Abstract:
Probucol, an agent characterized by lipid-lowering and antioxidant property, retards atherosclerosis effectively. To test the hypothesis that probucol might act its antiatherosclerotic role by suppressing immune maturation of dendritic cells (DCs), 7-week-old LDLR−/− mice were rendered diabetic with streptozotocin (STZ) and then fed either a high-fat diet only or added with 0.5% (wt/wt) probucol for 4 months, and human monocyte-derived dendritic cells were preincubated with or without probucol and stimulated by oxidized low-density lipoprotein. In STZ-induced diabetic LDLR−/− mice, probucol treatment significantly lowered plasma total cholesterol and high-density lipoprotein-cholesterol levels; regressed aortic atherosclerotic lesions; reduced splenic CD40, CD80, CD86, MHC-II expression, and plasma IL-12p70 production; and decreased the expression of CD11c+ DCs within atherosclerotic lesions. In vitro, oxidized low-density lipoprotein promoted human monocyte–derived dendritic cells maturation; stimulated CD40, CD86, CD1a, HLA-DR expression; increased tumor necrosis factor-α production; and decreased IL-4 production. However, these effects were obviously inhibited by probucol pretreatment. In conclusion, our study indicated that probucol effectively retarded atherosclerosis at least partly through lipid-lowering and inhibiting immune maturation of CD11c+ DCs in STZ-induced diabetic LDLR−/− mice.
Key Words: probucol, dendritic cells, atherosclerosis, streptozotocin, diabetes, LDLR−/− mice, ox-LDL
INTRODUCTION
The immunoinflammatory response plays a central role in the development of atherosclerosis. As the most potent antigen presentation cells, dendritic cells (DCs) are important players responsible for the induction of immunity.1–4 In the absence of inflammation, immature DCs located in peripheral tissues specialize in taking up innocuous and cell-associated self-antigens, whereas in the presence of danger signals, such as in early atherosclerotic lesions, immature DCs take up antigens such as oxidized low-density lipoprotein (ox-LDL) and heat shock protein, generate MHC–peptide complexes, migrate from the sites of antigen acquisition to secondary lymphoid organs, and fulfill the maturation. The mature DCs then physically interact with and activate T lymphocytes.5–7 It has been demonstrated that human monocyte–derived dendritic cell (h-monDC)-mediated lipid antigens delivery, and inflammatory cytokines are essential for the initiation and progression of atherosclerosis and that deficiency of antigen-presenting cell invariant chain retards atherosclerosis in mice.8–10 In mice with LDLR−/− background, hypercholesterolemia-induced atherosclerotic lesions could be significantly reduced by depleting intimal CD11c+ DCs.11
Probucol, a potent cholesterol-lowering and antioxidant drug, has diverse pharmacologic properties with therapeutic effects on the cardiovascular systems.12 Its molecular formula is C13H48O2S2, and Wu et al13 have demonstrated that the sulfur rather than the phenol moieties are critical for protection against atherosclerotic vascular disease by redox-active compounds. It retards atherosclerosis through a range of biological activities,14,15 such as exerting anti-inflammatory and antioxidant effects, affecting high-density lipoprotein (HDL) metabolism and maintaining endothelial cell function. A recent clinical study reported its potent antiatherosclerotic effect on improving long-term survival after complete coronary revascularization in patients with coronary artery disease,16 which inspired us to explore other possible pharmacologic mechanisms of probucol independent of its antioxidant and cholesterol-lowering activities. Besides, it was shown that probucol may suppress the progression of diabetes and ameliorate its complications.14 However, whether probucol affects the development of atherosclerosis in the setting of diabetes remains less clear.
Our previous reports17–20 indicated that immune maturation of DCs might play a significant role in atherogenesis, and suppressing DCs maturation might be a potential working mechanism of various antiatherogenic agents. Thus, this study was performed to investigate the exact role of probucol on the development of atherosclerosis in the setting of diabetes and to demonstrate whether probucol acted partially through suppression of the immune maturation of DCs.
MATERIALS AND METHODS
Materials
Human CD14+ and CD11c+ immunomagnetic microbeads were obtained from Miltenyi Biotech (Bergisch Gladbach, Germany); recombinant human granulocyte-macrophage colony-stimulating factor and recombinant human interleukin-4 from R&D Systems (Minneapolis, MN); probucol from Otsuka Pharmaceutical Ltd (Japan); low-density lipoprotein (LDL), ox-LDL, oil Red O, Triton X-100, and 4′,6-diamidino-2-phenylindole (DAPI) from Sigma (St Louis, MO); enzyme-linked immunosorbent assay kits for IL-12p70, tumor necrosis factor-α (TNF-α), and IL-4 from Invitrogen (Carlsbad, CA); total cholesterol (TC) and high-density lipoprotein-cholesterol (HDL-C) assay kits from BioSino (China); FITC- or PE- or APC-labeled monoclonal anti-CD40, anti-CD86, anti-CD1a, anti-HLA-DR, and anti-MHC-Ⅱ antibodies from BD Biosciences (San Jose, CA); primary rat anti-mouse CD11c monoclonal unconjugated antibody from Abcam (Cambridge, United Kingdom); secondary goat anti-rat antibody from Jackson ImmunoResearch (West Grove, PA).
Animals
Male LDLR−/− mice were provided by Institute of Cardiovascular Sciences and Key Laboratory of Molecular Cardiovascular Sciences of Peking University. All animals had free access to food and water in a 12-hour light/dark cycle and were housed in temperature-controlled animal room, and experimental protocols were approved by the Institutional Review Board of the Peking University Health Science Center (LA2010-061). At 7 weeks of age, the mice were rendered diabetic with streptozotocin (STZ) (60 mg/kg intraperitoneally, 5 times per week) until blood glucose reached 300 mg/dL. Then, 17 LDLR−/− mice were randomly divided into 2 groups. The mice were fed either a high-fat diet (15% fat, 0.1% cholesterol) for 4 months as a control (n = 9) or a high-fat diet supplemented with 0.5% probucol (n = 8).
Plasma Cholesterol Analysis
Mice were fasted for 4 hours, then blood samples were collected by retro-orbital bleeding after general anesthesia by intraperitoneal injection of 1% phenobarbital sodium (80 mg/kg). Plasma TC and HDL-C levels were measured by enzymatic method using commercial kits. HDL-C was determined after ApoB-lipoprotein was precipitated and removed by 20% polyethylene glycol solution.
Quantification of Atherosclerotic Lesions
After blood was collected from each mouse, the circulation system was washed with phosphate buffered saline (PBS) and then fixed with PBS containing 4% paraformaldehyde. The aorta was then excised from the root to the abdominal area. The aorta was cut longitudinally to expose the intimal surface and then observed with en face oil Red O staining. For the analysis of lesion formation in the aortic sinus, cryostat sections were prepared and embedded in OCT compound. Serial sections (7-μm thick) were collected from the level of the aortic valve leaflets up to approximately 560 μm above the leaflets in the aortic sinus. Every tenth sections were retrieved and placed onto slides; 8 sections were placed onto each slide for a total of 10 slides. Sections were stained with oil Red O and counterstained with hematoxylin. Atherosclerotic lesion areas were quantified using Leica QWin V3 software by a blinded observer.
Preparation of h-monDCs and Isolation of DCs from Spleen
Heparinized venous blood was taken from healthy volunteers from the blood bank of Zhongshan Hospital. Ethical approval was given by the Ethics Committee of Zhongshan hospital (2011–73). h-monDCs were obtained as previously described by Ge et al.18 In brief, blood was diluted in PBS and centrifuged for 30 minutes at 2000 rpm at room temperature. CD14+ peripheral blood mononuclear cells were purified by using CD14+ immunomagnetic microbeads (>98%) and incubated in RPMI-1640 medium supplemented with recombinant human granulocyte-macrophage colony-stimulating factor (100 ng/mL) and recombinant human interleukin-4 (50 ng/mL) in 6-well tissue culture plates at 37°C and at an atmosphere of 5% CO2. The medium was replaced every 2 days. On culture day 6, cells were pretreated with probucol (dissolved into 100% ethanol; 50 μg/mL) for 24 hours and then stimulated by addition of 50 μg/mL LDL or ox-LDL for another 24 hours. Cell viability was over 90% as assessed by Trypan blue staining. Untreated cells were used as controls. Above in vitro experiments were repeated for 3 times. CD11c+ DCs from spleen were separated as previously described.21 Briefly, mice spleen was crushed and treated with 1 mg/mL collagenase type 3 for 1 hour. CD11c+ DCs were isolated using antibodies coated with magnetic beads.
Flow Cytometric Measurement
h-monDCs were washed, resuspended in ice-cold PBS containing 5% fetal bovine serum (to prevent nonspecific binding), and then incubated with FITC- or PE-labeled monoclonal anti-CD40, anti-CD86, anti-CD1a, and anti-HLA-DR antibodies for 30 minutes at 4°C. Cells were washed twice and analyzed using flow cytometer. To assess CD11c+ DCs surface marker expression, DCs were isolated from spleens of all 17 LDLR−/− mice and then incubated with anti-class II major histocompatibility complex (MHC-Ⅱ)-APC, anti-CD80-FITC, anti-CD86-FITC, and anti-CD40-PE antibodies by 2- or 3-color fluorescence-activated cell sorter (FACS) analysis. Cells stained with the appropriate isotype-matched Ig were used as negative controls.
Measurement of Inflammatory Cytokines
TNF-α, IL-4 levels in supernatants of cultured h-monDCs, and plasma IL-12p70 levels of LDLR−/− mice were measured using enzyme-linked immunosorbent assay kits according to the manufacturer's instructions. The minimum of detectable dose is <10, <2, and <2 pg/mL, respectively.
Confocal Microscopy
Following previously established protocol,22 frozen 7-μm sections of aortic sinus were fixed for 60 minutes in PBS containing 4% formaldehyde, washed with deionized water, permeabilized with 0.5% Triton X-100, and washed with PBS. The primary rat anti-mouse CD11c monoclonal unconjugated antibody was diluted at the ratio of 1:50 in PBS and applied to the sections at 4°C overnight. After a wash in PBS, the secondary goat anti-rat antibody, diluted to a final concentration at 1:500 in PBS, was applied for 2 hours at room temperature. Sections were then washed with deionized water and mounted with mounting medium containing DAPI and photographed under a fluorescence confocal microscope.
Statistical Analysis
Data are presented as mean ± SD values. Comparison between 2 groups was performed by unpaired Student's t test. Comparisons between multiple groups were made using 1-way or 2-way analysis of variance, followed by Bonferroni post hoc tests. All statistical analyses were performed with SPSS 11.5 statistical software, and a P value <0.05 was considered statistically significant.
RESULTS
The Effect of Probucol on Plasma Cholesterol Levels in STZ-induced Diabetic LDLR−/− Mice
There were no significant differences in the body weight between the 2 groups during the experiment (data not shown). To determine the effect of probucol administration on cholesterol metabolism, plasma cholesterol levels were measured after a high-fat diet for 4 months. Compared with control mice, plasma TC and HDL-C levels (537.46 ± 167 vs. 2608.47 ± 524 mg/dL and 95.22 ± 12 vs. 243.64 ± 34 mg/dL, respectively, P < 0.01; Fig. 1) were markedly decreased in probucol-treated mice.
FIGURE 1.
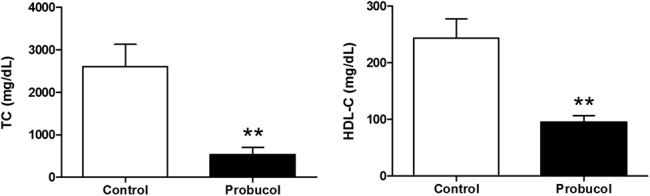
The effect of probucol on plasma levels of TC (left) and HDL-C (right) in STZ-induced diabetic LDLR−/− mice. Number of mice in the control group and probucol group was 9 and 8, respectively. **P < 0.01 versus control group.
Probucol Retards Atheroclerosis in STZ-induced Diabetic LDLR−/− Mice
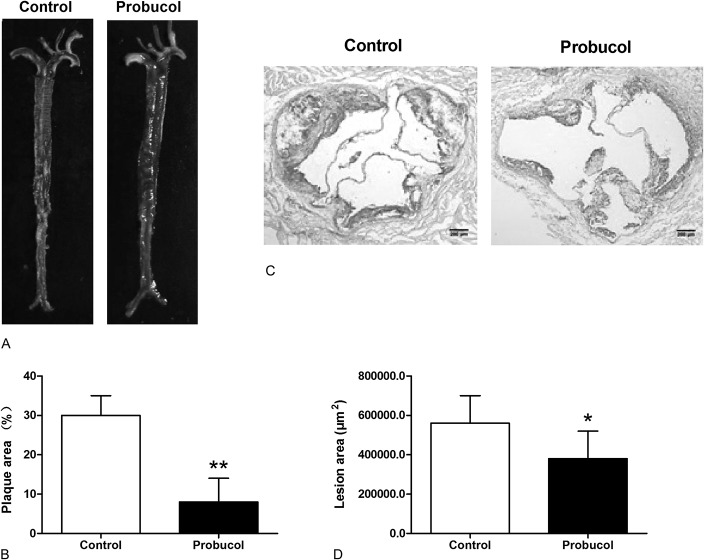
To determine the effect of probucol administration on the development of atherosclerosis, STZ-rendered LDLR−/− mice receiving a high-fat diet were orally treated with 0.5% (wt/wt) probucol every day for 4 months. In the control group, atherosclerotic plaques were obviously found on aortic arch, thoracic/abdominal aorta, opening of innominate, common carotid, and left subclavian arteries. In contrast, lesions on the descending aorta of probucol-treated mice were obviously smaller or absent (30% ± 5% vs. 8% ± 6%, P < 0.01; Figs. 2A–B). What's more, compared with the control mice, the atherosclerotic lesions in aortic sinus (Fig. 2C) were markedly reduced in probucol-treated mice (560,000 ± 140,000 μm2 vs. 380,000 ± 140,000 μm2, P < 0.05; Fig. 2D). These results ensured the antiatherogenic effect of probucol on reducing atherosclerotic lesions formation in STZ-induced LDLR−/− mice.
FIGURE 2.
The effect of probucol on atherosclerosis in STZ-induced diabetic LDLR−/− mice. Representative photographs stained with oil Red O in aorta (A) and aortic sinus (C) were shown. Mean plaque area in aorta (B) and mean atherosclerotic lesion area in the aortic sinus (D) were determined. Number of mice in the control group and probucol group was 9 and 8, respectively. *P < 0.05, **P < 0.01 versus control group.
Probucol Suppressed Immune Maturation of CD11c+ DCs from Spleen and Decreased Plasma IL-12p70 Concentration in STZ-induced Diabetic Mice
CD40, CD80, CD86, and MHC-II are recognized as important costimulatory molecules related to the maturation of DCs. FACS analysis results showed a significant decrease in the expression of CD40, CD80, CD86, and MHC-II of CD11c+ DCs from probucol-treated mice (Fig. 3A). Furthermore, we found a significant decrease in plasma IL-12p70 level in probucol-treated mice (21.2 ± 7.5 vs. 97.1 ± 3.0 pg/mL, P < 0.01; Fig. 3B). These results indicated that probucol significantly suppressed immune maturation of DCs in STZ-induced diabetic mice.
FIGURE 3.
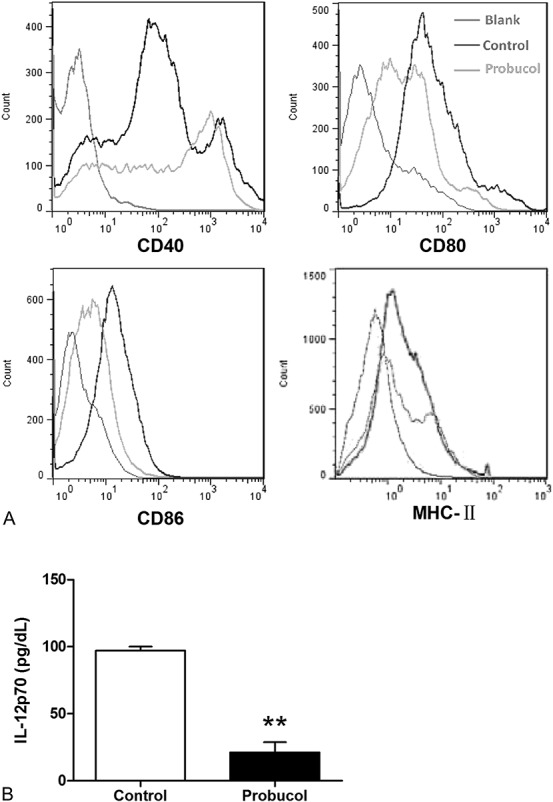
The effect of probucol on the immune maturation of CD11c+ DCs from spleen and plasma IL-12p70 concentration in STZ-induced diabetic mice. Cell surface markers (CD40, CD80, CD86, and MHC-II) of CD11c+ DCs from spleen were examined by flow cytometry (A). Plasma IL-12p70 concentration was measured by ELISA (B). Number of mice in the control group and probucol group was 9 and 8, respectively. **P < 0.01 versus control group. ELISA, enzyme-linked immunosorbent assay.
Probucol Inhibited CD11c+ DCs Expression in Atherosclerotic Plaques in STZ-induced Diabetic Mice
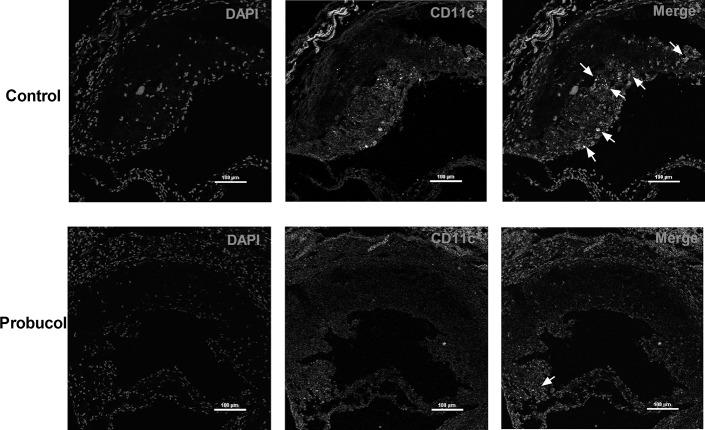
To further test the hypothesis that probucol retards atherosclerotic plaques' progression by inhibiting DCs expression inside, the markers of DCs (CD11c) in atherosclerotic lesions were assessed. As our results indicated, focal CD11c+ DCs accumulation within atherosclerotic plaques from the control group was markedly characterized under confocal microscopy. In contrast, CD11c+ DCs from the probucol-treated group were less or nearly absent within aortic atherosclerotic plaques (Fig. 4).
FIGURE 4.
The impact of probucol on the accumulation of CD11c+ DCs in the atherosclerotic plaques in aortic sinus. Representative sections were shown. DCs were stained with anti-CD11c (red) antibody and nuclei with DAPI (blue). The white arrows indicate CD11c+ DCs. Number of mice in the control group and probucol group was 9 and 8, respectively. Bars: 100 μm.
Probucol Suppressed Ox-LDL-induced h-monDCs Maturation
To further confirm the effect of probucol on suppressing DCs maturation, we designed the in vitro study using h-monDCs. Cell surface expressions of costimulatory molecules CD40, CD86, CD1a, and HLA-DR were examined in LDL or ox-LDL (both 50 mg/mL)-treated h-monDCs after treatment of probucol (50 μg/mL). FACS analysis indicated that ox-LDL-induced increased expressions of CD40, CD86, CD1a, and HLA-DR were significantly reduced by probucol (Fig. 5A). In addition, ox-LDL-induced increased TNF-α and decreased IL-4 concentration in supernatants of cultured h-monDCs was also significantly reversed by probucol (P < 0.01; Fig. 5B). These data suggested that probucol suppressed h-monDCs maturation and the subsequent inflammatory responses mediated by matured h-monDCs.
FIGURE 5.
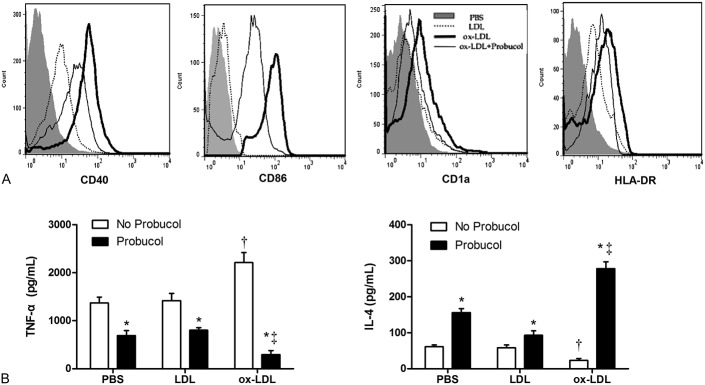
The effect of probucol on ox-LDL-induced h-monDCs maturation and inflammatory cytokines secretion. Surface molecular expressions (CD40, CD86, CD1a, HLA-DR) of h-monDCs were examined by flow cytometry (A). TNF-α and IL-4 in the supernatants of h-monDCs were collected and measured by ELISA (B). In each group, n = 3. *P < 0.01 versus corresponding non-probucol control groups, †P < 0.01 versus PBS or LDL groups without probucol treatment, and ‡P < 0.01 versus PBS or LDL groups with probucol treatment. ELISA, enzyme-linked immunosorbent assay.
DISCUSSION
Atherosclerosis is a complex disease accompanied by several pathological processes including endothelial injury, inflammation, and oxidative stress. Evidences have shown that β-cell damage in diabetes is partly contributed to oxidative stress.23 As a potent antioxidant and anti-inflammatory agent, probucol shows its effective impact on retarding atherosclerosis and serves as a potential therapeutic method for diabetes.14 But, it remains unknown what the exact role of probucol on the progression of atherosclerosis in the setting of diabetes is and whether DCs maturation is involved in the whole pathological process. In this study, we have revealed, for the first time to the best of our knowledge, that probucol effectively suppressed the progression of atherosclerosis in STZ-induced diabetic mice with LDLR−/− background. Also, we have demonstrated that the possible antiatherosclerotic effect of probucol might be mediated by suppressing DCs maturation and thus reducing subsequent inflammatory and immune responses mediated by matured DCs.
Probucol is a diphenolic compound with antioxidant and anti-inflammatory properties that protects against atherosclerosis.14 Unfortunately, the lowering of serum HDL-C and the cardiac electrophysiology (QT prolongation) has limited probucol's clinical application.12 In accordance with previous studies,24,25 our result showed that probucol has a distinct effect on lowering plasma TC and HDL-C levels. However, recent studies15,26 confirmed that the lowering of HDL-C level may not be a side effect of probucol, because probucol's impact on HDL metabolism may actually enhance the antioxidant property of HDL. And, Samia Mora et al27 showed that HDL particle number may be a better predictor of residual risk than chemically measured HDL-C concentration, although the study was conducted in the setting of potent statin therapy. Therefore, further study should be performed to better elucidate the potential and limitation of probucol's clinical application.
There are now considerable data indicating that adaptive immune responses significantly modulate atherogenesis. In particular, increasing evidences28–32 support the pivotal role of DCs, as the most potent antigen presentation cells, in inflammation and adaptive immunity and in pathogenesis of atherosclerosis. Liu et al33 had already indicated that decreased accumulation of DCs in the arterial intima can lead to attenuated atherosclerotic plaque progression in mice. Packard et al21 affirmed that CD11c+ DCs fully maintain their antigen proceeding presentation capabilities in LDLR−/− mice fed with a high-fat diet, in contrary to previous reports that questioned the occurrence of adequate and efficient immune responses under dyslipidemic conditions. Furthermore, a recent study from Sun et al10 also demonstrated that deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. These findings established solid background for us to interpret that probucol perform its antiatherosclerotic effect partially by suppressing maturation of DCs from an immunoinflammatory perspective.
The anti-inflammatory property of probucol has been reported previously.14 A recent study from Li et al34 also provided evidence that probucol possibly increases the stability of vulnerable plaques through its anti-inflammation and scavenger receptors suppression effects. Consistent with the above findings, our study provided evidence, for the first time, that probucol retarded atherosclerosis through reducing expression of CD40, CD80, CD86, and MHC-II, which reflected suppressed immune maturation of DCs from spleen. Besides the costimulatory molecules, inflammatory cytokines secreted by DCs also participate in DCs maturation and atherogenesis. As a cytokine released by activated DCs, IL-12 is a key mediator in triggering proliferation of natural killer cells and promoting T lymphocytes activation. We speculate that reduced plasma IL-12 level might also be one of the observed probucol's effect on inhibiting maturation of DCs. To further elucidate the effect of probucol on DCs maturation, we detected the effect of probucol on DCs accumulation within plaque microenvironment. Consistent with Taketa's recent reports35 concerning inhibitory effect of calcitriol on DCs in ApoE−/− mice, our results demonstrated that CD11c+ DCs within plaques in aortic sinus were less or nearly absent after treatment with probucol, suggesting that probucol tremendously decreased the number of DCs recruited into the plaque.
In the setting of diabetes, hyperglycemia and oxidative stress are recognized to be able to accelerate the development of atherosclerosis, which might be mediated through promotion of immune reactions. To further demonstrate the effect of probucol on the maturation of DCs, we designed the in vitro study using ox-LDL-incubated h-monDCs in the absence of hyperglycemia. The in vitro study further showed that probucol decreased the expression of costimulatory molecules (CD40, CD86, CD1a, and HLA-DR), suppressed TNF-α secretion, and increased IL-4 secretion in the supernatants of h-monDCs. Such results demonstrated that probucol effectively ameliorated the inflammatory state and suppressed the maturation of h-monDCs without the interference of hyperglycemia.
In summary, our study provided novel and plausible explanations for probucol on its anti-inflammatory role of suppressing DCs maturation in antiatherosclerotic process both in vitro and in vivo. By suppressing splenic DCs maturation and reducing accumulation of DCs within atherosclerotic plaques, probucol may restrain inflammation and thus stabilize atherosclerotic plaques in the setting of diabetes, features believed to account for the beneficial effects of probucol on cardiovascular morbidity and mortality.
Footnotes
Supported by the National Basic Research Program of China (2011CB503905), Chinese National Natural Science Foundation (Nos. 30900600 and 30971250), and the Research Fund for the Doctoral Program of Higher Education of China (No. 20090071120027).
The authors report no conflicts of interest.
H. Zhu and X. Jin have contributed equally.
REFERENCES
- 1.Bobryshev YV. Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur Heart J. 2005;26:1700–1704. [DOI] [PubMed] [Google Scholar]
- 2.Ludewig B, Zinkernagel RM, Hengartner H. Arterial inflammation and atherosclerosis. Trends Cardiovasc Med. 2002;12:154–159. [DOI] [PubMed] [Google Scholar]
- 3.Link A, Bohm M. Potential role of dendritic cells in atherogenesis. Cardiovasc Res. 2002;55:708–709. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz A, Lochno M, Traeg F, et al. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101–110. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. [DOI] [PubMed] [Google Scholar]
- 7.Austyn JM. Dendritic cells. Curr Opin Hematol. 1998;5:3–15. [DOI] [PubMed] [Google Scholar]
- 8.Lord RS, Bobryshev YV. Clustering of dendritic cells in athero-prone areas of the aorta. Atherosclerosis. 1999;146:197–198. [DOI] [PubMed] [Google Scholar]
- 9.Nickel T, Schmauss D, Hanssen H, et al. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205:442–450. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Hartvigsen K, Chou MY, et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 2010;122:808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulson KE, Zhu SN, Chen M, et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita S, Matsuzawa Y: Where are we with probucol: a new life for an old drug? Atherosclerosis. 2009;207:16–23. [DOI] [PubMed] [Google Scholar]
- 13.Wu BJ, Kathir K, Witting PK, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker R. Molecular mechanisms underlying the antiatherosclerotic and antidiabetic effects of probucol, succinobucol, and other probucol analogues. Curr Opin Lipidol. 2009;20:227–235. [DOI] [PubMed] [Google Scholar]
- 15.Zhong JK, Guo ZG, Li C, et al. Probucol alleviates atherosclerosis and improves high density lipoprotein function. Lipids Health Dis. 2011;10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasai T, Miyauchi K, Kubota N, et al. Probucol therapy improves long-term (>10-year) survival after complete revascularization: a propensity analysis. Atherosclerosis. 2012;220:463–469. [DOI] [PubMed] [Google Scholar]
- 17.Su W, Sun A, Xu D, et al. Tongxinluo inhibits oxidized low-density lipoprotein-induced maturation of human dendritic cells via activating peroxisome proliferator-activated receptor gamma pathway. J Cardiovasc Pharmacol. 2010;56:177–183. [DOI] [PubMed] [Google Scholar]
- 18.Ge J, Jia Q, Liang C, et al. Advanced glycosylation end products might promote atherosclerosis through inducing the immune maturation of dendritic cells. Arterioscler Thromb Vasc Biol. 2005;25:2157–2163. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Liang C, Xu C, et al. Ciglitazone inhibits oxidized-low density lipoprotein induced immune maturation of dendritic cells. J Cardiovasc Pharmacol. 2004;44:381–385. [DOI] [PubMed] [Google Scholar]
- 20.Sun A, Liu H, Wang S, et al. Salvianolic acid B suppresses maturation of human monocyte-derived dendritic cells by activating PPARgamma. Br J Pharmacol. 2011;164:2042–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Packard RR, Maganto-Garcia E, Gotsman I, et al. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–68. [DOI] [PubMed] [Google Scholar]
- 23.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choy K, Beck K, Png FY, et al. Processes involved in the site-specific effect of probucol on atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:1684–1690. [DOI] [PubMed] [Google Scholar]
- 25.Bird DA, Tangirala RK, Fruebis J, et al. Effect of probucol on LDL oxidation and atherosclerosis in LDL receptor-deficient mice. J Lipid Res. 1998;39:1079–1090. [PubMed] [Google Scholar]
- 26.Inagaki M, Nakagawa-Toyama Y, Nishida M, et al. Effect of probucol on antioxidant properties of HDL in patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2012;19:643–656. [DOI] [PubMed] [Google Scholar]
- 27.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 29.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 30.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kofler S, Sisic Z, Shvets N, et al. Expression of circulatory dendritic cells and regulatory T-cells in patients with different subsets of coronary artery disease. J Cardiovasc Pharmacol. 2011;57:542–549. [DOI] [PubMed] [Google Scholar]
- 32.Van Vre EA, Van Brussel I, Bosmans JM, et al. Dendritic cells in human atherosclerosis: from circulation to atherosclerotic plaques. Mediators Inflamm. 2011;2011:941396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu P, Yu YR, Spencer JA, et al. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol. 2008;28:243–250. [DOI] [PubMed] [Google Scholar]
- 34.Li T, Chen W, An F, et al. Probucol attenuates inflammation and increases stability of vulnerable atherosclerotic plaques in rabbits. Tohoku J Exp Med. 2011;225:23–34. [DOI] [PubMed] [Google Scholar]
- 35.Takeda M, Yamashita T, Sasaki N, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30:2495–2503. [DOI] [PubMed] [Google Scholar]