Abstract
As part of our research efforts in the area of titanium-based antitumor agents, we have investigated the cytotoxic activity of [Ti4(maltolato)8(μ-O)4], (Cp-R)2TiCl2 and (Cp-R)CpTiCl2 (R = CO2CH3 and CO2CH2CH3), and three water-soluble titanocene–amino acid complexes—[Cp2Ti(aa)2]Cl2 (aa = l-cysteine, l-methionine, and d-penicillamine)—on the human colon adenocarcinoma cell line, HT-29. The capacity of [Ti4(maltolato)8(μ-O)4] to donate Ti(IV) to human apo-transferrin and its hydrolytic stability have been investigated and compared to the previously reported data on modified titanocenes with either hydrophilic ancillary ligands or the functionalized cyclopentadienyl ligands. Notably, the titanium–maltolato complex does not transfer Ti(VI) to human apo-transferrin at any time within the first seven days of its interaction, demonstrating the inert character of this species. Stability studies on these complexes have shown that titanocene complexes decompose at physiological pH while the [Ti4(maltolato)8(μ-O)4] complex is stable at this pH without any notable decomposition for a period of ten days. The antitumor activity of these complexes against colon cancer HT-29 cells was determined using an MTT cell viability assay at 72 and 96 h. The titanocene–amino acid and the (Cp-R)2TiCl2/(Cp-R)CpTiCl2 (R = CO2CH3) complexes were not biologically active when human transferrin was absent; they also were inactive when human transferrin was present at dose-equivalent concentrations. (Cp-R)2TiCl2 and (Cp-R)CpTiCl2 (R = CO2CH2CH3) showed cytotoxic activity in HT-29 cells comparable to that which is displayed by titanocene dichloride. The titanium– maltolato complex had higher levels of cytotoxic activity than any other titanocene complex investigated. Transferrin may be important in protecting the titanium center from hydrolysis, but this may be achieved by selecting ligands that could result in hydrolytically stable, yet active, complexes.
Keywords: Titanocene dichloride, Maltol, Amino acid, Colon cancer, Antitumor activity, Apo-transferrin, HT-29, Titanium–maltolato
Introduction
The discovery of a metallocene-based anticancer agent, Cp2TiCl2, in 1979 by Köpf and Köpf-Maier stimulated much interest in investigating other non-platinum complexes with different mechanisms of antineoplastic activity [1–6]. Titanocene dichloride (Scheme 1) possesses cytotoxic properties that protect against a wide variety of cancer tumors, including Ehrlich ascites tumor, B16 melanoma, colon 38 carcinoma, and Lewis lung carcinoma, and exhibits less toxic effects than cis-platin [1–6]. The major drawback of titanocene dichloride is its lack of hydrolytic stability at physiological pH [7]. As a result, many of its mechanistic details are unknown, which hinders its use as a chemotherapeutic agent.
Scheme 1.
Structure of titanocene dichloride
Modification of ligands in titanocene dichloride is an active area of research since, among the metallocenes, titanocene dichloride is the most effective species [8–21]. As mentioned previously, the lack of hydrolytic stability has hampered its use as a chemotherapeutic drug because the chemical nature of the active species is unknown. Therefore, structure modification through the replacement of the chloride ligands by other ancillary ligands, particularly hydrophilic ligands, and the functionalization of the cyclopentadienyl rings will change the electronic and steric effects of the titanocenes, and can modify their antineoplastic activity. For example, the incorporation of polar electron-withdrawing groups on the Cp ligand affords titanocene species with a stronger Ti–Cp bond and a more electrophilic titanium center. These structural modifications would cause an increase in the Lewis acid character of the Ti(IV) center, leading to metal complexes with good binding properties to DNA and/or serum proteins such as transferrin. This last characteristic has been implicated in the delivery of Ti(IV) to cancer cells [22, 23]. On the other hand, this increased Lewis acid character could result in a decreased hydrolytic stability and, if the Ti(IV) is not coordinated by a carrier/stabilizing protein or molecule, could lead to the oligomerization of the titanocene species. The net result of this is the subsequent inactivation of the Ti(IV) complex [7]. Lately, we have been investigating other types of Ti(IV) complexes with bi- and multidentate oxygen ligands in order to produce more robust species at physiological pH and to circumvent the abovementioned problems [24, 25].
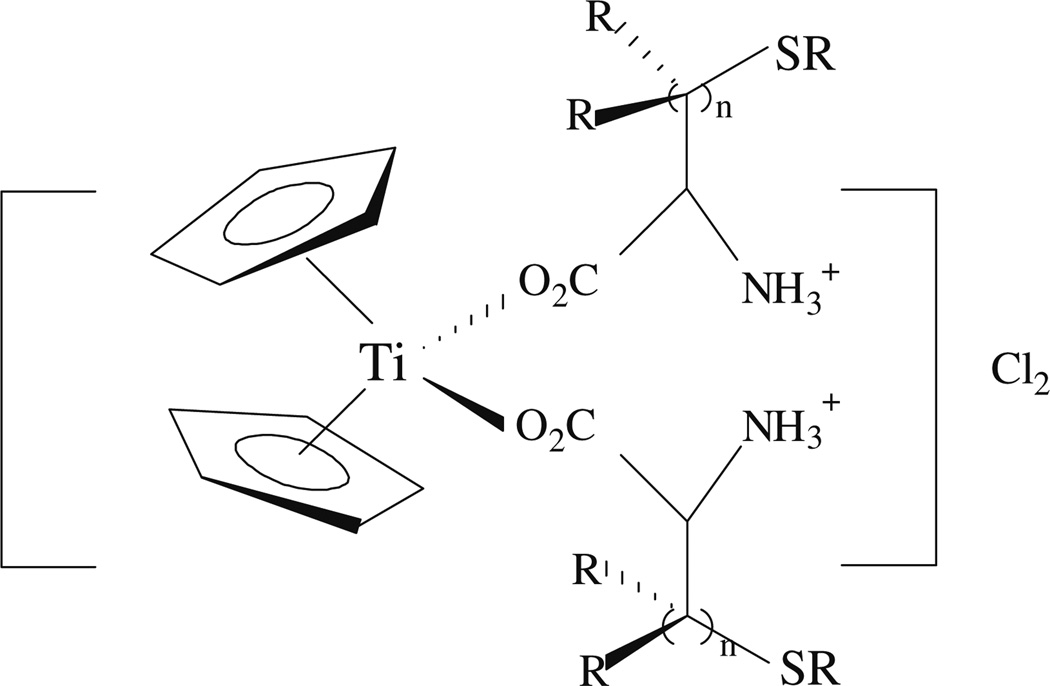
Our research in this area has focused on modifying the titanocene dichloride structure by replacing the chloride ligands with biologically important molecules (amino acids, thionucleobases) [19, 20], or by functionalizing Cp rings [21]. In addition, a new inorganic titanium complex has been synthesized which is hydrolytically stable at and and above physiological pH [24, 25]. In the case of [Cp2Ti(O-amino acid)2]Cl2, aa = l-cysteine(II), l-methionine(III), and d-penicillamine(IV), see Scheme 2, the amino acid ancillary ligands make their titanocene complexes more water-soluble. The functionalization of Cp ligands with polar groups (R = CO2CH3 and CO2CH2CH3) produces titanocenes with low aqueous solubility [21].
Scheme 2.
Structure of titanocene–amino acid complexes. R = H, CH3; n = 1, 2
On the other hand, the [Ti4(maltolato)8(μ-O)4] complex (see Fig. 1), in which Ti(IV) is coordinated to six oxygen atoms, is stable up to pH 8.4 [24, 25]—rather high when compared to Cp2TiCl2, which gets hydrolyzed at pH values of around 5.5 with subsequent precipitation [7]. We have investigated the cytotoxic properties of [Ti4(maltolato)8 (μ-O)4] and modified titanocene complexes on the colon adenocarcinoma cell line (HT-29) in the presence and the absence of transferrin in order to determine structure–activity relationships.
Fig. 1.
Structure of [Ti4(maltolato)8(μ-O)4]
Experimental
Materials and methods
Human apo-transferrin (98% pure, cell-culture tested, T2036 grade), was purchased from Sigma (St. Louis, MO, USA) and washed three times with KCl (0.1 M), using Centricon 30 (Amicon, Beverly, MA, USA) ultrafilters to remove impurities.
The purity of the titanocene complexes as well as that of the ligands was verified initially by 1H NMR spectroscopy. Water was double-distilled, deionized, and thoroughly saturated with dried nitrogen. All solvents for the NMR measurements were of 99.9% D purity grade and were obtained from Aldrich (Milwaukee, WI, USA).
The colon cancer cell line, HT-29, was purchased from American Type Culture Collection and was maintained at 37 °C and 95% air/5% CO2 in McCoy’s 5A (ATCC) complete medium, which had been supplemented with 10% (v/v) fetal bovine serum (ATCC) and 1% (v/v) antibiotic/antimycotic (Sigma). This medium is supplemented by the supplier to contain 1.5 mM l-glutamine and 2.2 g L−1 sodium bicarbonate. Both the MTT and the Triton X-100 used for the cytotoxic assay were obtained from Sigma. All MTT manipulations were performed in a dark room.
Physical measurements
1H and 13C NMR spectra were recorded on a 500 MHz Bruker Avance spectrometer under controlled temperature. Chemical shifts are referenced to TMS or TSSP. UV–vis spectroscopy measurements were recorded on a double-beam Lambda BIO 20 Perkin Elmer spectrophotometer thermostated at 25 °C. The system is interfaced with a 586 Nokia Computer System and the spectral handling was carried out using WinLab Software (Perkin Elmer). Mass spectral data was obtained on a Bruker Daltonics Esquire 6000. The complexes were dissolved in a mixture of water/methanol (1:1) prior to mass spectral analysis. The positive electrospray ionization mode was used during the MS experiment.
UV–vis spectroscopy titrations (for the binding interactions) were performed on 1.1–1.2 × 10−5 M solutions of human apo-transferrin in 10 mM NaCl/25 mM NaHCO3/100 mM Tris buffer at pH 7.4. The concentration of apo-transferrin was determined at 280 nm (ε280 93,000 M−1 cm−1). 1.0–2.0 × 10−3 M solutions of [Ti4(maltolato)8(μ-O)4] at 25 °C were used for the binding studies. Aliquots of 2.9–5.8 µL of [Ti4(maltolato)8(μ-O)4] were added sequentially to the apo-transferrin and to the reference cell (to correct for background/absorption interferences associated with the complexes). UV–vis spectra were recorded 60 min after the addition of [Ti4(maltolato)8(μ-O)4] for the titration or every 10 min for three days for kinetic experiments. The pH of the resulting solutions was measured, and no significant change from pH 7.4 was observed. Dilution factors were considered when calculating both the transferrin and the titanocene concentrations.
Stability studies were carried out under a nitrogen atmosphere at 25 °C. Solvents (D2O and doubly distilled and deionized H2O) were deoxygenated using a sparger and pre-purified nitrogen. NMR samples were prepared by transferring the weighted amount of the Ti complex into a 5-mm NMR tube and the solvent was added to form solutions of 1 × 10−2 M. The procedure was performed under an atmosphere of nitrogen, either in the glove box or using a double-manifold vacuum line. A sealed capillary tube containing TPPS was inserted into the NMR tube in order to ensure that the external reference would be isolated from the complex solution, thus avoiding possible secondary reactions with the complex.
Cytotoxic assay
Biological activity was determined using the MTT assay originally described by Mossman [30], but using 10% Triton in isopropanol as a solvent for the MTT formazan crystals [31]. Asynchronously growing cells were seeded at 1.0–1.5 × 104 cells per well in 96-well plates containing 100 µL of complete growth medium and allowed to recover overnight. For the assays in which transferrin was not present, various concentrations of the complexes (2–1,400 µM) were dissolved in 5% DMSO/95%. Medium was added to the wells (eight wells per concentration; experiments performed in quadruplicate plates), and cells were incubated for an additional 70, 94, or 118 h. For the assays in which transferrin was present, the original DMSO solution (into which the complexes had been dissolved) was slowly added to the corresponding amount of complete growth medium (supplemented so that it contained transferrin at a concentration equivalent to the final 5% DMSO/95% medium solution). The maximum transferrin concentration in the cell medium before supplementation was approximately 3 × 10−7 M. This concentration was calculated after dilution of 1:100 in the complete growth medium (assuming that all of the beta globulin concentration (0.26 g dL−1) present in the FBS F4135 from Sigma belongs to transferrin). The concentration of apotransferrin added to the cell medium ranged from 2 to 1,400 µM, according to the drug (dose) concentration. For instance, if the drug concentration in the medium was 1,400 µM, the transferrin concentration in the cell medium was 1,400 µM. Transferrin was diluted from a concentrated (spectrophotometrically determined) stock solution in PBS. This solution of the complex in the presence of transferrin was allowed to stand for the time required for the complete stripping of the Ti(IV) center and the subsequent uptake by transferrin (10 min for Cp2TiCl2 and the functionalized cyclopentadienyl complexes, 60 min for amino acid complexes), which had been determined previously [20, 21]. Although it is known that the [Ti4(maltolato)8(μ-O)4] complex does not transfer Ti(IV) to transferrin, it was also allowed to stand for 1 h prior to dosing the plates. After this time, MTT dissolved in complete growth medium was added to each well for a final concentration of 1.0 mg mL−1 and incubated for two additional hours for final compound exposure times of 72, 96, or 120 h, respectively. After this, all of the MTT containing medium was removed, and the cells were washed with cold PBS and dissolved with 200 µL of a 10% (v/v) Triton X-100 solution in isopropanol. After complete dissolution of the formazan crystals, well absorbances were recorded in triplicate on a 340 ATTC Microplate Reader (SLT Lab Instruments, Salzburg, Austria) at 570 nm with background subtraction at 630 nm. Concentrations of compounds required to inhibit cell proliferation by 50% (IC50) were calculated by fitting data to a four-parameter logistic plot by means of the SigmaPlot software from SPSS.
Analytical data for [Ti4(maltolato)8(μ-O)4] and Ti(maltolato)2(OH)2
[Ti4(maltolato)8(μ-O)4] IR(KBr disc): 3,418(br), 3,074(w), 2,920(vw), 1,619(s), 1,587(vs), 1,516(m), 1,472(s), 1,392(w), 1,368(w), 1,276(s), 1,243(m), 1,203(s), 1,092(w), 1,040(w), 928(w), 854(sh), 796(s), 722(s), 628(w), 610(w), 543(m), 469(w). 1H NMR (DMSO-d6, ambient) δ: 8.016 (d, 1H, J = 5 Hz), 6.350 (d, 1H, J = 5 Hz), 2.389 (s, 3H). 1H NMR (D2O, pH 8.4, ambient) δ: 7.835 (d, 1H, J = 5.5 Hz), 6.504 (d, 1H, J = 5.5 Hz), 3.42 (bs, H2O), 2.314 (s, 3H). ESI–MS (positive mode): m/z (relative intensity): [Ti(maltolato)2(OTi)2]+, 422.2 (12.1), 423.1 (100), 424.9 (31.1), 425.0 (13.6); [Ti(maltolato)2(OH)]+, 313.2 (5.8), 314.2 (7.3), 315.1 (100), 315.9 (46.3), 317.0 (8.5).
Ti(maltolato)2(OH)2 (monomer) IR(KBr disc): 3,396(br), 3,074(w), 1,620(m), 1,586(s), 1,518(m), 1,476(s), 1,276(s), 1,246(w), 1,204(m), 928(w), 854(sh), 796(m), 728(m), 630(w), 543(w), 475(w). 1H NMR (D2O, pH 8.4, ambient) δ: 8.066 (d, 1H, J = 5 Hz), 6.375 (d, 1H, J = 5 Hz), 2.457 (s, 3H). ESI–MS (positive mode), m/z (relative intensity): [Ti(η2-maltolato)2(OH)2–H]+ 327.2 (9.9), 328.2 (9.4), 329.1 (100), 330.1 (23), 331.1 (10.1); [Ti(η2-maltolato)2 (OH)]+, m/z (relative intensity): 313.1 (6.7), 314.1 (10.8), 315.0 (100), 316.0 (18.1), 317 (26.7).
Results and discussion
The syntheses of the titanocenes and titanium–maltolato complexes were reported previously [20, 21, 24, 25]. In addition, there are previous studies reporting the biological activity of modified titanocenes in different cell lines [8, 10–13, 21, 26–29], and there are reports regarding the ability of the Ti(IV)–transferrin complex to enter cells via the transferrin-receptor [22, 23]. But, to the best of our knowledge, this is the first report on the capacity of human transferrin to modify the cytotoxic activity of titanocenes and titanium–maltolato complexes on colon cancer cells.
Stability studies
Stability studies were performed on [Ti4(maltolato)8(μ-O)4] to establish the chemical nature of this species in aqueous solution. We used two spectroscopic techniques: UV–vis and 1H NMR spectroscopies.
In marked contrast to titanocene complexes, [Ti4 (maltolato)8(μ-O)4] is remarkably stable in aqueous solution at and above physiological pH, showing no notable decomposition over a period of ten days. Interestingly, after its dissolution in water at a pH of 8, [Ti4(maltolato) 8(μ-O)4] ([Ti(maltolato)2(μ-O)]4, tetramer) dissociates to some extent to form cis-Ti(maltolato)2(OH)2 (monomer) in a ratio of 7:1 (tetramer:monomer). The identities of the monomeric (at low pH) species and tetrameric (at high pH) species were characterized by 1H NMR and MS spectroscopies. As previously stated, the tetramer is the predominant species at high pH (>7), while the monomeric species predominates at low pH (<7), but none of the species degrade in aqueous solution at physiological pH.
The solid-state single-crystal X-ray analysis of [Ti4(maltolato)8(μ-O)4] has been discussed in detail in a previous publication [24, 25] and will not be discussed here. Bonding parameters are included in the “Electronic supplementary material.” Only a few highlights are emphasized. First, we observed that [Ti4(maltolato)8(μ-O)4] is a tetranuclear species with a Ti(IV) metal center coordinated by two chelating maltolatos and two bridging oxygens, in a pseudo-octahedral coordination geometry. Given that Ti(IV) is surrounded by six oxygens in the coordination sphere, this species is very stable in aqueous solution at high pH. Evidently, as discussed in the UV–vis spectroscopic titration section, this species is unable to donate Ti(IV) to apo-transferrin. Second, the maltol rings between adjacent titanium are coplanar, adopting a partially eclipsed configuration, and are separated by about 3.7 Å. This feature may be responsible for the cytotoxic activity as discussed below.
We have previously reported stability studies of titanocene dichloride and [Cp2Ti(O-amino acid)2]Cl2, aa = l-cysteine(II), l-methionine(III), and d-penicillamine(IV) complexes [20]. With regard to the ancillary ligand loss, these complexes have half-life times that ranged from 8.26 to 473 min, while for the Cp loss the t1/2 values ranged from 1.07 × 103 to 6.6 × 103 min. This clearly demonstrates the labile character of these species with regard to [Ti4(maltolato)8(μ-O)4]. The aqueous stability of titanocene dichloride with functionalized cyclopentadienyl (Cp-R)2TiCl2 and (Cp-R)CpTiCl2, R = CO2CH3 and CO2 CH2CH3 was not investigated due to their low solubility in water.
UV–vis spectroscopic titrations (for the binding interactions): Ti(IV) intake by apo-transferrin protein
A study was performed on Ti(IV) intake at iron-binding sites by human apo-transferrin. Our study was accomplished using electronic absorption (UV–vis) spectroscopy that utilized a Tris buffer solution containing bicarbonate, which acted as a synergistic anion. The objective of this study was to understand how the ligands surrounding Ti(IV) affect human apo-transferrin intake.
The binding of metal ions to the specific iron-binding sites of transferrin is commonly monitored by electronic absorption spectroscopy [20–23]. The coordination of metal ions by the phenolic groups of tyrosine residues located at the specific iron-binding sites of apo-transferrin produces two new absorption bands at 240 and 295 nm. These two absorption bands are attributed to the deprotonation and subsequent coordination of the tyrosine residues to the metal ion. In addition, when Ti(IV) is loaded into apo-transferrin, it produces a band at 321 nm that is attributed to ligand-to-metal charge transfer (LMCT).
The titration of human apo-transferrin (apo-Tr) with increasing amounts (aliquots) [Ti4(maltolato)8(μ-O)4] did not yield any of the characteristic absorption bands at 240, 295, or 321 nm in the UV difference spectra. At first, the transferrin was allowed to react for 60 min with the complex, since the time to reach equilibrium for Cp2TiCl2 and functionalized cyclopentadienyl titanocene dichlorides is about 2–10 min, whereas equilibrium for the titanocene–amino acid complex is reached in 45–60 min [20, 21]. Since the [Ti4(maltolato)8(μ-O)4] complex has a coordination sphere containing six oxygen ligands, it is not surprising that this complex is more stable than the titanocenes. Therefore, longer reaction times were utilized.
The interaction of transferrin with two molar equivalents of [Ti4(maltolato)8(μ-O)4] was allowed to react for three days, recording a full UV–vis spectrum every 10 min. The pH of the resulting solution was not altered after this period of time, and remained at 7.4, but the reaction did not proceed, and the absorption bands at 240, 295, and 321 nm were not observed. After the transferrin was allowed to react with the complex for a seven-day period, the changes in the 321 nm band were less than 0.1% of the original absorbance value. Thus, the oxygen-rich octahedral coordination environment makes a robust Ti(IV) complex, and the ligand-stripping process and the subsequent transfer of Ti(IV) to human apo-transferrin seems to be thermodynamically as well as kinetically unfavorable. Thus, there is a striking difference between [Ti4(maltolato)8(μ-O)4] and the titanocene complexes with regard to the intake of Ti(IV) by apo-transferrin protein.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay (for cytotoxicity analysis on the colon cancer cell line, HT-29)
The cytotoxicities of the functionalized titanocene, the titanocene–amino acid, and the titanium–maltolato complexes on the human colon cancer cell line, HT-29, were measured using a slightly modified MTT assay [30, 31]. Titanocene dichloride and the functionalized cyclopentadienyl complexes were re-tested in concentrations that ranged from 120 to 2,500 µM at 72 h [21]. Since titanocene dichloride has a longer intracellular activation period, it is usually tested at a time interval of 96 h, as was done in our study.
Table 1 summarizes the IC50 data for the titanocene complexes. There was increased cytotoxic activity at 96 h for titanocene dichloride and both carboethoxy-functionalized cyclopentadienyl titanocenes [being higher for the (Cp-COOCH2CH3)(Cp)TiCl2 complex], but the carbomethoxy-functionalized complexes remained inactive at concentrations below 0.9 mM. All titanocene–amino acid complexes were shown to be inactive at time intervals of 72 and 96 h, at concentrations below 0.7 mM. These complexes are more soluble in water than the parent complex, titanocene dichloride, with comparable aqueous stability to Cp2TiCl2; in addition, they are able to donate Ti(IV) ions to transferrin. Thus, it is unclear why they did not show biological activity against the HT-29 cell line.
Table 1.
Cytotoxicities of the complexes studied in the HT-29 colon cancer cell line, as determined by the MTT assay
| Complex | IC50 (× 10−4 M) | |||
|---|---|---|---|---|
| 72 h | 96 h (without Tr) | 96 h (with Tr) | 120 h | |
| Cp2TiCl2 | 4.5 (3) | 4.2 (3) | 4.1 (5) | – |
| [Ti4(maltolato)8(μ-O)4] | 2.8 (1) | 1.5 (1) | 1.7 (1) | 2.4 (1) |
| (Cp-COOEt)2TiCl2 | 5.8 (4) | 5.3 (3) | – | – |
| (Cp)(Cp-COOEt)TiCl2 | 6.3 (2) | 3.0 (3) | – | – |
| (Cp-COOMe)2TiCl2 | Na | Na | Na | – |
| (Cp)(Cp-COOMe)TiCl2 | Na | Na | Na | – |
| [Cp2Ti(l-cysteine)2]Cl2 | Na | Na | Na | – |
| [Cp2Ti(l-methionine)2]Cl2 | Na | Na | Na | – |
| [Cp2Ti(d-penicillamine)2]Cl2 | Na | Na | Na | – |
IC values are the average of four independent measurements
Na, not active
The titanium–maltolato complex was evaluated at time intervals of 72, 96, and 120 h to determine its optimal cytotoxic activity (Fig. 2). The complex displays its highest level of activity at 96 h, showing an IC50 value of 1.5 × 10−4 M, followed by 2.4 × 10−4 M at 120 h, and 2.8 × 10−4 M at 72 h (see Table 1). At all three time intervals [Ti4(maltolato)8(μ-O)4] was the most active species of all of the titanium complexes that were investigated in this study. By contrast, this complex is unable to donate Ti(IV) ions to human apo-transferrin.
Fig. 2.
Dose–response curves for [Ti4(maltolato)8(μ-O)4] against HT-29 cells at drug exposure times of 72, 96, and 120 h (diamonds, squares, and triangles), respectively
To study the role of transferrin in the cytotoxicity of these titanium complexes, we decided to retest both the titanocenes and the titanium–maltolate complexes, this time in a transferrin-enriched environment. The aim of these studies was to determine whether the presence of transferrin in a dose-equivalent concentration could stabilize the Ti(IV) ion, deliver it to the cells, and, perhaps, increase the cytotoxic activity against the HT-29 cell line. The titanocene dichloride, the carbomethoxy-functionalized titanocenes, [Cp2Ti(amino acid)2]Cl2 and the titanium–maltolato complexes were added to the cells in the presence of transferrin at a concentration equivalent to that of the Ti(IV) complex. Both the carbomethoxy-functionalized and the amino acid complexes remained inactive at concentrations below 0.5 mM. This rules them out as potential chemotherapeutic agents, at least for the HT-29 cell line. Both the titanocene dichloride and the titanium–maltolato complexes retained their IC50 values (Fig. 3), raising the issue of whether transferrin is a biologically favored route for Ti(IV) to exercise its toxic effect. While it cannot be generalized that transferrin is not a favorable route in Ti(IV)-based complexes for potential therapeutic use, it is highly probable that transferrin only has a stabilizing role in a hydrolytically hostile environment, such as plasma. In addition, the Ti(IV) that can enter the cell by transferrin-mediated transport will be “naked” and still prone to hydrolysis. The desired stabilizing role can be achieved by selecting the appropriate set of donors in polydentate ligands.
Fig. 3.
Dose–response curves for Cp2TiCl2 (diamonds) and [Ti4(maltolato)8(μ-O)4] (squares) in HT-29 cells at 96 h of drug exposure, with (dashed lines) and without (solid lines) transferring
Colon cancer cells typically show resistance to many cytotoxic drugs. In addition, titanocene dichloride is not very active in colon cancer cells and it is hydrolytically unstable [2, 6]. Therefore, [Ti4(maltolato)8(μ-O)4] represents a new type of Ti(IV) antineoplastic agent that possesses hydrolytic stability and a higher level of cytotoxicity than titanocene dichloride.
Our cytotoxic studies of the HT-29 cancer cell line have shown that the titanocene–amino acid complexes and the carbomethoxy-functionalized titanocenes have a lower or minimal activity compared to the titanocene dichloride and the titanium–maltolato complexes. In addition, transferrin is not a biologically favored route for these complexes to express their cytotoxic effect in HT-29 cells.
Concluding remarks
In this study, we have presented the aqueous stability, the properties of binding to human apo-transferrin, and the cytotoxicity on the HT-29 cell line of a hydrolytically stable Ti(IV) complex [Ti4(maltolato)8(μ-O)4] and compared it with series of modified titanocene complexes. While all titanocene complexes donate their Ti(IV) ion to human apo-transferrin, the presence of this protein in the cell medium does not enhance their cytotoxic activities in HT-29 cells. The titanium–maltolato complex, by contrast, showed a level of cytotoxic activity that exceeds those of titanocene dichloride and (Cp-COOEt)2TiCl2 and (Cp) (Cp-COOEt)TiCl2 but is still unable to donate Ti(IV) to apo-transferrin. All of the complexes which were inactive, in addition to titanocene dichloride and the maltolato, were evaluated in a transferrin-enriched environment, but this did not result in increased activity in any of the complexes studied. Of all the complexes studied, the titanium–maltolato complex is the most stable in water, being hydrolytically stable at and above physiological pH; it is also the most cytotoxic.
[Ti4(maltolato)8(μ-O)4] belongs to a prototype of coordination compounds with antitumor activity such as [Ti(phenyl-3,4-methyl-4-benzoylpyrazolon-5-ato)2(μ-O)]4 [32, 33] and the monomeric counterpart [Ti(maltolato)2(OH)2] to budotitane, cis-diethoxybis(1-phenylbutane-1,3-dionate)titanium(IV) [34]. Interestingly, while budotitane hydrolyzes extensively, making it unclear which species is biologically active, the titanium–maltolate complex overcomes such complications. On the other hand, [Ti(phenyl-3,4-methyl-4-benzoylpyrazolon-5-ato)2 (μ-O)]4 has low solubility in water and must be administered encapsulated in liposomes [33]. [Ti4(maltolato)8 (μ-O)4] is soluble and hydrolytically stable in water and buffer solutions at a pH of 7.4, circumventing the above complications.
It has been proven that the asymmetry of the β-diketone is responsible for the antitumor activities of these compounds [30, 31]. Likewise, in [Ti4(maltolato)8(μ-O)4], the maltolate ligand possesses the asymmetry needed for antitumor activity. It is particularly interesting that while several stereoisomers exist for budotitane complex, we have been able to isolate only one stereoisomer. In addition, the maltol rings of adjacent titaniums in [Ti4(maltolato)8(μ-O)4] adopt an eclipsed configuration and are separated by 3.7 Å. Thus it seems likely that these rings could intercalate between DNA bases (π–π stacking) and another possible mechanism of action could be invoked for [Ti4(maltolato)8(μ-O)4] that does not involve transferrin as a transport agent for Ti(IV) into the target place. At the present time, this issue is uncertain but deserves to be investigated in great detail.
Finally, the antitumor activity of [Ti4(maltolato)8(μ-O)4] raises a question about the role of transferrin. In this regard, transferrin may be important in protecting the titanium center from hydrolysis, but this could also be achieved by selecting ligands that result in hydrolytically stable, yet active, complexes.
Supplementary Material
Acknowledgments
Enrique Meléndez acknowledges the NIH-MBRS SCORE Programs at both the University of Puerto Rico Mayagüez and the Ponce School of Medicine for financial support via NIH-MBRS-SCORE Program grant #S06 GM008239-23 and the PSM-Moffitt Cancer Center Partnership 1U56CA126379-01. In addition, Enrique Meléndez thanks the NSF-MRI Program for providing funds for the purchase of the 500 MHz NMR instrument and to the Sloan Foundation for financial support in the form of a graduate fellowship to Ramón Hernández. Finally, Jaime Matta would like to thank Bob Ritchie, RCMI Program Grant #RR003050-23, for his help in the editing of this manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00775-008-0353-z) contains supplementary material, which is available to authorized users.
Contributor Information
Ramón Hernández, Department of Chemistry, University of Puerto Rico, Mayaguez, PR 00681, USA.
José Lamboy, Department of Chemistry, University of Puerto Rico, Mayaguez, PR 00681, USA.
Li Ming Gao, Department of Chemistry, University of Puerto Rico, Mayaguez, PR 00681, USA.
Jaime Matta, Department of Pharmacology, Toxicology and Physiology, Ponce School of Medicine, Ponce, PR 00732-7004, USA.
Félix R. Román, Department of Chemistry, University of Puerto Rico, Mayaguez, PR 00681, USA
Enrique Meléndez, Email: emelendez@uprm.edu, Department of Chemistry, University of Puerto Rico, Mayaguez, PR 00681, USA.
References
- 1.Köpf-Maier P. Eur J Clin Pharmacol. 1994;47:1. doi: 10.1007/BF00193472. [DOI] [PubMed] [Google Scholar]
- 2.Köpf-Maier P, Köpf H. Struct Bonding. 1988;70:103. [Google Scholar]
- 3.Köpf-Maier P, Köpf H. Chem Rev. 1987;87:1137. [Google Scholar]
- 4.Köpf-Maier P, Köpf H. In: Metal compounds in cancer therapy, organometallic titanium, vanadium, niobium, molybdenum and rhenium complexes—early transition metal antitumor drugs. Fricker SP, editor. London: Chapman & Hall; 1994. pp. 109–146. [Google Scholar]
- 5.Harding MM, Mokdsi G. Curr Med Chem. 2000;7:1289. doi: 10.2174/0929867003374066. [DOI] [PubMed] [Google Scholar]
- 6.Meléndez E. Crit Rev Oncol Hematol. 2002;42:309. doi: 10.1016/s1040-8428(01)00224-4. [DOI] [PubMed] [Google Scholar]
- 7.Toney JH, Marks TJ. J Am Chem Soc. 1985;107:947. [Google Scholar]
- 8.Boyles JR, Baird MC, Campling BG, Jain N. J Inorg Biochem. 2001;84:159. doi: 10.1016/s0162-0134(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 9.Valadares MC, Klein SL, Guaraldo AMA, Queiroz MLS. Eur J Pharmacol. 2003;473:191. doi: 10.1016/s0014-2999(03)01967-8. [DOI] [PubMed] [Google Scholar]
- 10.Allen OR, Croll L, Gott AL, Knox RJ, McGowan PC. Organometallics. 2004;23:288. [Google Scholar]
- 11.Causey PW, Baird MC, Cole SPC. Organometallics. 2004;23:4486. [Google Scholar]
- 12.Tacke M, Allen LT, Cuffe L, Gallagher WM, Lou Y, Mendoza O, Mueller-Bunz H, Rehmann F-JK, Sweeney N. J Organomet Chem. 2004;689:2242. doi: 10.1016/j.jinorgbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Rehmann F-JK, Cuffe LP, Mendoza O, Rai DK, Sweeney N, Strohfeldt K, Gallagher WM, Tacke M. Appl Organomet Chem. 2005;19:293. [Google Scholar]
- 14.Sweeney NJ, Mendoza O, Müller-Bunz H, Pampillón C, Rehmann F-JK, Strohfeldt K, Tacke M. J Organomet Chem. 2005;690:4537. [Google Scholar]
- 15.Gansäuer A, Franke D, Lauterbach T, Nieger M. J Am Chem Soc. 2005;127:11622. doi: 10.1021/ja054185r. [DOI] [PubMed] [Google Scholar]
- 16.Kelter G, Sweeney NJ, Strohfeldt K, Fiebig H-H, Tacke M. Anti Cancer Drugs. 2005;16:1091. doi: 10.1097/00001813-200511000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Valadares MC, Ramos AL, Rehmann F-JK, Sweeney NJ, Strohfeldt K, Tacke M, Queiroz MLS. Eur J Pharmacol. 2006;534:264. doi: 10.1016/j.ejphar.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner I, Pampillon C, Sweeney NJ, Strohfeldt K, Tacke M. Anti Cancer Drugs. 2006;17:333. doi: 10.1097/00001813-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Meléndez E, Marrero M, Rivera C, Hernández E, Segal A. Inorg Chim Acta. 2000;298:176. [Google Scholar]
- 20.Pérez Y, López V, Rivera-Rivera L, Cardona A, Meléndez E. J Biol Inorg Chem. 2005;10:94. doi: 10.1007/s00775-004-0614-4. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Hernández R, Matta J, Meléndez E. J Biol Inorg Chem. 2007;12:959. doi: 10.1007/s00775-007-0268-0. [DOI] [PubMed] [Google Scholar]
- 22.Guo M, Sun H, McArdle HJ, Gambling L, Sadler PJ. Biochemistry. 2000;39:10023. doi: 10.1021/bi000798z. [DOI] [PubMed] [Google Scholar]
- 23.Messori L, Orioli P, Banholer V, Pais I, Zatta P. FEBS Lett. 1999;422:157. doi: 10.1016/s0014-5793(98)01651-2. [DOI] [PubMed] [Google Scholar]
- 24.60987915. US Patent application number. 2007 Nov 14;
- 25.Lamboy JL, Pasquale A, Rheingold AL, Meléndez E. Inorg Chim Acta. 2007;360:2115. doi: 10.1016/j.ica.2006.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harstrick A, Schmoll H-J, Sass G, Poliwoda H, Rustum Y. Eur J Cancer. 1993;29A:1000. doi: 10.1016/s0959-8049(05)80210-2. [DOI] [PubMed] [Google Scholar]
- 27.Kurbacher CM, Bruckner HW, Andreotti PE, Kurbacher JA, Saβ G, Krebs D. Anti Cancer Drugs. 1995;6:697. doi: 10.1097/00001813-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Rehmann F-J, Rous AJ, Mendoza O, Sweeney NJ, Strohfeldt K, Gallagher WM, Tacke M. Polyhedron. 2005;24:1250. [Google Scholar]
- 29.Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M. J Organomet Chem. 2007;692:2153. [Google Scholar]
- 30.Mossman T. J Immunol Methods. 1983;65:55. [Google Scholar]
- 31.Denizot F, Lang R. J Immunol Methods. 1986;89:271. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 32.Caruso F, Rossi M. Mini Rev Med Chem. 2004;4:49. doi: 10.2174/1389557043487565. [DOI] [PubMed] [Google Scholar]
- 33.Caruso F, Rossi M, Tanski J, Sartori R, Sariego R, Moya S, Diez S, Navarrete E, Cingolani A, Marchetti F, Pettinari C. J Med Chem. 2000;43:3665. doi: 10.1021/jm990539b. [DOI] [PubMed] [Google Scholar]
- 34.Keppler BK, Friesen C, Moritz HG, Vongerichten H, Vogel E. Struct Bonding. 1991;78:98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.