Abstract
Recombinant Listeria monocytogenes has many attractive characteristics as a vaccine vector against human immunodeficiency virus (HIV). Wild-type and attenuated Listeria strains expressing HIV Gag have been shown to induce long-lived mucosal and systemic T-cell responses in mice. Using the feline immunodeficiency virus (FIV) model of HIV we evaluated recombinant L. monocytogenes in a challenge system. Five cats were immunized with recombinant L. monocytogenes that expresses the FIV Gag and delivers an FIV Env-expressing DNA vaccine (LMgag/pND14-Lc-env). Control cats were either sham immunized or immunized with wild-type L. monocytogenes (LM-wt). At 1 year after vaginal challenge, provirus could not be detected in any of the nine tissues evaluated from cats immunized with the recombinant bacteria but was detected in at least one tissue in 8 of 10 control animals. Virus was isolated from bone marrow of four of five LMgag/pND14-Lc-env-immunized cats by use of a stringent coculture system but required CD8+ T-cell depletion, indicating CD8+ T-cell suppression of virus replication. Control animals had an inverted CD4:CD8 ratio in mesenteric lymph node and were depleted of both CD4+ and CD8+ intestinal epithelial T cells, while LMgag/pND14-Lc-env-immunized animals showed no such abnormalities. Vaginal FIV-specific immunoglobulin A was present at high titer in three LMgag/pND14-Lc-env-immunized cats before challenge and in all five at 1 year postchallenge. This study demonstrates that recombinant L. monocytogenes conferred some control of viral load after vaginal challenge with FIV.
Because natural transmission of human immunodeficiency virus (HIV) occurs at the mucosa and because mucosa-associated lymphoid tissues may be the earliest site for virus replication (31, 74), successful induction of robust mucosal immunity may require vaccination by a mucosal route. Live vaccines tend to be more immunogenic than killed or subunit vaccines and are generally more effective because of the antigen load amplification by the replicating agent. Given the safety concerns regarding live attenuated HIV vaccines, vectored immunization strategies are under intense investigation as a means to deliver an adequately immunogenic antigen load. Many viral and bacterial vectors are being evaluated for use in HIV vaccine strategies, and each has unique benefits and drawbacks (76). Listeria monocytogenes has received relatively little attention as an HIV vaccine vector but possesses many characteristics thought to be necessary for a successful HIV vaccine strategy.
L. monocytogenes is a gram-positive, facultative intracellular bacterium that escapes the phagolysosome of host target cells, replicates in the cytosol, and efficiently delivers antigens to the major histocompatibility complex class I presentation pathway (54, 59, 67). The host response to L. monocytogenes has been studied for more than 40 years and is considered the paradigm for cell-mediated immunity induction (42). The natural route of infection by L. monocytogenes is at the mucosa upon oral ingestion, and targeted cells during infection include dendritic cells and macrophages (29, 38, 52, 55, 60). L. monocytogenes infection activates the innate immune system, induces a type 1 cytokine profile, and is cleared by a characteristically strong cytotoxic T lymphocyte (CTL) response (33, 50). As a vector, foreign genes can be stably inserted into the L. monocytogenes genome and express at high levels into the cytoplasm of host cells for processing and presentation by the major histocompatibility complex class I pathway (27). L. monocytogenes is also an effective delivery vehicle for plasmids designed for eukaryotic expression of immunogens (21, 32). With regard to safety, several strategies have been employed to restrict but not eliminate replication of L. monocytogenes, thereby allowing amplification of the antigenic load and induction of an immune response at the mucosa as well as systemically (25, 40). L. monocytogenes also has the advantage of being highly susceptible to many inexpensive and readily available antibiotics in the case of an adverse response to vaccination. Thus, L. monocytogenes can be delivered orally, infects antigen-presenting cells, and stimulates strong cell-mediated immunity. In addition, L. monocytogenes is easily engineered to express antigens of interest, is inexpensive to produce, and can be attenuated and controlled.
Recombinant L. monocytogenes bacteria have been successfully used as biologic vaccine vectors against lymphocytic choriomeningitis virus, cottontail rabbit papillomavirus, murine influenza, and murine neoplasms (40, 68, 77). Several studies using the mouse model have demonstrated the potential of L. monocytogenes as an HIV vaccine vector (24, 30, 43-45, 58, 62, 64). It has been shown that recombinant L. monocytogenes can induce a strong CTL response and long-lived memory response to HIV Gag and that oral administration with recombinant L. monocytogenes stimulates a strong intestinal mucosal cellular immune response (24, 45, 58, 62, 64). Given the potential of L. monocytogenes as a biologic vaccine vector against HIV, recombinant L. monocytogenes needs to be investigated in a challenge system.
In the present study, we employed the feline immunodeficiency virus (FIV) model of HIV to evaluate recombinant L. monocytogenes as a biologic vaccine vector. FIV is a pathogen of cats that induces a disease syndrome similar to that of HIV infection in humans (78). Like HIV, FIV infection leads to chronic immune dysfunctions, including depletion of CD4+ T cells, inversion of CD4/CD8 T-cell ratios, decreased lymphocyte proliferation, and increased susceptibility to opportunistic infections (2, 53, 72). The feline model of infection and disease progression is uniquely relevant for the evaluation of vaccine design and immune response upon challenge (22, 34, 61). Cats are the natural hosts for FIV and can be infected by the vaginal route with either cell-free or cell-associated virus, thereby mimicking the natural route of infection by HIV (11). We show here that a single oral immunization with a novel recombinant L. monocytogenes strain conferred some control of viral load after vaginal challenge with FIV.
MATERIALS AND METHODS
Animals, bacterial stock, and challenge inoculum.
A total of 15 female 16-week-old specific-pathogen-free (SPF) cats were randomly divided into three groups of 5 cats each. Data from three additional age-matched SPF cats from a separate study provided comparative normal values of intraepithelial lymphocyte (IEL) subset percentages (these animals were part of a larger study performed by K. Howard to fully characterize IEL subsets; unpublished data). Animals were housed and cared for in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care and with all guidelines of the Institutional Animal Care and Use Committee. L. monocytogenes strain 10403S was the wild-type (LM-wt) organism used in these studies. L. monocytogenes bacteria were grown in brain heart infusion medium (Difco, Detroit, Mich.). LMgag/pND14-Lc-env is the recombinant strain of L. monocytogenes that carries a full-length copy of the FIV molecular clone NCSU1 gag gene stably integrated into the L. monocytogenes genome. The LMgag/pND14-Lc-env strain also carries a DNA vaccine plasmid with the NCSU1 FIV envelope (surface glycoprotein and ectodomain of transmembrane glycoprotein) cloned into pND14-Lc for eukaryotic expression of the FIV Env under the control of a cytomegalovirus promoter. The construction and validation of LMgag/pND14-Lc-env have been previously described (G. A. Dean, A. LaVoy, R. Stevens, S. Nordone, M. J. Burkhard, unpublished data). Cats were either sham immunized with phosphate-buffered saline (PBS) or given a single oral immunization consisting of 5 × 106 CFU of LM-wt or LMgag/pND14-Lc-env. At 12 weeks postimmunization, all 15 cats were challenged by vaginal inoculation with 7.5 ×104 FIV-infected feline peripheral blood mononuclear cells (PBMC) and 7.5 × 104 TCID50 cell-free virus. The challenge virus was FIV-NCSU1, a pathogenic subgroup A molecular clone. Animals were sacrificed 52 weeks postchallenge.
Sample collection and processing.
Serum, saliva, and vaginal wash fluids for enzyme-linked immunosorbent assays (ELISA) were collected at 0, 4, 8, 16, 20, 24, and 60 weeks postimmunization and processed as previously described (10). PBMC prepared by centrifugation (600 × g, 30 min) over Histopaque (Sigma, St. Louis, Mo.) were washed in LBT medium (RPMI medium supplemented with 10% fetal bovine serum [FBS], 15 mM HEPES, 1 mM sodium pyruvate, 4 mM l-glutamine, 10 IU of penicillin/ml, and 10 μg of streptomycin/ml) and cryopreserved in 90% FBS-10% dimethyl sulfoxide. Retropharyngeal, mesenteric, and medial iliac lymph nodes as well as thymus, bone marrow, and spleen were harvested at necropsy and processed as previously described (16) and cryopreserved as for PBMC. Bronchial alveolar lavage was performed at necropsy. Alveolar macrophages were isolated by adherence to a plastic flask for 4 h at 37°C and 5% CO2. Nonadherent cells were washed off, and macrophages were gently removed with a 1:3 solution of lidocaine, washed, and pelleted for DNA extraction. IELs were isolated from a 20-cm section of distal jejunum. The intestine was flushed with wash medium (PBS supplemented with 20% FBS, 4 mM l-glutamine, 2 mM penicillin-streptomycin, 1.25% Fungizone [BioWhittaker, Walkersville, Md.], and 10 μg of gentamicin/ml) and cut into 0.5-cm strips following excision of Peyer's patches and lymphoid follicles. Cut intestinal sections were stirred vigorously for 30 min at 37°C in spin medium (Hanks balanced salt solution without Ca2+ or Mg2+ and supplemented with 20% FBS, 4 mM l-glutamine, 2 mM penicillin-streptomycin, 1.25% Fungizone [BioWhittaker], 10 μg of gentamicin/ml, 2 mM EDTA, and 2 mM dithiothreitol). The supernatant was collected and then centrifuged at 1,000 × g for 15 min at 16°C. The supernatant was removed, and the pellet was resuspended in 30% Percoll (Amersham Biosciences, Piscataway, N.J.) and then underlaid with 70% Percoll and centrifuged at 400 × g for 30 min at 25°C. Following centrifugation, the 30%-70% interface layer was collected and washed twice in wash medium and counting was performed.
Flow cytometric analysis.
PBMC, IEL, and mesenteric lymph node samples (106 cells) were stained with fluorescein isothiocyanate- or phycoerythrin-conjugated mouse monoclonal antibodies against feline CD8α (71) or CD4 (3-F4; Southern Biotechnology, Birmingham, Ala.) and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.) and CellQuest software (Becton Dickinson). Data were compared using analysis of variance (ANOVA) with Bonferroni posttest (GraphPad Prism; GraphPad Software, San Diego, Calif.).
FIV p24 antibody ELISA.
ELISA was performed as previously described (70), with minor modifications. Briefly, Immulon-2HB plates (Dynex Technologies, Chantilly, Va.) were coated with 1.0 μg of p24-GST fusion protein/ml (65). Antibody was detected with goat anti-cat immunoglobulin G (IgG) (Bethyl Labs, Montgomery, Tex.) or IgA (Serotec, Raleigh, N.C.) diluted 1:16,000 or 1:250, respectively, and incubated for 1 h at room temperature. Plates were developed with tetramethylbenzidine (Kirkegaard and Perry, Gaithersburg, Md.) for 10 min and stopped with 1 N H2SO4 before the optical density was read at 450 nm. Titers of antibody were considered positive when they were twofold higher than preimmune values for washes and threefold higher than preimmune values for serum. Statistical differences in antibody endpoint titers between immunization groups were determined using ANOVA, Duncan's multiple range test, and the ANOVA and DUNCAN procedure of SAS (version 8.02; SAS Institute, Inc., Cary, N.C.). Kinetics of antibody responses, including group and time interactions, were analyzed using regression analysis. Significance was defined as P ≤ 0.05.
Virus isolation.
Virus isolation and CD8+ T-cell depletion were performed as previously described (17), with minor modifications. Bone marrow mononuclear cells were stained for 20 min with a fluorescein isothiocyanate-conjugated anti-CD8 antibody cocktail consisting of monoclonal antibodies 357 (71) and fCD8 (Southern Biotechnology). Cells were washed with flow wash buffer (PBS supplemented with 10% horse serum and 0.1% sodium azide) and incubated with goat anti-mouse IgG beads (Miltenyi Biotec, Auburn, Calif.) for 20 min, washed again, and then run on an AutoMACS separation unit (Miltenyi Biotec) per the manufacturer's instructions. Sorting purity was higher than 98%, as determined by flow cytometric analysis (FACScan; Becton Dickinson). Unfractionated or CD8+-depleted bone marrow cells (2.5 × 106) were washed in LBT medium before being subjected to coculturing in triplicate with 1 × 106 FCD4E cells (a feline CD4+, T-cell line) (9) in the presence of interleukin-2 (National Institutes of Health AIDS Research and Reference Reagent Program). Supernatants were analyzed for FIV p24 levels by antigen capture ELISA every 2 to 3 days over the 21-day culture period.
FIV p24 antigen capture ELISA.
Maxisorp plates (Fisher Scientific, Houston, Tex.) were coated with 51G11.1 anti-p24 monoclonal antibody (a generous gift from Edward A. Hoover, Colorado State University) (0.45 μg/ml) and incubated overnight at 4°C. Plates were washed and blocked with TRis-EDTA-NaCl-2% bovine serum albumin overnight at 4°C. Plates were washed, and 100 μl of coculture supernatants was added to the plate. Positive and negative controls were run on each plate and consisted of supernatants collected from persistently FIV-NCSU1-infected Crandell feline kidney cells or uninfected Crandell feline kidney cells, respectively. The plates were incubated for 2 h at 37°C. After washing, 100 μl of CG5 antibody (DEAE-purified, S-300-sized IgG from FIV+ cat plasma; Custom Monoclonal Antibodies International, Sacramento, Calif.) (2 μg/ml) was added to each well and the plate was incubated at 37°C for 1 h. After washing, 100 μl of goat anti-cat IgG-horseradish peroxidase (Kirkegaard and Perry) diluted 1:1,000 in buffer containing 5% mouse serum and 2% goat serum was added to each well and incubated at room temperature for 45 min. Plates were developed with tetramethylbenzidine (Kirkegaard and Perry) for 10 min, and development was stopped with 1 N H2SO4 before the optical density was read at 450 nm.
Real-time DNA PCR.
Real-time PCR was performed to quantify FIV provirus as previously described (57). DNA was extracted from PBMC and tissue pellets by the use of a DNeasy tissue kit as directed by the manufacturer (QIAGEN, Valencia, Calif.). Each amplification reaction contained 0.5 μg of DNA. PCR primers and probe specific for the NCSU1 gag gene were designed using Primer Express (PE Applied Biosystems, Foster City, Calif.). The primers (FIVNC.491f and FIVNC.617r) and probe (FIVNC.555p) have previously been described (12). A single copy of the CCR5 sequence exists in the feline genome and was used to normalize the FIV copy number. The CCR5 primers and probe (University of California at Davis Taqman Service, Davis, Calif.) for quantification of the feline CCR5 gene were used in the PCR at 400 and 80 nM, respectively. All standards and controls were run in triplicate, and unknowns were run in duplicate and averaged. Standard curves for FIV and CCR5 were accepted when the slopes were between −3.74 and −3.32. The limit of detection was ≤10 copies FIV per μg of DNA.
RESULTS
Immunization and challenge strategy.
A total of 15 female 16-week-old cats were either sham immunized or orally inoculated with 5 × 106 CFU of wt-LM or LMgag/pND14-Lc-env bacteria. At 3 months postvaccination, all cats were vaginally challenged with FIV-NCSU1, a pathogenic subgroup A molecular clone. At 52 weeks postchallenge, all cats were sacrificed and multiple tissues were collected.
Assessment of proviral loads in tissues.
While sterilizing immunity may not be achievable, limiting proviral burden may provide protection against transmission and disease progression. To determine the effect of LMgag/pND14-Lc-env immunization on proviral burden after vaginal FIV challenge, we performed quantitative real-time PCR (sensitivity ≤ 10 provirus copies) on DNA extracted from PBMC at 4, 32, and 52 weeks postchallenge (Table 1). At 4 weeks postchallenge, two sham-immunized cats and one LM-wt-inoculated cat were PCR positive; at 32 weeks postchallenge, two of five sham-immunized and five of five LM-wt-inoculated cats were positive. Intestinal IELs and medial iliac lymph node, mesenteric lymph node, retropharyngeal lymph node, spleen, bone marrow, thymus, and bronchoalveolar lavage mononuclear cells (Table 1) were evaluated at the time of sacrifice (1 year postchallenge). By 52 weeks postchallenge, provirus was detected in the tissues of 8 of 10 control cats (3 of 5 sham-immunized cats and 5 of 5 LM-wt-immunized cats). Notably, provirus was not detected in any evaluated tissue from the LMgag/pND14-Lc-env-immunized cats.
TABLE 1.
Real-time PCR detection of proviral DNA in tissue
| Immunization group and cat | No. of cells with proviral DNA detecteda
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBMC at wk postchallenge:
|
Retrob | IEL | Balc | Thymus | BMd | MLNe | Spleen | Iliacf | |||
| 4 | 32 | 52 | |||||||||
| Sham | |||||||||||
| IIU3 | 8.20 × 101 | 8.00 × 102 | 5.48 × 102 | 6.63 | 1.07 | 2.78 × 101 | 5.48 | 1.29 × 104 | 4.03 × 104 | 1.32 × 102 | 1.70 × 103 |
| IIS2 | NDg | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| IIH4 | 2.42 × 103 | 5.75 × 104 | 2.07 × 104 | 1.20 × 103 | 5.70 × 104 | 1.39 × 109 | 2.24 × 109 | 5.74 × 109 | 7.22 × 106 | 7.24 × 105 | 1.16 × 106 |
| IIG5 | ND | ND | ND | ND | ND | ND | ND | 1.35 × 102 | ND | ND | ND |
| IIM3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Wt-LM | |||||||||||
| IIG4 | ND | 2.07 × 102 | ND | 9.37 | 5.42 × 102 | 3.28 × 103 | 7.11 × 104 | 5.29 × 101 | ND | 6.29 × 102 | 8.64 × 104 |
| IIS1 | ND | 3.88 × 102 | ND | 3.46 × 101 | 1.03 × 103 | 5.36 × 103 | 1.24 × 103 | 1.14 × 102 | 4.23 × 105 | 7.69 × 103 | 6.38 × 102 |
| IIH5 | ND | 5.03 × 102 | 3.60 × 102 | 5.60 × 102 | 5.28 × 103 | 3.14 × 102 | 1.20 × 105 | 1.30 × 103 | 3.93 × 102 | ND | 9.42 × 103 |
| IIP4 | 1.40 × 101 | 5.75 × 102 | 1.85 × 102 | 3.55 | 2.63 | 2.42 × 101 | 6.38 × 102 | 7.95 × 101 | 6.11 × 101 | 9.40 × 101 | 2.92 × 101 |
| IIM2 | ND | 1.88 × 102 | ND | 1.06 × 102 | ND | ND | ND | ND | ND | ND | ND |
| LMgag/envh | |||||||||||
| IIH2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| IIH3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| IIO5 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| IIO6 | 8.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| IIO7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Detection for all cell types other than PBMC at 52 weeks postchallenge.
Retro, retropharyngeal lymph node.
Bal, bronchoalveolar lavage mononuclear cells.
BM, bone marrow.
MLN, mesenteric lymph node.
Iliac, medial iliac lymph node.
ND, not detected.
LMgag/env, LMgag/pND14-Lc-env.
Virus isolation.
A maximum of 0.5 μg of DNA, representing approximately 5 × 105 cells, can be assessed per reaction with real-time PCR. To further search for viral reservoirs in a greater number of cells, virus isolation was performed in triplicate on 2.5 × 106 unfractionated or CD8+ T-cell-depleted bone marrow cells. Bone marrow mononuclear cells were cocultured with highly permissive FCD4E cells, and FIVp24 levels were measured in culture supernatants by antigen capture ELISA every 2 to 3 days. Cells were cocultured for a total of 21 days. Due to unavailability of bone marrow for cat IIM3, virus isolation was performed on mesenteric lymph node mononuclear cells. Virus isolation results were positive on day 14 for four of five sham-immunized cats by coculture in both unfractionated and CD8+-depleted cultures, while results for cat IIU3 were positive only when CD8+ T cells were depleted. Of the LM-wt-immunized cats, three of five gave positive results in unfractionated cultures and four of five gave positive results in CD8+ T-cell-depleted cultures. Thus, all 10 sham- and LM-wt-immunized cats were FIV positive by PCR and/or virus isolation. Only one of five LMgag/pND14-Lc-env-immunized cat was positive by virus isolation in unfractionated bone marrow cells. However, virus was isolated from all five LMgag/pND14-Lc-env-immunized cats when CD8+ T cells were depleted (Table 2). These results are consistent with suppression of virus replication by CD8+ T cells.
TABLE 2.
Virus isolation from unfractionated and CD8+ T-cell-depleted bone marrow 52 weeks after vaginal challenge with FIV
| Immunization group and cat | Day of virus isolation for indicated bone marrow typea
|
|
|---|---|---|
| Unfractionated | CD8 Depleted | |
| Sham | ||
| IIU3 | Neg | 14b |
| IIG5 | 14 | 14 |
| IIM3c | Neg | 9 |
| IIS2 | 14 | 14 |
| IIH4 | 7 | 7 |
| LM-wt | ||
| IIG4 | Neg | 21 |
| IIH5 | Neg | Neg |
| IIS1 | 19 | 19 |
| IIP4 | 8 | 8 |
| IIM2 | 19 | 19 |
| LM-gag/envd | ||
| IIH2 | Neg | 14 |
| IIH3 | Neg | 14 |
| IIO5 | Neg | 19 |
| IIO6 | 7 | 7 |
| IIO7 | Neg | 21 |
Neg, virus isolation negative after 21 days of coculture.
Day culture was FIVp24 positive by ELISA.
For cat IIM3 mesenteric lymph node was evaluated.
LM-gag/env, LMgag/pND14-Lc-env.
Lymphocyte subsets in lymph node and intestine.
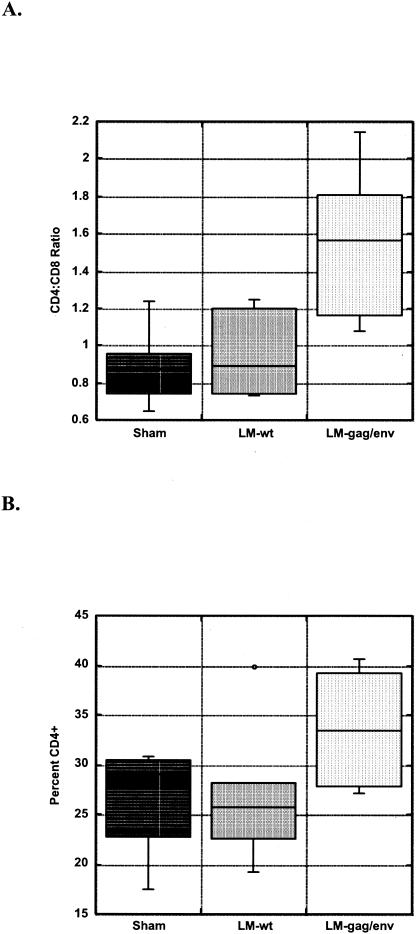
Peripheral blood lymphocyte numbers and phenotype subpopulations remained within normal limits for all cats throughout the study period (data not shown). However, significant differences in lymphocyte subsets in the mesenteric lymph node were observed among the three study groups. The mean CD4:CD8 ratio in the mesenteric lymph node of LMgag/pND14-Lc-env-immunized cats at 52 weeks post-vaginal FIV challenge was 1.55 ± 0.45. In contrast, sham- and LM-wt-immunized groups had inverted mean CD4:CD8 T-cell ratios of 0.89 ± 0.23 and 0.96 ± 0.25, respectively (Fig. 1A). These ratios for the sham- and LM-wt-immunized groups were significantly lower than those of the LMgag/pND14-Lc-env-immunized group (P = 0.04). The inverted ratios were the result of both CD4+ T-cell loss and increased CD8+ T-cell percentages (Fig. 1B and C).
FIG. 1.
Lymphocyte subsets in mesenteric lymph node. CD4:CD8 ratios (A) and percentages of CD4+ (B) and CD8+ (C) T cells are shown in a box (enclosing 50% of the data with the median value displayed as a line) and whisker (lines extending from the bottom and top of each box mark the minimum and maximum values) plot. An outlier is displayed as an individual point in panel B. The mean CD4:CD8 ratio for LMgag/pND14-Lc-env (LM-gag/env)-immunized group is significantly greater than that for the LM-wt- or sham-immunized group (P = 0.04 [ANOVA]).
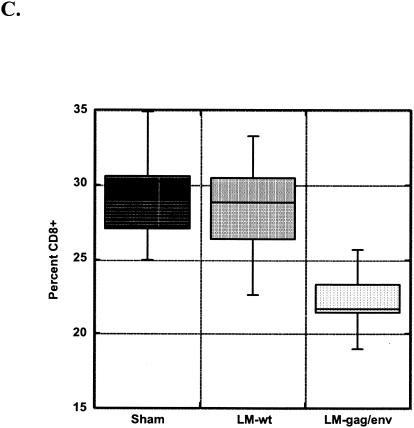
To determine whether oral immunization might prevent lymphocyte subset perturbations in the intestine, we determined the phenotype of IELs isolated at necropsy. We found immunophenotypic analysis by flow cytometry of cryopreserved IELs was not comparable to results obtained from freshly isolated IELs. Unfortunately, freshly isolated IELs were analyzed for just three LMgag/pND14-Lc-env-immunized and three sham-immunized animals. Data from three FIV-naïve cats are included for comparison. Two of three LMgag/pND14-Lc-env-immunized cats analyzed maintained normal (compared to FIV-naïve values) CD4+ percentages, while all three sham-immunized cats were CD4+ T cell depleted (Fig. 2A). The three sham-immunized cats also had significant loss of CD8+ Τ cells compared to LMgag/pND14-Lc-env-immunized cats (P = 0.0016), whose results were similar to FIV-naïve control values (Fig. 2B). CD4 and CD8 percentages in IELs of LMgag/pND14-Lc-env-immunized cats compared to those naïve control cats were not significantly different (P = 0.05).
FIG. 2.
Lymphocyte subsets in intestinal epithelium. Percentages of CD4+ (A) and CD8+ (B) T cells are shown for three FIV-naïve, SPF control cats, three sham-immunized cats, and three LMgag/pND14-Lc-env (LM-gag/env)-immunized cats.
Mucosal and systemic induction of Gag-specific IgG and IgA.
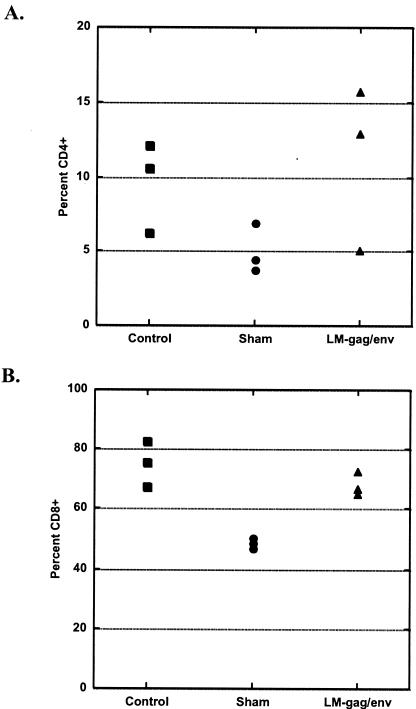
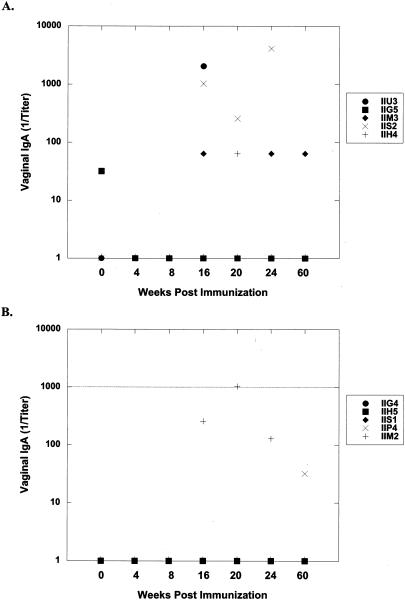
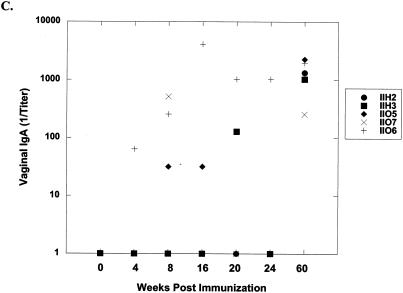
FIVp24-specific IgG and IgA levels in vaginal washes, saliva, and serum were measured by ELISA. Table 3 lists titers for each cat at 8 weeks postimmunization and at study termination (52 weeks postchallenge [60 weeks postimmunization]). Immunization with LMgag/pND14-Lc-env induced vaginal anti-p24 IgA responses in three of five cats. Vaginal IgA was found in all 5 immunized cats after challenge, while only 2 of 10 control cats mounted a minimal anti-p24 vaginal IgA antibody response upon vaginal FIV challenge (Fig. 3). Interestingly, four of five cats immunized with LMgag/pND14-Lc-env mounted a postchallenge anti-p24 IgG vaginal antibody response and by 52 weeks postchallenge there was a strong statistical difference between LMgag/pND14-Lc-env-immunized cats and LM-wt-immunized cats (P = 0.009). Two LMgag/pND14-Lc-env-immunized cats mounted anti-p24 IgG and IgA antibody responses in saliva; however, postchallenge antibody titers were not significantly different among the three groups. LMgag/pND14-Lc-env-immunized cats did not mount a prechallenge serum p24 IgG antibody response. However, by 52 weeks postchallenge the mean p24-specific serum IgG titer for LMgag/pND14-Lc-env-immunized cats was 3,270 ± 3,635 versus 716 ± 857 and 1,024 ± 1,024 for sham- and LM-wt-immunized cats, respectively. Although the mean titer of the LMgag/pND14-Lc-env-immunized group was 3 to 5 times greater than that of the control groups, this was not statistically significant due to variations among individual cats.
TABLE 3.
Anti-p24 IgG and IgA antibody response
| Immunization group and cat | Endpoint antibody titera
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal IgA
|
Vaginal IgG
|
Saliva IgA
|
Saliva IgG
|
Serum IgG
|
||||||
| 8 wpi | 52 wpc | 8 wpi | 52 wpc | 8 wpi | 52 wpc | 8 wpi | 52 wpc | 8 wpi | 52 wpc | |
| Sham | ||||||||||
| IIU3 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 64 | 0 | 2,048 |
| IIG5 | 0 | 0 | 0 | 128 | 0 | 4,096 | 0 | 512 | 0 | 0 |
| IIM3 | 0 | 64 | 0 | 64 | 0 | 128 | 0 | 64 | 0 | 512 |
| IIS2 | 0 | 0 | 32 | 128 | 64 | 0 | 0 | 0 | 0 | 1,024 |
| IIH4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LM-wt | ||||||||||
| IIG4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 512 | 0 | 1,024 |
| IIH5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2,048 |
| IIS1 | 0 | 0 | 0 | 0 | 0 | 0 | 128 | 64 | 0 | 0 |
| IIP4 | 0 | 32 | 0 | 0 | 0 | 4,096 | 0 | 128 | 0 | 2,048 |
| IIM2 | 0 | 0 | 0 | 0 | 0 | 1,024 | 0 | 512 | 0 | 0 |
| LM-gag/envb | ||||||||||
| IIH2 | 0 | 1,024 | 0 | 128 | 0 | 64 | 64 | 1,024 | 0 | 8,192 |
| IIH3 | 0 | 1,024 | 0 | 0 | 512 | 1,024 | 4,096 | 128 | 0 | 0 |
| IIO5 | 32 | 2,048 | 256 | 32 | 256 | 0 | 0 | 0 | 0 | 1,024 |
| IIO6 | 256 | 2,048 | 0 | 256 | 0 | 128 | 0 | 128 | 0 | 2,048 |
| IIO7 | 512 | 256 | 0 | 256 | 0 | 0 | 0 | 0 | 0 | 4,096 |
wpi, weeks postimmunization; wpc, weeks postchallenge.
LM-gag/env, LMgag/pND14-Lc-env.
FIG. 3.
Induction of FIV anti-p24 IgA in vaginal secretions. FIV Gag-specific IgA levels were measured in vaginal washes from sham-immunized (A), LM-wt-immunized (B), and LMgag/pND14-Lc-env-immunized (C) cats by ELISA at 0, 4, 8, 16, 20, 24, and 60 weeks postimmunization. Cats were vaginally challenged with FIV at week 12. Titers were determined by endpoint dilution.
DISCUSSION
To limit transmission of HIV and combat the virus at its earliest target, it may be essential for an efficacious vaccine to induce a strong mucosal immune response. Several studies have demonstrated the importance of mucosal CTL (3, 4, 35, 51) and suggest that mucosal CTL may be at least as important as systemic CTL (5). Another key component of the mucosal immune barrier is secretory IgA (sIgA). Mucosal IgA production has been correlated with protection (6, 18, 36, 49), and IgA-mediated virus neutralization has been demonstrated (7, 18, 56). While many parenteral vaccine strategies have induced mucosal immune responses, in general a more robust mucosal response is seen when immunization occurs at the mucosa (39). Oral immunization is therefore an attractive approach to target induction of mucosal immunity and has the practical benefit of ease of delivery.
In the present study, cats immunized with a single oral dose of recombinant L. monocytogenes bacteria showed some control of virus burden after vaginal challenge with pathogenic FIV. At 1 year after vaginal FIV challenge, immunized cats had undetectable provirus levels, as assessed by real-time PCR analysis of peripheral blood lymphocytes, multiple lymph nodes, intestinal mucosal lymphocytes, and several other tissues. Given the enormous difficulty in inducing sterilizing immunity, reducing or controlling the viral reservoirs may be the most important feature of a successful HIV vaccine. Prolonged virus isolation coculturing with large numbers of lymphocytes was required to reveal the presence of virus in the LMgag/pND14-Lc-env-immunized cats. Furthermore, CD8+ T-cell depletion was necessary for four of five of the cats before virus replication was detected. This result emphasizes the importance of CD8+ T cells in the control of viral replication, whether the control is mediated by direct cytotoxicity or through soluble factors.
Preventing lymphocyte depletion and dysfunction is another critical measure of vaccination success. Recent studies suggest that analysis of lymph nodes and mucosa-associated lymphocytes is a more sensitive approach to detecting alterations in lymphocyte subpopulations due to HIV or simian immunodeficiency virus infection (37, 47, 69, 73, 75). This was apparent in the present study, as no lymphocyte subset alterations were seen in the peripheral blood of any FIV-challenged animals. However, an inversion of the CD4:CD8 ratio in the mesenteric lymph node occurred in sham-immunized and LM-wt-immunized but not in LMgag/pND14-Lc-env-immunized cats. The loss of CD4+ T cells and increase of CD8+ T-cell levels observed is characteristic of FIV infection (78). Loss of CD4+ T cells from the intestinal immune system occurs very early in infection and is known to precede losses in lymph nodes of simian immunodeficiency virus-infected macaques and HIV-infected people (28, 37, 46, 47, 69, 73, 75). In fact, the intestine appears to be a primary target organ for virus infection and CD4+ T-cell depletion (28, 37, 47, 73, 75). We observed a loss of both CD4+ and CD8+ T cells in sham- and LM-wt-immunized cats, while LMgag/pND14-Lc-env-immunized cats maintained IEL subpopulations at FIV-naïve control levels. Oral immunization of mice with HIV-Gag recombinant L. monocytogenes has resulted in Gag-specific responses in about 35% of lamina propria CD8+ T cells (58). Induction of such a robust T-cell response in combination with sIgA may prevent the establishment of viral reservoirs and depletion of CD4+ T cells in the intestine. Given the pivotal role of the intestinal immune system in HIV pathogenesis, this type of protection may be sufficient to prevent disease.
Immunized cats demonstrated a durable vaginal IgA response to FIV Gag. Vaginal IgA has been well studied in exposed, uninfected individuals and has been shown to neutralize primary HIV-1 isolates from multiple clades (8, 18-20, 36, 41). The neutralizing effect may be due to inhibition of virus transcytosis across the epithelial membrane (8, 18). However, IgA titers have not necessarily been correlated with protection and this may be due to differences in epitope specificity or levels of the IgA (14, 48). Mapping of IgA-neutralizing activity has identified the coiled-coil pocket in the alpha-helical region of gp41, the transmembrane protein, as an important target (14). However, simply coating virus particles with sIgA, regardless of epitope specificity, may promote entrapment in the vaginal mucus (15). We did not determine whether IgA from immunized cats recognized the transmembrane glycoprotein or surface glycoprotein of FIV, so the role of IgA in the protection of the cats in this study is uncertain. L. monocytogenes is recognized as a strong inducer of cellular immunity and a weak inducer of humoral immunity. Our observation that recombinant L. monocytogenes induced mucosal sIgA that was enhanced upon vaginal challenge is significant and raises optimism that an oral vaccine strategy utilizing L. monocytogenes may be able to stimulate a useful antibody response at the vaginal mucosa.
Demonstration by ELISPOT of antigen-specific gamma interferon (IFN-γ) production in cats immunized with L. monocytogenes was confounded by dramatic cell activation associated with L. monocytogenes exposure (data not shown). We have previously observed high-level constitutive IFN-γ production by PBMC that persists 30 to 60 days postimmunization with L. monocytogenes (Dean et al., unpublished). This is not surprising given that L. monocytogenes is well known to be a strong inducer of cell-mediated immunity and as such is part of the appeal of L. monocytogenes as a vaccine vector. Some difficulty dissecting the antigen specificity of the IFN-γ response after immunization with recombinant L. monocytogenes has also been experienced in the mouse model but was nicely resolved using tetramer staining (58). Unfortunately tetramers are not available for the study of IFN-γ responses in cats. Several studies employing recombinant L. monocytogenes with HIV gag integrated into the bacterial genome have previously demonstrated induction of anti-Gag responses (23, 24, 44, 58, 62, 64). The CD8+ T-cell suppression of virus replication in the virus isolation assay provided some evidence for cell-mediated immunity in this study.
Bacteria have received much less attention than viruses as potential HIV vaccine vectors. However, bacteria have several attributes making them worthy of consideration in this respect. L. monocytogenes may have advantages over other bacterial vectors, since it replicates in the cytoplasm of the host cell and this improves the likelihood that antigens are protected against enzymatic degradation. Additional advantages of L. monocytogenes are that the natural route of infection is at the intestinal mucosa and that antigen-presenting cells are target cells. Furthermore, it has been proven that L. monocytogenes can transfer a plasmid to mammalian cells (21, 32) and, as reported here, can be employed to deliver an FIV Env-expressing DNA vaccine plasmid. As with any live vaccine vector, safety issues must be addressed. Towards this end, several promising and immunogenic attenuated L. monocytogenes have been reported previously (1, 13, 21, 24-26, 30, 63, 64, 66). With further development, recombinant L. monocytogenes may prove to be a safe and efficacious vaccine vector against HIV.
Acknowledgments
This work was supported by Public Health Service grants AI40407, AI47046, and AI01529.
We acknowledge the expert assistance of Ingrid Fisher, Julie McCormick, and Alora LaVoy.
REFERENCES
- 1.Angelakopoulos, H., K. Loock, D. M. Sisul, E. R. Jensen, J. F. Miller, and E. L. Hohmann. 2002. Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation. Infect. Immun. 70:3592-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlough, J. E., C. D. Ackley, J. W. George, N. Levy, R. Acevedo, P. F. Moore, B. A. Rideout, M. D. Cooper, and N. C. Pedersen. 1991. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J. Acquir. Immune Defic. Syndr. 4:219-227. [PubMed] [Google Scholar]
- 3.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Investig. 102:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., J. D. Ahlers, J. D. Clements, W. Strober, and J. A. Berzofsky. 2000. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J. Immunol. 165:6454-6462. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 6.Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, et al. 1999. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. J. Infect. Dis. 179:59-67. [DOI] [PubMed] [Google Scholar]
- 7.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 8.Broliden, K., J. Hinkula, C. Devito, P. Kiama, J. Kimani, D. Trabbatoni, J. J. Bwayo, M. Clerici, F. Plummer, and R. Kaul. 2001. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol. Lett. 79:29-36. [DOI] [PubMed] [Google Scholar]
- 9.Bucci, J. G., R. V. English, H. L. Jordan, T. A. Childers, M. B. Tompkins, and W. A. Tompkins. 1998. Mucosally transmitted feline immunodeficiency virus induces a CD8+ antiviral response that correlates with reduction of cell-associated virus. J. Infect. Dis. 177:18-25. [DOI] [PubMed] [Google Scholar]
- 10.Burkhard, M. J., C. K. Mathiason, T. Bowdre, and E. A. Hoover. 2001. Feline immunodeficiency virus Gag- and Env-specific immune responses after vaginal versus intravenous infection. AIDS Res. Hum. Retrovir. 17:1767-1778. [DOI] [PubMed] [Google Scholar]
- 11.Burkhard, M. J., L. A. Obert, L. L. O'Neil, L. J. Diehl, and E. A. Hoover. 1997. Mucosal transmission of cell-associated and cell-free feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 13:347-355. [DOI] [PubMed] [Google Scholar]
- 12.Burkhard, M. J., L. Valenski, S. Leavell, G. A. Dean, and W. A. Tompkins. 2002. Evaluation of FIV protein-expressing VEE-replicon vaccine vectors in cats. Vaccine 21:258-268. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty, T., F. Ebel, J. Wehland, J. Dufrenne, and S. Notermans. 1994. Naturally occurring virulence-attenuated isolates of Listeria monocytogenes capable of inducing long term protection against infection by virulent strains of homologous and heterologous serotypes. FEMS Immunol. Med. Microbiol. 10:1-9. [DOI] [PubMed] [Google Scholar]
- 14.Clerici, M., C. Barassi, C. Devito, C. Pastori, S. Piconi, D. Trabattoni, R. Longhi, J. Hinkula, K. Broliden, and L. Lopalco. 2002. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. AIDS 16:1731-1741. [DOI] [PubMed] [Google Scholar]
- 15.Cone, R. A. 1999. Mucus, p. 43-64. In P. L. Ogra, Mestecky, J., Lamm, M. E., Strober, W., Bienenstock, J., McGhee, J. R. (ed.), Mucosal Immunology, second ed. Academic Press, San Diego, Calif.
- 16.Dean, G. A., and N. C. Pedersen. 1998. Cytokine response in multiple lymphoid tissues during the primary phase of feline immunodeficiency virus infection. J. Virol. 72:9436-9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean, G. A., G. H. Reubel, P. F. Moore, and N. C. Pedersen. 1996. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J. Virol. 70:5165-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devito, C., K. Broliden, R. Kaul, L. Svensson, K. Johansen, P. Kiama, J. Kimani, L. Lopalco, S. Piconi, J. J. Bwayo, F. Plummer, M. Clerici, and J. Hinkula. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170-5176. [DOI] [PubMed] [Google Scholar]
- 19.Devito, C., J. Hinkula, R. Kaul, J. Kimani, P. Kiama, L. Lopalco, C. Barass, S. Piconi, D. Trabattoni, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2002. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 30:413-420. [DOI] [PubMed] [Google Scholar]
- 20.Devito, C., J. Hinkula, R. Kaul, L. Lopalco, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 14:1917-1920. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich, G., A. Bubert, I. Gentschev, Z. Sokolovic, A. Simm, A. Catic, S. H. Kaufmann, J. Hess, A. A. Szalay, and W. Goebel. 1998. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes [see comments]. Nat Biotechnol. 16:181-185. [DOI] [PubMed] [Google Scholar]
- 22.Elyar, J. S., M. C. Tellier, J. M. Soos, and J. K. Yamamoto. 1997. Perspectives on FIV vaccine development. Vaccine 15:1437-1444. [DOI] [PubMed] [Google Scholar]
- 23.Frankel, F. R., S. Hegde, J. Lieberman, and Y. Paterson. 1995. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J. Immunol. 155:4775-4782. [PubMed] [Google Scholar]
- 24.Friedman, R. S., F. R. Frankel, Z. Xu, and J. Lieberman. 2000. Induction of human immunodeficiency virus (HIV)-specific CD8 T-cell responses by Listeria monocytogenes and a hyperattenuated Listeria strain engineered to express HIV antigens. J. Virol. 74:9987-9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goossens, P. L., and G. Milon. 1992. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int. Immunol. 4:1413-1418. [DOI] [PubMed] [Google Scholar]
- 26.Goossens, P. L., G. Milon, P. Cossart, and M. F. Saron. 1995. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int. Immunol. 7:797-805. [DOI] [PubMed] [Google Scholar]
- 27.Goossens, P. L., C. Montixi, M. F. Saron, M. Rodriguez, F. Zavala, and G. Milon. 1995. Listeria monocytogenes: a live vector able to deliver heterologous protein within the cytosol and to drive a CD8 dependent T cell response. Biologicals 23:135-143. [DOI] [PubMed] [Google Scholar]
- 28.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman, C. A., M. Rohde, T. Chakraborty, E. Domann, M. Hudel, J. Wehland, and K. N. Timmis. 1995. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect. Immun. 63:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman, C. A., D. Saverino, E. Medina, D. Fenoglio, B. Gerstel, A. Merlo, G. Li Pira, F. Buffa, T. Chakraborty, and F. Manca. 1998. Attenuated Listeria monocytogenes carrier strains can deliver an HIV-1 gp120 T helper epitope to MHC class II-restricted human CD4+ T cells. Eur. J. Immunol. 28:1807-1814. [DOI] [PubMed] [Google Scholar]
- 31.Heise, C., C. J. Miller, A. Lackner, and S. Dandekar. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169:1116-1120. [DOI] [PubMed] [Google Scholar]
- 32.Hense, M., E. Domann, S. Krusch, P. Wachholz, K. E. Dittmar, M. Rohde, J. Wehland, T. Chakraborty, and S. Weiss. 2001. Eukaryotic expression plasmid transfer from the intracellular bacterium Listeria monocytogenes to host cells. Cell. Microbiol. 3:599-609. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages [see comments]. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 34.Jarrett, O., J. K. Yamamoto, and J. C. Neil. 1990. Feline immunodeficiency virus as a model for AIDS vaccination. AIDS 4(Suppl. 1):S163-S165. [PubMed] [Google Scholar]
- 35.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 36.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23-29. [DOI] [PubMed] [Google Scholar]
- 37.Kewenig, S., T. Schneider, K. Hohloch, K. Lampe-Dreyer, R. Ullrich, N. Stolte, C. Stahl-Hennig, F. J. Kaup, A. Stallmach, and M. Zeitz. 1999. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 116:1115-1123. [DOI] [PubMed] [Google Scholar]
- 38.Kolb-Maurer, A., I. Gentschev, H. W. Fries, F. Fiedler, E. B. Brocker, E. Kampgen, and W. Goebel. 2000. Listeria monocytogenes-infected human dendritic cells: uptake and host cell response. Infect. Immun. 68:3680-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozlowski, P. A., and M. R. Neutra. 2003. The role of mucosal immunity in prevention of HIV transmission. Curr. Mol. Med. 3:217-228. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman, J., and F. R. Frankel. 2002. Engineered Listeria monocytogenes as an AIDS vaccine. Vaccine 20:2007-2010. [DOI] [PubMed] [Google Scholar]
- 41.Lopalco, L., C. Pastori, A. Cosma, S. E. Burastero, B. Capiluppi, E. Boeri, A. Beretta, A. Lazzarin, and A. G. Siccardi. 2000. Anti-cell antibodies in exposed seronegative individuals with HIV type 1-neutralizing activity. AIDS Res. Hum. Retrovir. 16:109-115. [DOI] [PubMed] [Google Scholar]
- 42.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 43.Mata, M., and Y. Paterson. 2000. Listeria monocytogenes as an alternative vaccine vector for HIV. Arch. Immunol. Ther. Exp. (Warsaw) 48:151-162. [PubMed] [Google Scholar]
- 44.Mata, M., and Y. Paterson. 1999. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J. Immunol. 163:1449-1456. [PubMed] [Google Scholar]
- 45.Mata, M., Z. J. Yao, A. Zubair, K. Syres, and Y. Paterson. 2001. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine 19:1435-1445. [DOI] [PubMed] [Google Scholar]
- 46.Mattapallil, J. J., E. Reay, and S. Dandekar. 2000. An early expansion of CD8alphabeta T cells, but depletion of resident CD8αα T cells, occurs in the intestinal epithelium during primary simian immunodeficiency virus infection. AIDS 14:637-646. [DOI] [PubMed] [Google Scholar]
- 47.Mattapallil, J. J., Z. Smit-McBride, M. McChesney, and S. Dandekar. 1998. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1β expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J. Virol. 72:6421-6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Blé, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180:871-875. [DOI] [PubMed] [Google Scholar]
- 49.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 50.Mocci, S., S. A. Dalrymple, R. Nishinakamura, and R. Murray. 1997. The cytokine stew and innate resistance to L. monocytogenes. Immunol. Rev. 158:107-114. [DOI] [PubMed] [Google Scholar]
- 51.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540-549. [PubMed] [Google Scholar]
- 52.North, R. J. 1972. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J. Exp. Med. 135:1104-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novotney, C., R. V. English, J. Housman, M. G. Davidson, M. P. Nasisse, C. R. Jeng, W. C. Davis, and M. B. Tompkins. 1990. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS 4:1213-1218. [DOI] [PubMed] [Google Scholar]
- 54.Pamer, E. G., J. T. Harty, and M. J. Bevan. 1991. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353:852-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paschen, A., K. E. Dittmar, R. Grenningloh, M. Rohde, D. Schadendorf, E. Domann, T. Chakraborty, and S. Weiss. 2000. Human dendritic cells infected by Listeria monocytogenes: induction of maturation, requirements for phagolysosomal escape and antigen presentation capacity. Eur. J. Immunol. 30:3447-3456. [DOI] [PubMed] [Google Scholar]
- 56.Pastori, C., C. Barassi, S. Piconi, R. Longhi, M. L. Villa, A. G. Siccardi, M. Clerici, and L. Lopalco. 2000. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J. Biol. Regul. Homeost. Agents 14:15-21. [PubMed] [Google Scholar]
- 57.Pedersen, N. C., C. M. Leutenegger, J. Woo, and J. Higgins. 2001. Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CPGammar) in young adult specific pathogen free cats. Vet. Immunol. Immunopathol. 79:53-67. [DOI] [PubMed] [Google Scholar]
- 58.Peters, C., X. Peng, D. Douven, Z. K. Pan, and Y. Paterson. 2003. The induction of HIV Gag-specific CD8+ T cells in the spleen and gut-associated lymphoid tissue by parenteral or mucosal immunization with recombinant Listeria monocytogenes HIV Gag. J. Immunol. 170:5176-5187. [DOI] [PubMed] [Google Scholar]
- 59.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pron, B., C. Boumaila, F. Jaubert, P. Berche, G. Milon, F. Geissmann, and J. L. Gaillard. 2001. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell. Microbiol. 3:331-340. [DOI] [PubMed] [Google Scholar]
- 61.Pu, R., J. Coleman, M. Omori, M. Arai, T. Hohdatsu, C. Huang, T. Tanabe, and J. K. Yamamoto. 2001. Dual-subtype FIV vaccine protects cats against in vivo swarms of both homologous and heterologous subtype FIV isolates. AIDS 15:1225-1237. [DOI] [PubMed] [Google Scholar]
- 62.Rayevskaya, M., N. Kushnir, and F. R. Frankel. 2003. Anti-human immunodeficiency virus-gag CD8+ memory T cells generated in vitro from Listeria-immunized mice. Immunology 109:450-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rayevskaya, M., N. Kushnir, and F. R. Frankel. 2002. Safety and immunogenicity in neonatal mice of a hyperattenuated Listeria vaccine directed against human immunodeficiency virus. J. Virol. 76:918-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rayevskaya, M. V., and F. R. Frankel. 2001. Systemic immunity and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain of Listeria monocytogenes. J. Virol. 75:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reid, G., M. A. Rigby, M. McDonald, M. J. Hosie, J. C. Neil, and O. Jarrett. 1991. Immunodiagnosis of feline immunodeficiency virus infection using recombinant viral p17 and p24. AIDS 5:1477-1483. [DOI] [PubMed] [Google Scholar]
- 66.Saklani-Jusforgues, H., E. Fontan, N. Soussi, G. Milon, and P. L. Goossens. 2003. Enteral immunization with attenuated recombinant Listeria monocytogenes as a live vaccine vector: organ-dependent dynamics of CD4 T lymphocytes reactive to a Leishmania major tracer epitope. Infect. Immun. 71:1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen, H., J. F. Miller, X. Fan, D. Kolwyck, R. Ahmed, and J. T. Harty. 1998. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92:535-545. [DOI] [PubMed] [Google Scholar]
- 68.Shen, H., M. K. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Nat. Med. 1:471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Staats, H. F., W. G. Nichols, and T. J. Palker. 1996. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A). J. Immunol. 157:462-472. [PubMed] [Google Scholar]
- 71.Tompkins, M. B., D. H. Gebhard, H. R. Bingham, M. J. Hamilton, W. C. Davis, and W. A. Tompkins. 1990. Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet. Immunol. Immunopathol. 26:305-317. [DOI] [PubMed] [Google Scholar]
- 72.Torten, M., M. Franchini, J. E. Barlough, J. W. George, E. Mozes, H. Lutz, and N. C. Pedersen. 1991. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 65:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 74.Veazey, R. S., and A. A. Lackner. 1998. The gastrointestinal tract and the pathogenesis of AIDS. AIDS 12(Suppl. A):S35-S42. [PubMed] [Google Scholar]
- 75.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voltan, R., and M. Robert-Guroff. 2003. Live recombinant vectors for AIDS vaccine development. Curr. Mol. Med. 3:273-284. [DOI] [PubMed] [Google Scholar]
- 77.Weiskirch, L. M., and Y. Paterson. 1997. Listeria monocytogenes: a potent vaccine vector for neoplastic and infectious disease. Immunol. Rev. 158:159-169. [DOI] [PubMed] [Google Scholar]
- 78.Willett, B. J., J. N. Flynn, and M. J. Hosie. 1997. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today 18:182-189. [DOI] [PubMed] [Google Scholar]