Abstract
Objective
To determine the acute predictors associated with the development of postconcussion syndrome (PCS) in children and adolescents after mild traumatic brain injury.
Design
Retrospective analysis of a prospective observational study.
Setting
Pediatric emergency department (ED) in a children’s hospital.
Participants
Four hundred six children and adolescents aged 5 to 18 years.
Main Exposure
Closed head trauma.
Main Outcome Measures
The Rivermead Post Concussion Symptoms Questionnaire administered 3 months after the injury.
Results
Of the patients presenting to the ED with mild traumatic brain injury, 29.3% developed PCS. The most frequent PCS symptom was headache. Predictors of PCS, while controlling for other factors, were being of adolescent age, headache on presentation to the ED, and admission to the hospital. Patients who developed PCS missed a mean (SD) of 7.4 (13.9) days of school.
Conclusions
Adolescents who have headache on ED presentation and require hospital admission at the ED encounter are at elevated risk for PCS after mild traumatic brain injury. Interventions to identify this population and begin early treatment may improve outcomes and reduce the burden of disease.
More than 473 000 annual emergency department (ED) visits occur for children with traumatic brain injuries (TBIs) in the United States, and more than 75% of these injuries are defined as mild (mTBI).1–3 Mild TBI is a complex patho-physiologic process that can occur after trauma to the head. Some children with mTBI develop a cluster of cognitive, physical, and emotional problems commonly referred to as postconcussion syndrome (PCS).4–8 The incidence of PCS depends on the diagnostic criteria used and the population studied. The presence of at least 3 symptoms at 3 months is required to meet the diagnostic criteria for PCS as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).9
No tool can reliably predict development of PCS at the time of injury. Current ED evaluation for children focuses on symptoms that may predict abnormalities on computed tomography (CT), but cranial CT reveals normal findings in most patients with mTBI.10 Predicting PCS with acute symptoms of TBI has not been clearly established.11,12 Early administration of injury-specific information8,13 and provision of postinjury coping strategies in adults14–16 and children8 have been shown to improve post-mTBI functioning. The objectives of this study were to determine the incidence of PCS at 3 months and to identify predictors of PCS after mTBI in a cohort of children and adolescents who presented to the ED after the initial injury. We hypothesized that risk factors for the development of PCS would help to stratify the need for ongoing follow-up and resources for this population of children and adolescents who present to the ED.
METHODS
PARTICIPANTS AND SETTINGS
We performed a secondary analysis of a larger prospective cohort of consecutive patients with TBI who participated in a TBI registry study funded by the National Institutes of Health.16 The original cohort was recruited at the pediatric and adult EDs of the University of Rochester Medical Center from January 7, 2003, through September 6, 2004. Patients were eligible if they met the case definition of mTBI developed by the Mild Traumatic Brain Injury Committee of the American Congress of Rehabilitation Medicine, that is, a blow to the head or acceleration/deceleration movement of the head resulting in 1 or more of the following: loss of consciousness (LOC) of less than 30 minutes, amnesia of less than 24 hours or any alteration in mental state, and a Glasgow Coma Scale score of 13 or more measured 30 minutes or more after injury.17 This study cohort was limited to patients who completed follow-up and to verbal children and adolescents (age range, 5 to 18 years). The research subject review board at the University of Rochester approved the parent study and all secondary analyses. The institutional review board at Cincinnati Children’s Hospital Medical Center exempted this secondary analysis from review.
STUDY DESIGN
Research assistants identified participants who were confirmed by the attending emergency physician. Research assistants collected clinically relevant information from the participants and/or a parent or guardian on a standardized data collection form before ED discharge. Data included demographic factors (race, ethnicity, age, insurance information, and sex), history of TBI requiring an ED visit or associated with LOC, mechanism of injury, clinical signs and symptoms of TBI (LOC, amnesia, alteration of mental status, nausea/vomiting, and headache), physical examination factors (Glasgow Coma Scale score), results of neuroimaging (if performed), ED medication administration, receipt of injury-specific discharge instructions, referrals, and disposition. Age was further dichotomized into school-aged children (5–10 years) and adolescents (11–18 years). Severe mechanism of injury was extrapolated from the definition used in the neuroimaging prediction rule by Kuppermann et al.10,17 We defined this to include any of the following: a motor vehicle collision, a pedestrian struck by a motor vehicle, a bicyclist without a helmet, or a fall of more than 3 feet. Other diagnoses were determined by a post hoc review of billing records for non-TBI codes from the International Classification of Disease, Ninth Revision. An abnormal finding on cranial CT was defined by the presence of any intracranial injury and the presence of skull fractures.
Three months after the initial visit, participants or parents or guardians were interviewed by telephone. The following information was collected: number of days of school missed owing to the TBI, PCS symptom score using the Rivermead Post Concussion Symptoms Questionnaire (RPQ), and whether they were in the process of a lawsuit regarding the injury. Interviewers were blinded to the details of the initial ED presentation. The RPQ is used to assess common PCS symptoms in patients of all ages.18–20 Symptoms assessed include headaches, dizziness, nausea, noise sensitivity, sleep disturbance, fatigue, irritability, depression, frustration, poor memory, poor concentration, taking longer to think, blurred vision, light sensitivity, double vision, and restlessness. Participants or their parents or guardians rated the severity of each of the 16 symptoms during the past 24 hours compared with before the injury on a scale from 0 to 4, where 0 indicates absent; 1, the same; 2, mild; 3, moderate; and 4, severe. We defined PCS as the presence of 3 or more symptoms on the RPQ that were rated as worse (score of ≥2) than before the injury. This information was extrapolated from the diagnostic criteria for PCS set out by the DSM-IV, which requires the presence of at least 3 symptoms at 3 months after the injury.
STATISTICAL ANALYSIS
We compared variables between patients who completed the follow-up and those who were unavailable for follow-up by using χ2 tests for categorical data and unpaired 2-tailed t tests for continuous data. Frequencies of all the variables were computed for all those who completed follow-up and by the development of PCS. For all those completing follow-up, bivariate analysis using relative risk ratios for development of PCS was calculated for the independent variables, including demographic characteristics, symptoms, and treatment.
We developed a multivariable logistic regression model to identify significant predictors of PCS. Potential predictors were variables that could be observed during the ED visit and were included on the basis of clinical relevance, availability in the ED, and prior published work. The potential predictors included demographic characteristics and symptoms (ie, Glasgow Coma Scale score, LOC, headache, amnesia, nausea/vomiting, other mental status changes, CT results, history of TBI requiring an ED visit, and severe mechanism of injury). The person who completed the follow-up questions (a parent or guardian or the patient) and treatment variables, including analgesics administered in the ED, hospital admission, and discharge instructions, were included as potential confounders. We explored potential multicollinearity among the independent variables using a stepwise approach, examining changes in significance and odds ratios and exploring significant associations among the independent variables. Model goodness of fit was ascertained using the Hosmer-Lemeshow test.21 The C statistic (area under the receiver operating characteristic curve) was used to evaluate the predictive ability of the model. We used an a priori significance level of .05 for all statistical tests. Unless otherwise indicated, data are expressed as mean (SD). Data analysis was performed using commercially available software (SAS, version 9.22; SAS Institute Inc).
RESULTS
A total of 547 patients 5 years or older met criteria for enrollment and were approached during the initial study, of whom 66 (12.1%) refused participation, resulting in a total of 481 enrolled. Four hundred six children and adolescents (84.4%) completed the 3-month follow-up. Those who completed follow-up were more likely to be white (84.5% vs 54.7%; P<.001), to be non-Hispanic (94.1% vs 82.9%; P=.001), and to have private insurance (72.9% vs 44.0%; P<.001) compared with those who did not complete follow-up. Those who completed follow-up also presented less frequently with LOC (53.0% vs 69.3% yes or unsure; P=.009) but were more likely to have received specific mTBI discharge instructions (63.1% vs 50.0%; P=.04). No other significant differences were detected in the other clinically relevant variables captured in the ED.
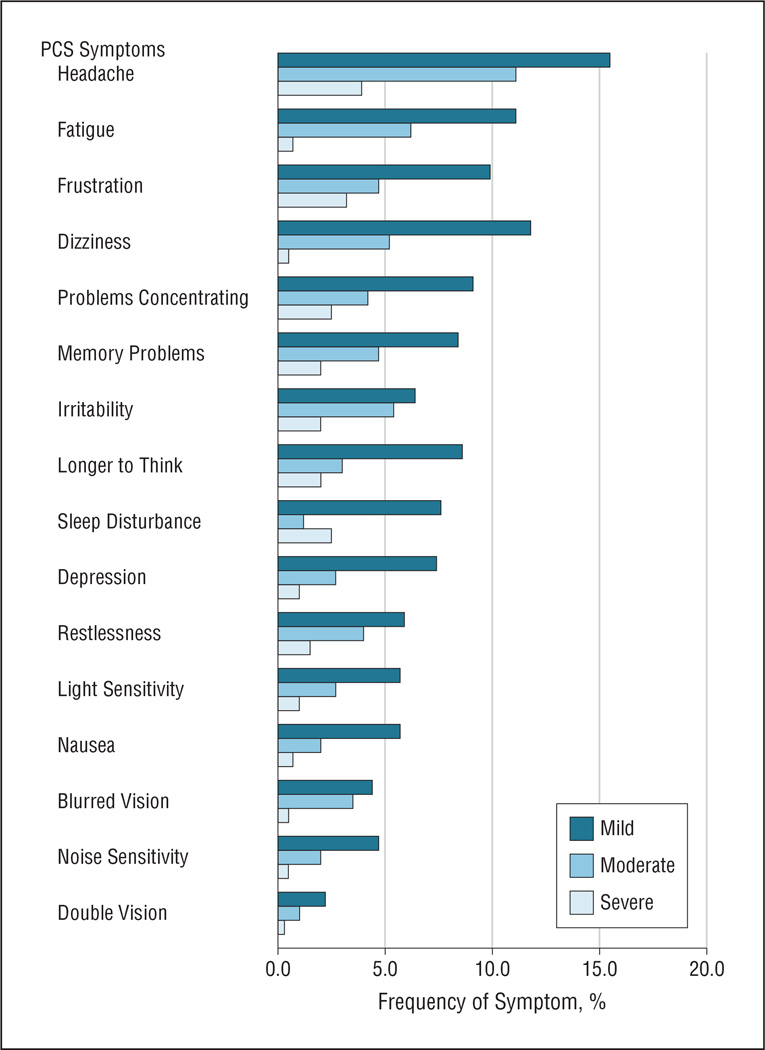
Of the 406 follow-up interviews, 145 (35.7%) were completed by the patient, whereas 261 (64.3%) were completed by a parent or guardian. Patients who self-completed the interview tended to be older than those whose parent or guardian did so (mean age, 14.8 [2.6] vs 12.3 [3.6] years; P<.001). For all patients who completed the 3-month follow-up, the mean score on the RPQ was 6.83 (9.78), with a median of 2.0 (range, 0–56). These data were skewed, with 34.2% of the patients having a score of 0. Headache was the most common symptom after the injury noted on the RPQ, reported by 30.5%. The frequency and severity of each PCS symptom are shown in the Figure.
Figure.
Frequency and severity of postconcussion syndrome (PCS) symptoms for all children and adolescents with mild traumatic brain injury who completed follow-up (n=406).
A total of 119 patients (29.3%) had PCS. The mean RPQ scores were significantly different between those who did and did not have PCS (18.0 [10.9] vs 2.2 [3.6]; P<.001). Table 1 provides descriptive statistics for each independent variable for the entire group and for the 2 groups of patients stratified by the development of PCS. For those who completed follow-up, a sport was the most common individual mechanism of injury (35.5%), followed by a motor vehicle collision (20.9%), a fall (16.5%), a cycling fall or crash (8.6%), assault (6.9%), other (6.9%), a pedestrian struck by a vehicle (2.5%), and a motorcycle crash (2.2%).
Table 1.
Characteristics of the Children and Adolescents Who Completed Follow-up and by Presence of PCS After mTBIa
| Characteristic | Completed Follow-up (n = 406) |
PCS (n = 119) |
No PCS (n = 287) |
|---|---|---|---|
| Demographic Variables | |||
| Age | |||
| Adolescents (11–18 y) | 79.6 | 87.4 | 76.3 |
| Mean (SD), y | 13.2 (3.5) | 14.1 (3.2) | 12.8 (3.6) |
| Median (range), y | 14.1 (5.0–18.0) | 14.9 (5.5–18.0) | 13.6 (5.9–18.0) |
| Male sex | 61.2 | 52.9 | 64.7 |
| Race/ethnicity | |||
| White | 84.5 | 82.4 | 85.4 |
| Non-Hispanic | 94.1 | 93.8 | 94.2 |
| Public insurance/self-pay | 27.1 | 32.8 | 24.7 |
| Prior TBI | 29.7 | 29.4 | 29.8 |
| Severe MOI | 39.2 | 45.4 | 36.6 |
| Arrival by EMS | 53.2 | 56.9 | 51.6 |
| Other diagnoses | 68.7 | 73.1 | 66.9 |
| ED Variables | |||
| GCS score <15 | 4.9 | 6.7 | 4.2 |
| LOC | 53.0 | 58.8 | 50.5 |
| Headache | 70.6 | 83.2 | 65.4 |
| Amnesia | 49.4 | 49.6 | 49.3 |
| Nausea/vomiting | 37.3 | 43.7 | 34.6 |
| Other MS changes | 91.9 | 91.6 | 92.0 |
| CT in ED | 54.2 | 63.0 | 50.5 |
| Abnormal CT finding | 3.5 | 3.4 | 3.5 |
| Analgesics administered in ED | 55.4 | 65.6 | 51.2 |
| Discharge instructions for mTBI | 63.1 | 57.9 | 65.2 |
| Hospital admission | 10.4 | 17.8 | 7.4 |
| 3-mo Follow-up Variables | |||
| Self-interview | 35.7 | 44.5 | 32.1 |
| Analgesics administered at home | 56.9 | 70.6 | 51.2 |
| Days of school missed >2 | 28.9 | 44.3 | 22.5 |
| Mean (SD) [range] | 3.8 (9.0) [0–75] | 7.4 (13.9) [0–75] | 2.2 (5.2) [0–50] |
| In process of/have intent to sue | 9.6 | 19.3 | 5.6 |
Abbreviations: CT, computed tomography; ED, emergency department; EMS, emergency medical services; GCS, Glasgow Coma Scale; LOC, loss of consciousness; MOI, mechanism of injury; MS, mental status; mTBI, mild traumatic brain injury; PCS, postconcussion syndrome.
Unless otherwise indicated, data are expressed as percentage of patients.
Table 2 provides the relative risk of developing PCS for each independent variable. Patients at risk of developing PCS were more likely to be adolescents, to be female, to present with headache in the ED, to have had cranial CT performed in the ED, to have received analgesics in the ED, to be admitted to the hospital for further treatment, and to be in the process or to have the intent of suing. All patients experienced considerable school absenteeism after the TBI, but those who developed PCS missed a mean of 7.4 (13.9) days. Patients who missed more than 2 days of school were at increased risk of developing PCS compared with those who missed fewer days. Because age was associated with the increased risk of developing PCS, additional exploratory bivariate analysis was performed for each age group. For patients aged 5 to 10 years, only those who were admitted (relative risk, 3.32 [95% CI, 1.37–8.01]) and in the process or with the intent of suing (4.94 [2.29–10.66]) had an increased risk of developing PCS. For adolescents, those at risk of developing PCS were more likely to be female (relative risk, 0.68 for being male [95% CI, 0.49–0.92]), to have sustained a severe mechanism of injury (1.38 [1.01–1.88]), to present with headache in the ED (2.33 [1.43–3.80]), to receive analgesics in the ED (1.47 [1.04–2.08]), to be admitted (1.67 [1.15–2.45]), to receive analgesics at home (1.90 [1.31–2.76]), to miss more than 2 days of school (1.89 [1.39–2.58]), and to be in process or to have the intent of suing (1.96 [1.40–2.75]). All other variables in the bivariate analysis for the age groups were not significant.
Table 2.
Risk for PCS in Children and Adolescents After mTBI Who Presented to the ED
| Characteristic | RR (95% CI) |
|---|---|
| Demographic Variables | |
| Age, 11–18 vs 5–10 y | 1.78 (1.10–2.89) |
| Sex, male vs female | 0.71 (0.53–0.96) |
| Race/ethnicity | |
| White vs nonwhite | 0.86 (0.58–1.26) |
| Non-Hispanic vs Hispanic | 0.95 (0.50–1.79) |
| Insurance, public/self-pay vs private | 1.31 (0.96–1.80) |
| Prior TBI, yes vs no | 0.99 (0.71–1.37) |
| Severe MOI, yes vs no | 1.29 (0.96–1.74) |
| Mode of arrival, EMS vs walk-in | 1.16 (0.85–1.59) |
| Other diagnoses | 1.24 (0.88–1.75) |
| ED Variables | |
| GCS scale <15, yes vs no | 1.39 (0.80–2.43) |
| LOC, yes vs no | 1.27 (0.93–1.73) |
| Headache, yes vs no | 2.06 (1.34–3.17) |
| Amnesia, yes vs no | 1.01 (0.75–1.36) |
| Nausea/vomiting, yes vs no | 1.31 (0.97–1.76) |
| Other MS changes, yes vs no | 0.97 (0.56–1.66) |
| CT in ED, performed vs not | 1.44 (1.05–1.98) |
| CT finding, abnormal vs normal or not performed | 0.97 (0.42–2.26) |
| Analgesics administered in ED, yes vs no | 1.53 (1.11–2.11) |
| Discharge instructions for mTBI, yes vs no | 0.80 (0.59–1.10) |
| Disposition, admitted vs discharged | 1.86 (1.32–2.63) |
| 3-mo Follow-up Variables | |
| Interviewee, self vs parent/guardian | 1.45 (1.07–1.95) |
| Analgesics administered at home, yes vs no | 1.82 (1.29–2.56) |
| Days of school missed, >2 vs ≥2 | 1.96 (1.46–2.64) |
| In process of/have intent to sue, yes vs no | 2.25 (1.65–3.08) |
Abbreviations: RR, relative risk. For other abbreviations, see Table 1.
A total of 402 participants (99.0%) had complete data for inclusion in the multivariable logistic regression model. Significant acute predictors for PCS were adolescent age (odds ratio, 2.00 [95% CI, 1.07–3.73]), presence of headache (2.63 [1.52–4.57]), and admission to the hospital (2.90 [1.48–5.68]). Although girls were at increased risk of developing PCS in the bivariate analysis, sex was not significant in the multivariable model. This finding could be explained, in part, because sex is significantly associated with a number of variables included in the model, such as prior TBI, severe mechanism of injury, and receiving analgesics in the ED. The multivariable model demonstrated goodness of fit as assessed by the Hosmer-Lemeshow test (P = .43) and moderate ability to predict PCS (C statistic, 0.66).
COMMENT
In this study, 29.3% of children and adolescents with mTBI who presented to the ED developed PCS, and those patients missed an average of more than 1 week of school as a result of their injury and the associated sequelae. Given the number of children and adolescents with mTBI3,22,23 who present to the ED and the high incidence of PCS in this population, the resultant school absenteeism implies that mTBI in this population imposes a significant public health burden. We developed a robust model identifying key variables at the initial ED visit that predict the development of PCS, including adolescent age, presence of headache, and admission to the hospital after the ED evaluation. Awareness of these factors can help the ED clinician recognize pediatric patients who are at elevated risk for persistent PCS and can deliver individualized post-mTBI care, such as symptom management, instructions for a stepwise return to activity, and arrangements for follow-up care. This tailored approach can potentially reduce the burden of this disease.8
Studies have shown a high incidence of symptoms immediately after injury that improve over time but still leave 11% to 17% of patients with impairment at 3 months.18,19,24 Our finding of a 29.3% incidence of PCS after ED presentation for mTBI is higher than what has been previously reported. Strict entrance criteria, presentation at an ED associated with a regional level I trauma center or pediatric tertiary referral center, and inclusion of patients (3.5%) with neuroimaging abnormalities may have contributed to the selection of children and adolescents with more severe initial symptoms that may lead to a higher symptom burden after injury. Despite these limitations and differences, our study supports the notion that a significant proportion of children and adolescents who sustain mTBI and present to the ED develop short-term sequelae.
Early identification of patients who will develop sequelae would assist in postinjury discharge planning in the ED. Although other factors thought to contribute to PCS, such as prior TBI, the mechanism of injury, LOC, nausea, amnesia, other mental status changes, and neuroimaging abnormalities, were not found to be associated with development of PCS in our study, we cannot infer that they are unrelated to the development of PCS.11,24–28 Our findings suggest that these factors do not contribute to the development of PCS after taking other factors into consideration. Controversy exists around the validity of the subjective symptoms within the definition of PCS and the notion of malingering for secondary gain. As was found in adults who sustained a TBI,11 the intent to sue was strongly associated with the development of PCS. For younger patients with mTBI, this association has not been previously reported.
The presence of headache in the ED was significantly related to the development of PCS. Headache was also the most frequently (30.5%) endorsed symptom at 3 months and was associated with risk of PCS for adolescents. Similarly, Blume et al29 found that 43% of children with mTBI reported having headaches at 3 months, and adolescents were particularly affected by headaches. As such, discharge instructions need to give specific guidance about the management of headache and about active interventions, such as the use of analgesics, activity restrictions, biofeedback, and other coping strategies. Simply reviewing discharge instructions detailing the symptoms and course of PCS has been shown to decrease the incidence and severity of symptoms.8 In addition to routine patient management, our finding of a mean of 7.4 days of school missed for more than one-quarter of the children and adolescents who present to the ED with mTBI underscores the need to explore the role of schools in the rehabilitation of students with mTBI.
Age at the time of injury may affect recovery for pediatric patients after mTBI.30 Adolescents in our sample were at greater risk of developing PCS than were younger children. Adolescents with PCS were more likely to be involved in severe mechanisms of injury associated with greater kinetic energies that may have resulted in brain injury on a cellular level. Adolescents may be able to better articulate their symptoms than younger children and tend to rate their symptoms as more severe than do their parents; thus, their responses on surveys may be more accurate than those completed by younger children or by parents.
In our cohort, only 10.4% of patients were admitted, but this percentage was independently associated with PCS. Similarly, Taylor et al27 found that hospital admission was associated with increased levels of PCS in a cohort of 186 children. Multiple factors enter the decision for admission and are related not only to the severity of symptoms but to concomitant injuries or illnesses, time of day, parental preference, and physician practice. Increased personal and family stress associated with admission to the hospital may contribute to the severity of PCS symptoms.31,32
Although we selected a large cohort of children and adolescents by using a rigorous definition of mTBI with a high participation rate at the 3-month follow-up, several constraints are imposed by the initial study design that limit the conclusions drawn from this secondary analysis. Some variables of interest at the time of ED presentation were not recorded prospectively (eg, other signs and symptoms associated with TBI and PCS and other diagnoses), and others were not recorded in a manner that would be clinically useful (severity indexes of presenting symptoms). Further limitations included the lack of inclusion of contributors to postinjury functioning, such as personal and family factors. The lack of premorbid data and a control comparison group does not allow for control of these factors. The calculation of severity of PCS with only 1 tool and at 1 time point does not allow for assessment of other neurocognitive domains affected by mTBI and does not further the understanding of the trajectory of recovery. Despite the use of the RPQ by other investigators for children and over the telephone, the instrument has not been validated in this population or for the definition of PCS used in this study.18–20 Recall bias of severity of symptoms before injury may have affected scoring on the RPQ. The variation in the person completing the interview may have also affected symptom recall. Last, considerable time has lapsed since initial data collection; however, similar data elements and procedures are conducted in present studies.
Symptoms at the time of injury presentation in the ED may not accurately predict all children and adolescents who develop PCS later, but they do provide a heuristic for identifying patients at elevated risk. Increasing resources directed at identifying children and adolescents with mTBI will ensure accurate incidence rates of the initial mTBI and help clarify the incidence and predictors of disability after injury. Variables found to accurately predict pediatric patients at risk for clinically significant intracranial injury need to be tested to assess their ability to predict the risk for PCS. Other objective testing at the time of injury, such as measures of reaction time, balance, and visual motor coordination, may be necessary adjuncts to routine clinical assessment in these patients.
Acknowledgments
Funding/Support: This study was supported in part by Career Development Award K23 NS41952-02 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (Dr Bazarian); by the KL2 Mentored Career Development Program in Clinical and Translational Research from the University of Cincinnati Center for Clinical and Translational Science and Training (Dr Babcock); and by the Division of Emergency Medicine at Cincinnati Children’s Hospital Medical Center.
For this work, Dr Babcock was awarded the Scholars Abstract Award at the 2011 Clinical and Translational Research and Education Annual Meeting. This study fulfilled Dr Babcock’s thesis requirements for a Master of Sciences in Clinical and Translational Science at the University of Cincinnati.
Footnotes
Author Contributions: All the authors are responsible for the reported research. Study concept and design: Babcock and Bazarian. Acquisition of data: Babcock, Mookerjee, and Bazarian. Analysis and interpretation of data: Babcock, Byczkowski, Wade, Ho, and Bazarian. Drafting of the manuscript: Babcock, Byczkowski, Wade, and Mookerjee. Critical revision of the manuscript for important intellectual content: Babcock, Wade, Ho, and Bazarian. Statistical analysis: Babcock, Byczkowski, and Ho. Obtain funding: Babcock and Bazarian. Administrative, technical, and material support: Babcock, Wade, and Mookerjee. Study supervision: Babcock, Wade, and Bazarian.
Paul Succop, PhD, Department of Environmental Health, provided statistical guidance and critique of the manuscript. Richard Ruddy, MD, Division of Emergency Medicine, Cincinnati Children’s Hospital Medical Center, provided mentorship and comments on the manuscript.
Conflict of Interest Disclosures: None reported.
Previous Presentations: Portions of this study were presented in abstract form at the Annual Meeting of the Pediatric Academic Societies; April 30, 2011; Denver, Colorado; the Annual Meeting of the Clinical and Translational Research and Education; April 28, 2011; Washington, DC; and the Annual Meeting of the Society of Academic Emergency Medicine; June 4, 2011; Boston, Massachusetts.
REFERENCES
- 1.Jager TE, Weiss HB, Coben JH, Pepe PE. Traumatic brain injuries evaluated in US emergency departments, 1992–1994. Acad Emerg Med. 2000;7(2):134–140. doi: 10.1111/j.1553-2712.2000.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Quality Improvement American Academy of Pediatrics. Commission on Clinical Policies and Research American Academy of Family Physicians. The management of minor closed head injury in children. Pediatrics. 1999;104(6):1407–1415. [PubMed] [Google Scholar]
- 3.Koepsell TD, Rivara FP, Vavilala MS, et al. Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics. 2011;128(5):946–954. doi: 10.1542/peds.2010-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc. 1997;3(6):581–591. [PubMed] [Google Scholar]
- 5.Fay GC, Jaffe KM, Polissar NL, et al. Mild pediatric traumatic brain injury: a cohort study. Arch Phys Med Rehabil. 1993;74(9):895–901. [PubMed] [Google Scholar]
- 6.Hooper SR, Alexander J, Moore D, et al. Caregiver reports of common symptoms in children following a traumatic brain injury. NeuroRehabilitation. 2004;19(3):175–189. [PubMed] [Google Scholar]
- 7.Beers SR, Berger RP, Adelson PD. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma. 2007;24(1):97–105. doi: 10.1089/neu.2006.0055. [DOI] [PubMed] [Google Scholar]
- 8.Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–1303. doi: 10.1542/peds.108.6.1297. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Kuppermann N, Holmes JF, Dayan PS, et al. Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinicallyimportant brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160–1170. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 11.Bazarian JJ, Wong T, Harris M, Leahey N, Mookerjee S, Dombovy M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999;13(3):173–189. doi: 10.1080/026990599121692. [DOI] [PubMed] [Google Scholar]
- 12.Savola O, Hillbom M. Early predictors of post-concussion symptoms in patients with mild head injury. Eur J Neurol. 2003;10(2):175–181. doi: 10.1046/j.1468-1331.2003.00552.x. [DOI] [PubMed] [Google Scholar]
- 13.Ponsford J. Rehabilitation interventions after mild head injury. Curr Opin Neurol. 2005;18(6):692–697. doi: 10.1097/01.wco.0000186840.61431.44. [DOI] [PubMed] [Google Scholar]
- 14.Mittenberg W, Tremont G, Zielinski RE, Fichera S, Rayls KR. Cognitive-behavioral prevention of postconcussion syndrome. Arch Clin Neuropsychol. 1996;11(2):139–145. [PubMed] [Google Scholar]
- 15.Wade DT, Crawford S, Wenden FJ, King NS, Moss NE. Does routine follow up after head injury help? a randomised controlled trial. J Neurol Neurosurg Psychiatry. 1997;62(5):478–484. doi: 10.1136/jnnp.62.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade DT, King NS, Wenden FJ, Crawford S, Caldwell FE. Routine follow up after head injury: a second randomised controlled trial. J Neurol Neurosurg Psychiatry. 1998;65(2):177–183. doi: 10.1136/jnnp.65.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nigrovic LE, Lee LK, Hoyle J, et al. Traumatic Brain Injury (TBI) Working Group of Pediatric Emergency Care Applied Research Network (PECARN). Prevalence of clinically important traumatic brain injuries in children with minor blunt head trauma and isolated severe injury mechanisms. Arch Pediatr Adolesc Med. 2012;166(4):356–361. doi: 10.1001/archpediatrics.2011.1156. [DOI] [PubMed] [Google Scholar]
- 18.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 19.Gioia GA, Schneider JC, Vaughan CG, Isquith PK. Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br J Sports Med. 2009;43(suppl 1):i13–i22. doi: 10.1136/bjsm.2009.058255. [DOI] [PubMed] [Google Scholar]
- 20.Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374–e381. doi: 10.1542/peds.2009-0925. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied Logistic Regression. Hoboken, NJ: John Wiley & Sons; 2000. [Google Scholar]
- 22.Faul M, Xu L, Wald MW, Coronado VG. [Accessed July 12, 2012];Traumatic Brain Injury in the US: Emergency Department Visits, Hospitalizations, and Deaths 2002–2006. 2010 http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf.
- 23.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Yeates KO, Kaizar E, Rusin J, et al. Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch Pediatr Adolesc Med. 2012;166(7):615–622. doi: 10.1001/archpediatrics.2011.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson VA, Morse SA, Klug G, et al. Predicting recovery from head injury in young children: a prospective analysis. J Int Neuropsychol Soc. 1997;3(6):568–580. [PubMed] [Google Scholar]
- 26.Yeates KO, Taylor HG, Rusin J, et al. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics. 2009;123(3):735–743. doi: 10.1542/peds.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor HG, Dietrich A, Nuss K, et al. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology. 2010;24(2):148–159. doi: 10.1037/a0018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311–2318. doi: 10.1177/0363546511410655. [DOI] [PubMed] [Google Scholar]
- 29.Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31–e39. doi: 10.1542/peds.2011-1742. [DOI] [PubMed] [Google Scholar]
- 30.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? a comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 31.Aitken ME, McCarthy ML, Slomine BS, et al. CHAT Study Group. Family burden after traumatic brain injury in children. Pediatrics. 2009;123(1):199–206. doi: 10.1542/peds.2008-0607. [DOI] [PubMed] [Google Scholar]
- 32.Ganesalingam K, Yeates KO, Ginn MS, et al. Family burden and parental distress following mild traumatic brain injury in children and its relationship to postconcussive symptoms. J Pediatr Psychol. 2008;33(6):621–629. doi: 10.1093/jpepsy/jsm133. [DOI] [PMC free article] [PubMed] [Google Scholar]