Abstract:
Ca2+ is a crucial factor in the regulation of smooth muscle contraction. Store-operated Ca2+ entry (SOCE) is one pathway that mediates Ca2+ influx and smooth muscle contraction. Vessel contraction function usually alters with aging to cause severe vascular-related diseases. However, the underlying mechanism is still not fully understood. Here, we assessed intracellular Ca2+ and vessel tension and found that SOCE and SOCE-mediated contraction of vascular smooth muscle cells (VSMCs) was reduced in aorta but increased in mesenteric arteries from aged rats. The results of Western blot and immunofluorescence staining show that the expression levels of Orai1, a store-operated Ca2+ channel, were increased in VSMCs of mesenteric arteries but were reduced in VSMCs of aorta with aging. In conclusion, we demonstrated that the changing pattern of SOCE and SOCE-mediated contraction of VSMCs is completely reversed in mesenteric arteries and aorta with aging, providing a potential therapeutic target for clinical treatment in age-related vascular diseases.
Key Words: store-operated Ca2+ entry, Orai1, vascular smooth muscle cell, aorta, mesenteric artery, age
INTRODUCTION
Aging is one of the strongest independent risk factors for vascular-related diseases, such as hypertension, diabetes mellitus, and atherosclerosis, through affecting vessel tone and arterial stiffness.1,2 Vascular smooth muscle cells (VSMCs) play a central role controlling the vascular tone and blood flow.3–5 In VSMCs, extracellular Ca2+ entry may be mediated through voltage-operated Ca2+ channels (VOCCs), receptor-operated Ca2+ channels,6 and/or store-operated Ca2+ channels (SOCCs). These pathways mediating Ca2+ entry regulate VSMCs contractility and vascular tone.7–9 In advanced aging, the balance of Ca2+ in cells is gradually lost, which may become a profound factor altering cellular physiological function.10–12
The activation of SOCCs is routinely evoked by Ca2+ store depletion, which is achieved by either Ca2+ release through IP3 receptors or by pharmacological inhibition of the sarco–endoplasmic reticulum (ER) Ca2+-ATPase.13 STIM1 and Orai1 have been well documented as the essential components of SOCCs.14–17 STIM1 serves as a Ca2+ store sensor located on the membrane of the ER. In the cell, store depletion induces STIM1 to oligomerize and move to ER/plasma membrane junctional domains, where it interacts with and activates Orai1 channels.18 Orai1 has been demonstrated to be a Ca2+-selective ion channel expressed in the plasma membrane. Opening Orai1 channels will mediate extracellular Ca2+ entry, which is commonly called store-operated Ca2+ entry (SOCE). In various tissue cells, SOCE is used as a key pathway to mediate longer-term cytosolic Ca2+ ([(Ca2+]i) signals and replenish intracellular Ca2+ stores.13,19,20 The down- or up-regulation of SOCE can result in the imbalance of intracellular Ca2+ homeostasis and subsequently alter blood vessel tone and diameter. Our previous study showed that changed SOCE in kidney mesangial cells with aging affected cell proliferation.12 However, whether SOCE and related vasoconstriction is changed in VSMCs from different vascular beds with aging remains to be determined.
In this study, we investigated the difference of SOCE and SOCE-induced vasoconstriction in mesenteric artery and the aorta with aging. The results showed that both SOCE and SOCE-induced vasoconstriction was enhanced in mesenteric arteries but was declined in the aorta with advancing age. Alterations of STIM1 and Orai1 protein expression were coincident with the vasoconstriction change in mesenteric arteries and the aorta with aging.
METHODS
Animal Preparation
All animal experiments were conducted in accordance with NIH publication no. 8523 and approved by the Animal Experimentation Ethics Committee of Anhui Medical University. Animals were housed in a temperature-controlled room with a 12:12 hour light-dark cycle. The thoracic aorta and mesenteric artery were isolated from male Sprague Dawley rats aged 3 or 22 months, which were classified into either the young or old group.
Aorta Tension Measurement in Organ Bath
Rats were killed by CO2 overdose. Then, thoracic aorta was removed and dissected from surrounding connective tissue and placed in an oxygenated ice-cold Krebs solution that contained (in mM) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4 (7 H2O), 25.2 NaHCO3, and 11.1 glucose. The endothelial layer was removed by gently scrubbing the luminal side of the ring with stainless steel wire. The vessel rings were mounted onto 2 thin stainless steel holders, one of which was connected to a force displacement transducer, and the other to a movable device that enabled the application of 1 g of passive tension. The mounted rings were kept in 5-mL organ baths containing Krebs solution at 37°C and continuously bubbled with a gas mixture of 95% O2 and 5% CO2 to maintain a pH of 7.4. The isometric tension was recorded and analyzed by a data acquisition and analysis system (BL-420E+, Chengdu Technology & Market Corp). The rings were equilibrated for 60 minutes, and the bathing solution was changed every 15 minutes. After the equilibration period, the contractile function of the vessel was tested by replacing the Krebs solution with a 60-mM K+ solution prepared by replacing NaCl with an equimolar amount of KCl. After the washout, the rings were challenged with 1 μM phenylephrine (Phe) to test their contractile responses and subsequently exposed to 1 μM acetylcholine to certify endothelial functional removal.
Mesenteric Artery Tension Measurement in a Myograph System
Mesenteric artery tension measurement was performed as reported previously.21 To obtain mesenteric arteries, the intestine together with the mesentery was excised, unfolded, extended, and then pinned to a Petri dish containing ice-cold Krebs solution. The mesenteric artery was dissected free of the surrounding connective tissue and fat and removed under microscopy. Dissection was focused on the third branch of the mesenteric artery. Then, 2-mm segments of the third branch of rat mesenteric arteries were mounted in Danish Myo Technology myograph organ baths filled with 5 mL Krebs solution, and changes in isometric tension or the diameter of arteries was measured. The endothelial layer was removed by slight rub with silk thread. The organ bath was then warmed to 37°C and bubbled with a mixture gas of 95% O2 and 5% CO2 to maintain a pH of 7.4. The rings were stretched to an optimal baseline force of 1 mN for 2 mm artery segments. All these procedures were performed with 4-channel myographs (model 610M, Danish Myo Technology, Aarhus, Denmark), and force was recorded on the PowerLab recording unit. For the diameter measurements, 50 mm Hg of intraluminal pressure was applied to the segments, and diameter was measured by a pressure myograph (Danish Myo Technology, Denmark, model 110P). After the equilibration period, the contractile function of the vessel was tested by replacing the Krebs solution with a 60-mM K+ solution prepared by replacing NaCl with an equimolar amount of KCl. After the following washout, the vessels were exposed to 1 μM Phe and 1 μM acetylcholine sequentially to examine endothelial removal.
[Ca2+]i Measurement
[Ca2+]i was measured, as described previously.22 The smooth muscle tissues of mesenteric arteries or aortas were loaded with 10 μmol/L Fluo-8/AM for 1 hour in the dark at 37°C. Ca2+ stores were depleted by treating smooth muscle tissues with 4 μM thapsigargin (TG) for 10 minutes in Ca2+-free phosphate buffer saline (PBS) (0Ca2+-PSS), which contained (in mmol/L) 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 0.2 EGTA, and 5 Hepes, pH 7.4. Ca2+ influx was initiated by applying 2.5 mM extracellular Ca2+. Fluorescence was recorded using a Leica TCS SP5 confocal laser system. Changes in [Ca2+]i were displayed as the ratio of fluorescence relative to the intensity before applying extracellular Ca2+ (F1/F0).
Western Blotting
Immunoblot was performed as reported previously.23 The proteins were extracted from the lysates of mesenteric artery or the aorta smooth muscle with detergent extracted buffer, which contained 1% Nonidet P-40, 150 mmol/L NaCl, and 20 mmol/L Tris-HCl, pH 8.0, plus protease inhibitor cock tail tablets. For the immunoblots, the poly (vinylidene difluoride) membrane carrying transferred proteins was incubated at 4°C overnight with the primary antibodies: anti-STIM1 (1:200, rabbit polyclonal, Abcam, Inc) or anti-Orai1 (1:200, rabbit polyclonal, Santa Cruz Biotechnology, Inc). Immunodetection was accomplished using horseradish peroxidase–conjugated secondary antibody, followed by processing through an ECL detection system. The optical density of each blot was normalized to that of β-tubulin analyzed within the same lane and represented as the relative optical density.
Immunofluorescence
The freshly isolated young and aged rat mesenteric artery or aorta were embedded in tissue freezing medium (Leica) and placed in nitrogen to solidify the medium and tissue. Then, 10 μm-thick sections were prepared. Freshly frozen samples were sectioned at 10 μm on a Leica CM1850 Cryostat (Leica Microsystems Inc), placed on 3-amino-alkyl ethoxy silane-coated slides, fixed in 4% formaldehyde solution for 30 minutes, washed in PBS, incubated in 0.1% Triton X-100 in PBS for 30 minutes, and then incubated in 5% BSA (Chemic on International) in PBS for 1 hour. Subsequently, STIM1 and Orai1 expression was determined by incubating the vascular sections with anti-STIM1 (1:50) or Orai1 antibody (1:50) overnight at 4°C. Vessel sections were then placed at room temperature, rinsed with PBS for 5 minutes, and probed with goat antirabbit Alexa Fluor 488 (1:200, green fluorescence, Invitrogen), followed by another rinse with PBS. Images were acquired with a confocal microscope (LSM 510 Meta 3.2, Zeiss). A negative control was performed by omitting primary antibodies, which showed no specific immunoreactivity.
Superoxide Dismutase Activity Assay
Superoxide dismutase (SOD) activity was detected according to the manual from the company. The rat serum samples were mixed with 0.5 mM hypoxanthine, 0.5 mM hydroxylamine, and 0.01 U xanthine oxidase in the buffer [104 mM potassium phosphate, 78 mM sodium borate, and 0.025 mM EDTA (pH 7.0)]. After 30 minutes at 37°C, the reaction was stopped by adding 0.2 mL of 16% (v/v) acetic acid solution containing 2.6 mM sulfanilic acid and 38.6 mM naphthyl ethylenediamine. To calculate SOD activity, the absorbance at 550 nm was recorded, and 1 nitroso unit of enzyme activity was calculated as that inhibiting 50% of the oxidation of hydroxylamine.
Statistics
All data are expressed as the mean ± SEM. Significance was assessed by a 2-tailed Student's t test. Differences between groups were considered significant at P < 0.05.
RESULTS
Changes of Weight, Blood Pressure, Blood Glucose, and Activities of SOD in Aged Rats
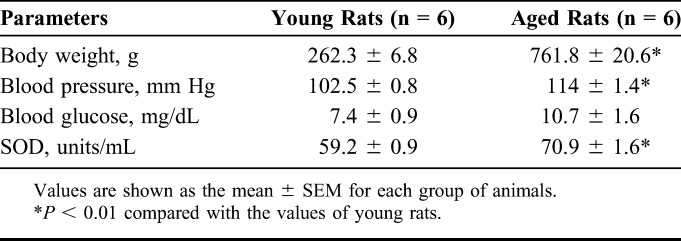
We compared several parameters of young and aged rats. The data indicate that weight and blood pressure were significantly higher. However, blood glucose was increased in aged rats, although the change was not significant (Table 1). Because many studies have reported that oxidative stress will occur with aging,24,25 we also compared the content of SOD in young and aged rat serum. It was found that SOD was significantly decreased in the aged rats (Table 1).
TABLE 1.
Weight, Blood Pressure, Blood Glucose, and Activities of SOD in Serum of the Young and Aged Rats
Change of SOCE in Aged VSMCs
TG, a Ca2+ pump inhibitor, was used to evoke SOCE by passive depletion of cellular internal Ca2+ stores. The vascular smooth muscle tissues of mesenteric arteries and aorta were treated with 4 μM TG in Ca2+-free Krebs solution to deplete Ca2+ stores for 10 minutes, after which 2.5 mM Ca2+ was added to the extracellular solution. Ca2+ influx through SOCE was attenuated in aortic muscle cells in the aged rats (Figs. 1A–D); however, SOCE was markedly increased in mesenteric artery muscle cells in aged rats (Figs. 1E–G).
FIGURE 1.
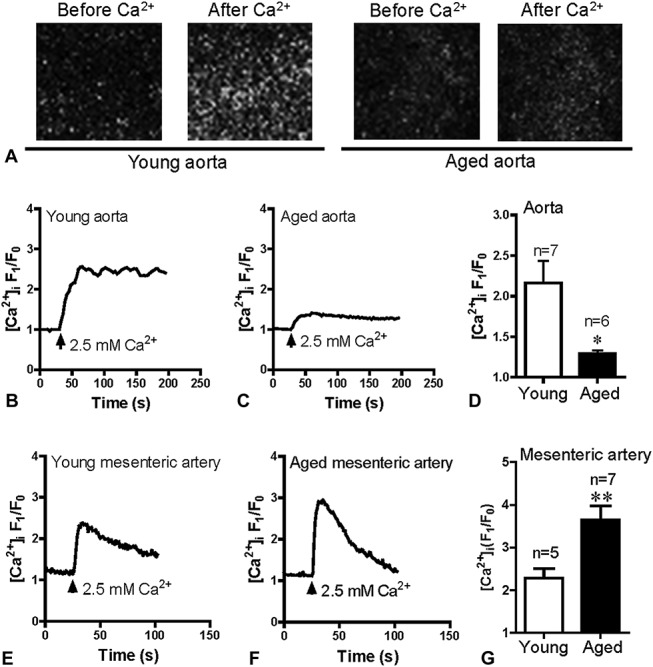
SOCE in smooth muscle tissue of the aorta and mesenteric arteries. Representative images and traces of 2.5 mM Ca2+ application-induced [Ca2+]i increase after 4 μM TG induced Ca2+ store depletion in smooth muscle tissue of the aorta (A–C) and mesenteric arteries (E and F) from Young and aged rats. Data showing that [Ca2+]i increases in response to extracellular Ca2+ application after TG treatment in the aorta (D) and mesenteric arteries (G). Values are presented as the mean ± SEM (n = 5–7); *P < 0.05, **P < 0.01. Young (3 months old) versus aged (22 months old).
Change of SOCE-induced Vasoconstriction in Aged Rats
Altered SOCE in VSMCs will affect blood vessel tone. Therefore, to evaluate the change of SOCE-induced vasoconstriction, vessel tension was measured. The mesenteric arteries and aorta were treated with 4 μM TG in Ca2+-free Krebs solution for 10 minutes with 1 μM nifedipine to block voltage-operated Ca2+ channels, after which 2.5 mM Ca2+ was added to the bath solution. SOCE-evoked vasoconstriction was significantly decreased in the aorta of aged rats (Figs. 2A–C). However, compared with young rats, SOCE-induced vasoconstriction was dramatically increased in the mesenteric arteries of the aged rats (Figs. 2D–F).
FIGURE 2.
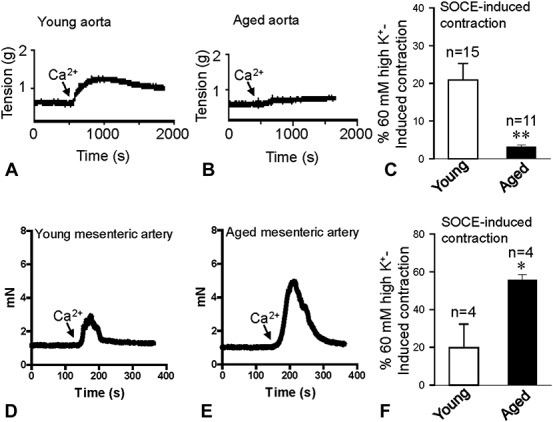
SOCE-induced vasoconstriction of the aorta and mesenteric arteries. Representative traces of 2.5 mM Ca2+ application-induced vessel contraction after 4 μM TG treatment for 10 minutes in the aorta (A and B) and mesenteric arteries (D and E) from Young and aged rats. Data showing vessel tension changes in response to extracellular Ca2+ application after TG treatment in the aorta (C) and mesenteric arteries (F) from young and aged rats. Values are presented as the mean ± SEM (n = 4–15); *P < 0.05, **P < 0.01. Young (3 months old) versus aged (22 months old).
To examine physiological responses, 2 agonists, including α-receptor agonist Phe and endothelin receptor agonist endothelin-1 (ET-1), were used. Phe and ET-1 can act on their receptors, which couple to G-proteins to induce IP3 production, leading to Ca2+ release from the ER.26 Therefore, Phe and ET-1 may deplete Ca2+ stores and evoke SOCE. After Phe or ET-1 treatment in Ca2+-free Krebs solution for 10–15 minutes with 1 μM nifedipine, after the addition of 2.5 mM Ca2+, a further sustained contraction was evoked, indicating SOCE-induced contraction. The data indicate that the SOCE-induced contraction in Phe and ET-1 pretreatment was significantly decreased in the aorta (Fig. 3) but was strongly enhanced in the mesenteric arteries of the aged rats (Fig. 4).
FIGURE 3.
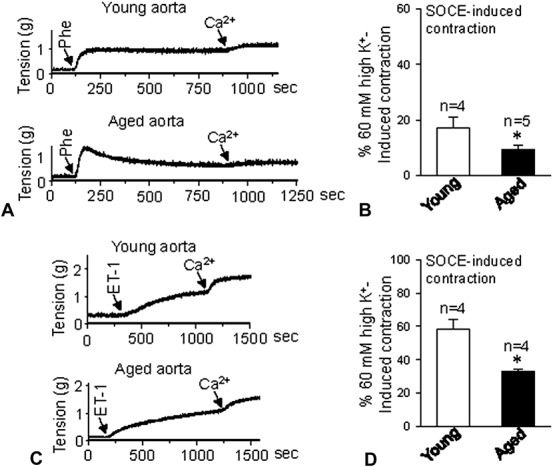
Agonist-induced contraction in the aorta. Representative traces of 2.5 mM Ca2+ application-induced vessel contraction after 1 μM Phe (A) or 10 nM ET-1 (C) treatment for 10 minutes in aorta from young and aged rats. B and D, show vessel tension changes in response to extracellular Ca2+ application after Phe (B) or ET-1 (D) treatment in aortas from young and aged rats. Values are presented as the mean ± SEM (n = 4–5); *P < 0.05. Young (3 months old) versus aged (22 months old).
FIGURE 4.
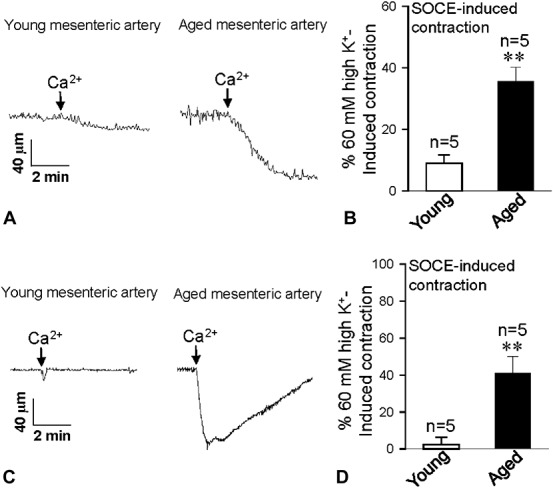
Agonist-induced contraction in mesenteric artery. Traces showing that after 1 μM Phe (A) or 10 nM ET-1 (C) treatment for 10 minutes, 2.5 mM Ca2+ application-induced vessel contraction in mesentery arteries from young and aged rats. B and D, present vessel tension changes in response to extracellular Ca2+ application after Phe (B) or ET-1 (D) treatment in mesentery arteries from young and aged rats. Values are presented as the mean ± SEM (n = 5); **P < 0.01. Young (3 months old) versus aged (22 months old).
Expression Profile of STIM1 and Orai1 in the VSMCs of Young and Aged Rats
STIM1 and Orai1 proteins are 2 essential components of SOCCs.27 To clarify why altered SOCE and SOCE-induced vasoconstriction was observed in mesenteric arteries and aorta with aging, we examined the expression levels of these 2 proteins in the VSMCs of young and aged rats. Interestingly, both Western blotting and immunofluorescence staining results show that Orai1 expression levels were dramatically increased but that STIM1 expression was significantly decreased in the VSMCs of the mesenteric arteries of aged rats (Fig. 5). However, in the aorta, only Orai1 expression level was significantly reduced in aged rats; no notable differences in STIM1 expression were observed between young and aged rats (Fig. 6).
FIGURE 5.
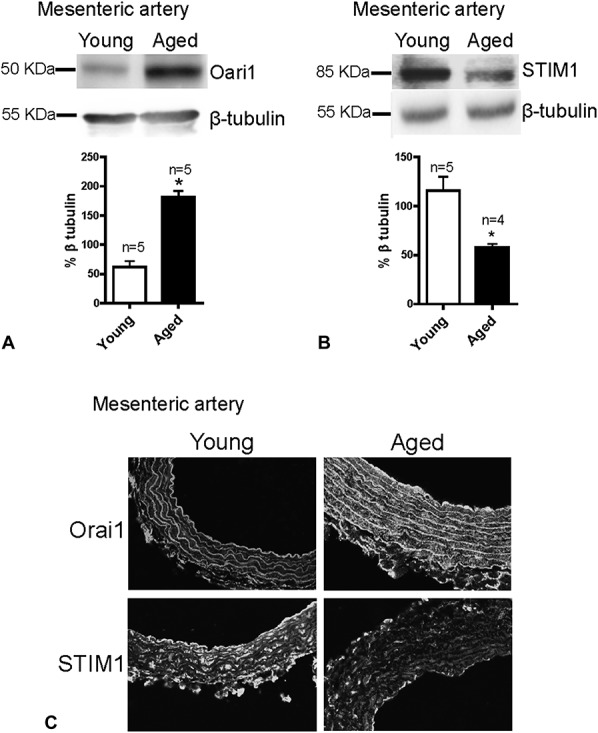
Expression levels of Orai1 and STIM1 in mesenteric arteries. Representative images and summarized data showing the expression levels of Orai1 (A and C) and STIM1 (B and C) in mesenteric arteries from young and aged rats. Protein expressions were normalized with β-tubulin. Protein levels are expressed as the relative optical density. Mean ± SEM (n = 4–5); *P < 0.05. Young (3 months old) versus aged (22 months old).
FIGURE 6.
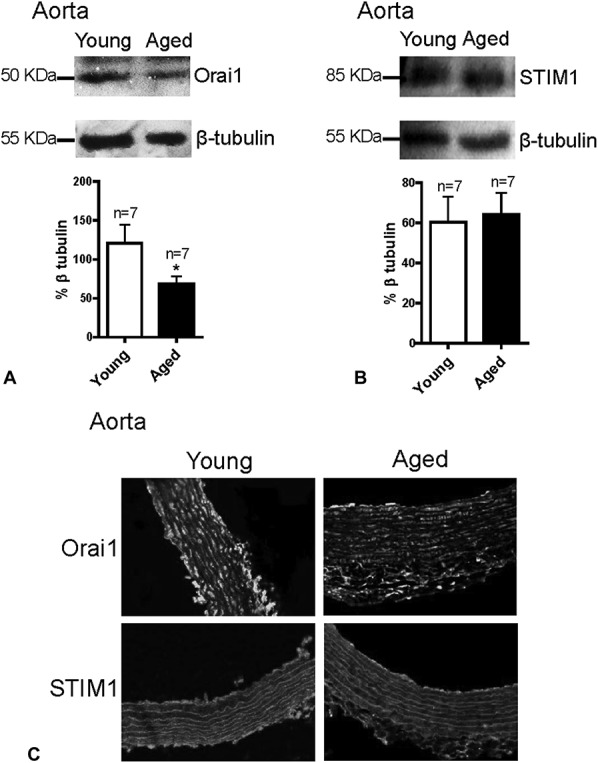
Expression levels of Orai1 and STIM1 in aorta. Western blot and inmmunofluorescence staining images and summarized data showing the expression levels of Orai1 (A and C) and STIM1 (B and C) in aorta from young and aged rats. The expression levels of proteins were normalized with β-tubulin and are expressed as the relative optical density. Mean ± SEM (n = 7); *P < 0.05. Young (3 months old) versus aged (22 months old).
DISCUSSION
The major findings of this study are as follows: 1) the Ca2+ image results demonstrated that TG-evoked SOCE in VSMCs was increased in the aged rat mesenteric arteries but decreased in the aorta of the aged rat; 2) SOCE-mediated vascular contraction of aged rats was increased in the mesenteric artery but decreased in the aorta compared with those of young rats; 3) Western blot and inmmunofluorescence staining show that Orai1 expression levels were increased in the aged mesenteric artery but decreased in the aged aorta.
This study suggests that age-related changes were largely different in mesenteric artery and aorta. Rubio et al11 reported that Phe-induced vasoconstriction of mesenteric arteries was increased with abnormal Ca2+ handling in aged rats. However, the molecular target destroying Ca2+ homeostasis with aging remained unknown. Our finding shows that Orai1 expression in VSMCs was enhanced in mesenteric arteries but decreased in aorta of the aged rats. Orai1 is a key Ca2+ channel protein that mediates Ca2+ influx through SOCE. Therefore, with advancing age, SOCE of VSMCs and SOCE-induced vasoconstriction were enhanced in mesenteric arteries but were decreased in aorta. Because SOCE links with G-protein-coupled receptor-induced intracellular Ca2+ release and vasoconstriction, mesenteric arteries with Orai1 overexpression in aged rats have an increased contractile response to the agonists, such as in this study with Phe and ET-1. Mesenteric arteries, one type of resistance artery, play an important role in controlling local blood flow and contribute to systemic blood pressure. A previous report and our data showed that systemic pressure increased in aged rats compared with young rats.28 Therefore, we first provide evidence that enhanced SOCE in VSMCs is likely the reason for the enhanced constriction of mesenteric arteries, which in turn contributes to the local hypoperfusion and increased systemic pressure with aging. However, in the young rats, our results (Figs. 3, 4) also indicate that the vasoconstriction evoked by Phe- and ET-1-induced SOCE were much weak in either mesenteric artery or aorta. These results suggest that SOCE-induced vasoconstriction might have limited role in the local hypoperfusion and systemic blood pressure controlling in the young rats.
The aorta is a large blood vessel that functions not only as a conduit for delivering blood to tissues but also as an important modulator of the entire cardiovascular system, buffering the intermittent pulsatile output from the heart to provide steady flow to capillary beds and reduce systolic blood pressure.29 By virtue of its elastic properties, aorta functioning influences left ventricular function and coronary blood flow.30,31 Morgan et al32 used Doppler to measure pulse wave velocity as a noninvasive, in vivo assessment of arterial stiffness, and found that pulse wave velocity was significantly faster in the aged rat arteries. That finding demonstrated that the arterial stiffness was significantly increased in the aged rats. In our study, SOCE-induced aorta contraction markedly decreased with aging. We presumed that the depressed constriction of the aorta contributes to the enlarged intermittent pulsatile with stiffness with aging.
With advancing age, Orai1 expression in aortic VSMCs was significantly decreased, which compromises the ability of SOCE in aged aorta. In contrast, Orai1 expression was elevated in VSMCs of aged mesenteric arteries compared with those of young mesenteric arteries. Therefore, the SOCE and SOCE-induced vasoconstriction was enhanced through increased Orai1 expression, which enables greater Ca2+ entry in VSMCs. However, the expression of STIM1, a Ca2+ sensor, was significantly decreased in aged mesenteric arteries but was not altered in aged aorta. These results may suggest that the age-associated SOCE variation is primarily induced by Orai1 channels and not caused by Ca2+ sensor through STIM1. In our previous study, we found that the expression level of Orai1 and STIM1 were both declined with the same pattern in glomerular mesangial cells in the aged rats.12 Although in this study, the change of expression level of Orai1 and STIM1 was opposite with aging. At this stage, we did not know the exact reason that the age-related expression changes of Orai1 and STIM1 are different in various tissues. Possibly, these changes are related to the functional adaptation with aging. The further study in future should be beneficial for clarifying the underling mechanism. Orai2 and Orai3 are homologues of Orai1 and have a structure similar to Orai1. They may be activated by Ca2+ store depletion when coexpressed with STIM1 in HEK293 cells, but the amplitude of the currents are smaller produced by Orai2 or Orai3.18 Potier et al33 reported that the knockdown of STIM1 and Orai1 in VSMCs markedly reduced SOCE, but the knockdown of Orai2 or Orai3 did not dramatically affect SOCE. Thus, we only tested Orai1 protein expression levels of 3 Orai isoforms. In addition, it is unclear whether canonical transient receptor potential (TRPC) channels function as SOCCs in smooth muscle and other tissues.19,34 Potier et al33 suggested that TRPC isoforms, including TRPC1, TRPC4, and TRPC6, do not affect SOCE in VSMCs. Here, we did not examine the role of TRPC in SOCE in VSMCs.
During the aging process, oxidative stress will occur, and the endogenously produced oxidants in the cells are usually enhanced.24,25 SOD is an important enzyme to catalyze the dismutation of the toxic superoxide radical into either ordinary molecular oxygen or hydrogen peroxide to remove reactive oxygen species. Our data show that serum SOD was significantly decreased in the aged rats compared with the young rats. Oxidative stress may act directly through modulation of gene expression such as Ca2+ channels.35 Therefore, Ca2+ homeostasis of the VSMCs will be out of balance. Augmented reactive oxygen species production may contribute to Ca2+ mishandling to enhance vasoconstriction.35 Over a long period, many signaling pathways linked to the maintenance of Ca2+ homeostasis might be altered. It is possible that SOCE is one of these pathways. Recently, studies in other group36 and our group (not published) indicated that SOCE was increased in the glomerular mesangial cells and ventricular muscle cells in the diabetic animals. Diabetes is also a disease having oxidative stress. Thus, increased oxidative stress is a possible reason to cause potentiated SOCE-induced vasoconstriction in the mesenteric artery in the aged rats. However, in response to the agonists the SOCE-induced vasoconstriction in the aorta was oppositely decreased. Maybe other unknown factors also affect the final outcome of SOCE-induced vasoconstriction with aging. Oxidative stress is not the only reason to explain this difference of the age-related behavior in the aorta or mesenteric arteries.
CONCLUSIONS
In this study, we demonstrated that SOCE function was altered in VSMCs in mesenteric arteries and aorta with aging. The changed Orai1 expression levels may contribute to the alteration of SOCE and vasoconstriction in aged aorta and mesenteric arteries. Because Ca2+ signaling plays crucial role in regulating vessel tone and blood pressure, our findings provide a therapeutic target in age-associated irregularities in the vascular system.
Footnotes
Supported by grants from the Natural Science Foundation of China (Grant Nos. 81371284 and 61273324); Anhui Provincial Natural Science Foundation (Grant Nos. 1208085MH181 and 1108085J11); Young Prominent Investigator Supporting Program from Anhui Medical University; Outstanding Young Investigator of Anhui Medical University; and Supporting Program for Excellent Young Talents in Universities of Anhui Province.
The authors report no conflicts of interest.
REFERENCES
- 1.Moore A, Mangoni AA, Lyons D, et al. The cardiovascular system. Br J Clin Pharmacol. 2003;56:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaboration APCS. The impact of cardiovascular risk factors on the age-related excess risk of coronary heart disease. Int J Epidemiol. 2006;35:1025–1033. [DOI] [PubMed] [Google Scholar]
- 3.Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. [DOI] [PubMed] [Google Scholar]
- 4.Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev. 2010;90:113–178. [DOI] [PubMed] [Google Scholar]
- 5.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. [DOI] [PubMed] [Google Scholar]
- 6.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. [DOI] [PubMed] [Google Scholar]
- 7.Brueggemann LI, Markun DR, Henderson KK, et al. Pharmacological and electrophysiological characterization of store-operated currents and capacitive Ca(2+) entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. [DOI] [PubMed] [Google Scholar]
- 8.Park KM, Trucillo M, Serban N, et al. Role of iPLA2 and store-operated channels in agonist-induced Ca2+ influx and constriction in cerebral, mesenteric, and carotid arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1183–H1187. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wier WG, Blaustein MP. Mg2+ blocks myogenic tone but not K+-induced constriction: role for SOCs in small arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2692–H2705. [DOI] [PubMed] [Google Scholar]
- 10.Lopes GS, Ferreira AT, Oshiro ME, et al. Aging-related changes of intracellular Ca2+ stores and contractile response of intestinal smooth muscle. Exp Gerontol. 2006;41:55–62. [DOI] [PubMed] [Google Scholar]
- 11.Rubio C, Moreno A, Briones A, et al. Alterations by age of calcium handling in rat resistance arteries. J Cardiovasc Pharmacol. 2002;40:832–840. [DOI] [PubMed] [Google Scholar]
- 12.Shen B, Zhu J, Zhang J, et al. Attenuated mesangial cell proliferation related to store-operated Ca2+ entry in aged rat: the role of STIM 1 and Orai 1. Age (Dordr). 2013;35:2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. [DOI] [PubMed] [Google Scholar]
- 14.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. [DOI] [PubMed] [Google Scholar]
- 17.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeHaven WI, Smyth JT, Boyles RR, et al. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam K, van Rossum DB, Patterson RL, et al. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–E272. [DOI] [PubMed] [Google Scholar]
- 20.Putney JW, Jr, Broad LM, Braun FJ, et al. Mechanisms of capacitate calcium entry. J Cell Sci. 2001;114(pt 12):2223–2229. [DOI] [PubMed] [Google Scholar]
- 21.Kwan HY, Shen B, Ma X, et al. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. [DOI] [PubMed] [Google Scholar]
- 22.Shen B, Kwan HY, Ma X, et al. cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway. J Biol Chem. 2011;286:19439–19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J, Ma X, Shen B, et al. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 2014;28:4677–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmy MM. Potential hepato-protective effect of alpha-tocopherol or simvastatin in aged rats. Pharmacol Rep. 2012;64:698–705. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Rong S, Xie B, et al. Procyanidins extracted from the lotus seedpod ameliorate age-related antioxidant deficit in aged rats. J Gerontol A Biol Sci Med Sci. 2010;65:236–241. [DOI] [PubMed] [Google Scholar]
- 26.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soltis EE. Effect of age on blood pressure and membrane-dependent vascular responses in the rat. Circ Res. 1987;61:889–897. [DOI] [PubMed] [Google Scholar]
- 29.Luchsinger PC, Sachs M, Patel DJ. Pressure-radius relationship in large blood vessels of man. Circ Res. 1962;11:885–888. [DOI] [PubMed] [Google Scholar]
- 30.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res. 1992;71:490–502. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsuka S, Kakihana M, Watanabe H, et al. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol. 1994;24:1406–1414. [DOI] [PubMed] [Google Scholar]
- 32.Morgan EE, Casabianca AB, Khouri SJ, et al. In vivo assessment of arterial stiffness in the isoflurane anesthetized spontaneously hypertensive rat. Cardiovasc Ultrasound. 2014;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potier M, Gonzalez JC, Motiani RK, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert AP, Saleh SN, Peppiatt-Wildman CM, et al. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583(pt 1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabet F, Savoia C, Schiffrin EL, et al. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–208. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhari S, Wu P, Wang Y, et al. High glucose and diabetes enhanced store-operated Ca(2+) entry and increased expression of its signaling proteins in mesangial cells. Am J Physiol Renal Physiol. 2014;306:F1069–F1080. [DOI] [PMC free article] [PubMed] [Google Scholar]


