Abstract
The murine leukemia virus (MLV) TR1.3 provides an excellent model to study the wide range of retrovirus-induced central nervous system (CNS) pathology and disease. TR1.3 rapidly induces thrombotic events in brain microvessels and causes cell-specific syncytium formation of brain capillary endothelial cells (BCEC). A single amino acid substitution, W102G, in the MLV envelope protein (Env) regulates the pathogenic effects. The role of Env in determining this disease phenotype compared to the induction of spongiform encephalomyelitis with a longer latency, as seen in several other MLV and in human retroviruses, was determined by studying in vitro-attenuated TR1.3. Virus cloned from this selection, termed TRM, induced progressive neurological disease characterized by ataxia and paralysis and the appearance of spongiform neurodegeneration throughout the brain stem and spinal cord. This disease was associated with virus replication in both BCEC and highly ramified glial cells. TRM did not induce syncytium formation, either in vivo or in vitro. Sequence and mutational analyses demonstrated that TRM contained a reversion of Env G102W but that neurological disease mapped to the single amino acid substitution Env S159P. The results demonstrate that single nucleotide changes within disparate regions of Env control dramatically different CNS disease patterns.
The retroviruses human immunodeficiency virus type 1 (HIV-1) and human T-lymphotropic virus 1 can induce debilitating neurological illness (2, 28). The impact of these diseases as a global health challenge continues to rise as both the spread of HIV and prolonged survival of infected individuals increases (53). Although in vivo and ex vivo research on HIV replication in the human central nervous system (CNS) has improved our knowledge base, supplemental models are needed. Murine leukemia viruses (MLV) provide a facile system for analysis of the complicated dynamics of retrovirus infections in the CNS (20, 28, 41, 52). MLV induce a variety of neurological deficiencies depending on the virus isolate and mouse strain (54). One common pathological feature is the induction of spongiform encephalomyelopathy in the CNS and linked deterioration of peripheral motor function (10, 11, 17, 25, 34, 54); characteristic examples of this include infection of mice with CasBrE, FrCasE, or Moloney ts1 virus (7, 33, 56) and of rats with PVC-211, NT-40, or A8 virus (5, 18, 50).
Although a common causative pathway has not been identified for MLV-induced neurological disease, each of these viral systems provides insights into disease processes. In the instance of PVC-211, disease severity correlates with an increased capacity for replication within CNS endothelial cells, which may be linked to oxidative damage (10, 15, 30, 31, 55). In contrast, with other MLV, there is excellent evidence that infection of either microglia (FrCasE and NT-40) or astrocytes (Moloney ts1) plays a pivotal role in disease expression (5, 12, 27, 31, 45). Despite these seemingly diverse mechanisms, strikingly similar neurodegenerative diseases result.
The Friend MLV TR1.3 is unique among the neuropathogenic MLV in the induction of acute vascular injury within the brain. TR1.3 infection of susceptible strains of neonatal mice results in intracerebral hemorrhage, stroke, and paralysis. These effects are directly attributed to TR1.3 infection and syncytium formation of brain capillary endothelial cells (BCEC) (35). This phenotype is the result of a single amino acid change of tryptophan to glycine at position 102 (W102G) in the SU domain of the envelope protein, which lies at the base of the putative receptor binding pocket (38). Interestingly, with other MLV the SU domain was also shown to contain the critical neurovirulence determinants that induce spongiform encephalomyelopathies (16, 41, 49), although the long terminal repeat (LTR) and other sequences may influence the temporal onset of disease and location of pathology within the CNS (7, 51).
These observations illustrate that while the biology of MLV enable interesting approaches to understanding the acute and more chronic effects of retrovirus infection within the CNS, many questions remain unanswered. The focus of the present analysis was to elucidate disease mechanisms by mapping regions of Env that regulate acute vascular disease versus slowly progressive neuronal degeneration on a common genetic background. To achieve this, we attenuated TR1.3 by means of selecting against syncytium-inducing capacity in cell culture. Virus cloned from this selection, termed TRM, was used to map disease phenotype and tissue pathology. The results demonstrate that single nucleotide changes within disparate regions of SU control dramatically different CNS disease patterns.
MATERIALS AND METHODS
Cells.
The mouse fibroblast cell lines SC-1 and XC were obtained from the American Type Culture Collection (Rockville, Md.). The Japanese quail fibrosarcoma cell line QT6 was obtained from the laboratory of Paul Bates (University of Pennsylvania). These cell lines were maintained in Dulbecco's modified Eagle medium (DMEM) (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (Invitrogen), 2 mM glutamine, and penicillin-streptomycin. Cells were grown at 37°C in 5% CO2 unless otherwise noted.
Viruses.
All viruses were prepared as previously described (36). Viral genomes were cloned into the modified pUC19B vector for growth and isolation in XL-1 Gold Ultracompetent cells (Stratagene, La Jolla, Calif.). Overnight HindIII (New England Biolabs, Beverly, Mass.) digestion, separation on a 1.2% agarose gel, and electroelution were used to purify a linear fragment of DNA encoding the full viral genome. Overnight ligation with T4 ligase was used to circularize the genome, which was then transfected into 105 SC-1 cells in a 100-mm Primaria dish (Becton Dickinson, Bedford, Mass.) by using a calcium phosphate transfection kit (Invitrogen). The day after transfection, the cells were dimethyl sulfoxide shocked according to the manufacturer's instructions. The following day, the cells were divided into two T150 flasks, and virus supernatants were harvested from confluent monolayers after changing the medium 24 h prior to harvest.
Biological attenuation of TR1.3 virus.
SC-1 cells (106 cells/plate) were seeded into T150 cell culture flask (Corning, Cambridge, Mass.) in 24 ml of DMEM (Gibco, Gaithersburg, Md.) containing 10% fetal calf serum (Gibco), 1% glutamine, and 1% penicillin and streptomycin. After 24 h of incubation, TR1.3 virus (multiplicity of infection of 15) and 8 μg of Polybrene (Sigma, St. Louis, Mo.)/ml were added to the cultures. The culture was then maintained for 3 months with regular passage, after which virus-infected SC-1 cells and supernatants were independently harvested and frozen for subsequent use. The presence of virus was confirmed by reverse transcriptase and XC plaque assay. High-molecular-weight DNA from cells was isolated for PCR cloning of virus by using a QIAamp tissue kit (QIAGEN, Santa Clarita, Calif.).
Molecular cloning of TRM virus.
The TRM genome was cloned by PCR amplification of high-molecular-weight DNA in three separate fragments (LTR-gag, pol, and env-LTR). DNA (250 ng) was amplified with Expand High-Fidelity PCR system (Roche, Indianapolis, Ind.) with 2.6 U of Pwo-Taq polymerase mixture and 300 nM of sense and antisense primers in PCR buffer consisting of 1.5 mM MgCl2 and 0.2 mM deoxynucleoside triphosphate. The PCR cycle consisted of denaturation at 94°C for 30 s which was followed by an annealing step at 65°C for 30 s and elongation at 68°C for 2.15 min for 10 cycles, followed by denaturation at 94°C for 30 s, an annealing step at 65°C for 30 s, and elongation at 68°C for 2.15 min, increasing by 20 s with each cycle, for 20 cycles. PCRs were performed with the following primers: LTR-gag (KpnI-EcoRI fragment, 2,927 bp) (sense, AAAAGAGCTCACAACCCCTCACTC; antisense, GGACAGGCCTATAATCATTAGTCCC), pol (EcoRI-HindIII fragment, 2,432 bp) (sense, CATAAAACAATACCCCATGTCACAA; antisense, AATCGGCTACTGTCTGACTTACCTT), and env-LTR (HindIII-KpnI fragment, 3,228 bp) (sense, ACCTGGCCTGTATGGGTATAAATA; antisense, GATGCAACAGCAAGAGGATTTATT). The amplicons were resolved by electrophoresis through 1% agarose gels and were gel isolated by using a QIAquick (QIAGEN) gel isolation kit according to the manufacturer's recommendations. The LTR-gag fragment was digested with KpnI and EcoRI restriction enzymes and cloned into pcDNA3.1(+) plasmid (Invitrogen), the pol fragment was digested with EcoRI and HindIII restriction enzymes and cloned into pcDNA3.1(−) plasmid (Invitrogen), and the env-LTR was digested by HindIII and KpnI and cloned into pcDNA3.1(+) plasmid containing the previously cloned LTR-gag fragment. In the next step, both the pcDNA3.1(+) plasmid containing fragment env-LTR-gag and the plasmid containing pol were digested with HindIII and NheI. Both products were ligated into the complementary sites yielding the full genome of the TRM virus cloned into the EcoRI site of the pcDNA3.1(+) plasmid.
Construction of TRM site-specific mutations.
The envelope gene was PCR amplified by using primers with restriction sites flanking the gene sequences 5′-GCGGGTACCTGCCCACGTAAAGGCTGCCG and 3′-CGCGAATTCCTGGCGCGCCGAGTGAGGGG. Following amplification, PCR products were digested and ligated into the pcDNA3.1 vector (Invitrogen) by using KpnI/EcoRI digestion in EcoRI buffer (New England Biolabs) and a rapid ligation kit (Roche). PCR was carried out with a reaction volume of 50 μl including 1× Pfu buffer, 1.0 mM MgCl2, 1 μM forward primer, 1 μM reverse primer, 1 μM deoxynucleoside triphosphate, 2.5 U of Pfu Turbo polymerase and 25 ng of FB29 whole-virus DNA. Reaction conditions were as follows: 1 cycle at 94° for 1 min; 35 cycles at 94° for 30 s, 70° for 1 min, and 68° for 3 min; and 1 cycle at 68° for 10 min. Once cloned into pcDNA3.1, site-directed mutagenesis (Stratagene) was used to introduce point mutations with the following primers: for S159P, 5′-GGAAGCCCTCCTCTCCTTGGGACTAC and 3′-GTAGTCCCAAGGAGAGGAGGGCTTCC; and for R190Q, 5′-GGCTATCCAGTTTACAAACGCCGGG and 3′-CCCGGCGTTTGTAAACTGGATAGCC. The PCRs were carried out according to the manufacturer's instructions (Stratagene). Enzymatic digest with AscI and BsaAI enzymes were used to isolate the envelope gene for religation into the respective parental virus vectors. The same procedure was used to clone TR1.3, W102G, and TRM envelope protein into pcDNA3.1. The P159S envelope protein construct was generated with the pcDNA3.1 plasmid containing TRM envelope protein and the primers 5′-GCCCTCCTCTTCTTGGGACTACATC and 3′-GATGTAGTCCCAAGAAGAGGAGGGC.
Mice.
Virus-free pregnant female BALB/c mice were obtained from Charles River (Wilmington, Mass.). Neonates (<48 h old) were inoculated either intracerebrally or intraperitoneally with 30 μl of virus supernatant containing between 4 × 104 and 4 × 106 PFU. Mice were monitored daily for symptoms of neurologic disease. Animal studies were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Histological analysis.
Tissue samples from brains were fixed overnight in 10% formalin. Samples were then transferred to 70% ethanol until they were embedded in paraffin. Sections of 6-μm thickness were stained with hematoxylin and eosin. For confocal analysis, deparaffinized, rehydrated sections were incubated for 1 h in 0.1% aqueous saponin. Sections were then incubated overnight in 1% donkey serum containing 1:2,000 Rauscher gp69/71 serum and 1:1,000 rabbit anti-human factor VIII-associated antigen (Von Willebrand factor [VWF]) antibody (Dako Corp., Carpinteria, Calif.). After three phosphate-buffered saline (PBS) washes, slides were incubated with the appropriate secondary antibodies (Jackson Immunoresearch) diluted 1:100 in 1% normal donkey serum for 1 h. Unbound antibody was removed with three final PBS washes, and the slides were mounted with Vectashield mounting medium (Vector Laboratories).
Cell fusion assay.
The cell fusion assay was modified from previously described protocols (4, 22). Briefly, QT6 cells were split into T25 flasks and grown to confluence for effector cells. QT6 cells were split from confluent T25 flasks equally into 24-well plates for target cells. The following day, effector cells were infected with vaccinia virus strain WR at a multiplicity of infection of 10 for 1 h in 2.5% DMEM. Following this step, the medium was aspirated and replaced with 10% DMEM. The cells were then transfected with 6 μg of Env plasmid and 6 μg of plasmid pSP64.vE/L.T7 RNAP encoding the T7 polymerase gene (a kind gift of James Hoxie). Target cells were transfected with 0.25 μg of pJET (a kind gift of James Cunningham)/well and 0.25 μg of luciferase-T7 plasmid (Promega, Madison, Wis.)/well. Transfections were incubated for 4 h, at which point the cells were refed with fresh medium: 10% DMEM for target cells and 10% DMEM with 100 μg of rifampin/ml for effector cells. Cells were incubated overnight at 37°C (target cells) or 32°C (effector cells). The following day, effector cells were detached with 0.5 mM EDTA in PBS without Ca2+ and Mg2+. These cells were then harvested at 800 rpm for 5 min and resuspended in 3.5 ml of 10% DMEM with 100 μg of rifampacin/ml. Medium from the target cells was aspirated, and each well was overlaid with 0.5 ml of effector cells. After 7.5 h of cocultivation, cells were lysed in 200 μl of 0.5% Triton X-100 in PBS. Twenty-five microliters of each well was mixed with 50 μl of luciferase substrate (Promega), and luciferase activity was immediately read on a Wallac luminometer.
RESULTS
Selection and characterization of MLV with altered neuropathology.
To isolate viruses that could cause an attenuated vascular disease, we selected for non-syncytium-forming TR1.3 variants by serial passage in vitro in SC-1 cells. After an initial period of high cytopathicity in culture, the appearance of syncytia diminished, and at 3 months of passage, no visible syncytia were present in the culture flasks. The continued presence of virus in these long-term cultures was verified by both reverse transcriptase assay and XC plaque assay. Subsequent to this process, viral DNA was cloned from both supernatant and cell lysates as described in Materials and Methods; the resultant molecular clone was termed TRM.
BALB/c neonatal mice were exposed to TRM (4 × 104 PFU) and monitored daily for clinical signs of disease to determine the in vivo pathogenicity. For comparison, age-matched litters were exposed to an identical infectious dose of parental TR1.3 or the closely related but nonpathogenic MLV FB29. As reported previously (35, 36), TR1.3-infected mice rapidly developed severe tremors and paralysis (day 10 onset), with death occurring by day 16, while animals infected with FB29 did not exhibit any symptoms of disease throughout the study period (90 days). In contrast to these observations, animals exposed to TRM displayed a form of disease that was attenuated in both temporal onset and severity of symptoms. As shown in Table 1, at the lowest concentration of TRM tested (4 × 104 PFU), clinical signs of disease onset were delayed until day 59. These symptoms included facial paralysis (uncontrollable clenching of one eye), gait disturbance, and diminished response in splay tests. Eventually, disease progressed to hind-limb paraparesis, hind-limb paralysis, and death (day 63). At higher concentrations of TRM (4 × 105 to 4 × 106 PFU), disease onset and death were more rapid; however, even at these doses, the transition of disease symptoms seen with TRM progressed more gradually than in TR1.3 infected mice.
TABLE 1.
Clinical disease in TRM-infected micea
| Virus | No. of Mice/Expt | Route of inoculation (titer [PFU]) | Range of onset of clinical symptoms (days) | Mean time to death (days) |
|---|---|---|---|---|
| TR1.3 | 8 | Intracranial (4 × 104) | 10-14 | 16 ± 1.5 |
| FB29 | 10 | Intracranial (4 × 106) | None detected | None detected |
| TRM | 7 | Intracranial (4 × 104) | 41-54 | 62.6 ± 6.9 |
| TRM | 4 | Intracranial (4 × 105) | 26-60 | 64.3 ± 5.8 |
| TRM | 10 | Intracranial (4 × 105) | 10-30 | 23.3 ± 10.9 |
| TRM | 8 | Intracranial (4 × 106) | 18-25 | 26.6 ± 6.5 |
| TRM | 4 | Intraperitoneal (4 × 106) | 15-26 | 64 ± 1.0 |
Mice were injected with 30 μl of TRM virus (4 × 104 to 4 × 106 PFU) within 48 h of birth. All injections were performed within the first 48 h after birth. Animal were monitored daily for clinical symptoms including facial paralysis, gait disturbance, and diminished response in splay tests.
Histological analysis of TRM pathology.
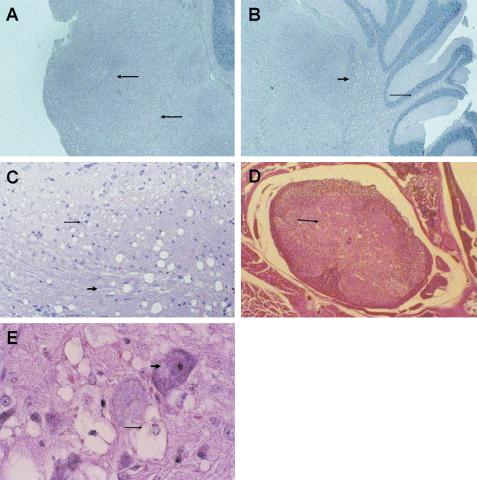
A detailed histological analysis of TRM-infected mice was conducted at various time points after inoculation to further characterize this neurological disease. Both gross and microscopic analysis of excised brain tissue failed to reveal evidence of vascular hemorrhage as routinely observed following TR1.3 infection (35, 37). Rather, as shown in Fig. 1, TRM-infected mice showed significant spongiform encephalopathy within the cerebellum, medulla, pons, and spinal cord. Spongiosis was most prevalent in the pons and medulla (Fig. 1A), and these regions were typically the first to show evidence of disease (day 18 postinfection). Within the cerebellum, these changes were evident around the cerebellar nuclei as well as in the molecular layer (Fig. 1B). Spongiform changes in the brain occurred within both gray and white matter tracts (Fig. 1C). Within the spinal cord, spongiform changes localized primarily to the gray matter of the anterior horns (Fig. 1D). These changes were evident throughout the spinal cord, with serial sections showing spongiform changes uninterrupted from cervical to sacral sections. Analysis under higher magnification demonstrated that neurons were the predominant cell type affected (Fig. 1E), with vacuolation of the cytoplasm, hyperchromatic picnotic nuclei, and nuclear condensation. None of the sections examined showed evidence of syncytium formation in endothelial cells or of any perturbation in vessel structures, which is characteristic of the parental virus TR1.3. There was no evidence of gross pathology in any tissues other than the CNS.
FIG. 1.
Spongiform encephalopathy in TRM-infected mice. Mice were inoculated with 3 × 105 PFU of TRM at birth, and tissue was extracted for analysis by hematoxylin- and eosin-stained sections (6 μm) fixed in 10% formalin at the indicated times. (A) Spongiform neurodegeneration in the pons and medulla, indicated by arrows, on day40 (magnification, ×8.5); (B) spongiosis in both the deep cerebral cortex (thick arrow) and the molecular layer (thin arrow) on day 40 (magnification, ×8.5); (C) brainstem revealing spongiform changes in both gray matter (thin arrow) and white matter (thick arrow) tracts on day 40 (magnification, ×17); (D) spinal cord with marked pathology in the anterior (thin arrow), but not ventral, horns on day 21 (magnification, ×8.5); (E) spinal cord with vacuolation of neuronal cytoplasm (thin arrow) and hyperchromatic and picnotic nuclei (thick arrow) on day 21 (magnification, ×34).
Cell tropism of TRM virus.
Immunohistochemical analysis was next performed to determine whether TRM virus infected BCEC, as seen with TR1.3 and/or other cell types. To identify infected cells, we conducted high-resolution confocal analysis on paraffin-embedded tissue from TRM-infected mice costained with the endothelial cell-specific marker factor VIII-related antigen (VWF) and the virus-specific marker gp70. As shown in Fig. 2, gp70 expression (green) colocalized primarily, although not exclusively, with VWF expression (red). The exception to this finding was the consistent observation of gp70 expression in a population of highly ramified cells that did not exhibit VWF antibody binding. These cells were located both within the brain tissue and in association with endothelial vessels and persisted throughout the course of disease progression (day 18 to 68). As reported in previous work, neither TR1.3 nor FB29 infection in cell types other than BCEC during this same time period has been observed (35, 37).
FIG. 2.
CNS cell tropism of TRM. Fixed thin sections of brain tissue from TRM-infected mice were analyzed for sites of infection by immunohistochemistry. Env and VWF expression in a paraffin section of brain tissue from a TRM-infected mouse on day 21 is shown (magnification, ×34). Virus Env expression appears in green, while VWF expression on endothelial cells appears in red. Colocalization of Env with VWF is indicated by the blue arrow, Env staining adjacent to endothelial cells is indicated by the purple arrow, and Env expression independent of endothelial cells is indicated with a white arrow.
Fusion potential of TRM.
Although histological analysis of TRM-infected mice did not reveal syncytium formation of endothelial cells or any other cell type, this does not eliminate the possibility that cell fusion might occur at a level below the threshold of detection by either light or fluorescence microscopy. To address this issue, we employed a sensitive in vitro cell-cell fusion assay that directly measures TRM Env fusion potential. Effector cells that express Env and T7 polymerase were cocultured with target cells that express the ecotropic receptor mCAT1 and a luciferase reporter gene under the control of a T7 polymerase promoter. Equivalent levels of Env expression on effectors was monitored by Western blot analysis. Cell fusion was measured following cocultivation of target and effector cells by luciferase activity. Results presented in Fig. 3 demonstrate that Env from syncytium-forming MLV, such as TR1.3 and the single-site mutant W102G (4), displayed enhanced fusion in this assay relative to the non-syncytium-forming MLV FB29. Most importantly for interpretation of the present analysis, the fusion potential of TRM Env was equivalent to that of FB29 and significantly below that of either TR1.3 or W102G. Although the background of cell fusion with FB29 and TRM Env was greater than that of Env negative controls, it has previously been shown that this result is a consequence of this assay (4).
FIG. 3.
Cell fusion potential of TRM Env. Env fusion potential was measured in an in vitro cell-cell fusion assay. Fusion is determined by luciferase activity (relative light units) after coculture of effector cells that express Env with target cells that express either the mCAT1 receptor protein (hatched bars) or no receptor (filled bars). The asterisk indicates statistically significant differences (P < 0.05) in fusion to a common receptor-bearing target, as determined by the unpaired Student's t test.
Mapping of TRM disease phenotype.
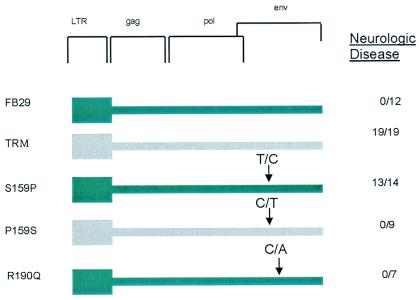
To identify the domain(s) of the viral genome that harbors the determinants of TRM neuropathogenicity, we mapped this disease phenotype in recombinant and point-mutated viruses by using restriction fragment exchange and DNA sequencing. As seen previously with the analysis of TR1.3, insertion of the full-length TRM env sequence into the background of the nonpathogenic virus FB29 was sufficient to confer the TRM disease phenotype. Nucleotide sequencing of TRM env revealed only a single predicted amino acid difference between TRM Env and the parental virus TR1.3 (Table 2): a conversion of glycine at position Env102 to tryptophan (G102W). As reported previously, the reciprocal conversion of tryptophan to glycine at Env102 (W102G) is the critical determinant of fusion in TR1.3 (38). Therefore, while it is not unexpected that the nonsyncytium phenotype of TRM be linked to the G102W conversion, the emergence of a spongiform disease phenotype indicates that other residues within TRM and TR1.3 must encode this disease.
TABLE 2.
Amino acid sequence comparison of FB29, TR1.3, and TRM MLV
| Position of sequence difference | Amino acid for MLV
|
||
|---|---|---|---|
| FB29 | TR1.3 | TRM | |
| 20 | Leucine | Isoleucine | Isoleucine |
| 80 | Valine | Isoleucine | Isoleucine |
| 102 | Tryptophan | Glycine | Tryptophan |
| 159 | Serine | Proline | Proline |
| 190 | Arginine | Glutamine | Glutamine |
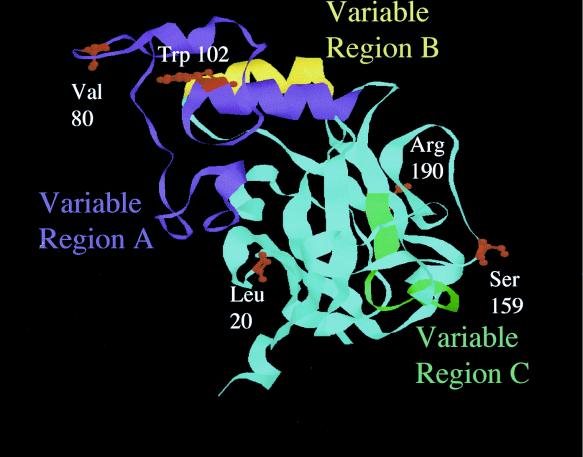
A comparison of env sequence between TRM and FB29 reveals four additional amino acid variations, L20I, V80I, S159P, and R190Q. The predicted locations of these mutations are shown in ball-and-stick form on the ribbon background of the MLV Friend 57 receptor binding domain crystal structure (Fig. 4). Amino acids L20I and V80I were previously introduced into the FB29 background in the analysis of TR1.3, with no evidence of spongiform disease (38). Accordingly, we separately introduced mutations S159P and R190Q into the FB29 background using site-directed mutagenesis. The results of this analysis, shown in Fig. 5, clearly showed that the conversion S159P, and not R190Q, was responsible for the TRM disease phenotype. This conclusion was confirmed by the loss of disease induced by TRM virus through the reciprocal conversion P159S. Histological analysis of S159P-infected mice also revealed spongiform changes in the brain stem and spinal cord tissues as seen with TRM. Although S159P was both necessary and sufficient for the disease phenotype, spongiform changes were less prevalent, and time to death was delayed compared to that of mice infected with an equivalent titer of TRM virus (61.0 ± 6 days for S159P versus 26.6 ± 6.5 days for TRM at 4 × 106 PFU). With the exception of W102G, none of the other single-site mutations showed evidence of syncytium formation in vivo or in vitro when inserted into the FB29 background (data not shown).
FIG. 4.
Identification of amino acid variations in MLV Env. The locations of differences in the amino acid sequences of TRM, TR1.3, and FB29 are highlighted on the ribbon model of Friend 57 Env receptor binding domain (9). Amino acids are numbered with the mature form of gp70 lacking a signal peptide.
FIG. 5.
Genetic mapping of TRM disease. TRM disease maps to Env159. The impact of individual TRM site mutants inserted into the backbone of FB29 on neurological disease was determined as described in Table 1. Arrows denote the site of nucleotide changes introduced to generate the mutation.
DISCUSSION
The causative mechanisms of neurological disease induced by human retroviruses remain poorly understood. MLV model systems provide important tools to dissect fundamental questions of CNS tropism, cell and tissue pathology, and the impact of the viral genome on disease type and course (54). A number of MLV strains have been utilized in this approach, and our laboratory has focused studies on the MLV TR1.3 (4, 35-38). Analysis of TR1.3 provides an excellent opportunity to study both the mechanism of retrovirus-induced syncytium formation in vivo and the consequences of acute vessel damage within the CNS (35); however, this infection triggers rapid disease progression that precludes analysis of the chronic effects of virus infection and, more specifically, of the role of the retroviral genome in regulating different forms of CNS disease. By attenuating TR1.3 through passage in cell culture we obtained a nonfusigenic variant (TRM) that induced slowly progressive spongiform neurodegeneration. This surprising discovery allowed us to map sequences that regulate acute vascular disease versus neuronal degeneration on a common genetic background. The non-syncytium-forming molecular clone TRM induced a noninflammatory spongiform encephalopathy that appears remarkably similar to that previously described for other neuropathogenic MLV (54).
The underlying molecular basis for neuronal degeneration, which is a hallmark of MLV pathology, remains undefined. Although there is scant evidence for direct infection of affected neurons, multiple other CNS cell types are infected by MLV (54). Thus, it is important to clarify whether infection of these cells triggers a common cascade of events that lead to neuronal degeneration or virus strain-specific inductive events. Several groups have shown that the incidence of neurological disease may be associated with alterations in the function of infected glial cells (23, 39, 43, 45). Microglial infection has been implicated in disease induced by CasBrE, FrCasE, and NT-40 MLV, whereas astrocyte infection has been suggested to play a role in disease induced by ts1 (12, 23, 26, 43-45). However, glial tropism is not itself sufficient to induce disease as the MLV F43 replicates in microglial cells without apparent pathology (1).
CNS pathology has also been directly linked to BCEC infection and/or alterations in vascular function during retrovirus infection. Infection of BCEC is an early uniform characteristic of MLV and is believed to be the primary entry portal to CNS infection (6, 24, 25, 40, 46). For MLV such as PVC-211 and TR1.3, where replication is observed only in BCEC, virus particles are readily detected budding from the abluminal BCEC surface and accumulate along the basal laminae (13, 37, 40). TR1.3 induces direct ultrastructural changes to BCEC that alter blood-brain barrier integrity (37), whereas with PVC-211, BCEC infection triggers transient reactive microglial response and subsequent oxidative damage that is hypothesized to effect neuronal integrity (15, 31, 32, 55). Despite these observations, as noted above with glial cells, BCEC tropism is not itself sufficient to induce disease, as the MLV FB29 replicates in mouse BCEC without evidence of pathology (35).
These results illustrate that factors other than cell tropism regulate the induction of CNS disease. There is a growing body of evidence suggesting that altered expression levels of virus or processing of MLV Env can have pathological effects across multiple cell types. For example, MLV PVC-211 displays an enhanced ability to replicate in BCEC, which correlates with pathogenicity (31). Similar findings with MLV A8 reiterate that high-level replication in the CNS is necessary, but not sufficient, for neurologic disease (50). Enhanced virus replication in the CNS can be achieved through greater replication within a particular cell type or expansion of the target cell population; PVC-211 achieves high levels of viremia through enhanced replication within endothelial cells, while NT-40 neurovirulence correlates with the ability to infect microglial cells in addition to BCEC.
The studies presented in this paper provide strong evidence that the pathogenic MLV TRM infects both BCEC and an unidentified glial cell population. Previous studies in our laboratory have used the BS-1 lectin to identify infected cell types in brain tissue from FB29 and TR1.3 MLV-infected mice. This marker has been reported to bind preferentially to both endothelial cells and microglial cells (19, 21, 47). This level of specificity was inadequate for the purposes of our present studies, and accordingly, we identified infected BCEC by double staining with gp70 and VWF antibodies. The results demonstrate that TRM, much like TR1.3 and FB 29, triggers widespread BCEC infection. However, these studies also showed TRM infection in a population of highly ramified cells that did not express VWF. In some instances, these cells were tightly associated with the endothelium; however, in other instances, these cells appeared distant from BCEC. Studies are currently under way to identify this infected cell type(s).
It has also been proposed that alterations in the processing of MLV Env may induce neurological disease. The most well-characterized example of this is Moloney ts1 MLV, where the single amino acid conversion of leucine to isoleucine at position Env 25 of wild-type Moloney MLV induces neuropathology (48, 49). This conversion affects the processing of the Env precursor protein, resulting in the accumulation of Env within the endoplasmic reticulum of infected astrocytes (45, 49). Envelope processing abnormalities have also been observed in microglial cells infected with FrCasE (8, 23). These cells express envelope protein, which appears by Western blot as a single species of approximately 90 kDa, suggesting that the signal sequence does not undergo appropriate cleavage in this cell type. Recent work has shown that the upregulation of gene transcripts associated with endoplasmic reticulum stress occurs in brain tissue of FrCasE-infected mice (8). This finding provides a potential link between protein misfolding and downstream toxic effects in that these could be dictated by specific Env sequences and/or could vary in vivo, depending on the infected cell type.
Our studies provide an analysis of the role of domains within the Env protein on CNS neuropathology. Through site-directed mutagenesis, we identified that the single amino acid conversion of serine to proline at Env159 regulates neuropathology of MLV TR. This mutation lies outside of the previously defined regions in Env known to be important for receptor binding as well as variable region A (VRA), VRB, and VRC (3). In addition to the Env25 mutation in Moloney ts1 mentioned above, other mapping studies have shown that mutations outside of the variable regions may regulate neurologic disease (17, 29, 31, 49, 50). PVC-211 contains two amino acid mutations critical for enhanced neuropathogenicity; one of these creates an additional heparin-binding domain which may facilitate enhanced replication in BCEC (14, 29, 31). Additional mutations dramatically accelerate the disease process (29, 31).
When the crystal structure of Friend 57 receptor binding domain is used as a model, Env159 appears to be a solvent exposed residue on the face opposite that of the putative receptor binding pocket. This residue lies in proximity to two amino acids that were previously shown to be crucial for neurovirulence in the polytropic MLV Fr98 (42). Thus, the comparison of TR1.3, TRM, and FB29 viruses illustrates that subtle changes in amino acid composition at disparate regions of a common MLV Env background can have a profound effect on induction, severity, and neurological disease phenotype. Whether the S159P conversion in TRM affects virus expression levels, Env processing, and/or other changes to the MLV life cycle, and how these changes might regulate the physiology of infected cells to cause neuronal degeneration, is the major focus of our continued studies.
REFERENCES
- 1.Askovic, S., F. J. McAtee, C. Favara, and J. L. Portis. 2000. Brain infection by neuroinvasive but avirulent murine oncornaviruses. J. Virol. 74:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barmak, K., E. Harhaj, C. Grant, T. Alefantis, and B. Wigdahl. 2003. Human T cell leukemia virus type I-induced disease: pathways to cancer and neurodegeneration. Virology 308:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Battini, J. L., O. Danos, and J. M. Heard. 1995. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 69:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung, M., K. Kizhatil, L. M. Albritton, and G. N. Gaulton. 1999. Induction of syncytia by neuropathogenic murine leukemia viruses depends on receptor density, host cell determinants, and the intrinsic fusion potential of envelope protein. J. Virol. 73:9377-9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czub, M., S. Czub, M. Rappold, S. Mazgareanu, S. Schwender, M. Demuth, A. Hein, and R. Dorries. 1995. Murine leukemia virus-induced neurodegeneration of rats: enhancement of neuropathogenicity correlates with enhanced viral tropism for macrophages, microglia, and brain vascular cells. Virology 214:239-244. [DOI] [PubMed] [Google Scholar]
- 6.Czub, S., W. P. Lynch, M. Czub, and J. L. Portis. 1994. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab. Investig. 70:711-723. [PubMed] [Google Scholar]
- 7.DesGroseillers, L., E. Rassart, Y. Robitaille, and P. Jolicoeur. 1985. Retrovirus-induced spongiform encephalopathy: the 3′-end long terminal repeat-containing viral sequences influence the incidence of the disease and the specificity of the neurological syndrome. Proc. Natl. Acad. Sci. USA 82:8818-8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimcheff, D. E., S. Askovic, A. H. Baker, C. Johnson-Fowler, and J. L. Portis. 2003. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 77:12617-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fass, D., R. A. Davey, C. A. Hamson, P. S. Kim, J. M. Cunningham, and J. M. Berger. 1997. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science 277:1662-1666. [DOI] [PubMed] [Google Scholar]
- 10.Gardner, M. B. 1978. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr. Top. Microbiol. Immunol. 79:215-259. [DOI] [PubMed] [Google Scholar]
- 11.Gardner, M. B., V. Klement, S. Rasheed, R. W. Rongey, J. C. Brown, M. Pike, B. E. Henderson, and R. J. Huebner. 1975. The pathogenesis of lymphoma and paralysis in wild mice. Bibl. Haematol. 43:204-208. [DOI] [PubMed]
- 12.Gravel, C., D. G. Kay, and P. Jolicoeur. 1993. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J. Virol. 67:6648-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman, P. M., E. F. Cimino, D. S. Robbins, R. D. Broadwell, J. M. Powers, and S. K. Ruscetti. 1992. Cellular tropism and localization in the rodent nervous system of a neuropathogenic variant of Friend murine leukemia virus. Lab. Investig. 67:314-321. [PubMed] [Google Scholar]
- 14.Jinno-Oue, A., M. Oue, and S. K. Ruscetti. 2001. A unique heparin-binding domain in the envelope protein of the neuropathogenic PVC-211 murine leukemia virus may contribute to its brain capillary endothelial cell tropism. J. Virol. 75:12439-12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinno-Oue, A., S. G. Wilt, C. Hanson, N. V. Dugger, P. M. Hoffman, M. Masuda, and S. K. Ruscetti. 2003. Expression of inducible nitric oxide synthase and elevation of tyrosine nitration of a 32-kilodalton cellular protein in brain capillary endothelial cells from rats infected with a neuropathogenic murine leukemia virus. J. Virol. 77:5145-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolicoeur, P. 1991. Neuronal loss in a lower motor neuron disease induced by a murine retrovirus. Can. J. Neurol. Sci. 18:411-413. [DOI] [PubMed] [Google Scholar]
- 17.Jolicoeur, P., E. Rassart, L. DesGroseillers, Y. Robitaille, Y. Paquette, and D. G. Kay. 1991. Retrovirus-induced motor neuron disease of mice: molecular basis of neurotropism and paralysis. Adv. Neurol. 56:481-493. [PubMed] [Google Scholar]
- 18.Kai, K., and T. Furuta. 1984. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J. Virol. 50:970-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kivela, T., and A. Tarkkanen. 1987. A lectin cytochemical study of glycoconjugates in the human retina. Cell Tissue Res. 249:277-288. [DOI] [PubMed] [Google Scholar]
- 20.Kolson, D. L., E. Lavi, and F. Gonzalez-Scarano. 1998. The effects of human immunodeficiency virus in the central nervous system. Adv. Virus Res. 50:1-47. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen, L. 1987. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem. J. 19:225-234. [DOI] [PubMed] [Google Scholar]
- 22.Lin, G., F. Baribaud, J. Romano, R. W. Doms, and J. A. Hoxie. 2003. Identification of gp120 binding sites on CXCR4 by using CD4-independent human immunodeficiency virus type 2 Env proteins. J. Virol. 77:931-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch, W. P., W. J. Brown, G. J. Spangrude, and J. L. Portis. 1994. Microglial infection by a neurovirulent murine retrovirus results in defective processing of envelope protein and intracellular budding of virus particles. J. Virol. 68:3401-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch, W. P., S. Czub, F. J. McAtee, S. F. Hayes, and J. L. Portis. 1991. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7:365-379. [DOI] [PubMed] [Google Scholar]
- 25.Lynch, W. P., and J. L. Portis. 1993. Murine retrovirus-induced spongiform encephalopathy: disease expression is dependent on postnatal development of the central nervous system. J. Virol. 67:2601-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch, W. P., S. J. Robertson, and J. L. Portis. 1995. Induction of focal spongiform neurodegeneration in developmentally resistant mice by implantation of murine retrovirus-infected microglia. J. Virol. 69:1408-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch, W. P., E. Y. Snyder, L. Qualtiere, J. L. Portis, and A. H. Sharpe. 1996. Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J. Virol. 70:8896-8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masliah, E., N. Ge, and L. Mucke. 1996. Pathogenesis of HIV-1 associated neurodegeneration. Crit. Rev. Neurobiol. 10:57-67. [DOI] [PubMed] [Google Scholar]
- 29.Masuda, M., C. A. Hanson, W. G. Alvord, P. M. Hoffman, and S. K. Ruscetti. 1996. Effects of subtle changes in the SU protein of ecotropic murine leukemia virus on its brain capillary endothelial cell tropism and interference properties. Virology 215:142-151. [DOI] [PubMed] [Google Scholar]
- 30.Masuda, M., C. A. Hanson, N. V. Dugger, D. S. Robbins, S. G. Wilt, S. K. Ruscetti, and P. M. Hoffman. 1997. Capillary endothelial cell tropism of PVC-211 murine leukemia virus and its application for gene transduction. J. Virol. 71:6168-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda, M., P. M. Hoffman, and S. K. Ruscetti. 1993. Viral determinants that control the neuropathogenicity of PVC-211 murine leukemia virus in vivo determine brain capillary endothelial cell tropism of the virus in vitro. J. Virol. 67:4580-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuda, M., S. K. Ruscetti, and P. M. Hoffman. 1997. Molecular mechanism for retroviral neuropathogenesis: possible involvement of capillary endothelial cells. Leukemia 11(Suppl. 3):233-235. [PubMed] [Google Scholar]
- 33.McCarter, J. A., J. K. Ball, and J. V. Frei. 1977. Lower limb paralysis induced in mice by a temperature-sensitive mutant of Moloney leukemia virus. J. Natl. Cancer Inst. 59:179-183. [DOI] [PubMed] [Google Scholar]
- 34.Oldstone, M. B., P. W. Lampert, S. Lee, and F. J. Dixon. 1977. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am. J. Pathol. 88:193-212. [PMC free article] [PubMed] [Google Scholar]
- 35.Park, B. H., E. Lavi, K. J. Blank, and G. N. Gaulton. 1993. Intracerebral hemorrhages and syncytium formation induced by endothelial cell infection with a murine leukemia virus. J. Virol. 67:6015-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, B. H., E. Lavi, and G. N. Gaulton. 1994. Intracerebral hemorrhages and infarction induced by a murine leukemia virus is influenced by host determinants within endothelial cells. Virology 203:393-396. [DOI] [PubMed] [Google Scholar]
- 37.Park, B. H., E. Lavi, A. Stieber, and G. N. Gaulton. 1994. Pathogenesis of cerebral infarction and hemorrhage induced by a murine leukemia virus. Lab. Investig. 70:78-85. [PubMed] [Google Scholar]
- 38.Park, B. H., B. Matuschke, E. Lavi, and G. N. Gaulton. 1994. A point mutation in the env gene of a murine leukemia virus induces syncytium formation and neurologic disease. J. Virol. 68:7516-7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson, K. E., S. J. Robertson, J. L. Portis, and B. Chesebro. 2001. Differences in cytokine and chemokine responses during neurological disease induced by polytropic murine retroviruses Map to separate regions of the viral envelope gene. J. Virol. 75:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitts, O. M., J. M. Powers, J. A. Bilello, and P. M. Hoffman. 1987. Ultrastructural changes associated with retroviral replication in central nervous system capillary endothelial cells. Lab. Investig. 56:401-409. [PubMed] [Google Scholar]
- 41.Portis, J. L., and W. P. Lynch. 1998. Dissecting the determinants of neuropathogenesis of the murine oncornaviruses. Virology 247:127-136. [DOI] [PubMed] [Google Scholar]
- 42.Poulsen, D. J., S. J. Robertson, C. A. Favara, J. L. Portis, and B. W. Chesebro. 1998. Mapping of a neurovirulence determinant within the envelope protein of a polytropic murine retrovirus: induction of central nervous system disease by low levels of virus. Virology 248:199-207. [DOI] [PubMed] [Google Scholar]
- 43.Robertson, S. J., K. J. Hasenkrug, B. Chesebro, and J. L. Portis. 1997. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J. Virol. 71:5287-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe, A. H., J. J. Hunter, P. Chassler, and R. Jaenisch. 1990. Role of abortive retroviral infection of neurons in spongiform CNS degeneration. Nature 346:181-183. [DOI] [PubMed] [Google Scholar]
- 45.Shikova, E., Y. C. Lin, K. Saha, B. R. Brooks, and P. K. Wong. 1993. Correlation of specific virus-astrocyte interactions and cytopathic effects induced by ts1, a neurovirulent mutant of Moloney murine leukemia virus. J. Virol. 67:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoica, G., O. Illanes, S. I. Tasca, and P. K. Wong. 1993. Temporal central and peripheral nervous system changes induced by a paralytogenic mutant of Moloney murine leukemia virus TB. Lab. Investig. 69:724-735. [PubMed] [Google Scholar]
- 47.Streit, W. J., and G. W. Kreutzberg. 1987. Lectin binding by resting and reactive microglia. J. Neurocytol. 16:249-260. [DOI] [PubMed] [Google Scholar]
- 48.Szurek, P. F., E. Floyd, P. H. Yuen, and P. K. Wong. 1990. Site-directed mutagenesis of the codon for Ile-25 in gPr80env alters the neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J. Virol. 64:5241-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szurek, P. F., P. H. Yuen, J. K. Ball, and P. K. Wong. 1990. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J. Virol. 64:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takase-Yoden, S., and R. Watanabe. 1997. Unique sequence and lesional tropism of a new variant of neuropathogenic Friend murine leukemia virus. Virology 233:411-422. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka, A., K. Saida, M. Andoh, K. Maeda, and K. Kai. 2000. At least four non-env factors that reside in the LTR, in the 5′-non-coding region, in gag and in part of pol affect neuropathogenicity of PVC-441 murine leukemia virus (MuLV). Virus Res. 69:17-30. [DOI] [PubMed] [Google Scholar]
- 52.Vitkovic, L., E. Stover, and S. H. Koslow. 1995. Animal models recapitulate aspects of HIV/CNS disease. AIDS Res. Hum. Retrovir. 11:753-759. [DOI] [PubMed] [Google Scholar]
- 53.Weiss, R. A. 2003. HIV and AIDS: looking ahead. Nat. Med. 9:887-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiley, C. A., and M. Gardner. 1993. The pathogenesis of murine retroviral infection of the central nervous system. Brain Pathol. 3:123-128. [DOI] [PubMed] [Google Scholar]
- 55.Wilt, S. G., N. V. Dugger, N. D. Hitt, and P. M. Hoffman. 2000. Evidence for oxidative damage in a murine leukemia virus-induced neurodegeneration. J. Neurosci. Res. 62:440-450. [DOI] [PubMed] [Google Scholar]
- 56.Wong, P. K., M. M. Soong, R. MacLeod, G. E. Gallick, and P. H. Yuen. 1983. A group of temperature-sensitive mutants of Moloney leukemia virus which is defective in cleavage of env precursor polypeptide in infected cells also induces hind-limb paralysis in newborn CFW/D mice. Virology 125:513-518. [DOI] [PubMed] [Google Scholar]