acetylation in pulmonary arteries of PAH rats. To further clarify the role of SIRT1 in the protective effects of CR, adenoviral vectors for overexpression of SIRT1 were administered intratracheally at 1 day before monocrotaline injection. Overexpression of SIRT1 exhibited similar beneficial effects on mPAP and endothelial function, and increased eNOS phosphorylation and reduced eNOS acetylation in the absence of CR. Moreover, SIRT1 overexpression attenuated the increase in mPAP in hypoxia-induced PAH animals. Overall, the present data demonstrate that CR may serve as an effective treatment of PAH, and targeting the SIRT1/eNOS pathway may improve treatment of PAH.
Key Words: calorie restriction, pulmonary arterial hypertension, endothelial dysfunction, sirtuin-1
INTRODUCTION
Pulmonary arterial hypertension (PAH) is an insidious disease characterized by progressively abnormal pulmonary vasoconstriction and vascular remodeling, and it has been deemed as “cancer of cardiovascular diseases” owing to its high mortality and morbidity. PAH can be triggered by many cardiopulmonary diseases or others.1,2 Although the pathogenic mechanisms of PAH are very complex, endothelial dysfunction has long been considered to play a crucial role in the initiation and development of PAH.3–6 As such, the discovery of effective interventions that attenuate endothelial dysfunction is potential strategies for the prevention of PAH.
Calorie restriction (CR) is defined as a low level of energy intake, whereas sufficient levels of protein and micronutrients intakes are maintained to avoid malnutrition. Since the first report of extended lifespan in rodents more than 70 years ago,7 there is growing evidence demonstrating that CR has a powerful ability to protect against many cardiovascular diseases that occur with increasing age.8,9 In clinical tests, the results of the first randomized trial of CR in humans suggest a decreased risk of age-related cardiovascular disease.10 Although typical CR protocols involve restricting the calorie intake to 40% of controls for at least 12 months,11 recent observations indicate that short-term CR for 4–5 weeks exhibits similar cardiovascular benefits as long-term CR.12,13 A component of the beneficial effects of short-term CR is mediated through the upregulation of sirtuin-1 (SIRT1).14 SIRT1 has been shown to associate with endothelial nitric oxide (NO) synthase (eNOS) in the cytoplasm of vascular endothelial cells, deacetylate eNOS, and increase the activity of eNOS.15 Because NO synthesis serves a crucial role in the maintenance of vascular function,16 short-term CR likely exerts its beneficial effects on the vascular system through an SIRT1/eNOS pathway. However, these preceding studies involved short-term CR on endothelial dysfunction and cardiovascular disease, such as hypertension in the systemic circulation,17,18 and it is unknown whether short-term CR can improve endothelial function in the pulmonary circulation and attenuates the progression of PAH.
Based on this rationale, the aim of this study was to explore the effects of short-term CR on PAH. Monocrotaline (MCT)-induced PAH in rats is a well-defined model in which the animals develop severe PAH after a single injection of MCT. We hypothesized that short-term CR could attenuate MCT-induced endothelial dysfunction and the increase in pulmonary artery pressure. We then tested whether CR exerted possible protective effects through SIRT1/eNOS signaling in the pulmonary vasculature. Finally, we evaluated whether the overexpression of SIRT1 with gene therapy, in the absence of CR, could attenuate the elevation of pulmonary artery pressure in the MCT-treated PAH rats.
METHODS
Animal Models
This study was performed in adherence with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. This animal experiment was approved by The Fourth Military Medical University Ethics Committee on Animal Research. Male Sprague–Dawley rats weighing 250 ± 10 g were obtained from the animal center of the Fourth Military Medical University. Rat PAH model was induced by injecting a single dose of MCT (60 mg/kg in 0.5 mL saline, purchased from Dalian Meilun Biotech Co, Ltd) subcutaneously as described previously.19,20
Thirty-two rats were randomly divided into 4 groups: (1) normal control (Ctr) group, the rats received a single injection of saline in the same way and were fed a standard chow diet ad libitum for 5 weeks, (2) Ctr + CR group, the animals had CR according to previous protocols,12,18 whereby the rats received 90% of the average caloric intake of ad libitum–fed animals for 2 weeks (10% restriction), followed by 65% of the caloric intake of the control rats for the 3 weeks (35% restriction). CR diets contained rich vitamins, minerals, and salts such that CR rats were not salt deficient or nutrient deficient compared with the control animals, (3) MCT group, MCT-treated animals for 3 weeks receiving a standard chow diet ad libitum, and (4) MCT + CR group, the same 10% CR was administrated before MCT injection for 2 weeks, and 35% CR was continued after MCT injection for 3 weeks.
Hemodynamic Measurements and Evaluation of Right Heart Hypertrophy
The rats were anesthetized with 3% pentobarbital sodium (60 mg/kg intraperitoneally). Anesthesia was then maintained with supplemental doses of sodium pentobarbital when needed. A polyethylene microcatheter was inserted into right ventricle and pulmonary artery from right external jugular vein as described previously.21 The mean pulmonary arterial pressure (mPAP) was recorded and analyzed by a hemodynamic analyzing system (RM-6200C, Chengdu Instrument Co, China). Cardiac output was measured by thermodilution as described previously.22 After finishing the hemodynamic measurements, left ventricle (LV), right ventricle (RV), and septum (S) were separated, and the ratios of RV/(LV + S) were used as RV hypertrophy index.
Assessment of Peripheral Pulmonary Arterial Morphology
The lung tissue was fixed overnight in 4% paraformaldehyde and was embedded in paraffin. The tissues sections (5 μm) were stained with hematoxylin and eosin. The pulmonary arterioles (external diameters of 50–150 μm) were randomly chosen at a magnification of ×400 and analyzed with an image-processing software (Image-Pro Plus; Media Cybernetics). The medial wall thickness (WT), external semidiameter, cross-sectional medial wall area (WA), and total arterial cross-sectional area of peripheral pulmonary artery were measured. The ratio of WA to total arterial cross-sectional area (WA %) and the ratio of WT to external semidiameter (WT %) were calculated to analyze the degree of pulmonary artery remodeling. For quantitative assessment, all morphological analyses were conducted in a double-blind manner.
Assessment of Endothelial Function
Endothelial function was assessed ex vivo in isolated pulmonary arteries as recently described.23 The secondary branches of the pulmonary arteries were carefully dissected and cleaned of perivascular fat connective tissue under a dissecting microscope. Then, they were cut into 3-mm segments. Pulmonary artery segments were subjected to isolated vascular perfusion system and were connected to the tension sensor. The tension of pulmonary rings was measured by multichannel physiological recording system. After equilibration, phenylephrine (PE, 1 μmol/L) was added to the bath. Once the stable contraction was reached, acetylcholine (ACh) or acidified NaNO2 was added to the bath. Endothelial function was examined by comparing the relaxation activity of pulmonary rings in response to ACh (an endothelium-dependent vasodilator) with that of acidified NaNO2 (an endothelium-independent vasodilator). Endothelial dysfunction was defined as a decreased relaxation activity in response to ACh with normal relaxation activity in response to acidified NaNO2.
Determination of eNOS Activity and NO Concentrations
Pulmonary artery tissues were minced and homogenized in lysis buffer (NaF 50 mM, NaCl 50 mM, sucrose 25 mM, Tris 20 mM, Na4P2O7·10H2O 5 mM, Na3VO4 2 mM, DTT 1 mM, and 1% protease inhibitor cocktail, pH 7.4). The homogenate was centrifuged at 12,000g for 10 minutes at 4°C, the supernatant was decanted, and eNOS activity was determined using an eNOS activity assay kit (Nanjing Jiancheng Bioengineering Institute) following the manufacturer instructions as previously described.24,25 Total NO production by pulmonary artery tissues was measured by NO assay kit (Nanjing Jiancheng Bioengineering Institute) strictly according to the instruction of production as previously reported.24,25
Immunoblot Analysis
Pulmonary artery tissues were lysed with lysis buffer. After sonication, the lysates were centrifuged, equal amounts of protein were electrophoresed on SDS-PAGE and electrophoretically transferred to PVDF (polyvinylidene difluoride)-Plus membranes (Millipore, Billerica, MA). After blocking with 5% skim milk at room temperature for 1 hour, the membranes were incubated with an antibody against SIRT1 (Santa Cruz Biotechnology), eNOS, phosphorylated eNOS (serine 1177, BD Bioscience) overnight at 4°C. The membranes were then washed with phosphate-buffered saline with 0.1% Tween 20 and incubated with corresponding horseradish peroxidase–conjugated IgG antibodies at room temperature for 1 hour. The immunoblotting was measured by using an enhanced chemiluminescence detection kit (Millipore, Billerica, MA) with ChemiDoc XRS (Bio-Rad, Hercules, CA), and the densities of the bands were analyzed using Quantity One software.
eNOS Acetylation Expression
There is no commercially available acetylated eNOS antibody until now. To determine acetylation state of eNOS, eNOS [1:1000 cross-linked to magnetic beads (Dynal, Invitrogen) for extraction] was immunoprecipitated from 25 μg of artery tissue lysate, and the association of the acetyllysine with eNOS was detected by immunoblotting with the primary antibody for acetyllysine (cell signaling) as described above.
Delivery of Adenovirus Vectors to the Lungs
Adenoviral vectors for the overexpression of SIRT1 (Ad. SIRT1) were used as previously described.26,27 Viral solution (7.5 × 109 pfu) was diluted before the use with sterile saline (pH 7.4) in a final volume of 0.1 mL. Rats were anesthetized with 3% pentobarbital sodium (60 mg/kg, intraperitoneally). Intratracheal instillation of 0.1 mL/rat of diluted Ad.SIRT1 was performed using a standard procedure, as previously described.28,29 Ad. GFP was used as a control vector.
Statistical Analysis
Values are presented as mean values ± standard error of the mean. Data were analyzed with unpaired Student 2-tailed t test, 2-way repeated-measures analysis of variance, Mann–Whitney U test, or 1-way analysis of variance followed by Bonferroni post hoc tests, when appropriate. A probability value of P < 0.05 was considered to be statistically significant.
RESULTS
Short-term CR Attenuated MCT-induced Pulmonary Hypertension and Vascular Remodeling
No differences in body weight existed among all the groups at baseline. The CR diet was fed to ensure that the rats in the CR groups received enough salt, minerals, and vitamins. As expected, 5 weeks of short-term CR reduced gain in body weight significantly (Fig. 1A, B), whereas it did not alter tibia length compared with controls (Fig. 1C), indicating that CR did not affect growth. The gain in body weight was also slightly decreased in MCT-treated control animals. All the rats survived until hemodynamic measurements 3 weeks after MCT injection.
FIGURE 1.
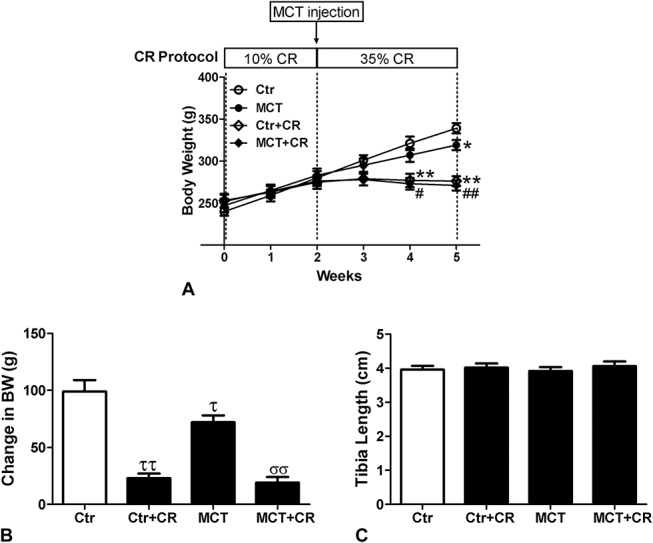
Physical parameters of Ctr and CR MCT-treated rats. A, Experiment protocol and body weight. B, Change in body weight. C, Tibia length. Values are mean ± standard error of the mean (n = 8). *P < 0.05, **P < 0.01 versus Ctr and #P < 0.05, ##P < 0.01 versus MCT using a 2-way repeated-measures analysis of variance followed by Bonferroni post hoc tests. τP < 0.05, ττP < 0.01 versus Ctr and σσP < 0.01 versus MCT using a Mann–Whitney U test.
MCT-treated rats exhibited a significant increase in mPAP (Fig. 2A), RV hypertrophy [RV/(LV + S)] (Fig. 2C), and pulmonary artery remodeling (WT%, WA%) (Fig. 2E, F). These changes were attenuated by short-term CR, whereas short-term CR had no effect on these parameters in control animals. There was a small trend toward decreased cardiac output that did not reach significance in control or MCT-treated animals receiving short-term CR (Fig. 2B). These data indicated that short-term CR could attenuate PAH and vascular remodeling, which may be mainly due to reduced pulmonary vascular resistance.
FIGURE 2.
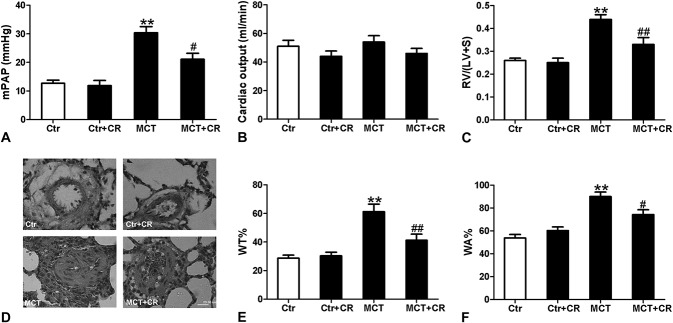
Effects of short-term CR on MCT-induced pulmonary hypertension and vascular remodeling and right ventricular hypertrophy. A, mPAP. B, Cardiac output. C, Right ventricle weight/left ventricle + septum weight ratio [RV/(LV + S)]. D, Representive histopathological changes in rat lung tissues (hematoxylin and eosin stain, × 400). The bar represents 20 μm. E, The ratio of the medial wall thickness (WT%). F, The ratio of the medial wall area (WA%). There were increased mPAP and vascular remodeling (WT%, WA%) and RV/LV + S in MCT-treated rats at 3 weeks after injection. Preventive administration of CR decreased mPAP and reduced vascular remodeling and RV hypertrophy index. Values are mean ± standard error of the mean (n = 8). **P < 0.01 versus Ctr and #P < 0.05, ##P < 0.01 versus MCT using a one-way ANOVA with Bonferroni multiple comparisons test.
Short-term CR Preserved ACh-induced Vasorelaxation in PAH Rats
Because endothelial dysfunction contributes to the initiation and development of PAH, we investigated whether endothelial function was improved by CR in vitro by comparing the relaxation activity of pulmonary rings in response to Ach with that of NaNO2 in different treatment groups. As shown in Figure 3B, vasorelaxation in response to NaNO2 was not changed in all the groups. However, concentration-dependent vasorelaxation responses to ACh were impaired in pulmonary arteries isolated from PAH rats compared with control group (Fig. 3A), which were consistent with previous results.30 Interestingly, short-term CR alleviated endothelial dysfunction, as demonstrated by a significant improvement of concentration-dependent vasorelaxation in response to ACh. The addition of Nω-nitro-l-arginine methylester (l-NAME; 0.5 mM) completely blocked ACh-induced vasorelaxation in those pulmonary arteries from MCT-treated rats receiving short-term CR.
FIGURE 3.
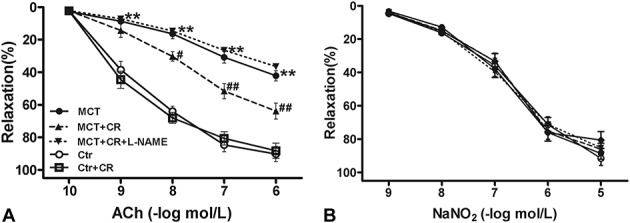
Concentration-dependent vasorelaxation in response to ACh (A) or NaNO2 (B) in pulmonary artery rings isolated from Ctr or MCT-treated rats without or with CR. Values are mean ± standard error of the mean (n = 10 vascular segments per group from 5 animals). **P < 0.01 versus Ctr and #P < 0.05, ##P < 0.01 versus MCT using a 2-way repeated-measures ANOVA followed by Bonferroni post hoc tests.
Short-term CR Increased NO Production and eNOS Activity While Did Not Alter eNOS Expression in PAH Rats
To reveal the potential mechanism of CR on endothelial dysfunction, NO production and eNOS expression and activity were measured. As shown in Figure 4, the production of NO and eNOS expression and activity in pulmonary arteries were significantly decreased in MCT-treated animals compared with that in normal control rats. Short-term CR increased eNOS activity and NO production but did not alter eNOS expression in pulmonary arteries of both control and PAH rats. These results suggested that improved endothelial function in response to CR might be attributed to the alteration of eNOS activity but not its expression.
FIGURE 4.
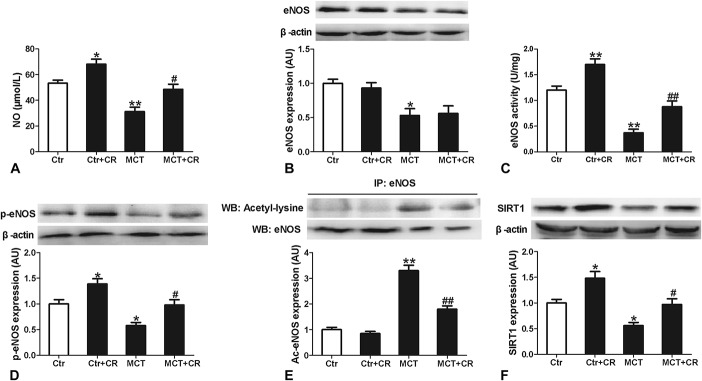
Analysis of NO production (A) and eNOS activity (C) and arterial signaling pathways in Ctr or MCT-treated rats without or with short-term CR. Protein expression is shown in pulmonary artery tissues for eNOS (B), eNOS phosphorylated at serine 1177 (p-eNOS) (D), acetylated eNOS (E), and SIRT-1 (F). Protein expression of eNOS, p-eNOS, and SIRT-1 is expressed relative to β-actin and immunoprecipitated acetylated eNOS is expressed relative to control eNOS expression. Representative images are shown above the summary data. Data are shown normalized to the control mean values. Values are mean values ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01 versus Ctr and #P < 0.05, ##P < 0.01 versus MCT using a 1-way ANOVA with Bonferroni multiple comparisons test.
Short-term CR Increased eNOS Phosphorylation and Reduced eNOS Acetylation in Association With Elevated SIRT1 Expression in PAH Rats
To elucidate the mechanism of CR-induced elevation of eNOS activity in PAH rats, we therefore measured phosphorylation (activation state) and acetylation (deactivation state) of eNOS and the expression of deacetylase SIRT1. As illustrated in Figure 4, the Western blot analysis of lung artery tissue demonstrated decreased eNOS phosphorylation and increased eNOS acetylation and reduced SIRT1 expression in MCT-treated rats. Short-term CR significantly increased eNOS phosphorylation and SIRT1 expression, and attenuated eNOS acetylation in these arteries. These results suggested that short-term CR might increase eNOS activity by the enhanced phosphorylation of eNOS and reduced acetylation of eNOS, which was associated with increased SIRT1 expression.
SIRT1 Is Involved in the Protective Effects of Short-term CR on PAH and Endothelial Dysfunction
To clarify the role of SIRT1 in the protective effects of CR on PAH, Ad.SIRT1 or Ad.GFP was administered intratracheally at 1 day before MCT injection. Eight days after the injection of Ad.SIRT1 (1 week after MCT injection), SIRT1 expression and phosphorylated eNOS in pulmonary arteries were significantly increased (Figs. 5A, B), and acetylated eNOS was decreased (Fig. 5C). These data indicated that increasing SIRT1 expression could enhance the phosphorylation level of eNOS and reduce the acetylation level of eNOS. Importantly, mPAP was significantly lower than Ad.GFP-treated controls at 3 weeks after MCT injection (Fig. 5D). Consistent with the reduction in mPAP in the Ad.SIRT1-treated PAH animals, endothelial function was also improved in pulmonary arteries of Ad.SIRT1-treated PAH rats compared with Ad.GFP-treated PAH animals at 3 weeks after MCT injection (Figs. 5E, F). In addition, body weight was not different between Ad.GFP- and Ad.SIRT1-treated rats (290.1 ± 6.3 vs. 298.4 ± 7.1 g). Therefore, SIRT1 overexpression in the MCT-induced PAH rats recapitulates the reduction in mPAP associated with CR, even in the absence of changed body weight.
FIGURE 5.
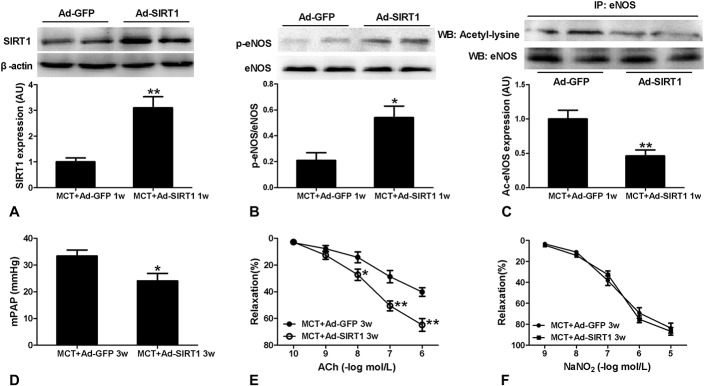
Effect of SIRT1 overexpression on MCT-induced pulmonary hypertension and endothelial dysfunction. Intratracheal administration of Ad.SIRT1 resulted in increased SIRT-1 expression (A) and phosphorylated eNOS (B) and decreased acetylated eNOS (C) in pulmonary artery tissues form MCT-treated rats at 8 days after transfection compared with Ad.GFP administration (1 week after MCT injection). SIRT-1 is expressed relative to β-actin. Phosphorylated eNOS and immunopreciptated acetylated eNOS are expressed relative to total or control eNOS expression. Data are shown normalized to the control mean values. Overexpression of SIRT1 decreased mPAP (D) and improved concentration-dependent vasorelaxation responses to Ach (E) at 3 weeks after MCT injection. No significant difference existed in concentration-dependent vasorelaxation responses to NaNO2 (F) between Ad.SIRT1 and Ad.GFP administration at 3 weeks after MCT injection. Values are mean values ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01 versus MCT + Ad.GFP 1w or 3w using an unpaired Student 2-tailed t test.
To further confirm the effect of SIRT1 overexpression on PAH, we tested the effect of SIRT1 overexpression on another type of PAH induced by hypoxia. The rats were treated with Ad.SIRT1 or Ad.GFP intratracheally 1 day as previously described before being exposed to hypobaric and hypoxic conditions (air pressure 50 kPa, oxygen concentration 10%) for 8 hours every day. Eight days after injection of Ad.SIRT1 (1 week of hypoxia), SIRT1 expression in pulmonary arteries was significantly increased (Fig. 6A). Moreover, mPAP was significantly lower in Ad.SIRT1-treated rats compared with Ad.GFP-treated controls after 2 weeks of hypoxia (Fig. 6B). Taken together, these data imply that increasing SIRT1 expression is sufficient to attenuate both MCT-induced and hypoxia-induced pulmonary hypertension in the absence of CR.
FIGURE 6.
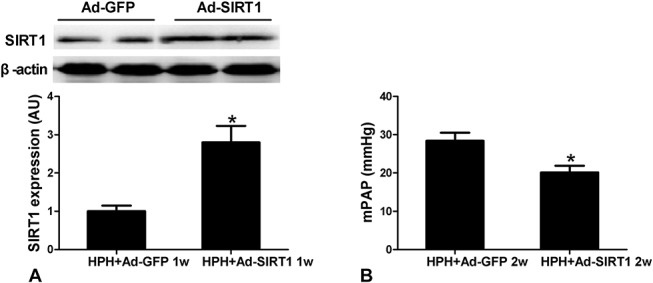
Effect of SIRT1 overexpression on hypoxia-induced pulmonary hypertension (HPH) rats. SIRT-1 expression (A) in pulmonary artery tissues form HPH rats was increased significantly at 8 days after intratracheal administration of Ad.SIRT1 or Ad.GFP (1 week of hypoxia). SIRT-1 is expressed relative to β-actin. Overexpression of SIRT1 decreased mPAP (B) in HPH rats. Values are mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01 versus HPH + Ad.GFP 1w or 2w using an unpaired Student 2-tailed t test.
DISCUSSION
We have made several novel observations in this study. First, we demonstrated for the first time that short-term CR ameliorates MCT-induced endothelial dysfunction and pulmonary hypertension. Second, the mechanisms of these actions are associated with the activation of SIRT1/eNOS signaling pathway. More importantly, the data indicate that SIRT1 overexpression increases eNOS phosphorylation and exhibits similar protective effects on PAH in the absence of CR, which provides a new potential therapeutic target to prevent the development of PAH.
Calorie restriction is one of the most effective nonpharmacological interventions that extends lifespan and protects against cardiovascular disease. In particular, long-term CR has been considered to be a blood pressure lowering strategy for both human and animal models of hypertension.31–33 Recent evidence showed that short-term CR has similar benefits for the cardiovascular system. Rippe et al17 found that short-term CR initiated in old age reversed age-associated endothelial dysfunction. Dolinsky et al18 demonstrated that CR improved vascular function and prevented hypertension in the spontaneously hypertensive rat model. However, these previous studies mainly focused on the effects of CR on the systemic circulation while provided little information on its pulmonary effects. MCT injury rat model is one of the most commonly used animal models of PH, which develops severe PH with increased right ventricular systolic pressures after several weeks. The severity of this model and the rapid progression of the disease process are comparable to those of human PAH (equivalent to New York Heart Association functional classes III–IV) and makes it an attractive model to test therapeutic agents or strategies. In this study, we found a prominent attenuation of MCT-induced endothelial dysfunction and pulmonary hypertension in response to CR, as evidenced by improved endothelial-dependent vasodilation and decreased mPAP. Accordingly, reducing pulmonary hypertension attenuated right ventricle hypertrophy as evidenced by decreased RV/(LV + S).
Several lines of evidence have suggested that obesity plays a pathogenic role in the development of PAH. Specifically, autopsy studies indicate a higher prevalence of PAH in obese subjects when compared with nonobese historical controls.34 Moreover, findings from the REVEAL registry, the largest pulmonary hypertension database in the United States, indicate a higher prevalence of overweight and obese individuals among those with idiopathic forms of PAH.35 It is important to note that an obesity paradox exists in which it has been demonstrated that obese patients with established PAH seem to have a lower mortality than leaner patients.36 Nevertheless, the constellation of data support dramatic functional improvement after weight loss surgery in PAH patients,37–39 which is consistent with our study that CR exhibits similar beneficial effects on the development of PAH.
In the pathogenesis of PAH, reduced NO-mediated vasodilation plays a crucial role in the impairment of endothelial-dependent vasodilation.40 Impaired endothelial function is a consequence of reduced NO production in PAH. However, impaired vascular endothelium may also lead to reduced production of NO.41,42 Thus, it is believed that endothelial dysfunction and impaired NO produce may share reciprocal causation relationship in the development of PAH. Consistent with previous study, our study showed that the level of NO in PAH rats was reduced, and in the meantime, isolated pulmonary vascular perfusion result demonstrated that endothelial dysfunction was also induced in PAH rats. Short-term CR is demonstrated to increase NO reduction and to ameliorate endothelial dysfunction in PAH rats. Moreover, the relaxing effect of CR on pulmonary artery was blunted by an eNOS inhibitor l-NAME, indicating that the elevation of NO induced by CR may contribute to the improvement of endothelial dysfunction.
Endothelial NO is formed from l-arginine through enzymatic conversion by eNOS. It has been demonstrated that PAH is associated with decreased eNOS expression in rats.43,44 Consistent with previous study, we found reduced eNOS protein expression and activity in MCT-treated rats. However, CR did not alter the expression of eNOS, whereas it increased eNOS activity in PAH rats. These studies suggest that the development of PAH is not only due to the decreased expression of eNOS but also may be related to a decreased activity of eNOS to produce sufficient NO.
Modification of protein is involved in the alteration of eNOS activity. SIRT1 is a histone deacetylase that plays an important role in regulating eNOS activity. It has been reported that SIRT1 and eNOS colocalize and coprecipitate in endothelial cells, and deacetylation by SIRT1 enhances eNOS activity and NO production.15 In this study, pulmonary SIRT1 expression in PAH rats was lower than in normal controls, which is associated with increased acetylated eNOS. This is in agreement with the fact that viral (siRNA) or pharmacological (sirtinol) inhibition of SIRT-1 activity directly acetylates eNOS and inhibits eNOS activity in cultured endothelial cells.15,45 Moreover, we found that reduced SIRT-1 expression and increased acetylation of eNOS were accompanied with a reduction in eNOS phosphorylation, indicators of eNOS activation state. Interestingly, short-term CR increased SIRT1 expression and reduced eNOS acetylation in PAH animals, which is associated with enhanced eNOS phosphorylation. These results indicate that reduced pulmonary SIRT-1 expression is associated with acetylation and deactivation of eNOS and impaired NO-mediated vascular endothelial dysfunction in the development of PAH.
To further clarify the role of SIRT1 in the protective effects of CR on MCT-induced endothelial dysfunction and pulmonary hypertension, we used adenoviral vectors to upregulate SIRT1 expression in the lung tissue. It was showed that SIRT1 overexpression restored the eNOS phosphorylation and endothelial function of pulmonary artery and attenuated the increase in mPAP in MCT-induced animals, even in the absence of changed body weight. Although different mechanisms, such as different neointimal proliferative changes, are involved in the development of MCT-induced and hypoxia-induced PAH, similar molecular mechanisms, such as decreased eNOS phosphorylation, have been reported to play an important role in the development of both MCT-induced and hypoxia-induced PAH.25,30,46 It was demonstrated that SIRT1 overexpression also attenuated the increase in mPAP in hypoxia-induced PAH animals in which the mechanisms may be related to the restoration of eNOS phosphorylation. One of the limitations of this study was that we could not prevent the increase of SIRT1 expression in the CR rats to confirm that the protective effects of CR on PAH are dependent on SIRT1. However, taken together, our study suggests that short-term CR restored SIRT1/eNOS signaling in pulmonary vasculature of the PAH rats, thus attenuates endothelial dysfunction and pulmonary hypertension.
CONCLUSIONS
In summary, our study indicated that short-term CR attenuates the development of PAH in a recognized animal model of this disease. The possible molecular mechanisms of this beneficial effect seemed to be related to the restoration of SIRT/eNOS signaling and improvement of endothelial function. This study highlights CR as a potential nonpharmacological intervention against PAH in clinical practice. However, despite the protective vascular benefits of short-term CR reported herein, compliance can be a major hindrance because the lifestyle changes associated with CR need considerable patient commitment. Based on our findings, pharmacological treatment or gene therapy strategies that stimulate the SIRT1/eNOS signaling pathway could provide an alternative approach to retard the development of PAH.
Footnotes
Supported by grants from National Natural Science Foundation of China (No. 81270124).
The authors report no conflicts of interest.
M. Ding and J. Lei contributed equally to this study.
REFERENCES
- 1.McLaughlin V. Managing pulmonary arterial hypertension and optimizing treatment options: prognosis of pulmonary artery hypertension. Am J Cardiol. 2013;111:10C–15C. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Souza R, Galie N, et al. Pulmonary arterial hypertension: bridging the present to the future. Eur Respir Rev. 2012;21:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tual-Chalot S, Guibert C, Muller B, et al. Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction. Am J Respir Crit Care Med. 2010;182:261–268. [DOI] [PubMed] [Google Scholar]
- 4.Ito KM, Sato M, Ushijima K, et al. Alterations of endothelium and smooth muscle function in monocrotaline-induced pulmonary hypertensive arteries. Am J Physiol Heart Circ Physiol. 2000;279:H1786–H1795. [DOI] [PubMed] [Google Scholar]
- 5.Mam V, Tanbe AF, Vitali SH, et al. Impaired vasoconstriction and nitric oxide-mediated relaxation in pulmonary arteries of hypoxia- and monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Exp Ther. 2010;332:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou ZJ, Wei W, Huang DD, et al. L-arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol Endocrinol Metab. 2010;298:E1131–E1139. [DOI] [PubMed] [Google Scholar]
- 7.McCay C, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 8.Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–H1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohrbach S, Aslam M, Niemann B, et al. Impact of caloric restriction on myocardial ischaemia/reperfusion injury and new therapeutic options to mimic its effects. Br J Pharmacol. 2014;171:2964–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinmura K, Tamaki K, Saito K, et al. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. [DOI] [PubMed] [Google Scholar]
- 13.Kondo M, Shibata R, Miura R, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitada M, Kume S, Takeda-Watanabe A, et al. Calorie restriction in overweight males ameliorates obesity-related metabolic alterations and cellular adaptations through anti-aging effects, possibly including AMPK and SIRT1 activation. Biochim Biophys Acta. 2013;1830:4820–4827. [DOI] [PubMed] [Google Scholar]
- 15.Mattagajasingh I, Kim CS, Naqvi A, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509. [DOI] [PubMed] [Google Scholar]
- 17.Rippe C, Lesniewski L, Connell M, et al. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolinsky VW, Morton JS, Oka T, et al. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Niu W, Xu D, et al. Oxymatrine prevents hypoxia- and monocrotaline-induced pulmonary hypertension in rats. Free Radic Biol Med. 2014;69:198–207. [DOI] [PubMed] [Google Scholar]
- 20.Baber SR, Deng W, Master RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–H1128. [DOI] [PubMed] [Google Scholar]
- 21.Xu D, Niu W, Luo Y, et al. Endogenous estrogen attenuates hypoxia-induced pulmonary hypertension by inhibiting pulmonary arterial vasoconstriction and pulmonary arterial smooth muscle cells proliferation. Int J Med Sci. 2013;10:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogaard HJ, Natarajan R, Mizuno S, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–660. [DOI] [PubMed] [Google Scholar]
- 23.Kassan M, Sevilla MA, Gonzalez-Santos JM, et al. Pravastatin improves endothelial function in arteries used in coronary bypass grafting. J Cardiovasc Pharmacol. 2013;61:513–519. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Sun J, Xue F, et al. Effect of 3,4-Dihydroxyacetophenone on endothelial dysfunction in Streptozotocin-induced type 2 Diabetic rats. J Cardiovasc Pharmacol. 2015;65:22–27. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Wang HY, Li J, et al. Kappa-opioid receptor stimulation improves endothelial function in hypoxic pulmonary hypertension. PLoS One. 2013;8:e60850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Song MY, Song EK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S, Chen HZ, Wan YZ, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. [DOI] [PubMed] [Google Scholar]
- 28.Partovian C, Adnot S, Raffestin B, et al. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol. 2000;23:762–771. [DOI] [PubMed] [Google Scholar]
- 29.Pascaud MA, Griscelli F, Raoul W, et al. Lung overexpression of angiostatin aggravates pulmonary hypertension in chronically hypoxic mice. Am J Respir Cell Mol Biol. 2003;29:449–457. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Lu W, Cai WW, et al. Telmisartan attenuates monocrotaline-induced pulmonary artery endothelial dysfunction through a PPAR gamma-dependent PI3K/Akt/eNOS pathway. Pulm Pharmacol Ther. 2014;28:17–24. [DOI] [PubMed] [Google Scholar]
- 31.Young JB, Mullen D, Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1978;27:1711–1714. [DOI] [PubMed] [Google Scholar]
- 32.Overton JM, VanNess JM, Casto RM. Food restriction reduces sympathetic support of blood pressure in spontaneously hypertensive rats. J Nutr. 1997;127:655–660. [DOI] [PubMed] [Google Scholar]
- 33.Meyer TE, Kovacs SJ, Ehsani AA, et al. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. [DOI] [PubMed] [Google Scholar]
- 34.Haque AK, Gadre S, Taylor J, et al. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med. 2008;132:1397–1404. [DOI] [PubMed] [Google Scholar]
- 35.Burger CD, Foreman AJ, Miller DP, et al. Comparison of body habitus in patients with pulmonary arterial hypertension enrolled in the Registry to Evaluate Early and Long-term PAH Disease Management with normative values from the National Health and Nutrition Examination Survey. Mayo Clin Proc. 2011;86:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zafrir B, Adir Y, Shehadeh W, et al. The association between obesity, mortality and filling pressures in pulmonary hypertension patients; the “obesity paradox”. Respir Med. 2013;107:139–146. [DOI] [PubMed] [Google Scholar]
- 37.Mathier MA, Zhang J, Ramanathan RC. Dramatic functional improvement following bariatric surgery in a patient with pulmonary arterial hypertension and morbid obesity. Chest. 2008;133:789–792. [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Lobato S, Navarro JG, Perez-Rodriguez E. Dramatic functional improvement following bariatric surgery in a patient with pulmonary arterial hypertension and morbid obesity. Chest. 2008;134:670. [DOI] [PubMed] [Google Scholar]
- 39.Pugh ME, Newman JH, Williams DB, et al. Hemodynamic improvement of pulmonary arterial hypertension after bariatric surgery: potential role for metabolic regulation. Diabetes Care. 2013;36:e32–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–646. [DOI] [PubMed] [Google Scholar]
- 42.Hagan G, Pepke-Zaba J. Pulmonary hypertension, nitric oxide and nitric oxide-releasing compounds. Expert Rev Respir Med. 2011;5:163–171. [DOI] [PubMed] [Google Scholar]
- 43.Kanno S, Wu YJ, Lee PC, et al. Angiotensin-converting enzyme inhibitor preserves p21 and endothelial nitric oxide synthase expression in monocrotaline-induced pulmonary arterial hypertension in rats. Circulation. 2001;104:945–950. [DOI] [PubMed] [Google Scholar]
- 44.Csiszar A, Labinskyy N, Olson S, et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ota H, Eto M, Kano MR, et al. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30:2205–2211. [DOI] [PubMed] [Google Scholar]
- 46.Kuriyama S, Morio Y, Toba M, et al. Genistein attenuates hypoxic pulmonary hypertension via enhanced nitric oxide signaling and the erythropoietin system. Am J Physiol Lung Cell Mol Physiol. 2014;306:L996–L1005. [DOI] [PubMed] [Google Scholar]


