Abstract
Three ferrocene complexes vectorized with estrogens and vitamin D2 were synthesized and fully characterized by spectroscopic, electrochemical and computational methods. The synthesis of these esters was accomplished by reacting ferrocenoyl chloride with the corresponding ROH groups (R = ergocalciferol, estradiol, estrone). The cytotoxicity of these complexes in HT-29 colon cancer and MCF-7 breast cancer cell lines was investigated in vitro. Only ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate showed good cytotoxic activity in both cell lines, exceeding those of ferrocenium and ferrocene. In MCF-7, ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate exhibited remarkable IC50, in the low micromolar range. This may be attributed to the presence of the estradiol vector. Docking studies between alpha-estrogen receptor ligand binding site and ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate revealed some key hydrophobic interactions that might explain the cytotoxic activity of this ester.
Introduction
Metals have a long story of being used as therapeutic agents in the treatment of fungal diseases, arthritis, ulcers and other ailments. However, the interest of metals in medicine was awakened by the accidental discovery of cis-platin, Pt(NH3)2Cl2, as a potential anticancer agent in 1970.1 Many coordination compounds, mainly derivatives of cis-platin, were investigated but the use of transition metal organometallic compounds in biology was basically unexplored. It was surprising that ferrocene (I) was not investigated as a potential drug since it had many applications in organic synthesis, catalysis and material science.2–5 Its thermodynamic and kinetic stabilities as well as the redox properties makes ferrocene an excellent candidate to be investigated in the biological arena.
 |
In 1984 Köpf–Maier and co-workers reported for the first time the anticancer properties of ferrocenium in Ehrlich ascite tumor.6 Subsequently, ferrocenium, ferrocene and functionalized-ferrocenes have been studied in biological and medicinal applications.7–10 While initially the ferrocenium complex was reported to be the active species responsible for the formation of oxygen free radical species, inducing oxidative damage to DNA,11,12 on a recent report ferrocene was found to possess anticancer activity due to the metabolic formation of ferrocenium.13,14 As a consequence, functionalized ferrocene has become an area of interest for many investigators in cancer research.7–10
We have recently reported the cytotoxic activity of ferrocenyl ester derivatives in HT-29 colon cancer and MCF-7 breast cancer cell lines.15 Our in vitro cytotoxicity study showed that as we increase the lipophilic character of the functionalized ferrocene, the cytotoxicity improves, approaching the cytotoxic activity of ferrocenium.15 In order to continue our assessment on the role of the pendant groups on the cyclopentadienyl (Cp) ligand, herein we report our finding on vectorized ferrocenoyl esters with functional groups of biological importance.
2. Results and discussion
2.1 Synthesis and structure
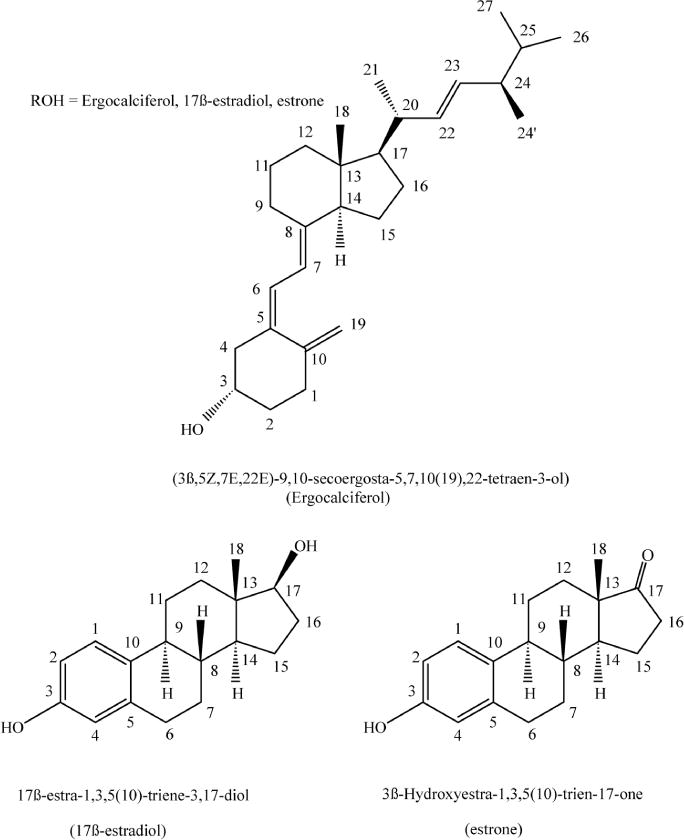
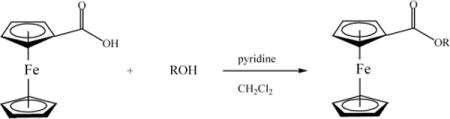
The synthesis of ferrocenyl esters was achieved using ferrocenoyl chloride as the starting material and by reaction of this species with the corresponding ROH groups (R = ergocalciferol, estradiol, estrone, Scheme I), eqn (1). This is a well-known route for the synthesis of ferrocenyl esters.16 The selection of the pendant groups is based on the following criteria. Estradiol and estrone are estrogens which can be recognized by the estrogen receptors and potentially make the ferrocenyl group selective for hormone dependent breast cancer. Ergocalciferol is vitamin D2.17 Ergocalciferol undergoes enzymatic hydroxylation to yield active vitamin D.17 Recently, it has been reported that vitamin D can help to prevent colon, breast and ovarian cancers.18,19 In addition, vitamin D receptor (VDR), as well as glucocorticoids (GR), mineralocorticoids (MR), progesterone (OR), androgen (AR) and estrogen (ER) receptors, belong to the nuclear hormone receptor superfamily.20 Thus, in principle this complex can lead to a receptor specific drug.
Scheme 1.
The new species were characterized by analytical and spectroscopic methods. The structures of these new species, ferrocenoyl (3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-olate (ergocalciferol ferrocenoylate), ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate (estradiol ferrocenoylate) and ferrocenoyl 3β-estra-1,3,5(10)-trien-17-one-3-olate (estrone ferrocenoylate), have been determined by NMR methods. The 3D structure of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate has been determined by DFT calculations using GAUSSIAN 03.21 The lowest energy structure of the ferrocenoyl is depicted in Fig. 2a and will be discussed in the next section.
 |
(1) |
Fig. 2.
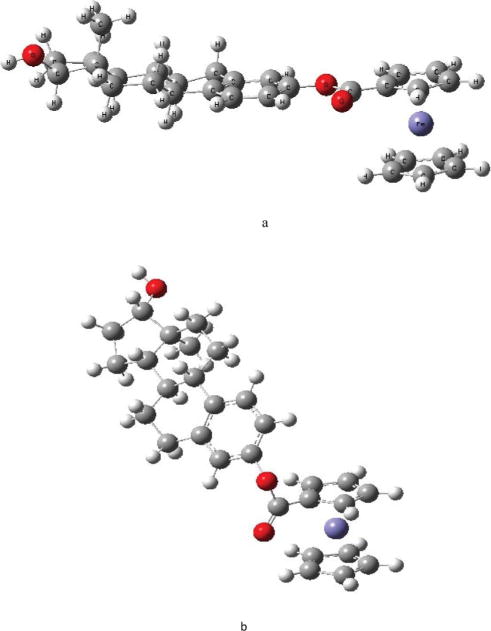
Conformations of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate a) lowest energy conformer b) conformation inside the receptor.
The IR spectral data showed v(C=O) broad bands in the 1710–1726 cm−1 range, corresponding to the carbonyl groups of the esters. In the 13C NMR spectra, the ferrocenoyl esters showed a signal at about 170 ppm, attributed to the carbonyl carbons of the ester groups. In the 1H NMR spectra, the complexes showed one signal (singlet) at about 4.1–4.3 ppm, corresponding to the unsubstituted Cp rings. The functionalized Cp ring showed two sets of multiplets between 4.4 to 5 ppm, corresponding to an AA′BB′ splitting pattern of the four Cp protons. In general, the proton signals of the pendant groups shifted downfield when compared to the free hormones. In particular, the proton signals of the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate (estradiol ferrocenoylate) was assigned using previously published assignments on estradiol.22
2.2 Calculated structure by density functional theory
In the absence of a single crystal for X-ray structure analysis, we calculated the optimum structure for ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate using GAUSSIAN 03.21 The purpose of calculating the optimum structure (conformation) is to compare it with the structure adopted in the estrogen receptor ligand binding domain (ER LBD). For β-estradiol, we used the X-ray structure of β-estradiol found in the ERα (code: 1A52 pdb) and created a separate file with its coordinates and then the hydrogen at C-3 was replaced by ferrocenoyl. The resulting structure was energy minimized using density function theory (DFT) with the B3LYP hybrid functional and the 6–31G** basis set. Once the optimum structure was calculated (Fig. 2a), single point energies for nine conformations at different torsion angles (a and b angles) were calculated, see Table 1. Torsion angles are depicted in Fig. 1 and are defined as follows. Torsion angle a is the angle between the Cp ring and the C=O group while torsion angle b is between the C–O (of the ester group) and the phenyl ring. Fig. 2a depicts the lowest energy conformation while Fig. 2b is the conformation adopted inside the estrogen receptor, ERα. The selected bond distances and angles of 2a are presented in Table 2.
Table 1.
Single point energies for nine conformations at different a and b torsion angles of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate
| Complex ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate | Torsion angle between Cp and (C=O) group, a (°) | Torsion angle between C–O and phenyl ring, b (°) | Energy (eV) |
|---|---|---|---|
| 1 (most stable 2a) | 0.4 | 2.6 | −7.11118 × 104 |
| 2 (conformation in the receptor 2b) | 90.0 | 62.6 | −7.110954 × 104 |
| 3 | 0 | 62.6 | −7.110951 × 104 |
| 4 | 90 (below Cp ring) | 62.6 | −7.110990 × 104 |
| 5 | 40 (below Cp ring) | 62.6 | −7.110981 × 104 |
| 6 | 0 | 62.6 | −7.110973 × 104 |
| 7 | 7.7 | 62.7 | −7.110815 × 104 |
| 8 | 180 | 62.7 | −7.110822 × 104 |
| 9 | 90 (below Cp ring) | 90 | −7.110811 × 104 |
| 10 | 0 | 90 | −7.110826 × 104 |
Fig. 1.
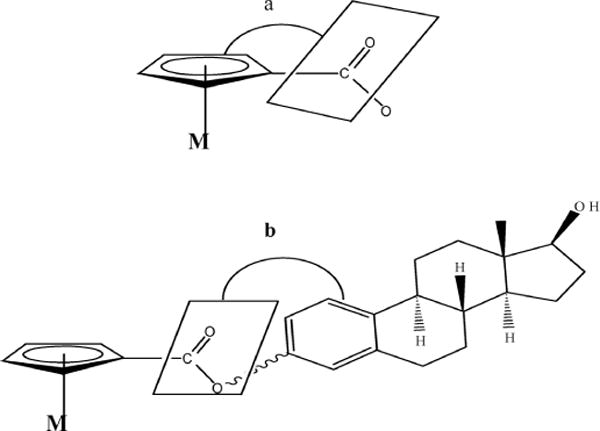
Torsion angles a and b in ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate structure.
Table 2.
Selected bond distances and torsion angles of 2a.
| Bonds | Bond distance (Å) |
|---|---|
| C(Cp)–C(CO2) | 1.459 |
| C=O | 1.238 |
| C–O (CO2) | 1.389 |
| O(CO)–C(Ph) | 1.418 |
| Fe–C(Cp) average | 2.081 |
| Fe–C*(Cp–CO2) | 2.064 |
| Fe–C(substituted Cp) average | 2.077 |
|
| |
| Groups | Torsion angle (°) |
|
| |
| Cp and (C=O) group a | 0.4 |
| C–O and phenyl ring b | 2.6 |
indicates substituted Cp ring
The ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate structure (Fig. 2a) showed that the two Cp rings of ferrocene are eclipsed and the angles between the substituted Cp and the C=O group of the estradiol are co-planar with a torsion angle of only 0.4°. This is not very different from what has been observed for ferrocene-1-1′-dicarboxylic acid, which has tilt angles of 0.4° and 4.1° between the Cp plane and the carboxy plane.23 This co-planarity between the Cp and the C=O groups is favored to allow efficient overlap between the two π-systems.23 The torsion angle between the C–O (CO2 group) and the phenyl ring is almost co-planar, with a deviation from planarity of 2.6°. Delocalization of electrons between the Cp and aromatic ring through the CO2 group is not observed since the Cp–C(CO2) bond distance is 1.459 Å whereas the C–O(CO2) and O–C(phenyl) bond distances are 1.389 Å and 1.418 Å respectively, all corresponding to single bonds. The last two bond distances are important since the estradiol can rotate around these bonds, potentially leading to different conformations. However, the conformation of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate inside the LBD is represented by Fig. 2b which has higher energy than the one in Fig. 2a, with a difference of 2.26 eV (3.7 × 10 −22 kJ). Thus, the hydrophobic interactions in the LBD between the protein and ferrocenoyl ester might be responsible for this change in the ferrocenoyl conformation, as discussed later.
2.3 Cytotoxic studies on HT-29 and MCF-7 cell lines
In order to elucidate and develop structure–activity relationships, the cytotoxicity properties of the ferrocenyl esters were determined on hormone-dependent breast cancer MCF-7 and colon cancer HT-29 cell lines using the MTT cell viability assay.24 Table 3 shows the IC50 values on both cell lines after 72 h of drug exposure.
Table 3.
Cytotoxicities of the ferrocenoyl esters studied on MCF-7 breast cancer and HT-29 colon cancer cell lines, as determined by MTT assay after 72 h of drug exposure. IC50 values based on quadruplicate experiments and standard deviation in parenthesis. n/a is non-active
| Complex | IC50 × 10−6 M, MCF-7 | IC50 × 10−6 M, HT-29 |
|---|---|---|
| Ferocenoyl (3β,5Z,7E,22E)-9,10-secoergosta5,7,10(19),22-tetraen-3-olate | 1326(110) | 346(50) |
| Ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate | 9(2) | 24.4(6) |
| Ferrocenoyl 3β-estra1,3,5(10)-trien-17-one-3-olate | 108(7) | n/a |
| Ferrocene | 1500 | 360 |
| Ferrocenium tetrafluoroborate | 150 | 180 |
In the MCF-7 cell line, the ferrocenoyl esters proved more cytotoxic than ferrocene and ferrocenium, except for ferrocenoyl (3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-olate which demonstrated poor cytotoxic properties. Substantial improvement is evident on ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate with IC50 values in the micromolar range, about two orders of magnitude more cytotoxic than ferrocenium tetrafluoroborate. The introduction of this type of hormone vector appears to be a convenient strategy to develop highly active target specific species in breast cancer.
On the HT-29 colon cancer cell line, the response was somewhat similar. Ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate exhibited better cytotoxicity than ferrocene and ferrocenium, whereas the ergocalciferol substituent yielded an ester with lower cytotoxic activity than ferrocenuim. The low solubility of ferrocenoyl 3β-estra-1,3,5(10)-trien-17-one-3-olate hindered us to determine the IC50 values on HT-29 cell line. Notably, ferrocenoyl (3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-olate has better cytotoxicity in the colon cancer HT-29 than in the breast cancer MCF-7 cell line.
2.4 Molecular modeling – docking studies
In order to explain the cytotoxic effect of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate on the MCF-7 breast cancer cell line, we performed docking studies between the ERα and the above complex using VINA program.25 It is known that the receptor agonist must fit the ligand binding pocket of the receptor, whereas the antagonist may reside either in the pocket or in another location, but must induce a conformational change in the protein structure, inactivating the receptor. Thus to elucidate this, docking studies were appropriate to understand the structural factors involved in this interaction.
As a starting point, the ERα structure (code: 1A52 pdb) initially occupied by β-estradiol was selected. This is a dimeric structure, thus only half of it was used for the studies. The β-estradiol inside the binding pocket was replaced by ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate with the most stable conformation (Fig. 2a). As stated before, this structure has lengths in the Cp–C(CO2), O–C(CO2) and O–C(phenyl) bonds corresponding to single bonds. Thus, the estradiol is able to rotate around these bonds, forming different conformations. In order to establish the ideal position for the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate complex, two sets of calculations were performed: a) with the amino acids lining the ligand binding domain (LBD) fixed but the ferrocenoyl ester flexible; b) the amino acids lining the (LBD) as well as the ferrocenoyl ester were set flexible, allowing them to move freely in the ligand binding domain (LBD). The initial structure was the ferrocenoyl ester inside the β-estradiol binding pocket. This structure was found to be unstable and did not reach a minimum in energy. Instead, ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate moved away from this pocket apparently due to steric hindrance of the Cp rings inside the pocket. Several structures were calculated, but the minimum energy structure of the ligand–receptor obtained is depicted below, as shown in Fig. 3.
Fig. 3.
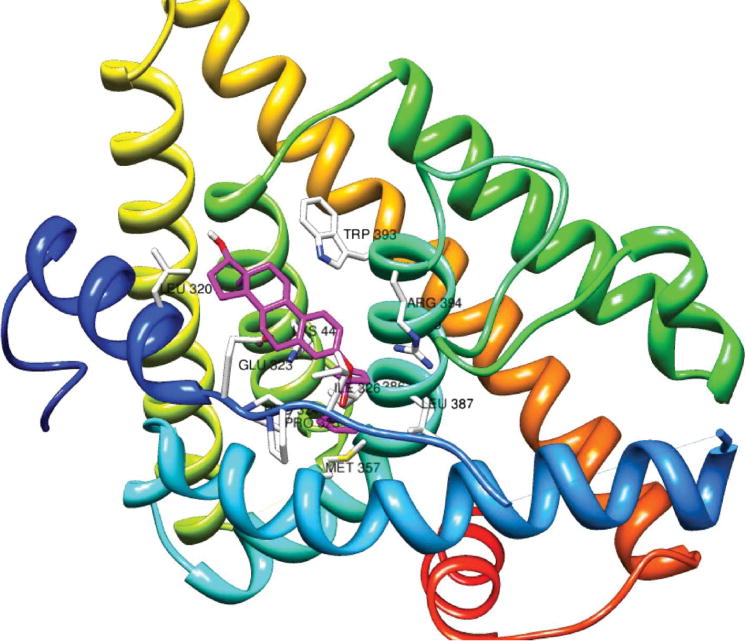
Representation of the interaction of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate in the ERα LBD. The complex structure is colored violet.
Our docking studies demonstrated that our lowest energy structure (conformation) is when ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate moves away from the β-estradiol binding pocket. It docks between helix 1 and helix 5 and cannot fit into the pocket due to the steric hindrance with the ferrocenoyl moiety. The structure where the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate is residing in the β-estradiol binding pocket is highly energetic. For comparison, E2 (17β-estradiol) docks between helix 3 and helix 5.26 The amino acids surrounding the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate are different to those of the β-estradiol binding pocket, as described below. Particularly important is that the conformation adopted by ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate (Fig. 2b) inside the LBD is not the lowest energy conformation calculated in the previous section with Gaussian 3.0.21
The ferrocenoyl Cp rings get involved in hydrophobic interactions with PRO 324, PRO 325, ILE 326, GLU 353, MET 357, ILE 386, GLY 390 and ARG 304 but the 17β-hydroxy-estra-1,3,5(10)-trien-3-olate group is not sitting in the typical E2 ligand binding pocket (LBP), see Fig. 3.
The 17β-hydroxy-estra-1,3,5(10)-trien-3-olate group (pendant group) is surrounded by LEU 320, GLU 323, TRP 393, ARG 394, and LYS 449. The amino acids mentioned above are the closest to the ferrocenoyl ester complex, at a distance of 3.5 Å. In contrast, in β-estradiol (E2, natural estrogen ligand) the phenol gets involved in hydrogen bonding with GLU 353 and ARG 394, whereas the C-17 hydroxyl group hydrogen bonds with HIS 524. In addition, β-estradiol is surrounded by LEU 346, LEU 349, ALA 350, LEU 354, LEU 387, MET 388, LEU 391, PHEN 404, GLU 419, LEU 428, LEU 525 and LEU 540.26 Thus, the ferrocenoyl 17b-hydroxy-estra-1,3,5(10)-trien-3-olate complex gets involved in hydrophobic interactions using a different set of amino acids to β-estradiol and does not involve hydrogen bonding with GLU 353 and ARG 394 or HIS 524. This suggests that since ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate docks in a different place on the receptor, the position of the ferrocenoyl complex in the LBD causes changes in the protein conformation. This may inactivate the estrogen receptor and explain the high cytotoxic activity of this ferrocenyl ester complex in the MCF-7 cell line, if indeed it behaves as an antagonist. But the genotoxic properties of these species due to the redox behavior of ferrocene group (see next section) cannot be ignored.
To put in perspective ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate and in order to explain its docking interactions, we compared it with other metal complexes containing estradiol as a pendant group.27 Docking studies on 17α-ruthenocenylethyleneestradiol and 17α-ruthenocenylmethyleneestradiol have been performed with ERα to explain why the former complex has an affinity to the estrogen receptor and not the 17α-ruthenocenylmethyleneestradiol. They determined that 17α-ruthenocenylethyleneestradiol can fit into the E2 binding pocket, allowing the ruthenocenyl group passing through the bottleneck of the pocket. In contrast, 17a-ruthenocenylmethyleneestradiol exhibits steric hindrance between the ruthenocenyl group and the aminoacids in the bottleneck of the receptor.27
Our result is somewhat different, as is the structure of ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate. In ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate the hydrogen of the hydroxyl group in position C-3 (in β-estradiol) is substituted by a ferrocenecarbonyl group and the hydrogen bonds with GLU 353 and ARG 394 are absent. As a result, the ferrocenenoyl group encounters steric hindrance with the amino acids lining the LBD and do not allow the complex to fit into the ligand binding pocket. In fact, the steroid is rotated by about 180° (as compared to E2) but the position of the C-17 hydroxyl group is not close enough to the GLU353 and ARG 394 amino acids to be able to engage in hydrogen bonding.
2.5 Electrochemical characterization
The redox behavior of functionalized ferrocenes is of fundamental importance since the facile oxidation to ferrocenium is attributed to be a key step inducing genotoxicity. Thus, the subject complexes were investigated by cyclic voltammetry. Epa and Epc are presented in Table 4 and compared to the Fc/Fc+ redox couple. The cyclic voltammograms are depicted in Fig. 4.
Table 4.
Redox potential of ferrocenoyl esters in CH3CN 0.1 M [NBun4]PF6 at a scan rate of 100 mV s−1, using Ag/AgCl saturated as standard electrode. The Ferrocenoyl concentration was 2 × 10−3 M
| Complex: Ferrocenoyl − | Epa(mV) | Epc(mV) | ΔE(mV) | E1/2(mV) |
|---|---|---|---|---|
| 17b-hydroxy-estra-1,3,5(10)-trien-3-olate | 801 | 711 | 90 | 756 |
| 3β-estra1,3,5(10)-trien-17-one-3-olate | 827 | 735 | 92 | 781 |
| (3β,5Z,7E,22E)-9,10-secoergosta5,7,10(19),22-tetraen-3-olate | 770 | 656 | 90 | 713 |
| Fc/Fc+ | 494 | 414 | 84 | 452 |
Fig. 4.
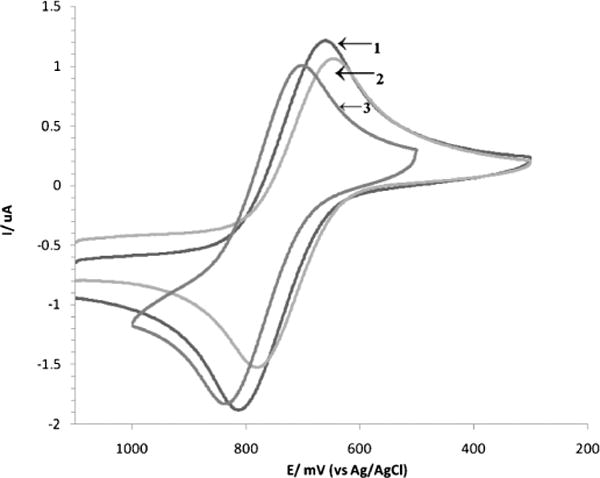
Cyclic voltammograms of estradiol (1), ergocalciferol (2) and estrone (3) ferrocenoylates in CH3CN with 0.1 M of [NBun4]PF6 as supporting electrolyte and ferrocene complex concentration of 2 × 10−3 M at room temperature. The working electrode was a platinum disk, the reference electrode was Ag/AgCl and the scan rate was 100 mv s−1.
Upon examination of Table 4 it is evident that all three functionalized ferrocenoyls undergo reversible redox processes with an ipa/ipc ratio close to one and demonstrate oxidation potentials, Epa, higher than ferrocene. This is the result of the electrowithdrawing (inductive) effect of the ester groups on the Cp rings. In principle, the ferrocenoyl esters should generate Fc+ radical species but less readily than ferrocene. The Fc+ radical is a key species which generates oxo radicals, inducing oxidative stress in the cell and causing cell death. Interestingly, ferrocenoyl (3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-olate has the lower oxidation potential but it is not the most cytotoxic complex, which may suggest that the cytotoxic activity of these functionalized ferrocenes, in particular ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate (estradiol ferrocenoylate), does not rely only on its redox behavior but in a greater extent on the vector (E2 hormone). However, our electrochemical data was obtained under non-physiological conditions (organic solvent) thus, the redox behaviors of the ferrocenoyl esters and the amount of ROS formed under physiological conditions cannot be ruled out. In any event and very importantly, we can envision that the role of the vector is important in two ways, most likely targeting the receptor as well as increasing the lipophilic character of the ferrocene.
3. Concluding remarks
We have synthesized three functionalized ferrocenes with estrogens and vitamin D2 as vectors intended to be potentially target and receptor specific drugs. The ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate showed the highest cytotoxic activity, in particular to the hormone dependent MCF-7 breast cancer cell line. There are few ferrocenes reported with high cytotoxic activity against breast cancer.28–30 In particular, Jaouen and co-workers synthesized a series of ferrocenes with selective receptor modulator (SRM) as a pendant group to yield antagonist to estrogen hormone receptor.28,29 For instance, ferrocifens (ferrocene containing tamoxifen) have IC50 values in the micromolar range as a result of binding the estrogen receptor and serving as antagonists.28,29 Thus, the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate could potentially bind the estrogen receptor and behave as an antagonist. To investigate this possibility, we performed docking studies between ERα and ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate as well as determining the lowest energy conformation of the ferrocenoyl ester. The ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate does not fit into the E2 binding pocket and the conformation adopted in the LBD (Fig. 2b) is not the lowest energy conformation calculated by using DFT methods. The difference in energy between 2a and 2b is 2.26 eV. This indicates that conformation 2b is adopted inside the protein, although it is not the most stable conformation, as a result of the hydrophobic interactions between the amino acids surrounding the LBD and the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate. These hydrophobic interactions provide sufficient energy to the ferrocenoyl ester to overcome this energy barrier and change the conformation.
Other functionalized ferrocenes with diphenols have been prepared and reported to have good antiproliferative activity against hormone dependent MCF-7 and hormone independent MDA-MB231 breast cancer cells with IC50 values of 0.7 and 0.6 μM, respectively.30 It is hypothesized that the high cytotoxic activity of these ferrocenes is attributed to the facile oxidation to yield ferrocenium radical cations, activating the redox process in the cell. While this possibility cannot be ruled out, our electrochemical studies strongly suggest that the cytotoxic activity of the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate does not rely only on redox behavior, but in the capacity of the vector (E2 hormone) to target most likely the estrogen receptor. Thus, ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate showed good antineoplastic activity probably due to the hormone vector making this species potentially a target specific drug.
4. Experimental section
4.1 General procedure
All reactions were performed under an atmosphere of dry nitrogen using schlenk glassware or a glovebox, unless otherwise stated. Reaction vessels were flame dried under a stream of nitrogen, and anhydrous solvents were transferred by oven-dried syringes or cannula. Tetrahydrofuran was dried and deoxygenated by distillation over K-benzophenone under nitrogen. Infrared spectra were recorded on a Brucker Vector-22 spectrometer with the samples as compressed KBr discs. The NMR spectra were obtained on a 500 MHz Bruker spectrometer. Elemental analyses were obtained from Atlantic Microlab Inc. Silica gel was heated at about 200 °C while a slow stream of dry nitrogen was passed through it.
The colon cancer cell line HT-29 and the breast adenocarcinoma cell line MCF-7 were purchased from American Type Culture Collection and were maintained at 37 °C and 95% Air–5% CO2. Growth medium for HT29 was McCoy’s 5A complete medium supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) antibiotic/antimycotic. Growth medium for MCF7 was Eagle’s Minimum Essential Media supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) antibiotic/antimycotic, non-essential amino acids and 0.01 mg mL−1 bovine insulin. MTT and Triton X-100 used for the cytotoxic assay were obtained from Sigma. All MTT manipulations were performed in a dark room.
4.2 Synthesis
General synthesis of ferrocenoyl esters
In a 50 mL three necked round bottom flask and under nitrogen atmosphere, 1 mmol of ferrocenecarboxylic acid was dissolved in 15 mL of dry dichloromethane at room temperature. To this solution, 170–200 μL of oxalyl chloride was added dropwise and stirred overnight. The solution changed from orange to dark red. The reaction mixture was filtered in a fritted funnel and the filtrate collected. In a separate 100 mL three necked round bottom flask under nitrogen, 1.0 mmol of the hormone, and 1.0 mmol of pyridine were dissolved in 10–15 mL of dichloromethane. To this solution, the ferrocenecarbonyl chloride solution prepared previously was added dropwise. The solution was stirred for 6–12 h in the dark under a nitrogen atmosphere and at room temperature. Thin layer chromatography (TLC) was used to monitor the reaction. After the reaction was finished, the mixture was filtered in a fritted funnel with a pad of celite. The filtrate collected was washed with 3 × 5 mL 1 N HCl to remove pyridine and other by-products. The organic layer containing the compound was purified by column chromatography, using Silica gel and eluted with dichloromethane and isolated in 40–45% yield.
Ferrocenoyl 3β-estra1,3,5(10)-trien-17-one-3-olate
1H NMR (500 MHz, CDCl3) δ (ppm): 7.34 (d, 1H, 3J = 8.44 Hz, 1H), 6.96 (d, 1H, 3J = 8.57 Hz, 2H), 6.92 (s, 1H, 4H), 4.96 (s, 2H; Cp), 4.50 (s, 2H; Cp), 4.31 (s, 5H; Cp), 2.96 (m, 2H, 6Hαβ), 2.53 (dd, 3J = 18.92, 1H, 3J = 8.69, 16-βH), 2.44 (m, 1H, 11Hα), 2.33 (t, 1H, 9H), 2.19−1.98 (m, 4H, 16Hα, 12Hβ, 7Hβ, 15Hα), 1.65−1.49 (m, 6H, 11Hβ, 8H, 15Hβ, 7Hα, 12Hα, 14H), 0.94 (s, 3H, 18-CH3). 13C NMR (125 MHz, CDCl3), δ (ppm): 220.9 (C=O), 170.7, 149.0, 138.2, 137.3, 126.6, 121.9, 119.1, 72.1, 70.9, 70.5, 70.2, 50.7, 48.2, 44.4, 38.3, 36.1, 31.8, 29.7, 26.6, 26.0, 21.8, 14.1. IR (KBr, cm−1): 3104, 2939, 2869, 1726, 1707, 1494, 1454, 1376, 1269, 1221, 1107. Anal. Calc. for C29H30O3Fe*1/8(CH2Cl2): C, 69.61; H, 6.44. Found: C, 70.90; H, 6.54.
Ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate
1H NMR (500 MHz, DMSO-d6) δ (ppm): 7.33 (d, 1H, 3J = 8.55 Hz, 1H), 6.93 (dd, 1H, 3J = 6.27, 4J = 2.07 Hz, 2H), 6.87 (s, 1H, 4H), 4.89 (s, 2H; Cp), 4.59 (s, 2H; Cp), 4.52 (d, 1H, OH), 4.34 (s, 5H; Cp), 3.54 (m, 1H, 17H), 2.83 (m, 2H, 6Hαβ), 2.32 (dd, 1H, 11Hα), 2.18 (dt, 1H, 9H), 1.90−1.81 (m, 3H, 16Hα, 12Hβ, 7Hβ), 1.60 (q, 1H, 15Hα), 1.34 (m, 1H, 16Hβ), 1.31 (m, 1H, 11Hβ), 1.29−1.10 (m, 5H, 8H, 15Hβ, 7Hα, 12Hα, 14H), 0.66 (s,3H, 18H (CH3)). 13C NMR (125 MHz, DMSO-d6), δ (ppm): 169.7 (C=O), 148.2, 137.8, 137.5, 126.2, 121.5, 118.9, 80.0, 71.9, 70.2, 69.8, 69.6, 49.5, 43.7, 42.8, 40.0, 38.2, 36.5, 29.9, 28.9, 26.6, 25.9, 22.8, 11.2. IR (KBr, cm−1): 3427, 3098, 2924, 2867, 1724, 1453, 1267, 1108. Anal. Calc. for C29H32 O3Fe: C, 71.9; H, 6.65. Found: C, 71.76; H, 6.11.
Ferrocenoyl (3β,5Z,7E,22E)-9,10-secoergosta5,7,10(19),22-tetraen-3-olate
1H NMR (500 MHz, CDCl3) δ (ppm): 6.28 (d, 1H, 3J = 11.25 Hz, 6H), 6.08 (d, 1H, 3J = 11.22 Hz, 7H), 5.20 (m, 2H, 22/23H), 5.13 (m, 1H, 3-βH), 5.10 (s, 1H, 19H), 4.88 (s, 1H, 19′H), 4.81 (s, 1H; Cp), 4.78 (s, 1H; Cp), 4.37 (s, 2H; Cp), 4.19 (s, 5H; Cp), 2.81 (dd, 1H, H9), 2.66 (dd, 1H, H4), 2.52 (m, 1H, H1,1′), 2.29 (m, 1H, H4′), 1.02 (d, 3H, 3J = 6.62 Hz, 21-CH3), 0.927 (d, 3H, 3J = 6.84 Hz, 24′-CH3), 0.840 (t, 3J = 7.31 Hz, 27,26-CH3), 0.572 (s, 3H, 18-CH3). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.24 (C=O), 145.1, 142.5, 135.8, 134.7, 132.2, 122.7, 117.7, 112.8, 71.9, 71.4, 71.3, 70.4, 70.3, 69.9, 56.7, 46.0, 43.0, 42.6, 40.6, 40.6, 33.3, 32.5, 32.4, 29.9, 29.3, 28.0, 23.8, 22.5, 21.3, 19.9, 17.8, 12.47. IR (KBr, cm−1): 3023, 2954, 2925, 2869, 2851, 1712, 1459, 1274, 1138. Anal. Calc. for C38H52O2Fe: C, 76.96; H, 8.61. Found: C, 77.38; H, 8.89.
4.3 Cytotoxic assay
Biological activity was determined using the MTT assay originally described by Mossman24a but using 10% Triton in isopropanol as a solvent for the MTT formazan crystals.24b HT29 and MCF7 cells were maintained at 37 °C and 95% Air–5% CO2 in McCoy’s 5A (ATCC) complete medium, which had been supplemented with 10% (v/v) fetal bovine serum (ATCC) and 1% (v/v) antibiotic/antimycotic (Sigma). Asynchronously growing cells were seeded at 1.5 × 104 cells per well in 96-well plates containing 100 μL of complete growth medium, and allowed to recover overnight. Various concentrations of the complexes (10–1300 μM) dissolved in 5% DMSO–95% medium were added to the wells (eight wells per concentration; experiments performed in quadruplicate plates). The complex solutions were prepared first by dissolving the corresponding ferrocene in DMSO and then medium was added to a final composition of 5% DMSO–95% medium. In addition to the cells treated with the ferrocenes, two controls experiments were run: one without any addition of solvent mixture (5% DMSO–95% medium) and one adding 5% DMSO–95% medium to the cells. Both control experiments behaved identically, showing that 5% of DMSO in the medium did not render toxic to these types of cells. The cells were incubated for an additional 70 h. After this time, MTT dissolved in complete growth medium was added to each well to a final concentration of 1.0 mg mL−1 and incubated for two additional hours. After this period of time, all MTT containing medium was removed, cells were washed with cold PBS and dissolved with 200 μL of a 10% (v/v) Triton X-100 solution in isopropanol. After complete dissolution of the formazan crystals, absorbances were recorded in triplicate on a 340 ATTC microplate reader (SLT Lab Instruments) at 570 nm with background subtraction at 630 nm. Concentrations of compounds required to inhibit cell proliferation by 50% (IC50) were calculated by fitting data to a four-parameter logistic plot by means of SigmaPlot software from SPSS.
4.4 Electrochemistry
Cyclic voltammetric experiments were performed in deoxygenated CH3CN solution of ferrocene complexes with 0.1 M of [NBun4]PF6 as supporting electrolyte and ferrocene complex concentration of 2 × 10−3 M. The three electrodes used were platinum disk as the working electrode, Ag/AgCl as a reference electrode, and Pt wire as an auxiliary electrode. The working electrode was polished with 0.05 mm alumina slurry for 1–2 min, and then rinsed with double-distilled and deionized water. This cleaning process is done before each cv experiment and a sweep between 0 and 2000 mV is performed on the electrolyte solution to detect any possible deposition of ferrocene on the electrode surface.
4.5 Docking studies
Docking studies between the ERα and the complex were performed using VINA program. The ERα dimeric structure (code: 1A52 pdb) initially occupied by β-estradiol was selected but only half of it was used for the studies. The β-estradiol inside the binding pocket was replaced by ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate with the most stable conformation (Fig. 2a). Several structures were calculated to obtain the minimum energy structure of the ligand–receptor by adjusting the following parameters. The rotation of the ester group relative to the Cp ring was either fixed (restricted) or flexible, as well as the amino acids lining the ligand binding domain. The amino acids in the ligand binding pocket were flexible. Among these possibilities, either flexible or fixed amino acids in the LBD with flexible ferrocenoyl complex yielded the same lowest energy structure.
The initial search with the VINA docking calculation was done on the active pocket where β-estradiol binds. To get a better sampling of all possible binding sites on the ERα, the search was expanded to include the whole protein. This resulted in a different conformation with a higher ligand–receptor binding energy. Exhaustiveness was set to the default value. The grid box was centred on the ERα macromolecule and the size of the grid box adjusted to completely include the macromolecule. All rotatable bonds on the ligand were allowed to rotate during the molecular simulation. Regarding the ERα receptor, flexibility in the residues that bind the β-estradiol or the residues that surround the ferrocenoyl 17β-hydroxy-estra-1,3,5(10)-trien-3-olate ligand did not create significant changes in the ligand–receptor binding energy compared to the situation where the macromolecule is completely rigid.
Supplementary Material
Acknowledgments
E.M. acknowledges the NIH-MBRS SCORE Programs at the University of Puerto Rico Mayagüez for financial support via the NIH-MBRS-SCORE Program grant #S06 GM008103-37. J.M. acknowledges support through award 1SC1CA157250-01 through the National Cancer Institute and the PSM-Moffitt Cancer Center Partnership U56 CA126379-05 grant. In addition, EM thanks the NSF-MRI Program for providing funds for the purchase of the 500 MHz NMR instrument.
Footnotes
Electronic supplementary information (ESI) available. See DOI:10.1039/c1dt10995b
References
- 1.Clarke MJ, Clarke F, Frasca DR. Chem Rev. 1999;99:2511–2533. doi: 10.1021/cr9804238. [DOI] [PubMed] [Google Scholar]
- 2.Togni A, Hayashi T, editors. Ferrocenes: Homogenoeous Catalysis-Organic Synthesis-Material Science. VCH; Weinheim, Germany: 1995. [Google Scholar]
- 3.Arrayas RG, Adrio J, Carretero JC. Angew Chem, Int Ed. 2006;45:7674–7715. doi: 10.1002/anie.200602482. [DOI] [PubMed] [Google Scholar]
- 4.Togni A, Halterman RL, editors. Metallocenes. Wiley-VCH; Weinheim, Germany: 1998. [Google Scholar]
- 5.Atkinson RCJ, Gibson VC, Long NJ. Chem Soc Rev. 2004;33:313–328. doi: 10.1039/b316819k. [DOI] [PubMed] [Google Scholar]
- 6.Köpf-Maier P, Köpf H. J Cancer Res Clin Oncol. 1984;108:336–340. doi: 10.1007/BF00390468. [DOI] [PubMed] [Google Scholar]
- 7.Jaouen G, Metzler-Nolte N, editors. Medicinal Organometallic Chemistry. Springer-Verlag; Berlin, Heidelberg: 2010. pp. 81–117. [Google Scholar]
- 8.Jaouen G, editor. Bioorganometallics. Wiley-VCH; Weinheim, Germany: 2006. pp. 65–95. [Google Scholar]
- 9.van Staveren DR, Metzler-Nolte N. Chem Rev. 2004;104:5931–5985. doi: 10.1021/cr0101510. [DOI] [PubMed] [Google Scholar]
- 10.Fouda MFR, Abd-Elzaher MM, Abdelsamaia RA, Labib AA. Appl Organomet Chem. 2007;21:613–625. [Google Scholar]
- 11.Tabbi G, Cassino C, Cavigiolio G, Colangelo D, Ghiglia A, Viano I, Osella D. J Med Chem. 2002;45:5786–5796. doi: 10.1021/jm021003k. [DOI] [PubMed] [Google Scholar]
- 12.Osella D, Mahboobi H, Colangelo D, Cavigiolio G, Vessie’res A, Jaouen G. Inorg Chim Acta. 2005;358:1993–1998. [Google Scholar]
- 13.Miwa M. Chem Lett. 1997;11:1177–1178. [Google Scholar]
- 14.Kovjazin R, Eldar T, Patya M, Vanichkin A, Lander HM, Novogrodsky A. FASEB. 2003;17:467–469. doi: 10.1096/fj.02-0558fje. [DOI] [PubMed] [Google Scholar]
- 15.Gao LM, Hernández R, Matta J, Meléndez E. Met-Based Drugs. 2009 doi: 10.1155/2009/420784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrick RS, Jarret RM, Curran TP, Dragoli DR, Flaherty MB, Lindyberg SE, Slate RA, Thornton LC. Tetrahedron Lett. 1996;37:5289–5292. [Google Scholar]
- 17.Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry. John Wiley and Sons; New York: 2001. p. 230. [Google Scholar]
- 18.Gorham ED. J Steroid Biochem Mol Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Garland CF. Am J Pub Health. 2006;96:9–18. [Google Scholar]
- 20.Mangeisdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Gonzalez C, Pople JA. GAUSSIAN 03 (Revision B.04) Gaussian, Inc.; Wallingford, CT: 2004. [Google Scholar]
- 22.Guo J, Duclos RI, Jr, Vemuri VK, Makriyannis A. Tetrahedron Lett. 2010;51:3465–3469. doi: 10.1016/j.tetlet.2010.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling L, Berces A, Kraatz H-B. J Organomet Chem. 1998;556:11–20. [Google Scholar]
- 24.(a) Mossman T. J Immunol Methods. 1983;65:55–63. [Google Scholar]; (b) Denizot F, Lang R. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 25.Trott O, Olson AJ. J Comp Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wurtz JM, Egner U, Heinrich N, Moras D, Muller-Fahrnow A. J Med Chem. 1998;41:1803–1814. doi: 10.1021/jm970406v. [DOI] [PubMed] [Google Scholar]
- 27.Top S, Hafa HE, Vessiéres A, Huché Ml, Vaissermann J, Jaouen G. Chem–Eur J. 2002;8:5241–5249. doi: 10.1002/1521-3765(20021115)8:22<5241::AID-CHEM5241>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Jaouen G, Top S, Vessières A, Leclercq G, McGlinchey MJ. Curr Med Chem. 2004;11:2505–2517. doi: 10.2174/0929867043364487. [DOI] [PubMed] [Google Scholar]
- 29.Vessières A, Top S, Beck W, Hillard E, Jaouen G. Dalton Trans. 2006:529–541. doi: 10.1039/b509984f. [DOI] [PubMed] [Google Scholar]
- 30.Vessières A, Top S, Pigeon P, Hillard E, Boubeker L, Spera D, Jaouen G. J Med Chem. 2005;48:3937–3940. doi: 10.1021/jm050251o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


