Abstract
Aims
To assess shift work in relation to incident type 2 diabetes among African American women.
Methods
In the Black Women's Health Study (BWHS), an ongoing prospective cohort study, we followed 28,041 participants for incident diabetes during 2005-2013. They answered questions in 2005 about having worked the night shift. We estimated hazard ratios (HR) and 95% confidence intervals (CI) for incident diabetes using Cox proportional hazards models. The basic multivariable model included age, time period, family history of diabetes, education, and neighborhood SES. In further models, we controlled for lifestyle factors and body mass index (BMI).
Results
Over the 8 years of follow-up, there were 1,786 incident diabetes cases. Relative to never having worked the night shift, HRs (95% CI) of diabetes were 1.17 (1.04, 1.31) for 1-2 years of night shift work, 1.23 (1.06, 1.41) for 3-9 years, and 1.42 (1.19, 1.70) for ≥ 10 years (P-trend < 0.0001). The monotonic positive association between night shift work and type 2 diabetes remained after multivariable adjustment (P-trend = 0.02). The association did not vary by obesity status, but was stronger in women aged < 50 years.
Conclusions
Long durations of shift work were associated with an increased risk of type 2 diabetes. The association was only partially explained by lifestyle factors and BMI. A better understanding of the mechanisms by which shift work may affect risk of diabetes is needed in view of the high prevalence of shift work among U.S. workers.
Keywords: sleep, circadian, shift work, diabetes, black, African-American
Over 8 million Americans are employed in shift work [1], which is that occurring outside of typical daytime work hours. Shift workers may have hours that change periodically, or may have a permanent work pattern that occurs at unusual times of the day, such as afternoon or night. These atypical work patterns may perturb the circadian system, which is entrained most powerfully by the solar light/dark cycle and modulates daily rhythms in alertness, core body temperature, heart rate, blood pressure, and neurotransmitter and hormone secretion [2, 3]. Even in the short-term, misalignment of endogenous circadian rhythms with the actual sleep-wake cycle leads to disrupted melatonin and cortisol secretion, decreased leptin, and increased glucose and insulin [4-6]. A meta-analysis showed a modest association between shift work and vascular events [7], and there is strong evidence linking shift work to metabolic syndrome [8-12] and obesity [13-15], but there are far fewer prospective data on the association between shift work and incident diabetes [16-20]. A recent meta-analysis of twelve studies found a pooled adjusted odds ratio of 1.09 (95% CI 1.05,1.12) for ever having done shift work [21], highlighting the importance of better understanding how exposure to this highly prevalent, modifiable risk factor relates to the risk of diabetes.
The largest prospective cohort study to examine this relationship, the Nurses’ Health Study, reported an increasing risk of incident type 2 diabetes with more years of rotating night shift work. Among those with the longest duration of shift work, ≥ 20 years, there was a hazard ratio of 1.58 (1.43, 1.74) for diabetes [17]. This cohort consisted of female nurses, nearly all white. Two separate longitudinal studies among male Japanese shift workers reported an adjusted odds ratio of 1.35 (1.05, 1.75) for alternating shift work compared to day-shift work [20], and non-significant adjusted relative risks of 1.73 (0.85, 3.52) for two-shift workers and 1.33 (0.74, 2.36) for three-shift workers as compared to fixed-shift daytime workers [19]. It is unknown whether these findings extend to other population groups.
In the U.S., the prevalence of diabetes among black women (12.2%) is more than twice that of white women (4.5%) [22]. In the present study, we examine the relationship of night shift work to incident type 2 diabetes among U.S. black women. In the Nurses’ Health Study, BMI explained much, but not all, of the relationship between rotating shift work and incident diabetes [17]. In a study of Swedish women [16], the association between shift work and diabetes became non-significant after multivariable adjustment for factors including BMI. Because black women have a particularly high prevalence of obesity, at nearly 60% [23], we examined the degree to which this risk factor explains any observed association between shift work and diabetes in our study.
Research Design and Methods
Study population
The Black Women's Health Study (BWHS), started in 1995, is an ongoing prospective cohort study which examines the determinants of health and disease in African-American women [24]. Participants enrolled by responding to questionnaires mailed to subscribers of Essence (a popular magazine targeted to African American women), to members of several African American professional organizations, and to friends or relatives of early respondents. The final cohort included 59,000 women across the United States, aged 21 through 69 years at baseline. Follow-up questionnaires are mailed every two years to collect updated information that includes lifestyle factors, medication use, and medical problems. After 8 cycles, follow-up of the baseline cohort is 80%. The institutional review boards of Boston University, Boston, Massachusetts, and Howard University, Washington, DC approved the study protocol. All study participants indicated consent by filling out and returning baseline and follow up questionnaires.
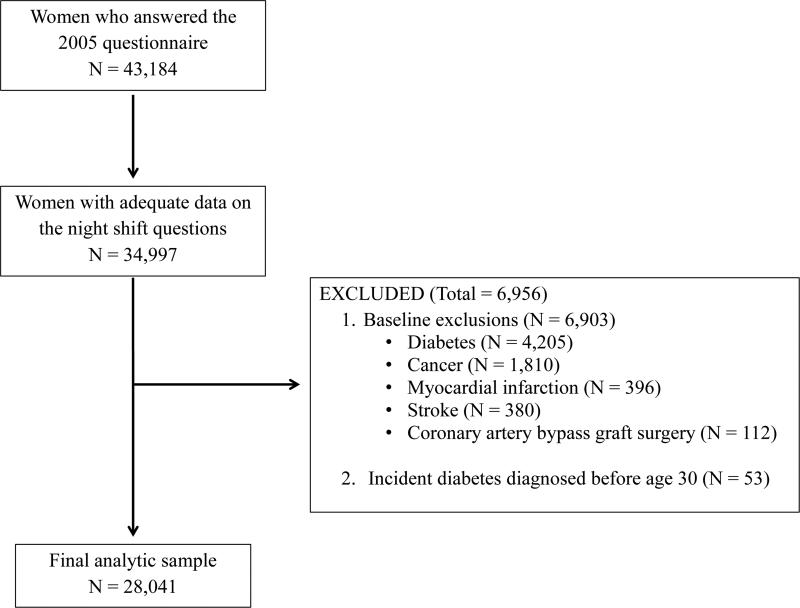
Questions about night shift work were first asked on the 2005 questionnaire. The present analysis includes women who answered the night shift work questions and who did not have diabetes at that time. Among the 34,997 women who provided adequate data on the night shift questions, we excluded women with diabetes in 2005 (i.e. baseline in the present analysis); with diabetes diagnosed at ≤ age 30; or with history of cancer, myocardial infarction, stroke, and/or coronary artery bypass graft surgery at baseline. This resulted in a final analytic sample of 28,041 women (Figure 1). Women excluded from the present analyses because they did not answer the night shift questions were similar to the ones who answered the questions with regard to age, BMI, energy intake, family history of diabetes, alcohol, coffee and soda consumption, vigorous exercise, and dietary patterns. They had a higher frequency of smoking, less years of education, and more frequently lived in neighborhood with low SES (Supplementary Table 1).
Figure 1.
Final analytic sample after exclusions. Among the 34,997 women who provided adequate data on the night shift questions, we excluded those with diabetes (N=4,205), cancer (N=1,810), myocardial infarction (N=396), stroke (N=380), or coronary artery bypass graft surgery (N=112) at baseline, or incident diabetes diagnosed before age 30 (N=53), which resulted in a final analytic sample of 28,041 women.
Assessment of night shift work and covariates
In 2005, participants were asked, “Have you ever worked a night shift (graveyard shift, Midnight to 8 AM)?”. Women who responded affirmatively to this then described how many years they worked the night shift. Responses were categorized into Never, 1-2 years, 3-9 years, and ≥ 10 years, as was done in a previous study among women [17]. We assessed reproducibility of self-reported years of night shift work among 379 BWHS participants who completed the 2005 questionnaire two times. The weighted kappa coefficient was 0.81 for the categories used in the analysis.
Current body mass index (BMI, kg/m2) was calculated from adult height as reported in 1995, and current weight, which is updated in every questionnaire cycle. In a validation study of anthropometric measures conducted among 115 BWHS participants, Spearman correlations for self-reported versus technician-measured weight and height were 0.97, and 0.93 respectively [25]. Data on vigorous physical activity were obtained from the 2001 questionnaire and updated in follow-up questionnaires. Smoking and alcohol drinking were obtained from the baseline questionnaire for the present analysis (2005) and updated in follow-up questionnaires. Coffee and soda drinking were obtained from 1995 and 2001 food frequency questionnaires (FFQs) [26, 27]. Energy intake (calories/day) was estimated from the 1995 and 2001 FFQs using the DIET*CALC software, version 1.4.1 from the National Cancer Institute [28]. Vegetables/fruit and meat/fried foods dietary patterns were calculated from the 1995 and 2001 FFQs as previously described [29]. The vegetable/fruit dietary pattern is high in whole grains, vegetables, fruit, legumes, and fish. The meat/fried foods pattern is high in red meat, processed meat, French fries, fried chicken, and added fat. First-degree family history of diabetes was ascertained in 1995 and 1999. Educational attainment was asked in the 1995 and 2003 questionnaires. We measured neighborhood socio-economic status (SES) as previously described [30, 31]. Geocoding (Mapping Analytics, Rochester, New York) was used to link participants’ current addresses to 2000 US Census block groups. We then used factor analysis to calculate an index of neighborhood SES.
Assessment of diabetes
The presence of diabetes was ascertained through self-report on biennial follow-up questionnaires between 2005 and 2013. Cases were classified as incident if there was no report of a previous diagnosis. We conducted a validation study among a random sample of 656 women who reported a diagnosis of diabetes after 1995 (baseline of the BWHS cohort). Of the 293 women providing permission to contact their physicians, a completed questionnaire was returned by the physicians for 229 and a diagnosis of diabetes was confirmed in 220 (96%) of these cases. Of the 9 participants without a confirmed type 2 diabetes diagnosis, there were 2 with type 1 diabetes, 1 with metabolic syndrome, 1 with steroid-induced diabetes, 2 with gestational diabetes, and 3 who did not have diabetes.
Undiagnosed diabetes
To estimate the prevalence of undiagnosed diabetes in our cohort, we used data from blood samples that have been collected from BWHS participants in the last year. The BWHS has been funded to request a blood draw from each participant for long-term storage, with hemoglobin A1C assayed on every sample. Samples are drawn at Quest Diagnostics Patient Service Centers and processing and assays are perfomed at Quest Diagnositics Regional Laboratories. Among the 2449 women who provided a sample in the first year of collection, 1873 had never reported diabetes. Among this group of 1873, 120 women (6.4%) had A1C levels of 6.5% or higher (Supplementary Table 2) (i.e. A1C criteria of the International Expert Committee appointed by the American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation) [32].
Statistical Analysis
Person-years were measured from baseline (2005) until the diagnosis of type 2 diabetes, loss to follow-up, death, or end of follow-up in 2013, whichever came first. We used Cox proportional hazards regression stratified by age and questionnaire cycle to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of years of night shift work with incident type 2 diabetes. We used the Andersen-Gill approach to update time-varying exposures and covariates over follow-up.
In the basic multivariate model, we adjusted for age, questionnaire cycle, first-degree family history of diabetes, education (≤12, 13-15, 16, ≥17 years), and quintiles of the neighborhood SES index. Next we added a group of lifestyle factors [(vigorous physical activity (hours/week), smoking (never smoked, past smoker, current smoker), alcohol drinking (never drank, past drinker, current drinker), dietary energy intake (kilocalories/day), vegetable/fruit and meat/fried foods dietary pattern scores (continuous), regular coffee consumption (never,<1 cup/month, 1 cup/month-6 cups/week, 1, 2-3, ≥4 cups/day), decaffeinated coffee consumption (never-<1 cup/month, 1 cup/month-6 cups/week, 1, ≥2 cups/day), regular soda consumption (<1cup/month, 1-7 cups/month, 2-6 cups/week, 1, ≥2 cups/day), and diet soda consumption (<1cup/month, 1-7 cups/month, 2-6 cups/week, 1, ≥2 cups/day). We then compared results without and with adjustment for BMI (<25, 25-29, 30-34, 35-39, ≥40 kg/m2) to assess how much of the association is explained by BMI. We performed tests for trend across categories of years of night shift work by using the median value for each of the night shift work categories. We also conducted BMI-stratified (non-obese BMI <30, and obese BMI ≥30 kg/m2) and age-stratified (<50 and ≥50 years) analyses to assess potential effect modifications by obesity and age. We controlled for BMI as a continuous variable in the BMI-stratified analysis.
Results
Table 1 shows baseline characteristics of the participants by categories of years of night shift work. Of the 28,041 women included in the study, 21% worked the night shift for 1-2 years, 11% worked the night shift for 3-9 years, and 5% worked the night shift for ≥ 10 years. Compared to the group who never worked the night shift, the group that worked ≥ 10 years was older, had a higher mean BMI and energy intake, and more frequently had a family history of diabetes. They also had a higher frequency of smoking and more coffee and soda consumption, In addition, the group with ≥ 10 years of night shift work also had lower levels of alcohol use, education, exercise, and healthy dietary patterns, and more frequently lived in neighborhoods within the lowest quintile of SES.
Table 1.
Age-adjusted baseline (2005) characteristics by years of night shift work*
| Characteristic | Night shift work (years) |
|||
|---|---|---|---|---|
| Never | 1 - 2 | 3 - 9 | ≥10 | |
| Number of women | 17,722 | 5,791 | 3,149 | 1,379 |
| Mean age, years | 47.5 | 46.9 | 47.8 | 50.9 |
| Mean BMI, kg/m2 | 29.0 | 29.9 | 30.2 | 30.6 |
| Mean energy intake, kcal/day | 1,435 | 1,501 | 1,570 | 1,603 |
| Family history of diabetes, % | 34 | 36 | 37 | 41 |
| Current smokers, % | 9 | 12 | 14 | 13 |
| Current alcohol drinkers, % | 26 | 25 | 25 | 23 |
| Regular coffee consumption >1 cup/day, % | 8 | 9 | 11 | 11 |
| Decaf coffee consumption >1 cup/day, % | 2 | 2 | 2 | 1 |
| Regular soda consumption >1 cup/day, % | 5 | 6 | 7 | 9 |
| Diet soda consumption >1 cup/day, % | 2 | 1 | 2 | 2 |
| Education ≤12 years, % | 10 | 13 | 14 | 18 |
| Vigorous exercise ≥5 hours/week, % | 9 | 7 | 9 | 8 |
| Dietary patterns, % | ||||
| 5th quintile of vegetable/fruit (healthiest) | 20 | 19 | 18 | 18 |
| 5th quintile of meat/fried foods (unhealthiest) | 19 | 21 | 21 | 22 |
| Neighborhood socioeconomic status, % | ||||
| 1st quintile (poorest neighborhood) | 17 | 20 | 22 | 24 |
| 5th quintile (wealthiest neighborhood) | 21 | 16 | 14 | 12 |
All of the characteristics vary significantly across years of shift work categories at the alpha=0.05 level.
Over 189,710 person-years of follow-up there were 1,786 cases of incident diabetes (Table 2). In a multivariate model without adjustment for lifestyle factors or BMI, the HR increased as duration of night shift work increased (P-trend <0.0001), with an HR (95%CI) of 1.42 (1.19, 1.70) for ≥ 10 years of working relative to never working the night shift. Adjustment for lifestyle factors and BMI attenuated the rate ratios, though a trend of increasing risk with more years of night shift work persisted (P-trend = 0.02). Among the group with the longest duration of night shift work there was an HR (95%CI) of 1.23 (1.03, 1.47). The HR (95%CI) for ever vs. never having worked the night shift was 1.22 (1.11, 1.34), and this remained significant after adjustment for lifestyle factors and BMI [1.12 (1.01, 1.23)].
Table 2.
Association of night shift work with incidence of type 2 diabetes in the Black Women's Health Study, 2005-2013
| Night shift work (years) | Trend test | Ever worked night shift | ||||
|---|---|---|---|---|---|---|
| Never | 1 – 2 years | 3 – 9 years | 10+ years | P-value | HR (95% CI) | |
| Cases/Person-years | 1,014/120,899 | 399/39,095 | 235/20,817 | 138/8,899 | 772/68,811 | |
| Basic multivariatea | 1.00 (reference) | 1.17 (1.04,1.31) | 1.23 (1.06,1.41) | 1.42 (1.19,1.70) | <0.0001 | 1.22 (1.11,1.34) |
| + Lifestyle factorsb | 1.00 (reference) | 1.14 (1.01,1.28) | 1.18 (1.02,1.36) | 1.35 (1.13,1.62) | 0.0007 | 1.18 (1.08,1.30) |
| + Body mass index | 1.00 (reference) | 1.09 (0.97,1.22) | 1.11 (0.96,1.28) | 1.23 (1.03,1.47) | 0.022 | 1.12 (1.01,1.23) |
Basic covariates: age, questionnaire cycle, family history of diabetes, education, and neighborhood socioeconomic status
Lifestyle factors: vigorous activity levels, smoking, alcohol, energy intake, dietary pattern, and coffee, decaffeinated coffee, soda consumption, and diet soda consumption
Stratified analyses showed that the association between night shift work and diabetes did not significantly vary by obesity status (P-interaction = 0.12) (Table 3). On the other hand, the association was stronger among women younger compared to older women. Working ≥10 years in the night shift relative to never working the night shift was associated with 39% higher risk of diabetes among women aged <50 years as compared to just 17% higher risk in older women aged ≥50 years (Table 3) (P-interaction = 0.028).
Table 3.
Association of night shift work with incidence of type 2 diabetes by obesity and age in the Black Women's Health Study, 2005-2013
| Night shift work (years) | Trend test | Ever worked night shift | ||||
|---|---|---|---|---|---|---|
| Never | 1 – 2 years | 3 – 9 years | 10+ years | P-value | HR (95% CI) | |
| Non-Obese | ||||||
| Cases/Person-years | 333/73,933 | 125/21,472 | 48/10,993 | 32/4,325 | 205/36,790 | |
| Multivariate Modela | 1.00 (reference) | 1.17 (0.95,1.45) | 0.81 (0.60,1.10) | 1.16 (0.80,1.68) | 0.79 | 1.06 (0.89,1.27) |
| Obese | ||||||
| Cases/Person-years | 653/45,405 | 261/17,054 | 174/9,439 | 98/4,364 | 533/30,857 | |
| Multivariate Modela | 1.00 (reference) | 1.05 (0.91,1.21) | 1.20 (1.01,1.42) | 1.23 (0.99,1.53) | 0.029 | 1.12 (1.00,1.26) |
| < 50 years | ||||||
| Cases/Person-years | 339/62,394 | 163/21,205 | 100/10,433 | 40/3,253 | 303/34,891 | |
| Multivariate Modela | 1.00 (reference) | 1.19 (0.99,1.44) | 1.38 (1.10,1.73) | 1.39 (0.99,1.94) | 0.012 | 1.27 (1.09,1.49) |
| ≥ 50 years | ||||||
| Cases/Person-years | 675/58,505 | 236/17,890 | 135/10,384 | 98/5,646 | 469/33,921 | |
| Multivariate Modela | 1.00 (reference) | 1.02 (0.88,1.18) | 0.97 (0.80,1.16) | 1.17 (0.94,1.45) | 0.20 | 1.03 (0.91,1.16) |
Adjusted for age, questionnaire cycle, family history of diabetes, education, neighborhood socioeconomic status, body mass index (continuous in the obesity-stratified analysis, and categorical in the age-stratified analysis), and lifestyle factors (vigorous activity levels, smoking, alcohol, energy intake, dietary pattern, and coffee, decaffeinated coffee, soda consumption, and diet soda consumption)
Discussion
In this large prospective cohort study of U.S. black women, we found that increasing years of night shift work were associated with greater risk of incident type 2 diabetes, even after adjustment for lifestyle factors and BMI. Those with the longest duration of night shift work had a 23% increased incidence of type 2 diabetes. The association was stronger among women less than 50 years of age than among older women.
Our findings are consistent with the few epidemiologic studies of which we are aware that examine the impact of increasing duration of shift work on diabetes risk. In a cross-sectional telephone survey of 1,111 retired older adults in Western Pennsylvania, the prevalence of diabetes was doubled among those with any history of night shift work. Even after adjustment for BMI, there was increasing diabetes risk with increased exposure to the night shift; those with 20+ years of night shift work had a two-fold increased risk [33]. In contrast, an early Nurses Health Study (NHS) paper based on 356 cases of diabetes found that an association between increasing years of shift work and incident diabetes was explained away completely in a multivariable analysis that included BMI [18]. This study examined rotating night shift work (3 nights/month in addition to days and evenings in that month). An updated analysis from the NHS included more than 177,000 women from NHS (older women, 6,165 incident diabetes cases) and NHS II (younger women, 3,961 cases) [17]. Over 20 years of follow-up, these authors found a positive association, which was attenuated but still present after adjustment for BMI. For example, among women in the NHS with 10-19 years and ≥ 20 years of night shift work, the pooled hazard ratios for incident diabetes were 1.40 (95% CI, 1.30, 1.51) and 1.58 (1.43, 1.74), respectively. Adjustment for BMI decreased the HR to 1.10 (95% CI, 1.02, 1.18) and 1.24 (1.13, 1.37), respectively. In both models, there was a significant trend for increasing diabetes risk with increasing years of shift work (P-trend <0.001). Our results on the association of night shift work with incident type 2 diabetes in a population of black women are consistent with those based on data from white women. In addition, our finding that adjustment for BMI substantially attenuated relative risk, as in the NHS, indicates that BMI could be a major mediator for the observed association between night shift work and incident diabetes. NHS investigators found the relationship between night shift work and diabetes was similar in younger and older women. In contrast, we found that the association between years of shift work and diabetes was stronger among younger women (<50 years). This may be because the effect of shift work is more easily detected among those who are at lower risk of diabetes, who are the younger women.
Even though lifestyle factors and BMI explained a major part of the association of shift work with incident diabetes, women with a long duration of shift work had an increased risk of diabetes after control for those factors, suggesting the presence of additional causal pathways. Shift work is associated with disrupted circadian rhythms and reduced total hours of sleep [34]. Similar to the effects of jet lag, which are short-term, shift workers experience fatigue, sleepiness during scheduled awake periods, and poor sleep during scheduled sleeping periods [4]. These alterations in the normal sleep-wake cycle have profound effects on metabolism [6]. Leproult et al. reported that circadian misalignment is associated with increased insulin resistance and inflammation, independent of sleep loss [35]. In another study, Scheer at al. demonstrated that circadian misalignment leads to decreased leptin, increased glucose, insulin, and mean arterial pressure, and inversion of the normal diurnal cortisol secretion pattern [4]. The precise mechanisms by which these changes occur are still unclear. In animal models, circadian disruption in susceptible rats led to more rapid loss of beta-cell function and increased beta-cell apoptosis, resulting in decreased beta-cell mass, decreased glucose-stimulated insulin secretion, and accelerated development of diabetes [36]. Even after many years of night shift work, circadian rhythms do not fully adjust to the shifted sleep-wake cycle [37]. The metabolic effects of long-term shift work likely underlie a part of the association with diabetes that we and others describe, and which strengthens with years of exposure to sleep disruption.
The present study has several strengths, including its large size, high rates of follow-up, prospective data on shift work and on many important potential confounders, and a high-risk population group not previously studied with regard to shift work and diabetes. However, we do note some limitations. We relied on self-reported measures at one point in time of cumulative exposure to night shift work and did not have data on specific schedules or dates, which introduces the possibility of exposure misclassification. We did not collect data on shift work status subsequently. We also relied on a self-reported diabetes diagnosis rather than clinical data. However, in an earlier validation study among 229 participants whose physicians returned a completed questionnaire, we found 96% of self-reported diabetes cases to be confirmed in the medical record. Prevalence of undiagnosed diabetes in the BWHS (6.4%) was similar to what has previously been reported in African-American women [22]. We found no major difference in prevalence of undiagnosed diabetes between night shift workers (6.1%) vs. non night shift workers (6.5%) (p = 0.72 for difference of proportions), or between women who answered the night shift questions (6.3%) vs. women who did not answer the night shift questions (6.9%) (p = 0.55 for difference of proportions) (Supplementary Table 2). We estimated that the observed prevalence of undiagnosed diabetes in the BWHS, given non-differential misclassification between night shift workers and non night shift workers, would result in an underestimation of about 5% of the true association of night shift work with diabetes (see online appendix for calculations). While we did find significant associations between night shift work and diabetes, the true association may be stronger. A certain degree of selection bias is also possible given that not all women who answered the 2005 questionnaire did answer the night shift questions. Although we are unable to quantify the degree of bias, the fact that women who did not answer the night shift questions had similar characteristics regarding major risk factors for type 2 diabetes (i.e. age, BMI, family history of diabetes, diet, and physical activity) (Supplementary Table 1) as well as similar prevalence of undiagnosed diabetes (Supplementary Table 2) compared to women who did answer the night shift questions suggests that selection bias might have a minor effect in the present results. We cannot completely rule out the presence of residual confounding due to measurement error of covariates, or failure to control for other confounders. With regard to BMI, a previous validation study found that self-report of height and weight in the BWHS is highly accurate [25]. In addition, the association with night shift work was present even among obese women. Also, previously reported associations of BMI [38] and behavior variables [39] with diabetes in BWHS suggest that our covariate measures capture biologically-relevant information.
In summary, we found that black women with long-duration night shift work had a higher risk of incident diabetes. The fact that the association remained, though attenuated, after adjustment for lifestyle factors and BMI suggests that additional pathways such as disruption of the circadian system may be playing a role. In view of the high prevalence of shift work among U.S. workers (35% among non-Hispanic blacks and 28% in non-Hispanic whites) [40], an increased diabetes risk among this group has important public health implications. There is a need for continued research into facilitating circadian adaption to shift work [3] and consideration of avoiding shift work in favor of other work arrangements when possible [37].
Supplementary Material
Acknowledgements
We thank the Black Women's Health Study participants for their continuing participation in this research effort. We also thank Dr. Eva Schernhammer for her help in designing the questions on night shift work.
Funding
This work was supported by National Institute on Minority Health and Health Disparities (NIMHD) grant R01MD007015 and by National Cancer Institute (NCI) grant R01CA058420 and UM1CA164974. ERN is supported by grant 11SDG7390014 from the American Heart Association. Additionally, the material is based upon work or supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
Abbreviations
- BMI
Body-mass index
- BWHS
Black Women's Health Study
- CI
Confidence interval(s)
- FFQ
Food frequency questionnaire
- HR
Hazard ratios
- NHS
Nurses’ Health Study
- SES
Socio-economic status
Footnotes
Contribution statement
All authors made substantial contributions to one or more of: the study conception and design, acquisition of data, and analysis and interpretation of the data. All authors contributed to drafting and/or revising the article critically for important intellectual content, and all authors provided their final approval of the version to be published. Dr. Ruiz Narváez is the guarantor of this work.
Duality of interest
The authors have no conflicts of interest.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the United States government, the National Institute on Minority Health and Health Disparities, the National Cancer Institute, the National Institutes of Health, or the American Heart Association.
REFERENCES
- 1.Beers TM. Flexible schedules and shift work: replacing the 9-to-5 workday. Monthly Lab Rev. 2000;123:33. [Google Scholar]
- 2.Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. J Neuroendocrinol. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 3.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puttonen S, Harma M, Hublin C. Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;36:96–108. doi: 10.5271/sjweh.2894. [DOI] [PubMed] [Google Scholar]
- 6.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. Bmj. 2012:345. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 9.Pietroiusti A, Neri A, Somma G, et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–57. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 10.Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int. 2009;26:740–755. doi: 10.1080/07420520902929029. [DOI] [PubMed] [Google Scholar]
- 11.Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–559. doi: 10.1080/07420520902821176. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23:155–168. doi: 10.1017/S0954422410000016. [DOI] [PubMed] [Google Scholar]
- 14.Kim MJ, Son KH, Park HY, et al. Association between shift work and obesity among female nurses: Korean Nurses' Survey. BMC Public Health. 2013;13:1204. doi: 10.1186/1471-2458-13-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Amelsvoort LG, Schouten EG, Kok FJ. Duration of shiftwork related to body mass index and waist to hip ratio. Int J Obes Relat Metab Disord. 1999;23:973–978. doi: 10.1038/sj.ijo.0801028. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson AK, van den Donk M, Hilding A, Ostenson CG. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care. 2013;36:2683–2689. doi: 10.2337/dc12-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa Y, Nakagawa H, Miura K, et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- 20.Suwazono Y, Sakata K, Okubo Y, et al. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. J Occup Environ Med. 2006;48:455–461. doi: 10.1097/01.jom.0000214355.69182.fa. [DOI] [PubMed] [Google Scholar]
- 21.Gan Y, Yang C, Tong X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2014 doi: 10.1136/oemed-2014-102512. [DOI] [PubMed] [Google Scholar]
- 22.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 23.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50:56–58. [PubMed] [Google Scholar]
- 25.Carter-Nolan PL, Adams-Campbell LL, Makambi K, Lewis S, Palmer JR, Rosenberg L. Validation of physical activity instruments: Black Women's Health Study. Ethn Dis. 2006;16:943–947. [PubMed] [Google Scholar]
- 26.Kumanyika SK, Mauger D, Mitchell DC, Phillips B, Smiciklas-Wright H, Palmer JR. Relative validity of food frequency questionnaire nutrient estimates in the Black Women's Health Study. Ann Epidemiol. 2003;13:111–118. doi: 10.1016/s1047-2797(02)00253-3. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 28.ARP N. Diet*Cal Analysis Program. Bethesda MD.: 2005. [Google Scholar]
- 29.Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in African American women. Am J Clin Nutr. 2011;94:86–94. doi: 10.3945/ajcn.111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women's Health Study. Am J Epidemiol. 2010;171:564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coogan PF, Cozier YC, Krishnan S, et al. Neighborhood socioeconomic status in relation to 10-year weight gain in the Black Women's Health Study. Obesity (Silver Spring) 2010;18:2064–2065. doi: 10.1038/oby.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Axelsson J, Puttonen S. Night shift work increases the risk for type 2 diabetes. Evid Based Med. 2012;17:193–194. doi: 10.1136/ebmed-2012-100649. [DOI] [PubMed] [Google Scholar]
- 34.Pilcher JJ, Lambert BJ, Huffcutt AI. Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep. 2000;23:155–163. [PubMed] [Google Scholar]
- 35.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan S, Rosenberg L, Djousse L, Cupples LA, Palmer JR. Overall and central obesity and risk of type 2 diabetes in U.S. black women. Obesity (Silver Spring) 2007;15:1860–1866. doi: 10.1038/oby.2007.220. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan S, Rosenberg L, Palmer JR. Physical activity and television watching in relation to risk of type 2 diabetes: the Black Women's Health Study. Am J Epidemiol. 2009;169:428–434. doi: 10.1093/aje/kwn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Prevalence rates of work organization characteristics among workers in the U.S.: data from the 2010 National Health Interview Survey. Am J Ind Med. 2013;56:647–659. doi: 10.1002/ajim.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.