Abstract:
Vascular injury after chronic hypoxia leads to endothelial injury and structural damage to tight junctions (TJs), thereby resulting in a variety of cardiovascular diseases. Thus, attenuating hypoxia-induced damage has great significance for the prevention and treatment of cardiovascular disease. The aim of this study was to investigate whether the endothelial protection conferred by tongxinluo (TXL), a traditional Chinese medicinal compound, is related to its regulation of TJ protein expression. In vivo, we found that TXL could promote hypoxia-induced angiogenesis in lung and liver tissue. In vitro, we found that CoCl2 treatment significantly reduced the expression of the TJ proteins occludin, claudin-1, VE-cadherin, and beta-catenin in cultured human cardiac microvascular endothelial cells. TXL pretreatment abrogated the CoCl2-induced downregulation of these TJ proteins. Conversely, overexpression of Krüppel-like factor 4 (KLF4) inhibited the expression of TJ proteins in human cardiac microvascular endothelial cells, an effect that was reversed by TXL pretreatment. Further experiments showed that TXL could promote endothelial cell proliferation by increasing KLF4 phosphorylation, thereby reversing the effect of KLF4 on the expression of TJ proteins. These findings provide a new molecular mechanism for the TXL-induced increase in TJ protein expression.
Key Words: TXL, human cardiac microvascular endothelial cells, chronic hypoxia, tight junction protein, KLF4
INTRODUCTION
Hypoxia, the decrease in oxygen bioavailability, is involved in a diverse range of physiological and pathophysiological processes, including development, wound healing, inflammation, vascular disease, and cancer.1,2 Hypoxia-induced endothelial dysfunction is an early event that leads to a host of severe cardiovascular diseases.3 Through the regulation of gene expression by key oxygen-sensitive transcriptional regulators, such as hypoxia-inducible factor (HIF-1α) and nuclear factor-κB, hypoxia accelerates the apoptosis and inflammation of endothelial cells.4,5 Clinically, several disease states, including obesity, ischemic injury, and hypertensive diseases, have connections to hypoxia and the vascular endothelial inflammatory response. However, the mechanism that regulates hypoxia-mediated endothelial dysfunction remains elusive, as does an effective treatment.
Vascular endothelial cells line the walls of blood vessels and control the transport of substances between the blood and tissue through formation of tight junction (TJ) complexes. The proper arrangement and function of TJ complexes are critical for maintaining the integrity of the blood–tissue barrier.6,7 Hypoxia, ie, impaired O2 delivery, acts as an initial trigger for pathophysiological changes in endothelial cells. Such changes, including altered distribution of water and ions, oxidative stress, and inflammatory event, are bound to affect the TJs between endothelial cells and hence their secretory function. Therefore, the search for effective drugs targeting hypoxia-induced vascular endothelial dysfunction and blood–tissue barrier impairment, and the molecular mechanisms by which they occur, is significant for preventing cardiovascular diseases.
Tongxinluo (TXL), a traditional Chinese medicine, is composed of Radix ginseng, Buthus martensi, Hirudo, Eupolyphaga seu steleophaga, Scolopendra subspinipes, Periostracum cicadae, Radix paeoniae rubra, Semen ziziphi spinosae, Lignum dalbergiae odoriferae, Lignum santali albi, and Borneolum syntheticum. TXL was registered in the State Food and Drug Administration of China in 1996,8 and it has been used in the treatment of cardiovascular and cerebrovascular diseases in China.9,10 Pharmacological research has revealed that TXL has pleiotropic effects, including improvement of endothelial cell function, reduction of lipid levels, vasodilation, anti-inflammation, antiapoptosis, and enhancement of angiogenesis.11–14 However, the effects of TXL on the hypoxia-induced expression of TJ proteins and on their function in endothelial cells have not been reported in the literature. In this study, we investigate the effects of TXL on the TJs of endothelial cells and study its mechanism of action both in vivo and in vitro.
MATERIALS AND METHODS
Animals
Adult male C57BL/6J mice with a mean weight of 25 g were obtained from the Experimental Animal Center of Hebei Medical University, which is fully accredited by the Institutional Animal Care and Use Committee (IACUC). C57BL/6J mice were randomly assigned to one of the following 3 groups: control group, hypoxia group, and TXL preincubation (6 mice per group). The last 2 groups were placed in a hypobaric chamber (barometric pressure [PB] = 404 mm Hg, oxygen pressure [PO2] = 84 mm Hg) to simulate an altitude of 5000 m (6 h/d, 28 days). TXL (7.5 mg/10 g) was intragastrically administered 3 days before hypoxic treatment in the hypobaric chamber (TXL preincubation group) and continued for 28 days, until finished in the hypobaric chamber. At the end of the exposure period, we anesthetized each mouse with a pentobarbital injection and then removed the thoracic aorta, lung, and liver, fixing them by immersion in 4% paraformaldehyde for 24 hours.
Immunohistochemistry
Tissues were dehydrated, cleared, and embedded in paraffin wax. The paraffin blocks were then cut into 4-µm-thick sections. For immunohistochemistry, sections were deparaffinized and rehydrated in graduated alcohol. Next, they were treated in a 0.1 mol/L sodium citrate buffer and heated for 30 minutes to retrieve antigens. The sections were then incubated overnight with antibodies against anti–HIF-1α (1:100; Abcam), anti-CD31 (1:50; Anbo), antioccludin (1:50; Epitomics), anti–claudin-1 (1:50; Novus), or anti–Krüppel-like factor 4 (KLF4) (1:50; Epitomics) and subsequently incubated with the appropriate secondary antibodies (Abcam). Sections were visualized with 3,3′-diaminobenzidine (DAB) and counterstained using hematoxylin. Brown and yellow coloring indicated positive stains.
Western Blotting
Human cardiac microvascular endothelial cell (HCMECs) line (Cat 6110; ScienCell) was bought from ScienCell company and cultured in endothelial cell medium with 10% fetal bovine serum and maintained in 5% CO2 at 37°C in a humidified atmosphere. Cells were treated with TXL, CoCl2, pAd-GFP, or pAd-KLF4 for 24 hours and then harvested with lysis buffer containing 1% NP-40, 150 mM NaCl, 50 mM Tris-Cl pH 7.5, 10% glycerin, 1 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride (PMSF), and 1 mM dithiothreitol (DTT). The tissues were lysed with the above lysis buffer. Proteins were isolated from HCMECs, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were blocked with 5% milk in Tris-HCl–Tween buffer solution for 2 hours at 37°C and incubated overnight at 4°C with specific antibodies [anti–HIF-1α (1:1000; Abcam), anti–VE-cadherin (1:1000; Abcam), anti–beta-catenin (1:10,000; Abgent), antioccludin (1:100,000; Epitomics), anti–claudin-1 (1:500; Novus), and anti-KLF4 (1:1000; Epitomics)]. After incubation with appropriate secondary antibodies, the membranes were developed using the chemiluminescence plus Western blot analysis kit (Millipore).
Immunofluorescence Staining
To observe the localization of occludin, claudin-1, and ZO-1 proteins, cultured cells were washed with 0.1 M phosphate-buffered saline 3 times before being fixed in 4% paraformaldehyde for 10 minutes. After that, the cells were incubated with antioccludin (1:100; Epitomics), anti–claudin-1 (1:100; Novus), anti–ZO-1 (1:100; Abcam) antibodies overnight at 4°C. After washing with phosphate-buffered saline, stained coverslips were observed by Leica SP5 confocal microscope.
Cell Viability Assay
HCMECs were seeded into 96-well plates with 5 replicates for each group. The next day, the cells were pretreated with different concentrations of TXL for 24 hours and exposed to 100 µM CoCl2, or different concentrations of CoCl2, respectively. After incubating for 24 hours, the cells were inoculated with 10 µL of CCK-8 solution (Dojindo, Kumamoto, Japan) and incubated again for 2 hours at 37°C, after which absorbance was measured at an optical density of 450 nm.
Co-immunoprecipitation Assay
Cell lysates were immunoprecipitated with anti-KLF4 or anti–serine phosphate antibodies for 1 hour at 4°C and then incubated with protein A–agarose overnight at 4°C. Protein A–agarose–antigen-antibody complexes were collected and washed 4 times with IPH buffer for 20 minutes each time. Bound proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Western blotting.
MTT Assay
Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Endothelial cells were cultured in a 96-well culture plate and then subjected to growth arrest for 24 hours before treatment with different drugs according to experimental group. At the end of the treatment, the cells were incubated for 4 hours in a medium containing 0.5% MTT, the yellow mitochondrial dye. The reaction was terminated by adding 150 μL DMSO to the cells and incubating for 10 minutes. Absorbance at 490 nm was recorded with an enzyme-linked immunosorbent assay plate reader.
Tube Formation Assay
Ninety-six–well culture plates were coated with 30 μL of growth factor–reduced Matrigel and solidified for 30 minutes at 37°C. Endothelial cells were trypsinized and resuspended at 1 × 104 cells per milliliter, and 100 μL of this cell suspension was added to each well and treated with TXL or CoCl2 according to experimental group. Tube formation was observed under an inverted microscope (×4). Three random frames from each well were used to observe the formation of blood vessels.
Statistical Analysis
All results are expressed as mean ± SD from 3 or more independent experiments. Statistical significance was assessed by 1-way analysis of variance for comparison of different time points within a group. P values below 0.05 were considered significant.
RESULTS
TXL Promotes Hypoxia-induced Angiogenesis in Lung and Liver Tissue
To detect the effect of TXL on chronic hypoxia, the expression of HIF-1α was examined in the liver. As shown in Figure 1A, the chronic hypoxia group showed higher HIF-1α expression than the control group, suggesting that the chronic hypoxia model was successful. Among the 3 groups, HIF-1α–positive cells were most obvious in the TXL preincubation group, indicating that the TXL preincubation mice were highly adaptable to hypoxia. Western blot got the same results (Fig. 1B). CD31 is a marker of vascular endothelial cells. As shown in Figure 1C, the number of CD31-positive cells of liver in the TXL preincubation group was higher than in the hypoxia group. We further detected the CD31 expression in vascular tissue using Western blot (Fig. 1D) and found that CD31 was upregulated in hypoxia and TXL preincubation groups, suggesting that TXL could promote angiogenesis in liver tissue subjected to hypoxia.
FIGURE 1.
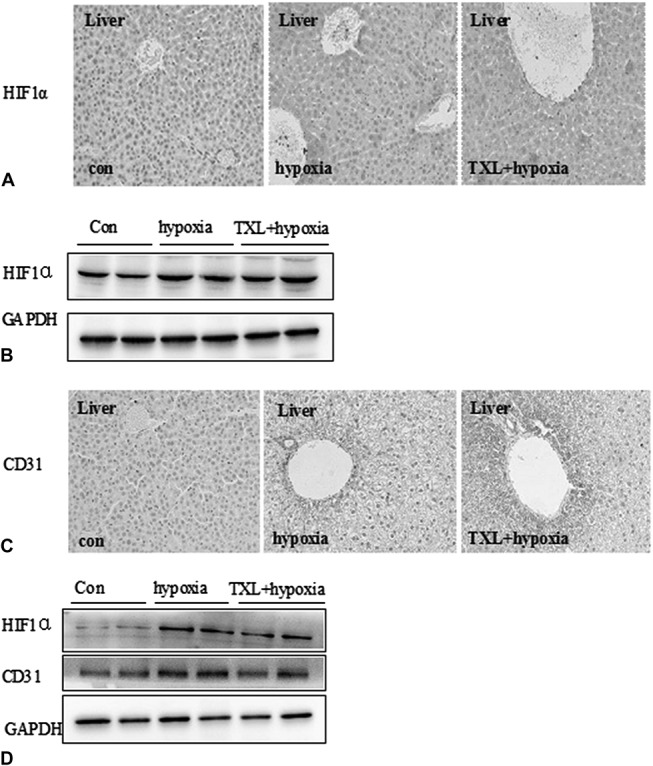
HIF-1α and CD31 expression in lung and liver tissue. A, HIF-1α expression in liver tissue was evaluated by immunohistochemical staining using anti–HIF-1α antibody. B, HIF-1α expression in liver tissue was evaluated by Western blotting using anti–HIF-1α antibody. C, CD31 expression in liver tissue was evaluated by immunohistochemical staining using anti-CD31 antibody. D, HIF-1α and CD31 and expression in vascular tissue were evaluated by Western blotting using anti–HIF-1α and anti-CD31 antibodies.
TXL Promotes Angiogenesis Under Hypoxia
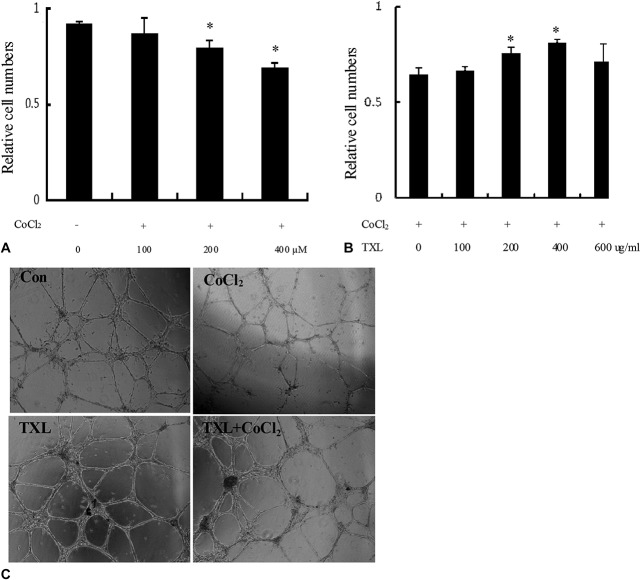
To further investigate the molecular mechanism by which TXL promoted angiogenesis, CoCl2 was used to establish a cell culture model of hypoxia for this study. Proliferation of endothelial cells was observed using a CCK-8 assay. As shown in Figure 2A, CoCl2 significantly inhibited the proliferation of endothelial cells compared with the control group, suggesting that the CoCl2-induced hypoxia accelerated the apoptosis of endothelial cells. In contrast, TXL preincubation significantly reversed CoCl2-induced endothelial cell death in a dose-dependent manner (Fig. 2B). These results indicated that TXL promotes hypoxia-induced angiogenesis by means of a mechanism that may be partially related to promoting the proliferation of endothelial cells. To further study the effect of TXL on endothelial cell function, a tube formation assay was used. As shown in Figure 2C, in the hypoxia group, tubules were short and relatively homogeneous, whereas those formed in the TXL preincubation group were significantly stronger, consisting of both short and long interconnecting tubules that more closely resembled capillaries, suggesting that TXL promoted tubule morphology and angiogenesis. These results suggest that TXL-induced angiogenesis is mediated by hypoxia-induced endothelial proliferation and tube formation.
FIGURE 2.
TXL promotes angiogenesis under hypoxia. A, Vascular endothelial cells were treated with different doses of CoCl2 as a hypoxia inducer, and an MTT assay was used to observe the effect of CoCl2 on the proliferation of endothelial cells. Values are given as mean ± SD; *P < 0.05 versus other groups. B, Vascular endothelial cells were treated with different doses of TXL, and an MTT assay was used to observe the effect of TXL on the proliferation of endothelial cells. Values are given as mean ± SD; *P < 0.05 versus other groups. C, A tube formation assay demonstrated that TXL promoted tubule morphology and angiogenesis, as observed by microscopy.
TXL Increases the Expression of TJ Proteins and KLF4 in Hypoxic Aorta
TJs are one mode of cell–cell adhesion in endothelial cellular sheets. They act as a primary barrier to the diffusion of solutes through the intercellular space. Physicochemical conditions such as chronic hypoxia can disturb microcirculation by accelerating endothelial cell apoptosis and damaging the endothelial structure. Therefore, we further studied the influence of TXL on the expression of endothelial TJ proteins in the hypoxic aorta. As expected, CoCl2 decreased the gene expression of endothelial TJ proteins such as VE-cadherin, occludin, and claudin-1 (Fig. 3A); this decrease was also associated with reductions of KLF4 at the protein (Fig. 3A) and messenger RNA (mRNA) (Fig. 3B) levels. In contrast, TXL treatment significantly induced the expression of the above proteins, especially occludin, claudin-1, and KLF4. Immunohistochemical staining (Fig. 3C) and Western blotting (Fig. 3D) showed that TXL preincubation significantly increased the expression of occludin, claudin-1, and KLF4, suggesting that the increased expression of TJ proteins induced by TXL might be related to KLF4. In human umbilical vein endothelial cells (HUVECs) under normoxia condition, cells exhibited continuous membranous staining of ZO-1 and occludin, whereas claudin-1 scarcely expressed on the membrane (Fig. 3E). After hypoxia, HUVEC showed a discontinuous staining of claudin-1, ZO-1, and occludin (Fig. 3E). Interestingly, TXL treatment restored the linear distribution and expression of claudin-1, ZO-1, and occludin (Fig. 3E). These results suggest that the increased expression and distribution of TJ proteins induced by TXL might be related to KLF4.
FIGURE 3.
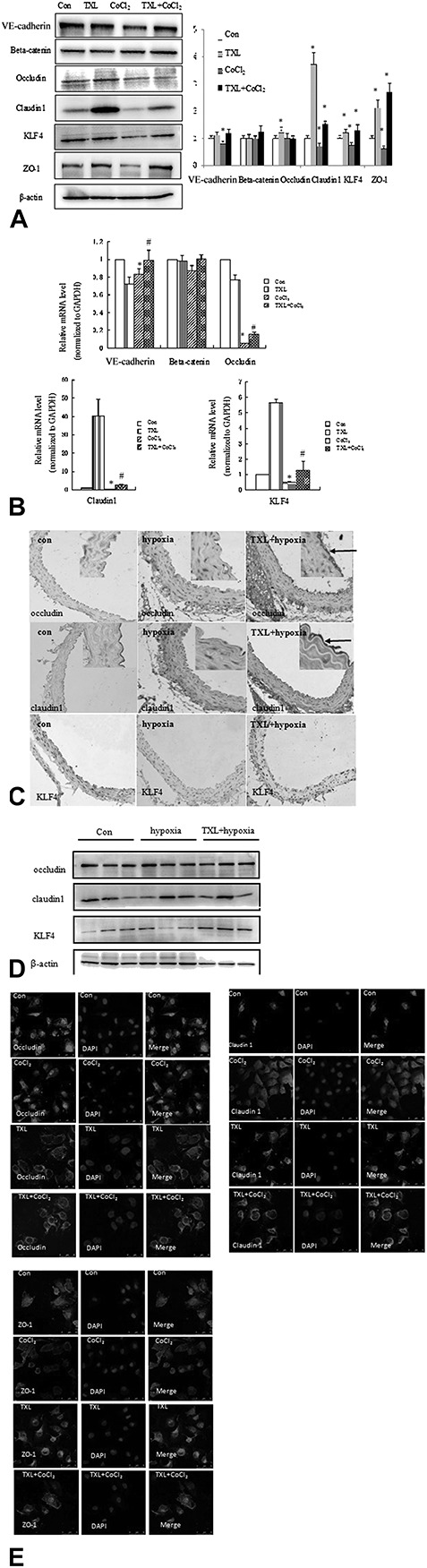
TXL increases the expression of tight junction proteins and KLF4 in hypoxic aorta. A, Endothelial cells were treated with the indicated stimuli for 24 hours. Western blot analysis of VE-cadherin, beta-catenin, occludin, claudin-1, ZO-1, and KLF4 expression is shown (left panel). Densitometric scanning (right panel). Values are the mean ± SD from 3 independent experiments. *P < 0.05, compared with the Con. B, Endothelial cells were treated with the indicated stimuli for 24 hours. Total RNA was prepared, and the mRNA levels of VE-cadherin, beta-catenin, occludin, claudin-1, and KLF4 were examined by quantified RT-PCR and normalized to GAPDH. The data are presented as mean ± SD. *P < 0.05, #P < 0.05. C, The thoracic aortas of the 3 groups were isolated. TJ protein and KLF4 expression levels were evaluated by immunohistochemical staining using antioccludin, anti–claudin-1, and anti-KLF4 antibodies (×100). Arrows indicate localization of TJ protein in the enlarged panels (×200). D, The thoracic aortas of the 3 groups were isolated. TJ protein and KLF4 expression levels were evaluated by Western blotting using antioccludin, anti–claudin-1, and anti-KLF4 antibodies. E, Endothelial cells were treated with the indicated stimuli for 24 hours. Distribution of occludin, claudin-1, and ZO-1 expression was detected using immunofluorescence staining.
KLF4 May Mediate TXL-induced Expression of the TJ Proteins Occludin and Claudin-1
KLF4, a zinc finger-type transcription factor, plays an important role in endothelial cells. Hale et al15 showed that sustained expression of KLF4 promotes ineffective angiogenesis, leading to diminished tumor growth independent of effects on endothelial cell proliferation or the cell cycle. Such tumors feature increased vessel density, but they are hypoperfused, leading to tumor hypoxia. These results prompted us to believe that KLF4 was one possible target of TXL during angiogenesis. Thus, we investigated whether KLF4 participates in the reduced expression of TJ proteins that is reversed by TXL. As expected, overexpression of KLF4 decreased the expression of VE-cadherin, beta-catenin, claudin-1, and occludin, with the greatest effect on claudin-1 and occludin. Interestingly, overexpression of KLF4 after incubation with TXL significantly increased the expression of occludin and claudin-1 (Fig. 4A), and real-time polymerase chain reaction produced similar results (Fig. 4B). As shown in Figure 4C, HUVECs-transfected pAd or pAd-KLF4 exhibited relatively discontinuous membranous staining of ZO-1 and claudin-1. TXL treatment restored the linear distribution and expression of claudin-1 and ZO-1 (Fig. 3E). These results suggest that the increased expression and distribution of TJ proteins induced by TXL might be related to the modification of KLF4. Zheng et al16 showed that overexpression of KLF4 in endothelial cells significantly impaired tube formation and endothelial cell proliferation. These conflicting results suggested that TXL-promoted angiogenesis and function might be related to increasing the expression of KLF4 while reversing its function in endothelial cells. To test this hypothesis, an immunoprecipitation assay was used to detect the phosphorylation of KLF4. As shown in Figure 4D, TXL significantly promoted KLF4 phosphorylation, suggesting that TXL-induced expression of occludin and claudin-1 is associated with KLF4 phosphorylation.
FIGURE 4.
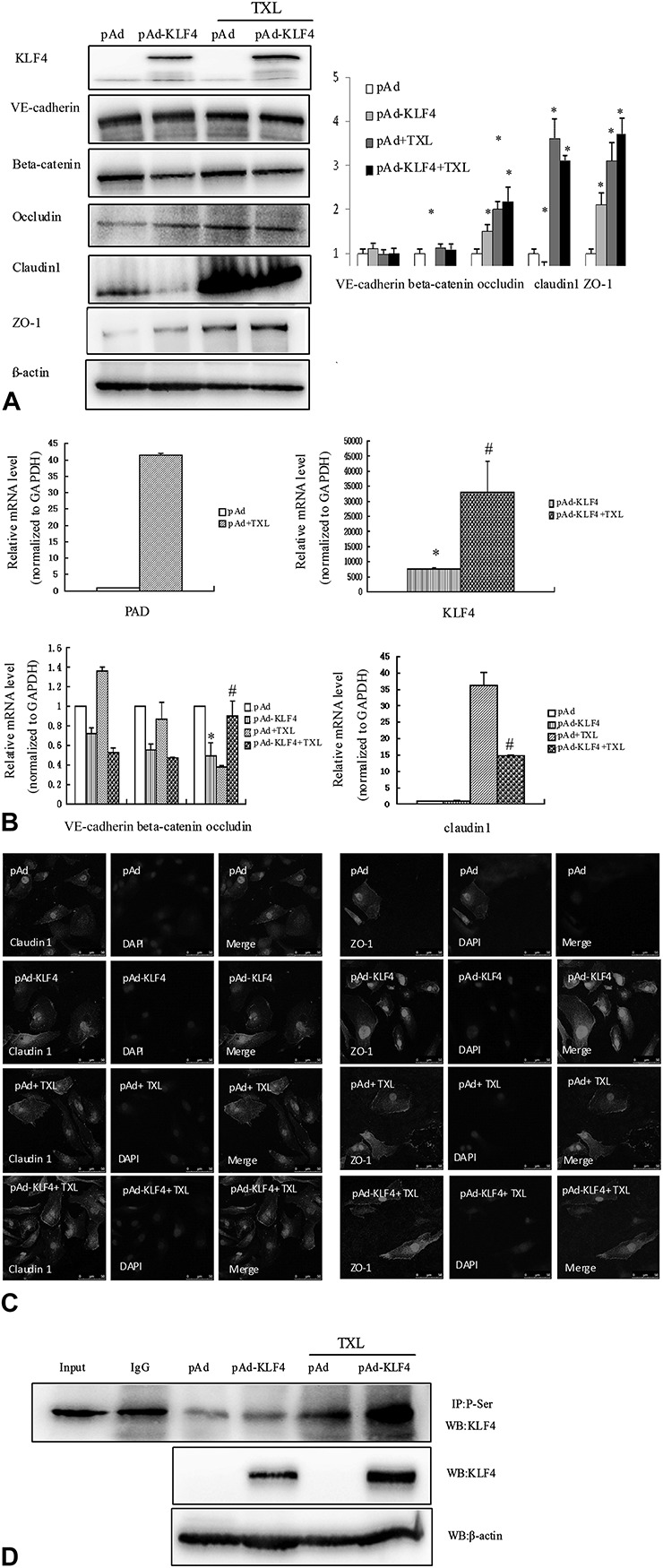
KLF4 may mediate TXL-induced expression of the tight junction proteins occludin and claudin-1. A, Endothelial cells were subjected to various stimuli for 24 hours. Western blot analysis was used to detect the expression of KLF4, VE-cadherin, beta-catenin, occludin, ZO-1, and claudin-1. Densitometric scanning (right panel). Values are the mean ± SD from 3 independent experiments. *P < 0.05, compared with the pAd group. B, Endothelial cells were subjected to various stimuli for 24 hours. Total RNA was prepared, and the mRNA levels of KLF4, VE-cadherin, beta-catenin, occludin, and claudin-1 were examined by qRT-PCR and normalized to GAPDH. The data are presented as mean ± SD from 3 independent experiments. *P < 0.05, #P < 0.05. C, Endothelial cells were treated with the indicated stimuli for 24 hours. Distribution of claudin-1 and ZO-1 expression was detected using immunofluorescence staining. D, TXL-induced KLF4 phosphorylation was detected by immunoprecipitation using an anti-phosphoserine antibody and immunoblotting with the anti-KLF4 antibody.
DISCUSSION
Chronic hypoxia is regarded as a common pathological stimulus for cardiovascular diseases; its first effects are on vascular endothelial cells, where it causes abnormal secretion of various bioactive substances that cause endothelial dysfunction.17 Thus, the study of impaired endothelial function and structure and the development of effective intervention measures are very important for the development of cardiovascular disease therapies. Numerous studies have indicated that hypoxia is a major stress factor inducing TJ disruption. The 3 major types of transmembrane proteins are located at the TJ: occludin, claudin, and junction adhesion molecules.6 Hypoxia modulates protein expression levels and the subcellular redistribution of occludin, claudin-5, and ZO-1. The protein redistribution hypercritically regulates TJ integrity and is most likely mediated through changes in the phosphorylation status of threonine, serine, and tyrosine residues that determine their localization at the plasma membrane and their interactions with other proteins at the TJ.15,18
TXL is a Chinese herbal compound based on the meridian theory of traditional Chinese medicine, and it is officially approved for the treatment of cardiovascular and cerebrovascular diseases in China.9,10 Numerous clinical and animal studies have shown that TXL can significantly improve vascular function, that it possesses antioxidant and antithrombosis properties, and that it reduces myocardial ischemic reperfusion injury. TXL can upregulate the expression of endothelial nitric oxide synthase (eNOS) through the PI-3K/Akt/HIF-dependent signaling pathway and thus improve vascular function.19 In this study, we found that that the expression levels of vascular endothelial occludin and claudin-1 tended to be lower than in the normal group after chronic hypoxia, whereas the occludin and claudin-1 expression levels in the TXL preincubation group were clearly elevated. These findings indicate that TXL may play a specific role in the function of damaged vascular endothelial cells after chronic hypoxia.
KLF4 is a zinc finger transcription factor that participates in a variety of cellular functions by activating or repressing the transcriptional activity of multiple genes through its associations with numerous partner proteins.20 KLF4 plays a critical role in the regulation of gene transcription in the cardiovascular system, and it is constitutively expressed in vascular endothelial cells.21,22 Several recent studies have shown that overexpression of KLF4 increased the expression of anti-inflammatory and antithrombotic factors, including eNOS and thrombomodulin, whereas endothelial cell–specific KLF4 deletion led to the enhancement of tumor necrosis factor α–induced expression of vascular cell adhesion molecule 1 (VCAM-1) and tissue factor in cultured HUVECs. We found that the expression levels of the TJ proteins VE-cadherin, claudin-1, and KLF4 were significantly higher in the TXL group than in the hypoxia group. Overexpression of KLF4 reduced the expression of occludin and claudin-1, whereas overexpression of KLF4 after TXL incubation significantly increased the expression of occludin and claudin-1. Therefore, we speculate that TXL may induce KLF4 modifications that reverse its function. Protein phosphorylation is an important molecular mechanism in the recruitment and derecruitment of other transcription factors; for example, KLF4 phosphorylation by Smad and PKC-δ plays a key role in regulating AT1 receptor expression and vascular smooth muscle cell proliferation.23 Immunoprecipitation assay in our experiment showed that TXL significantly promoted KLF4 phosphorylation, suggesting that TXL-induced expression of occludin and claudin-1 is associated with KLF4 phosphorylation.
In conclusion, we demonstrate that TXL can improve endothelial function after chronic hypoxia both in vivo and in vitro. The molecular mechanism of this effect is related to the ability of TXL to promote endothelial cell proliferation and to increase the phosphorylation of the transcription factor KLF4, reversing its effect on TJ protein expression. This finding provides a new molecular mechanism for the TXL-induced increase in TJ protein expression. However, these findings are inconclusive and require further study. For example, whether KLF4 phosphorylation leads to changes in TJ proteins independent of TXL? How TXL affects KLF4 phosphorylation? What is the relationship between effect of TXL on TJ proteins and the effect of TXL on KLF4 phosphorylation. It is hoped that we are able to address this question in the future.
Footnotes
Supported by the National Basic Research Program of China (No. 2012CB518601), the National Natural Science Foundation of China (No. 31271396, No. 31271224), and Fok Ying Tung Education Foundation (131037).
The authors report no conflicts of interest.
C-Y. Zheng and L-L. Song have contributed equally (co-first authors).
REFERENCES
- 1.Yang C, Jiang L, Zhang H, et al. Analysis of hypoxia-induced metabolic reprogramming. Methods Enzymol. 2013;542:425–455. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation inhealth and disease: a focus on hypoxia. Br J Pharmacol. 2014;171:1210–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moccia F, Dragoni S, Cinelli M, et al. How to utilize Ca2+ signals to rejuvenate the repairative phenotype of senescent endothelial progenitor cells in elderly patients affected by cardiovascular diseases: a useful therapeutic support of surgical approach? BMC Surg. 2013;13(suppl 2):S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelhardt S, Al‐Ahmad AJ, Gassmann M, et al. Hypoxia Selectively Disrupts brain microvascular endothelial tight Junction complexes through a Hypoxia‐Inducible Factor-1 (HIF‐1) dependent mechanism. J Cell Physiol. 2014;229:1096–1105. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK, Lee SH, Hong KW; Inflammation and Immunopharmacology. Rebamipide inhibits neutrophil adhesion to hypoxia/reoxygenation-stimulated endothelial cells via nuclear factor-kB-dependent pathway. J Pharmacol Exp Ther. 2000;294:864–869. [PubMed] [Google Scholar]
- 6.Günzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol. 2012;2:1819–1852. [DOI] [PubMed] [Google Scholar]
- 7.Ding L, Lu Z, Lu Q, et al. The claudin family of proteins in human malignancy: a clinical perspective. Cancer Manag Res. 2013;5:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu GC, Gao RL, Wu YL, et al. Clinical study on tongxinluo capsule in treatment of patients with angina pectoris caused by coronary heart disease. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17:414–416. [PubMed] [Google Scholar]
- 9.Chen WQ, Zhong L, Zhang L, et al. Chinese medicine tongxinluo significantly lowers serum lipid levels and stabilizes vulnerable plaques in a rabbit model. J Ethnopharmacol. 2009;124:103–110. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Liu Y, Lu XT, et al. Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: a comparison with a high-dose simvastatin therapy. Am J Physiol Heart Circ Physiol. 2009;297:H2004–H2014. [DOI] [PubMed] [Google Scholar]
- 11.Zhao MZ, Gao CM, Zhang YY, et al. Effects of Tongxinluo capsule on myocardial apoptosis and apoptosis-related gene expression after ischaemia-reperfusion. Chin J Cardiol (Chin). 2000;28:206. [Google Scholar]
- 12.Wang WJ, Fu XD, Chen WH, et al. The experiment research of angiogenesis of Tongxinluo capsule on the chick embryo chorioallantoic membrane model. Chin J Diffic Complicat Cases (Chin). 2003;2:2–5. [Google Scholar]
- 13.Xu XL, Zhang ZR, Huang Y. Optimization of the separation conditions of fingerprint for rhubarb by high performance liquid chromatography. Sichuan Da Xue Xue Bao Yi Xue Ban (Chin). 2004;35:559–562. [PubMed] [Google Scholar]
- 14.Zhang YH, Ji M, Zhang YL, et al. Effects of Tongxinluo capsule on endothelial function of patients with dysfunction of blood lipid profile. Chin J Diffic Complicat Cases (Chin). 2005;4:92–93. [Google Scholar]
- 15.Hale AT, Tian H, Anih E, et al. Endothelial Krüppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J Biol Chem. 2014;289:12016–12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng B, Han M, Shu Y, et al. HDAC2 phosphorylation-dependent Klf5 deacetylation and RARα acetylation induced by RAR agonist switch the transcription regulatory programs of p21 in VSMCs. Cell Res. 2011;21:1487–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu K, Zheng B, Han M, et al. ATRA activates and PDGF-BB represses the SM22α promoter through KLF4 binding to, or dissociating from, its cis-DNA elements. Cardiovasc Res. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Han M, Bernier M, et al. Krüppel-like factor 4 promotes differentiation by transforming growth factor-β receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J Biol Chem. 2010;285:17846–17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng X, Li A, Zhao L, et al. Key role of microRNA-15a in the KLF4 suppressions of proliferation and angiogenesis in endothelial and vascular smooth muscle cells. Biochem Biophys Res Commun. 2013;437:625–631. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Zhang H, Pan Q, et al. Hypoxia/Aglycemia-Induced endothelial barrier dysfunction and tight Junction protein downregulation can Be Ameliorated by Citicoline. PLoS One. 2013;8:e82604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zehendner CM, Librizzi L, Hedrich J, et al. Moderate hypoxia followed by reoxygenation results in blood-brain barrier breakdown via oxidative stress-dependent tight-junction protein disruption. PLoS One. 2013;8:e82823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, Dohgu S, Takata F, et al. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell 4. Biol Pharm Bull. 2013;36:492–495. [DOI] [PubMed] [Google Scholar]
- 23.Liang JQ, Wu K, Jia ZH, et al. Chinese medicine Tongxinluo modulates vascular endothelial function by inducing eNOS expression via the PI-3K/Akt/HIF-dependent signaling pathway. J Ethnopharmacol. 2011;133:517–523. [DOI] [PubMed] [Google Scholar]