Abstract
Although treatment of drug-susceptible tuberculosis (TB) under ideal conditions may be successful in ≥95% of cases, cure rates in the field are often significantly lower due to the logistical challenges of administering and properly supervising the intake of combination chemotherapy for 6–9 months. Success rates are far worse for multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB cases. There is general agreement that new anti-TB drugs are needed to shorten or otherwise simplify treatment for drug-susceptible and MDR/XDR-TB, including TB associated with HIV infection. For the first time in over 40 years, a nascent pipeline of new anti-TB drug candidates has been assembled. Eleven candidates from 7 classes are currently being evaluated in clinical trials. They include novel chemical entities belonging to entirely new classes of antibacterials, agents approved for use against infections other than TB, and an agent already approved for limited use against TB. In this article, we review the current state of TB treatment and its limitations and provide updates on the status of new drugs in clinical trials. In the conclusion, we briefly highlight ongoing efforts to discover new compounds and recent advances in alternative drug delivery systems.
Keywords: tuberculosis, anti-tubercular agents, drug therapy, multidrug-resistant tuberculosis, investigational drugs
CURRENT TREATMENT OF TUBERCULOSIS AND ITS LIMITATIONS
Chemotherapy for tuberculosis (TB) was possible only after the discovery of streptomycin in 1944. The introduction of isoniazid constituted the basis of primary anti-TB chemotherapy in the 1950s to 1960s. At that time, the anti-tuberculosis drug regimen generally comprised streptomycin, isoniazid and para-aminosalicylic acid in the initial months, followed by isoniazid and para-aminosalicylic acid in subsequent months, requiring a total duration of administration of 18 months. In 1965, rifampicin was discovered. In the 1970s, short-course treatment for TB was commenced. The important drug components for a short-course regimen are shown in Table 1 as Category A drugs. Properly administered short-course chemotherapy for TB confers bactericidal and sterilizing actions against Mycobacterium tuberculosis and prevents emergence of drug resistance.
Table 1.
Categories of Anti-TB Drugs
| Category A: first-line oral drugs |
| Isoniazid |
| Rifampicin |
| Ethambutol |
| Pyrazinamide |
| Category B: injectable agents |
| Streptomycin (*) |
| Amikacin |
| Kanamycin |
| Capreomycin |
| Category C: fluoroquinolones |
| Ofloxacin |
| Levofloxacin |
| Moxifloxacin |
| Category D: oral bacteriostatic second-line agents |
| Ethionamide |
| Prothionamide |
| Cycloserine |
| Terizidone |
| Para-aminosalicylic acid |
| Category E: agents with efficacy that is not totally clear/certain |
| Clofazimine |
| Amoxicillin clavulanate |
| Thiacetazone |
| Linezolid |
| Imipenem/cilastatin |
| Clarithromycin |
first-line drug
Drug-susceptible tuberculosis
The Directly Observed Treatment, Short-course (DOTS) strategy, when properly implemented with the necessary infrastructure, produces high TB cure rates and curtails the development of acquired drug-resistant TB. Although treatment of drug-susceptible TB under ideal conditions by DOTS may be successful in ≥95% of cases,1 cure rates in the field are often significantly lower.2 Indeed, in practice, the DOTS strategy has proven difficult for many national TB control programmes to sustain adequately over long periods of time. In particular, the failure to ensure patient adherence to the recommended chemotherapeutic regimen not only lowers cure rates, but leads to the development of drug-resistant TB, inclusive of the formidable scenarios of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB), which are escalating worldwide.3,4 MDR-TB denotes drug-resistant TB with bacillary resistance to at least isoniazid and rifampicin. XDR-TB denotes MDR-TB with additional bacillary resistance to fluoroquinolones and one or more of the 3 second-line injectables – amikacin, kanamycin and capreomycin. Without a means to rapidly identify MDR- and XDR-TB at the point of care, the DOTS strategy alone is inadequate for treating new cases of TB in settings with a high prevalence of initial drug resistance.
Currently, the standard regimen for treating drug-susceptible pulmonary TB comprises the use of rifampicin, isoniazid and pyrazinamide in combination with either ethambutol or streptomycin for 2 months, followed by continuation of rifampicin and isoniazid for another 4 months – totally 6 months. This is the drug regimen recommended by the World Health Organization (WHO) for the treatment of smear-positive pulmonary TB in newly diagnosed patients.5 On the whole, daily administration of anti-TB medications in smear-positive TB, especially with cavitation, is associated with lower relapse rate,6 and thus it should generally be advocated during the first 2 months, followed by three-times weekly administration in the subsequent 4 months. The presence of initial cavitation on chest radiograph and 2-month culture positivity would generally favour prolongation of the continuation phase to 7 months for a total of 9 months of treatment to minimize relapse of TB.7
Thus, the current therapy of drug-susceptible TB appears complex and lasts at least 6 months. Furthermore, the drug components of the current regimens (which include rifamycins) have the additional disadvantage of drug-drug interaction with antiretroviral agents,8 primarily protease inhibitors and non-nucleoside reverse transcriptase inhibitors (at the cytochrome P-450 level), which may produce a significant problem given the high incidence and prevalence of HIV/M. tuberculosis co-infection in some parts of the world.
Drug resistance in M. tuberculosis arises from man-made selection of mutants that result from spontaneous chromosomal alterations. Selective amplification of drug-resistant mutants is facilitated by patient non-adherence as well as inappropriately prescribed regimens, poor drug quality, and erratic drug supply, oftentimes reflecting failure in the implementation of TB control programmes.9
While DOTS is highly effective in the treatment of drug-susceptible TB, it is generally regarded as insufficient for controlling established MDR-TB.10 Alternative specific chemotherapy is required using second-line drugs (See Table 1). Among these agents, the fluoroquinolones and the aminoglycosides (and the allied injectable peptide antibiotics) are the most potent.9,11
Multidrug-resistant tuberculosis
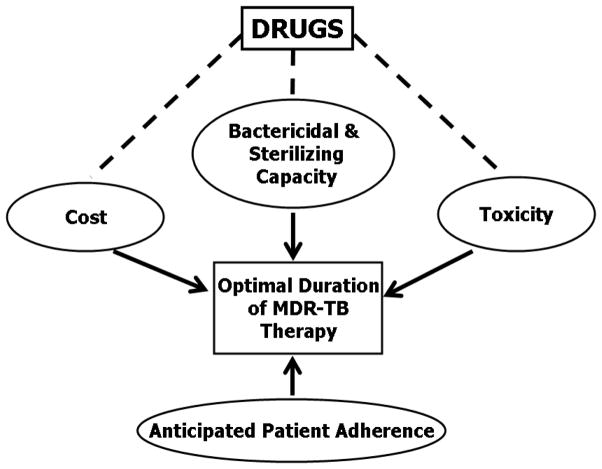
The treatment regimen for MDR-TB should typically include 5–6 drugs, with either certain or almost certain efficacy based on drug susceptibility testing results and/or previous treatment history. For example, a combination of an aminoglycoside, a fluoroquinolone, ethambutol, pyrazinamide, and ethionamide or prothionamide can be used for MDR-TB with dual bacillary resistance to isoniazid and rifampicin only, but recourse to a regimen of an aminoglycoside, fluoroquinolone, para-aminosalicylic acid, cycloserine, and ethionamide or prothionamide is required for disease with bacillary resistance to isoniazid, rifampicin, ethambutol and pyrazinamide.9 There are basically 2 treatment approaches. The standardized regimens are designed on the basis of representative drug-resistance surveillance data of specific treatment categories. The individualized regimens are designed on the basis of previous history of anti-TB treatment and results of individual drug susceptibility testing. The delivery of the regimens must be made on a programmatic basis,12 with the 5 key components as shown in Figure 1.
Figure 1.
DOTS Framework Applied to the Management of Drug-Resistant TB
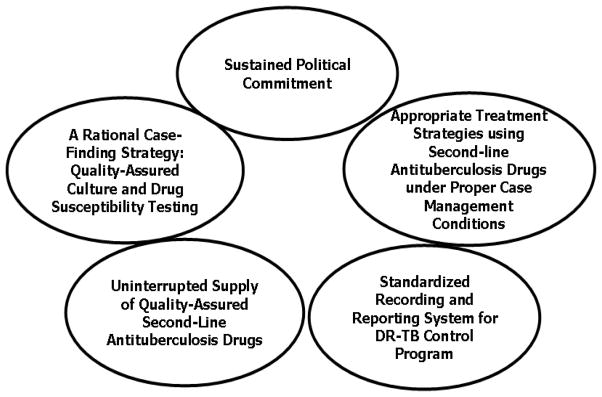
The WHO recommends treatment for at least 18 months after smear conversion to negativity, even in HIV-negative patients. However, some limited experience has also suggested that, with an intensive combination of active drugs, and the inclusion of fluoroquinolones to which the bacilli are still susceptible, the total treatment duration for some patients may be shortened to 12 – 15 months or one year after sputum culture conversion.13 Nevertheless, this preliminary impression needs further delineation. For patients with diabetes mellitus, silicosis, slow sputum culture conversion, extensive drug resistance or extensive radiographic disease, a longer duration of therapy is generally required. The prolonged administration of the more “toxic” second-line drugs poses concerns for safety, tolerability and cost. It appears that the time is now right for conducting randomized controlled studies on treatment of MDR-TB.14 Figure 2 serves to summarize the pertinent issues.
Figure 2.
Major Determinants of Optimal Treatment for Multidrug-Resistant TB
CURRENT TREATMENT OF LATENT TUBERCULOSIS INFECTION AND ITS LIMITATIONS
It is estimated that about 2 billion people in the world are infected with M. tuberculosis.2 While the reader is referred to the recent article by LoBue and Menzies15 for a more comprehensive review of latent TB infection (LTBI), several aspects related to treatment of this condition are reviewed here. Currently recommended treatment of LTBI to prevent endogenous reactivation consists of 6–12 months of isoniazid.16–18 Due to the long duration of therapy and potential for hepatic toxicity, nonadherence to the regimen remains a significant problem. Operational barriers to implementing such treatment regimens on a widespread scale also exist. Shorter and more intermittent rifamycin-containing regimens have been investigated.19,20 Rifampicin for 4 months is an alternative in the United States and Canada.17,18,21,22 In the United Kingdom, a 3-mo regimen of rifampicin plus isoniazid is used with success.20,23 After clinical trials proved its efficacy among HIV-infected individuals with LTBI,24,25 a 2-month rifampicin-pyrazinamide regimen was briefly in clinical use before being abandoned due to hepatotoxicity concerns.22 Finally, a 3-month once-weekly regimen combining isoniazid and rifapentine, a rifamycin with a half-life 5 times longer than that of rifampicin, has shown promising efficacy in humans.26 However, regimens containing rifamycins might still pose difficulties for administration in HIV-positive subjects taking antiretroviral medications.17 Furthermore, as the prevalence of MDR-TB and XDR-TB increases, so does the prevalence of drug-resistant latent TB infections, creating an increasing need for LTBI regimens based on novel drugs. At the moment, the used combination of pyrazinamide and a fluoroquinolone proves trying due to the associated toxic effects, especially on the liver and joints.27
THE NEED FOR NEW ANTI-TUBERCULOSIS DRUGS AND REGIMENS
Thus, there exists a clear and pressing need to develop new anti-TB drugs. They are needed to (i) shorten and simplify treatment of drug-susceptible TB, (ii) provide shorter, safer, more effective and cheaper treatment alternatives for MDR-TB (and XDR-TB), (iii) abolish obstacles to effective treatment of TB in HIV-positive individuals, and (iv) shorten treatment of LTBI.
The desired attributes of a new anti-TB drug are evident from the preceding discussion. In order to shorten the duration of treatment in a meaningful way, the new drug should have “sterilizing” activity, that is bactericidal activity against the small sub-population(s) of drug-tolerant bacilli which persist in a viable state despite exposures to existing drugs that are lethal for the bacterial population at-large, for these microbial “persisters” provide the genesis for relapse after treatment of inadequate duration.28,29 One or more new drugs capable of shortening the duration of treatment of both drug-susceptible and MDR/XDR-TB are highly desirable. However, given the substantial sterilizing activity of the first-line regimen and the poor sterilizing activity of second- and third-line regimens, it is possible that a new drug be capable of shortening the duration of treatment of MDR/XDR-TB without improving the treatment of drug-susceptible TB.
Certain pharmacological attributes are desirable in order to facilitate use of the new drug in the field, including oral bioavailability and pharmacokinetic/pharmacodynamic (PK/PD) properties enabling once daily (or less frequent) administration. However, where the need is greatest, as in treating XDR-TB, the requirement for such desirable features may be waived. Moreover, advances in drug delivery technology may someday enable alternative routes of administration and improved pharmacokinetic profiles for drug candidates which have unfavorable bioavailability, rapid clearance or other adverse attributes.30
Due to the requirement for combination chemotherapy in the treatment of active TB and the common necessity of treating TB and HIV simultaneously, the problem of potential drug-drug interactions is extremely important in the development of new anti-TB drugs.8 This issue is highlighted in perhaps the most important present class of anti-TB drugs, the rifamycins. Drug-drug interactions have plagued the use of rifampicin for TB. As a potent inducer of cytochrome P450 enzymes (especially CYP3A4), it accelerates the metabolism of HIV protease inhibitors resulting in sub-therapeutic drug concentrations. Rifampicin also induces the activity of efflux transporters such as p-glycoprotein and of Phase II enzymes such as glucuronosyltransferase and sulfotransferase. These drug-drug interactions affect new anti-TB drugs as well. For example, co-administration of TMC207 and rifampicin reduces the TMC207 AUC by approximately 50%.31 Co-administration of moxifloxacin and rifampicin reduces the moxifloxacin AUC by 27%.32 Rifapentine induces the cytochrome P450 enzymes at approximately 85% of rifampicin’s effect and appears to have a lesser effect on the Phase II enzymes as well, but such interactions remain problematic.33,34 Rifabutin has a lower inductive effect than either rifampicin or rifapentine and is the rifamycin of choice for co-administration with HIV protease inhibitors.8 But dosing recommendations are not always evidence based and rifabutin has its share of adverse effects which limit dose increases.
THE CURRENT CLINICAL PIPELINE
New chemotherapeutic advances may arise from optimizing the use of existing anti-TB drugs, re-purposing existing antibiotics for use as anti-TB drugs, or the discovery and development of new chemical entities. Among the drugs currently in clinical trials for TB are examples from each category (Table 2). The progress of the new drugs in the registration process is illustrated in Figure 3.
Table 2.
New anti-TB drug candidates in clinical development
| Molecular structure | Drug | Class | Mechanism(s) of action | Target |
|---|---|---|---|---|
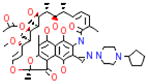
|
rifapentine | rifamycin | Inhibition of RNA synthesis | DNA-dependent RNA polymerase |
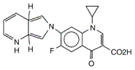
|
moxifloxacin | fluoroquinolone | Inhibition of DNA synthesis | DNA gyrase |
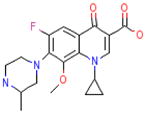
|
gatifloxacin | fluoroquinolone | Inhibition of DNA synthesis | DNA gyrase |
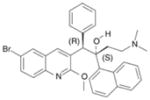
|
TMC207 | diarylquinoline | Inhibition of ATP synthesis | F1F0 proton ATP synthase |
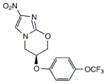
|
PA-824 | nitroimidazo-oxazine | Inhibition of cell wall lipid synthesis, inhibition of protein synthesis, indirect effects of nitric oxide generation (?) | unknown |
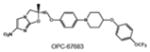
|
OPC-67683 | nitroimidazo-oxazole | Inhibition of cell wall lipid synthesis, inhibition of protein synthesis, indirect effects of nitric oxide generation (?) | unknown |
|
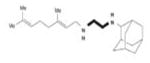
SQ109 | diethylamine | Inhibition of cell wall synthesis | unknown |
|
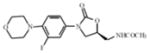
linezolid | oxazolidinone | Inhibition of protein synthesis | ribosomal initiation complex |
|
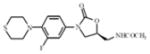
PNU-100480 | oxazolidinone | Inhibition of protein synthesis | ribosomal initiation complex |
| Not available | AZD5847 | oxazolidinone | Inhibition of protein synthesis | ribosomal initiation complex |
|
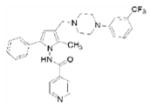
LL-3858 | pyrrole | unknown | unknown |
Figure 3.
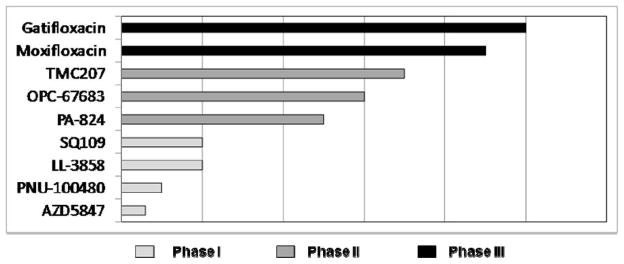
The global clinical portfolio: New anti-TB drugs in registration programs
Rifamycins
Recent renewed interest in high-dose rifamycins, including daily rifapentine therapy, provides a compelling example of the importance of integrating PK/PD principles into TB drug development. Since the introduction of rifampicin four decades ago, the rifamycins have been cornerstone agents in the modern short-course regimen due to their relatively strong sterilizing activity.28,35 The widely recommended 10 mg/kg dose of rifampicin was established early on as the minimum effective dose capable of producing treatment-shortening effects while keeping the drug acquisition costs as low as possible in order to facilitate global distribution of what was then an expensive new drug. However, despite the critical importance of rifampicin and its relatively low cost today, dosing recommendations have not been seriously re-examined. Fear of an influenza-like syndrome associated with twice- and once-weekly administration of higher rifampicin doses has contributed to the inertia despite the absence of evidence that the syndrome is more frequent when higher doses are administered daily.36,37 In fact, the prevailing theory of rifampicin antibodies and immune complex-mediated hypersensitivity suggests that daily drug administration should reduce the risk of this adverse reaction.37,38
Data from in vitro systems and animal models provide compelling evidence that rifampicin’s activity against M. tuberculosis is concentration-dependent and correlates best with the quotient of the area under the concentration-time curve and the minimum inhibitory concentration (AUC/MIC).39,40 According to a study in mice, the 10 mg/kg dose of rifampicin in humans falls at the low end of a steep and tall dose-response curve,40 indicating that increasing doses may produce log linear increases in bactericidal activity. This premise is supported by existing data from trials examining the early bactericidal activity of rifampicin.41,42 Limited data from treatment of other infectious diseases with rifampicin suggest that daily doses as high as 1200 mg may be well tolerated.43,44 Whether such dose increases have the potential to increase the sterilizing activity of rifampicin-containing regimens and thereby shorten treatment remains an important question, although long-term studies in the murine model suggest they would.45 Taken together, the existing data on the pharmacodynamics of rifampicin strongly support a series of sequential clinical trials to establish the highest well tolerated dose of rifampicin and investigate its treatment-shortening potential.
Rifapentine is a congener with more potent in vitro activity (MIC 0.06 vs. 0.25 μg/ml)46 and a longer serum half-life (11–18 hr vs. 2–4 hr)47,48 compared to rifampicin. Its development as an anti-TB drug was limited solely to providing an option for once-weekly supervision of therapy during the continuation phase. Although a rifapentine dose of 600 mg is effective in this regard, it has seen little clinical use because it is less effective than conventional rifampicin-based regimens, especially in patients at higher risk of relapse or acquired rifamycin monoresistance.7,49–52 Encouraged by the concentration-dependent activity of rifamycins and data suggesting higher and/or more frequent doses of rifapentine may be well tolerated in humans,47,51,53,54 a series of long-term murine model experiments established that some regimens containing rifapentine, pyrazinamide and either isoniazid or moxifloxacin were significantly more effective than the standard daily 1st-line regimen of rifampicin, pyrazinamide and isoniazid.55–57 Use of rifapentine at 7.5 or 10 mg/kg (corresponding to typical human doses of 450 or 600 mg) in daily regimens or 15 mg/kg (corresponding to typical human doses of 900 mg) in a thrice weekly regimen in combination with isoniazid and pyrazinamide shortened the treatment duration needed to prevent relapse by ≥ 50%.56 Higher rifapentine doses were even more effective.45,56 Similar results have been observed with daily rifapentine-containing regimens in a murine model of LTBI.58 Based on these results, four Phase II and III trials are now underway to evaluate various daily and intermittent rifapentine-based treatment regimens.
The lone Phase III trial involving rifapentine, the RIFAQUIN trial, includes two experimental arms in which the initial phase treatment is with rifampicin (10 mg/kg), moxifloxacin (400 mg), pyrazinamide and ethambutol and the continuation phase treatment is either twice weekly rifapentine (15 mg/kg) and moxifloxacin for 2 months or once weekly rifapentine (20 mg/kg) and moxifloxacin for 4 months. Three Phase II trials will evaluate daily rifapentine-based regimens. A randomized, double blind trial conducted by the U.S. Centers for the Disease Control and Prevention TB Trials Consortium (TBTC) will evaluate the safety, tolerability and efficacy of replacing rifampicin 10 mg/kg with rifapentine 10 mg/kg administered 5 days per week during the first 8 weeks of daily treatment (ClinicalTrials.gov identifier: NCT00694629). Enrollment is expected to be completed in the second quarter of 2010 (S. Dorman, personal communication). In a second trial conducted in South Africa, investigators from Johns Hopkins University and Cape Town University will evaluate the dose-response of two daily rifapentine doses (7.5 and 10 mg/kg) administered 7 days per week in place of rifampicin during the first 8 weeks of treatment (ClinicalTrials.gov identifier: NCT00814671). In a third trial, investigators from Johns Hopkins University and the Federal University of Rio de Janeiro will evaluate the efficacy of a daily regimen of rifapentine 7.5 mg/kg combined with moxifloxacin, pyrazinamide and ethambutol administered 7 days per week for the first 8 weeks of treatment in a trial conducted in Brazil (ClinicalTrials.gov identifier: NCT00728507).
Fluoroquinolones
The fluoroquinolones are broad-spectrum anti-bacterial drugs that are currently marketed for a variety of infectious indications other than TB. Due to their excellent oral bioavailability, bactericidal activity against M. tuberculosis, lack of cross-resistance with existing anti-TB drugs and favourable safety and tolerability profile, they have been employed off-label against MDR-TB and are now regarded as cornerstone agents for this disease.9,12,21 Newer generation fluoroquinolones such as moxifloxacin and gatifloxacin have potent bactericidal activity which rivals that of isoniazid in animal models59–61 as well as EBA studies.62–64 At a dose of 1000 mg, levofloxacin has an EBA at least as strong as that of the 400 mg dose of moxifloxacin or gatifloxacin,63 which is the highest recommended dose of the latter 2 drugs. These results and additional animal model and human observational data establish these three fluoroquinolones as the preferred agents for treatment of MDR-TB.13,65–67
Their favourable profiles have also made the fluoroquinolones the first new antibiotic class to be considered for 1st-line usage against TB since the introduction of rifampicin in 1968. Long-term murine model studies suggest that replacement of ethambutol or isoniazid with moxifloxacin or gatifloxacin in the 1st-line regimen has the potential to improve treatment.68,69 Replacement of ethambutol with moxifloxacin or gatifloxacin, each at 400 mg, is further supported by the results of three recent Phase II trials. The first study published was TBTC Study 27 in which substitution of moxifloxacin for ethambutol resulted in a higher rate of sputum culture conversion at 4 and 6 weeks but not at 8 weeks.70 A trial with the same treatment groups conducted by investigators from Johns Hopkins University in Rio de Janeiro found that the moxifloxacin group experienced a higher rate of sputum culture conversion beginning at week 2 and persisting to week 8.71 The OFLOTUB study evaluated the replacement of ethambutol by ofloxacin 800 mg, moxifloxacin 400 mg or gatifloxacin 400 mg using a new microbiologic outcome measure of serial sputum colony counting.72 Whereas replacement of ethambutol with ofloxacin resulted in no measureable improvement, use of moxifloxacin or gatifloxacin was associated with more rapid clearance of bacteria from the sputum. In the case of replacing isoniazid with moxifloxacin, which was the substitution associated with the greatest benefit in the murine model,68 the lone corresponding Phase II trial demonstrated a statistically insignificant 5.5% increase in the 2-month sputum conversion rate in the moxifloxacin arm.73 The fluoroquinolones have been safe and well-tolerated in these studies. Although it is well known that moxifloxacin produces a small mean increase in the QTc interval, no clinically significant adverse events or arrhythmias have been reported among subjects receiving moxifloxacin-containing regimens. Careful consideration of the potential for additive effects on the QTc interval will be required before moxifloxacin is combined with new agents which may also prolong the QTc interval (e.g., TMC207). Rifamycins induce the metabolism of moxifloxacin but the clinical significance of the rather modest reductions in AUC is uncertain.32,34
Two independent Phase III trials are ongoing to determine whether use of moxifloxacin or gatifloxacin in combination with first-line drugs enables shortening the duration of treatment to 4 months without sacrificing efficacy. The OFLOTUB consortium is evaluating a 4-month regimen based on 2 months of rifampicin-isoniazid-pyrazinamide-gatifloxacin followed by 2 months of rifampicin-isoniazid-gatifloxacin (ClinicalTrials.gov identifier: NCT00216385). The REMoxTB trial is evaluating two 4-month regimens: 2 months of rifampicin-isoniazid-pyrazinamide-moxifloxacin followed by 2 months of rifampicin-isoniazid-moxifloxacin or 2 months of rifampicin-moxifloxacin-pyrazinamide-ethambutol followed by 2 months of rifampicin-moxifloxacin (ClinicalTrials.gov identifier: NCT00864383).
TMC207
TMC207 was the first truly novel chemical entity to enter Phase II clinical trials for a TB indication in 40 years. This diarylquinoline has potent anti-TB activity in vitro (MIC, 0.03–0.12 μg/ml) exerted through a novel mechanism: inhibition of the mycobacterial F1F0 proton ATP synthase, an essential enzyme responsible for ATP synthesis.74,75 This activity is selective for mycobacteria.74 Resistant mutants selected in vitro harbor mutations in atpE, which encodes part of the F0 subunit and appear at a frequency of 5 × 10−8 but other resistance mechanisms exist.74,76 To date, cross-resistance between TMC207 commonly used first- or second-line anti-TB drugs has not been described,74 making TMC207 potentially useful in both drug-susceptible and drug-resistant TB. That TMC207 is active against non-replicating persisters under anaerobic conditions suggest TMC207 may have valuable sterilizing activity as well.77,78
Studies in animal models have demonstrated potent bactericidal activity at drug exposures that appear achievable in humans.74,79,80 For example, daily treatment with TMC207 alone at 25 mg/kg has a greater bactericidal effect than treatment with the standard first-line regimen of rifampicin, isoniazid and pyrazinamide over the first 2 months of treatment.74 Moreover, an impressive synergisitic bactericidal effect has been observed when TMC207 is combined with pyrazinamide.81 Long-term mouse model studies using relapse after treatment as the measure of sterilizing activity have demonstrated that combinations of TMC207 and pyrazinamide with rifampicin, isoniazid or moxifloxacin may be capable of shortening the duration of treatment to less than 6 months.82,83 Combinations with pyrazinamide and rifapentine may be even more effective. The sterilizing potential of TMC207 in combinations that do not contain pyrazinamide has yet to be evaluated and remains an important question given the unreliability and common omission of pyrazinamide susceptibility testing as well as the high frequency of pyrazinamide resistance reported among MDR M. tuberculosis isolates in some hot spots.67,84,85
Phase I pharmacokinetic and safety studies in healthy volunteers revealed the drug to be well tolerated with dose-proportional pharmacokinetic parameters over the dose range and time intervals studied.74 In the single ascending dose study, TMC207 was given in a dose range of 10 to 700 mg. In the multiple ascending dose study (once daily doses of 50, 150 and 400 mg per day for 14 days), accumulation was observed with a doubling of the area under the time-concentration curve (AUC) by the 14th day.
An open-label randomized trial has been conducted to study the dose-ranging early bactericidal activity (EBA) of TMC207.80 Newly diagnosed patients with smear-positive pulmonary TB were randomized (15 per arm) to receive daily therapy with isoniazid 300 mg, rifampicin 600 mg, or TMC207 at 25, 100 or 400 mg for 7 days before receiving standard combination therapy. Although TMC207 at 25 and 100 mg had no measurable effect on sputum CFU counts, a bactericidal effect was observed for the 400 mg dose beginning around the fourth day of treatment. The overall reduction in CFU counts with TMC207 was considerably smaller than that produced by isoniazid or rifampicin but several caveats must be considered in the interpretation of these data. First, TMC207 concentrations did not achieve steady state during this trial, so its maximal effect was probably not observed. Second, TMC207 has consistently demonstrated time-dependent bactericidal effects in vitro74 and in mice31 so it should not be surprising that the same would be observed in the EBA study. Future evaluations of novel TMC207-containing regimens should be extended over a minimum of 14 days to capture this drug’s time-dependent activity. A 14-day EBA trial is currently being planned to better understand the dose-response curve for this compound (M. Spigelman, personal communication).
Interim results of a randomized, double blind, placebo-controlled Phase IIb study of TMC207 in newly diagnosed MDR-TB patients have recently been reported.79 In Stage I of this trial, all patients received an optimized background regimen (typically kanamycin, ofloxacin, ethionamide, pyrazinamide and ethambutol, cycloserine or terizidone) plus either placebo or TMC207 at 400 mg daily for 2 weeks followed by 200 mg thrice weekly for 6 weeks. TMC207 appeared to be well tolerated, although nausea was more common among patients in the TMC207 group. In addition, there was some prolongation of the QT interval noted in the group receiving TMC207; this was of unclear clinical significance. The primary efficacy outcome of time to sputum culture conversion was significantly shorter among patients receiving TMC207. Using liquid culture in the MGIT system, 10 (48%) of 21 evaluable patients receiving TMC207 converted their sputum cultures to negative by 8 weeks compared to 2 (9%) of 23 evaluable patients receiving placebo. These results raise hopes that TMC207-containing regimens may significantly improve the treatment of MDR/XDR-TB. Stage 2 of this trial will evaluate the same treatment arms after 6 months of treatment (ClinicalTrials.gov identifier: NCT00449644). A second open label trial is evaluating the safety and efficacy of the same dose of TMC207 administered for 6 months as part of an individualized regimen for patients with MDR/XDR-TB not responding to current therapy (ClinicalTrials.gov identifier: NCT00910871).
A development program to evaluate the potential of TMC207 in combination with first-line drugs is being pursued in parallel with the development program in MDR-TB. One significant obstacle is the induction of TMC207 metabolism by rifampicin resulting in a 50% reduction in TMC207 AUC, likely through the induction of CYP3A4.31 Results from murine model experiments suggest that combining rifampicin and TMC207 would still have a net additive effect despite the drug-drug interaction.31 Additional measures to improve the activity of the combination could include using other rifamycins with weaker induction of CYP3A4, such as rifabutin and, to a lesser extent, rifapentine. Once-weekly combinations containing TMC207, pyrazinamide and rifapentine have significant bactericidal activity in mice suggesting their potential for widely spaced intermittent ultra-short course regimens but their sterilizing activity and ability to prevent the selection of drug-resistant mutants have not been evaluated.86,87
Nitroimidazole derivatives (OPC-67683, PA-824)
The activity of nitroimidazole derivatives against M. tuberculosis was first reported over 15 years ago.88 Two members of this class are currently in Phase II clinical trials for a TB indication and seem devoid of the mutagenicity that plagued many compounds in the class.89,90
OPC-67683 is a nitroimidazo-oxazole developed after members of the class were identified in a screen for mycolic acid synthesis inhibitors. Like the related nitroimidazo-oxazine, PA-824, it appears to undergo nitroreductive activation91 and inhibits ketomycolic acid synthesis as at least one of its mechanisms of action.89 Cross-resistance to both drugs occurs through mutations in ddn, the enzyme responsible for activation.92 Whether cross-resistance occurs with mutations in fgd and fbiA, fbiB, or fbiC which confer resistance to PA-824, has not been reported. OPC-67683 is more potent than PA-824 in vitro and in vivo with an MIC range of 0.006–0.024 μg/ml and minimum bactericidal dose (MBD), resulting in a 2 log10 reduction in CFU, of 2.5 mg/kg in mice (compared to 50 mg/kg for PA-824 in a similar model [E. Nuermberger, unpublished observations]).89 As with PA-824, combination of OPC-67683 at the MBD with rifampicin and pyrazinamide resulted in more rapid attainment of negative cultures in the lungs of mice.89
In Phase I multi-dose studies with 2 different formulations, OPC-67683 was administered in doses up to 400 mg. A 14-day extended EBA trial has been performed with the newer formulation of OPC-67683 but the results have not been reported in detail. A Phase IIb trial is currently underway in MDR-TB patients randomized to receive an optimized background regimen with either OPC-67683 at 100 or 200 mg twice daily or placebo (ClinicalTrials.gov identifier: NCT00685360).
PA-824 was selected from a library of newly synthesized nitroimidazo-oxazines (also described as nitroimidazopyrans) as the compound having the most potent activity in a murine model.90 Its MIC ranges from ≤ 0.015 to 0.25 μg/ml against susceptible strains, as well as strains resistant to the commonly used first- and second-line drugs.90 Activity is limited to members of the M. tuberculosis complex, with the exception of Helicobacter pylori and some anaerobic bacteria. PA-824 is a pro-drug which is activated by a bacterial deazaflavin (F420)-dependent nitroreductase named Ddn (Rv3547).91,93 Mutations in the genes encoding the F420 biosynthetic enzymes (fbiA, fbiB and fbiC), the F420-dependent glucose-6-phosphate dehydrogenase responsible for reducing F420 (fgd) as well as the activating enzyme itself (ddn) confer resistance to PA-824.90,93–95 The reactive intermediate(s) of PA-824 likely exert anti-TB activity through one or more novel mechanisms including inhibition of ketomycolic acid (cell wall) synthesis, inhibition of protein synthesis and, under certain conditions, generation of intracellular nitric oxide.90,91 Like TMC207, PA-824 is active against non-replicating persisters in the in vitro Wayne model90,96 and those selected by treatment of 100-day-old cultures with or without high concentrations of rifampicin,97 indicating it may have significant sterilizing activity.
PA-824 demonstrates dose-dependent bactericidal and sterilizing activity in a murine model of TB.96,98 At a daily dose of 100 mg/kg in mice, it has bactericidal activity which approaches that of isoniazid and sterilizing activity comparable to that of rifampicin. Combination of PA-824 with rifampicin and pyrazinamide results in more rapid lung culture conversion than the standard first-line regimen of isoniazid, rifampicin and pyrazinamide.99,100 The combination of PA-824 with moxifloxacin and pyrazinamide cures mice more rapidly than the first-line regimen.101 These data suggest PA-824 may be capable of shortening the duration of treatment of drug-susceptible as well as MDR/XDR-TB.
Phase I studies demonstrated less-than-dose proportional increases in PA-824 exposure which, in the multi-dose study, reached a plateau at the 600 mg dose.102 Maximal serum concentrations were 3.8 μg/ml and the elimination half-life was 16–20 hours.
A randomized partially blinded Phase IIa extended EBA trial has been conducted in South Africa to evaluate the dose-response of PA-824 in newly diagnosed patients with smear-positive pulmonary TB.103 Subjects received PA-824 in doses of 200, 600, 1000 and 1200 mg or the 4-drug combination of rifampicin, isoniazid, pyrazinamide and ethambutol (Rifafour®) for 14 days. All were blinded as to the dose of PA-824, but no blinding was employed to mask which patients received standard therapy. While not as active as Rifafour over the first 2 days, PA-824 had considerable bactericidal activity, resulting in an average reduction of 0.1 log10 CFU/ml of sputum per day which was sustained over the entire 14 day period. Remarkably, there was no dose-response observed over the range of doses tested. While the lack of dose-response between the 600–1200 mg doses may be explained by the above-mentioned plateau in drug exposure, the lack of dose effect over the 200–600 mg dose range is surprising. However, it may be reconciled with the dose-dependent activity observed in the murine model if the principal pharmacodynamic index correlating with the bactericidal effect is the proportion of the dosing interval in which active drug concentrations exceed the MIC, as recently demonstrated in mice.104 A second 14-day dose-ranging EBA trial evaluating PA-824 doses of 50, 100, 150 and 200 mg will be completed in the first quarter of 2010 (ClinicalTrials.gov identifier: NCT00944021).
SQ109
SQ109 is an ethylenediamine identified by screening a library of ethambutol derivatives.105 It has an MIC of 0.16–0.64 μg/ml which does not appear to be affected by resistance to ethambutol.105,106 The mechanism of action of SQ109 remains to be fully elucidated. Inhibition of cell wall synthesis is implicated by the induction of Rv0341 but, in addition to the lack of evidence of cross-resistance, gene expression analysis suggests it may not be the same as that of ethambutol.105,106 A remarkable synergistic effect is evident when sub-MIC concentrations of SQ109 and rifampicin are combined106 and a synergistic effect of combining SQ109 and TMC207 has also been described recently.107
In animal models, SQ109 shows extensive tissue distribution and concentration which may explain how the drug maintains activity in mice even when serum concentrations do not exceed the MIC.105,108 SQ109 in daily doses ranging from 1 to 25 mg/kg is similar in activity to ethambutol at the presumed human-equivalent dose of 100 mg/kg in mice, but combination studies suggest replacement of ethambutol with SQ109 at 10 mg/kg increases the bactericidal activity of the first-line regimen of rifampicin, isoniazid and pyrazinamide.105,109
In a Phase I study, SQ109 was safe and well tolerated in single doses up to 300 mg (http://www.sequella.com/docs/Sequella_1sheet09v2_SQ109.pdf, accessed January 2, 2010). The long half-life of 61 hours suggests the potential for widely spaced intermittent therapy. A multi-dose dose escalation study is currently recruiting subjects (ClinicalTrials.gov identifier: NCT00866190). In vitro metabolism studies using recombinant cDNA-expressed human CYPs in conjunction with specific CYP inhibitors indicated that CYP2D6 andCYP2C19 were the predominant CYPs involved in SQ109 metabolism, with little effect of CYP3A4.110
Oxazolidinones
The oxazolidinones exert their anti-TB activity by inhibiting protein synthesis through a novel mechanism by blocking formation of the ribosomal initiation complex. The only currently marketed oxazolidinone, linezolid, has an MIC range of 0.125–1 μg/ml, with a MIC50 of 0.5 μg/ml and an MIC90 of 1 μg/ml.111–113 As a result, it has been used outside of labeled indications in difficult-to treat cases of MDR- and XDR-TB.114–122 While there is evidence linezolid may contribute to sputum culture conversion in such cases, its individual activity in TB patients and its precise contribution to combination regimens remain unclear. These studies also demonstrate that the duration of linezolid administration is commonly limited by hematologic and neurologic toxicity that can occur with long-term administration.114,115,117–119 Dosage reductions appear to reduce the incidence of hematologic but perhaps not the neuropathic side effects116,117,120,121 and the effect on drug efficacy is uncertain.123,124 In an EBA study comparing linezolid with isoniazid, once and twice daily administration of linezolid 600 mg resulted in a modest bactericidal effect of 0.18 and 0.26 log10 CFU/ml sputum/day (compared to 0.67 for isoniazid) over the first 2 days of treatment, but more limited effects of 0.04–0.09 over the next 5 days.125 A double blind randomized controlled trial is underway to evaluate low-dose linezolid (e.g., 600 mg daily) vs. placebo added to an optimized background regimen in South African patients with MDR/XDR-TB (ClinicalTrials.gov identifier: NCT00664313). A second open label randomized trial is evaluating linezolid in South Korean patients with XDR-TB (ClinicalTrials.gov identifier: NCT00727844). Nevertheless, new oxazolidinones with more potent in vivo activity against M. tuberculosis and/or lower risk of toxicity with prolonged administration are desirable.
Oxazolidinones with more potent activity against M. tuberculosis have been described. 126–128 The anti-TB activity of PNU-100480 was first reported in 1996.126 Recent experiments in the murine model show it to be more active than linezolid even when serum concentrations are considerably lower.129 A long-term experiment demonstrated that the addition of PNU-100480 at 160 mg/kg shortened the duration of treatment necessary to prevent relapse, suggesting that PNU-100480 has sterilizing activity that could improve the treatment of drug-susceptible as well as drug-resistant TB.130
PNU-100480 is currently in Phase I, where single doses of 600 and 1000 mg were well tolerated and bactericidal drug concentrations were maintained in whole blood samples for 12 and 24 hours post-dose, respectively.131 A multi-dose study of PK, safety, tolerability and whole blood bactericidal activity is currently underway (ClinicalTrials.gov identifier: NCT00990990).
Another oxazolidinone, AZD5847, has entered Phase I with an ascending dose study of the PK, safety and tolerability of the compound (ClinicalTrials.gov identifier: NCT01037725). No information from the pre-clinical evaluation of the compound is publicly available.
Carbapenems
The β-lactams are among the most successful antibiotic classes in clinical use on the basis of their bactericidal activity and favorable safety and tolerability profile. Yet this class has demonstrated little utility against M. tuberculosis, primarily because of resistance mediated by BlaC, a bacterial β-lactamase capable of hydrolyzing penicillins, cephalosporins and carbapenems.132–134 For penicillins and cephalosporins, MICs against M. tuberculosis typically exceed the range of clinically achievable drug concentrations.135,136 Amoxicillin MICs, for example, are typically >16 μg/ml.135,137 But amoxicillin MICs are lower in the presence of clavulanate, which irreversibly inhibits BlaC.134,135,137 Clinical experience with this combination in TB patients has been mixed. While anecdotal successes have been reported138,139 and one extended EBA study demonstrated activity at an oral dose of 1000 mg/250 mg thrice daily,140 a second study found no EBA with a single daily dose of 3000 mg/750mg.141 These results may indicate that the principal pharmacodynamic parameter correlating with activity against M. tuberculosis is time above MIC (T>MIC), as it is for β-lactams against other pathogens and that multiple daily doses are required for activity. An additional finding in the former study was that the EBA over the first 2 days of treatment (0.34 log10 CFU/ml sputum/day) was comparable to that of ofloxacin 600 mg daily but the EBA over the subsequent 5 days (0.02 log10 CFU/ml sputum/day) was very limited, which may indicate limited “sterilizing” activity against more slowly multiplying or non-multiplying organisms.
The carbapenems may hold more promise as anti-TB drugs. In general, they are not as susceptible to hydrolysis as the penicillins and cephalosporins.134 For example, imipenem MICs are 1.25 to 10 and this drug has activity in a murine model of TB.136,142,142,143 A small case series suggested that imipenem at a dose of 1 g intravenously every 12 hours contributed activity to combination chemotherapy of MDR-TB patients but the study design did not permit measurement of its individual contribution.142 Meropenem may be more effective than imipenem. It is hydrolyzed by BlaC so slowly that it functions almost like a β-lactamase inhibitor rather than a substrate.21,134,136 Against the limited number of strains studied, meropenem was more consistently active than imipenem in the presence of clavulanate (MIC90 0.94 μg/ml for meropenem vs. 10 μg/ml for imipenem).136 Data from a pulmonary PK study in healthy volunteers suggest that, at a dose of 1 g IV every 8 hours, meropenem would attain T>MIC values in plasma and alveolar epithelial lining fluid of 64–100% and 38–100% against organisms with MIC values of 2–0.12 μg/ml, respectively, where 40% is the T>MIC target associated with maximal bactericidal activity against bacterial pathogens other than M. tuberculosis.144,145 Efforts are reportedly underway to launch a trial to evaluate the efficacy of meropenem/clavulanate in patients with MDR/XDR-TB.146
The principal drawbacks to clinical usage of meropenem and imipenem for TB are the need for intravenous access and, likely, multiple daily doses for maximal efficacy. These are major disadvantages for use in the field. Ertapenem is a marketed carbapenem approved for intramuscular administration. It also has a half-life of 4 hrs (vs. 1 hr for meropenem), which makes it suitable for once daily administration. However, the susceptibility of M. tuberculosis to ertapenem has not been described. Oral carbapenems have been developed. Faropenem advanced to Phase II trials against bacterial respiratory tract infections but was not advanced beyond this stage. An oral pro-drug of sulopenem is currently being evaluated in Phase II trials for community-acquired pneumonia (ClinicalTrials.gov identifier: NCT00797108), but its activity against M. tuberculosis has not been described.
LL-3858
The anti-TB activity of the pyrrole class was first described in 1998.147 The discovery of LL-3858 was reported in 2004.148 While the mechanism of action remains unknown, early reports described an MIC range of 0.06–0.5 μg/ml that was not affected by resistance to isoniazid and rifampicin.148 Additive activity in combination with first-line drugs in the murine model was also described.148 To date however, the data remain unpublished and few other details about this compound have been made publicly available. It has reportedly completed Phase I clinical testing and is awaiting regulatory approval to enter Phase II.
DISCOVERY PIPELINE
Although the current clinical pipeline for potential new anti-TB drugs is more robust than ever, given the expected attrition rate in clinical drug development, there is a need for many more candidate compounds. The discovery pipeline for new anti-TB drugs has also grown considerably, especially over the past five years with over 30 discovery and preclinical projects presently being pursued. Although beyond the scope of this review to describe these discovery projects in detail, they derive primarily from two sources, phenotypic screening and pursuit of specific molecular targets.
Phenotypic screening, that is screening general or specific compound libraries to see which compounds will kill M. tuberculosis in vitro, has been employed widely to derive “hits” that can be used as starting points in the search for new drug candidate molecules. The advantage of the phenotypic screening approach is that the hits will have already demonstrated the ability to kill the mycobacterium. Literally millions of compounds have been screened over the past 5 to 10 years against M. tuberculosis. One of the present rate-limiting steps in this approach is the relative lack of sophisticated medicinal chemistry capability to modify the hits, an expensive and time-consuming step usually necessary for incorporating good drug-like qualities into a candidate molecule.
Pursuing specific targets within M. tuberculosis is another commonly used approach in the search for new anti-TB drugs. At present, there are a variety of targets being pursued. These include various cell wall synthetic enzymes, M. tuberculosis-specific kinases, proteases, energy metabolism intermediaries, and what are felt to be persistence-specific enzymes that are used by M. tuberculosis to maintain dormancy. One advantage of target-specific discovery programs is that it is relatively easy to make sure the compounds that are being synthesized continue to hit the desired target and one has a better understanding of the mechanism of cell death.
Of note, there have been relatively few discovery efforts that seek to improve TB therapy through immunomodulatory effects on the host. Such agents would primarily act by influencing the patient’s immune system to kill M. tuberculosis or prevent reactivation from latency.
NOVEL APPROACHES TO DRUG DELIVERY
Other approaches to improving drug therapy of TB involve optimizing the delivery systems for drug administration, be they for use with novel or existing therapeutics. Various techniques are under investigation oftentimes with two new methods being used simultaneously.149
One of the most promising approaches is the use of nanoparticles.30,150,151 These are drug carriers that may have the following desirable attributes: high stability, high capacity, feasibility of incorporation of both hydrophilic and hydrophobic substances, and feasibility of oral and inhalational administration in addition to parenteral. As nanoparticles can be designed to yield a sustained release from the matrix, they also have the possibility to reduce dosing frequency. Although liposomal formulations have also been considered for similarly novel delivery systems of TB drugs, the potential flexibility with nanoparticles appears much greater than for liposome-encapsulated drugs.
Nanoparticles may also be useful for targeted therapies aiming to deliver drugs selectively to intracellular sites, such as those within monocytes, macrophages or the reticuloendothelial system. The rationale for this approach is that the persistent organisms responsible for lengthy treatment periods may be intracellular.152–154 However, this assumption has never been well validated.155
Inhalational approaches for TB treatment are also being tested.156,157 One of the presently used parenterally administered second-line drugs, capreomycin, is being tested in human volunteers in a Phase I study as an inhalational product. The formulation being used with capreomycin is large porous particles.158 Many of the other commonly used first-line drugs, as well as some investigational agents, have also been formulated and tested in an inhalational delivery system. Inhalational approaches deliver much higher doses of drug to the lung, but the exact histological localization of increased delivery is not clear.
One overriding aspect of novel delivery systems that always needs to be taken into consideration is cost of goods. Because of the limited resources available in virtually all TB endemic countries and because of the presently very inexpensive cost of goods for first-line drugs, all new delivery systems, as well as new compounds, are subject to cost considerations. This can be a limiting factor for the feasibility of using some of the novel delivery approaches.
References
- 1.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3:S231–S279. [PubMed] [Google Scholar]
- 2.World Health Organization. WHO/HTM/TB/2009.411. Geneva: World Health Organization; 2009. Global tuberculosis control: epidemiology, planning, financing: WHO report 2009. [Google Scholar]
- 3.Zignol M, Hosseini MS, Wright A, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006;194:479–85. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- 4.Raviglione MC, Smith IM. XDR tuberculosis--implications for global public health. N Engl J Med. 2007;356:656–9. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Treatment of tuberculosis: Guidelines for national programmes. Geneva: WHO; 2003. Publication no. WHO/CDS/TB/2003.313. [Google Scholar]
- 6.Chang KC, Leung CC, Yew WW, Chan SL, Tam CM. Dosing schedules of 6-month regimens and relapse for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:1153–8. doi: 10.1164/rccm.200605-637OC. [DOI] [PubMed] [Google Scholar]
- 7.Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–34. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 8.Burman WJ. Issues in the management of HIV-related tuberculosis. Clin Chest Med. 2005;26:283–94. doi: 10.1016/j.ccm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Yew WW, Leung CC. Management of multidrug-resistant tuberculosis: Update 2007. Respirology. 2008;13:21–46. doi: 10.1111/j.1440-1843.2007.01180.x. [DOI] [PubMed] [Google Scholar]
- 10.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 11.Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008;21:587–95. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO/HTM/TB/2006361. Geneva: WHO; 2006. Guidelines for the programmatic management of drug-resistant tuberculosis. [Google Scholar]
- 13.Yew WW, Chan CK, Chau CH, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–51. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 14.Mitnick CD, Castro KG, Harrington M, Sacks LV, Burman W. Randomized trials to optimize treatment of multidrug-resistant tuberculosis. PLoS Med. 2007;4:e292. doi: 10.1371/journal.pmed.0040292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LoBue PA, Menzies D. Latent TB infection. Respirology. 2010 doi: 10.1111/j.1440-1843.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- 16.British Thoracic Society. Control and prevention of tuberculosis in the United Kingdom: code of practice 2000. Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 2000;55:887–901. doi: 10.1136/thorax.55.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention and American Thoracic Society. . Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 18.Long R. Canadian Tuberculosis Standards. 6. Ottawa: Canadian Lung Association and Public Health Agency of Canada; 2007. [Google Scholar]
- 19.Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179:1055–60. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spyridis NP, Spyridis PG, Gelesme A, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis. 2007;45:715–22. doi: 10.1086/520983. [DOI] [PubMed] [Google Scholar]
- 21.Assessment of a daily combined preparation of isoniazid, rifampin, and pyrazinamide in a controlled trial of three 6-month regimens for smear-positive pulmonary tuberculosis. Singapore Tuberculosis Service/British Medical Research Council. Am Rev Respir Dis. 1991;143:707–12. doi: 10.1164/ajrccm/143.4_Pt_1.707. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection--United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:735–9. [PubMed] [Google Scholar]
- 23.Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax. 1998;53:536–48. [PMC free article] [PubMed] [Google Scholar]
- 24.Gordin F, Chaisson RE, Matts JP, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. JAMA. 2000;283:1445–50. doi: 10.1001/jama.283.11.1445. [DOI] [PubMed] [Google Scholar]
- 25.Halsey NA, Coberly JS, Desormeaux J, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351:786–92. doi: 10.1016/S0140-6736(97)06532-X. [DOI] [PubMed] [Google Scholar]
- 26.Schechter M, Zajdenverg R, Falco G, et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med. 2006;173:922–6. doi: 10.1164/rccm.200512-1953OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papastavros T, Dolovich LR, Holbrook A, Whitehead L, Loeb M. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ. 2002;167:131–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Grosset J. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin Chest Med. 1980;1:231–41. [PubMed] [Google Scholar]
- 29.Mitchison DA. The Garrod Lecture. Understanding the chemotherapy of tuberculosis--current problems. J Antimicrob Chemother. 1992;29:477–93. doi: 10.1093/jac/29.5.477. [DOI] [PubMed] [Google Scholar]
- 30.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005;172:1487–90. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lounis N, Gevers T, Van Den Berg J, Andries K. Impact of the interaction of R207910 with rifampin on the treatment of tuberculosis studied in the mouse model. Antimicrob Agents Chemother. 2008;52:3568–72. doi: 10.1128/AAC.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner M, Burman W, Luo CC, et al. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother. 2007;51:2861–6. doi: 10.1128/AAC.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet. 2001;40:327–41. doi: 10.2165/00003088-200140050-00002. [DOI] [PubMed] [Google Scholar]
- 34.Dooley K, Flexner C, Hackman J, et al. Repeated administration of high-dose intermittent rifapentine reduces rifapentine and moxifloxacin plasma concentrations. Antimicrob Agents Chemother. 2008;52:4037–42. doi: 10.1128/AAC.00554-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4:796–806. [PubMed] [Google Scholar]
- 36.Girling DJ. Adverse reactions to rifampicin in antituberculosis regimens. J Antimicrob Chemother. 1977;3:115–32. doi: 10.1093/jac/3.2.115. [DOI] [PubMed] [Google Scholar]
- 37.Grosset J, Leventis S. Adverse effects of rifampin. Rev Infect Dis. 1983;5(Suppl 3):S440–S450. doi: 10.1093/clinids/5.supplement_3.s440. [DOI] [PubMed] [Google Scholar]
- 38.Martinez E, Collazos J, Mayo J. Hypersensitivity reactions to rifampin. Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine (Baltimore) 1999;78:361–9. doi: 10.1097/00005792-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Gumbo T, Louie A, Deziel MR, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51:3781–8. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47:2118–24. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–49. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 42.Diacon AH, Patientia RF, Venter A, et al. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother. 2007;51:2994–6. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kochar DK, Saini G, Kochar SK, et al. A double blind, randomised placebo controlled trial of rifampicin with omeprazole in the treatment of human cutaneous leishmaniasis. J Vector Borne Dis. 2006;43:161–7. [PubMed] [Google Scholar]
- 44.Solera J, Rodriguez-Zapata M, Geijo P, et al. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrob Agents Chemother. 1995;39:2061–7. doi: 10.1128/aac.39.9.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosset JH, Rosenthal I, Zhang M, Williams K, Nuermberger EL. Low doses of rifapentine versus high doses of rifampin in the mouse model of tuberculosis. Int J Tuberc Lung Dis. 2008;12(11):S63. [Google Scholar]
- 46.Heifets LB, Lindholm-Levy PJ, Flory MA. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141:626–30. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 47.Keung A, Eller MG, McKenzie KA, Weir SJ. Single and multiple dose pharmacokinetics of rifapentine in man: part II. Int J Tuberc Lung Dis. 1999;3:437–44. [PubMed] [Google Scholar]
- 48.Langdon G, Wilkins JJ, Smith PJ, McIlleron H. Consecutive-dose pharmacokinetics of rifapentine in patients diagnosed with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2004;8:862–7. [PubMed] [Google Scholar]
- 49.Tam CM, Chan SL, Kam KM, Goodall RL, Mitchison DA. Rifapentine and isoniazid in the continuation phase of a 6-month regimen. Final report at 5 years: prognostic value of various measures. Int J Tuberc Lung Dis. 2002;6:3–10. [PubMed] [Google Scholar]
- 50.Vernon A, Burman W, Benator D, Khan A, Bozeman L. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Tuberculosis Trials Consortium. Lancet. 1999;353:1843–7. doi: 10.1016/s0140-6736(98)11467-8. [DOI] [PubMed] [Google Scholar]
- 51.Anonymous. Rifapentine (Priftin) data on file [package insert] Hoechst Marion Roussel; Kansas City, MO: 1998. [Google Scholar]
- 52.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 53.Bock NN, Sterling TR, Hamilton CD, et al. A prospective, randomized, double-blind study of the tolerability of rifapentine 600, 900, and 1,200 mg plus isoniazid in the continuation phase of tuberculosis treatment. Am J Respir Crit Care Med. 2002;165:1526–30. doi: 10.1164/rccm.200201-047OC. [DOI] [PubMed] [Google Scholar]
- 54.Sirgel FA, Fourie PB, Donald PR, et al. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:128–35. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- 55.Rosenthal IM, Williams K, Tyagi S, et al. Potent Twice-Weekly Rifapentine-containing Regimens in Murine Tuberculosis. Am J Respir Crit Care Med. 2006;174:94–101. doi: 10.1164/rccm.200602-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal IM, Zhang M, Almeida D, Grosset JH, Nuermberger EL. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am J Respir Crit Care Med. 2008;178:989–93. doi: 10.1164/rccm.200807-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med. 2009;180:1151–7. doi: 10.1164/rccm.200905-0795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–9. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenaerts AJ, Gruppo V, Brooks JV, Orme IM. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob Agents Chemother. 2003;47:783–5. doi: 10.1128/AAC.47.2.783-785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyazaki E, Miyazaki M, Chen JM, Chaisson RE, Bishai WR. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–9. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gosling RD, Uiso LO, Sam NE, et al. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;168:1342–5. doi: 10.1164/rccm.200305-682OC. [DOI] [PubMed] [Google Scholar]
- 63.Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:605–12. [PubMed] [Google Scholar]
- 64.Pletz MW, De Roux A, Roth A, Neumann KH, Mauch H, Lode H. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother. 2004;48:780–2. doi: 10.1128/AAC.48.3.780-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veziris N, Truffot-Pernot C, Aubry A, Jarlier V, Lounis N. Fluoroquinolone-containing third-line regimen against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother. 2003;47:3117–22. doi: 10.1128/AAC.47.10.3117-3122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rieder HL. Fourth-generation fluoroquinolones in tuberculosis. Lancet. 2009;373:1148–9. doi: 10.1016/S0140-6736(09)60559-6. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization. WHO/HTM/TB/2008402. Geneva: WHO; 2008. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update. [Google Scholar]
- 68.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing Regimen Greatly Reduces Time to Culture Conversion in Murine Tuberculosis. Am J Respir Crit Care Med. 2004;169:421–6. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 69.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170:1131–4. doi: 10.1164/rccm.200407-885OC. [DOI] [PubMed] [Google Scholar]
- 70.Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:331–8. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 71.Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373:1183–9. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rustomjee R, Lienhardt C, Kanyok T, et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:128–38. [PubMed] [Google Scholar]
- 73.Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180:273–80. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- 74.Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–7. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 75.Koul A, Dendouga N, Vergauwen K, et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol. 2007;3:323–4. doi: 10.1038/nchembio884. [DOI] [PubMed] [Google Scholar]
- 76.Huitric E, Verhasselt P, Koul A, Andries K, Hoffner S, Andersson DI. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.01611-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koul A, Vranckx L, Dendouga N, et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem. 2008;283:25273–80. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- 78.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2008;105:11945–50. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 80.Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:2831–5. doi: 10.1128/AAC.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibrahim M, Andries K, Lounis N, et al. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother. 2007;51:1011–5. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ibrahim M, Truffot-Pernot C, Andries K, Jarlier V, Veziris N. Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis. Am J Respir Crit Care Med. 2009;180:553–7. doi: 10.1164/rccm.200807-1152OC. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim M, Truffot-Pernot C, Andries K, Jarlier V, Veziris N. Sterilizing activity of a second line regimen including R207910 (TMC207) in murine tuberculosis. Abstracts of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the Infectious Diseases Society of America (IDSA) 46th Annual Meeting; 2008. [Google Scholar]
- 84.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–9. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 85.Mphahlele M, Syre H, Valvatne H, et al. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol. 2008;46:3459–64. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nuermberger E, Mitchison DA. Once-weekly treatment of tuberculosis with the diarylquinoline R207910: a real possibility. Am J Respir Crit Care Med. 2009;179:2–3. doi: 10.1164/rccm.200808-1333ED. [DOI] [PubMed] [Google Scholar]
- 87.Veziris N, Ibrahim M, Lounis N, et al. A once-weekly R207910-containing regimen exceeds activity of the standard daily regimen in murine tuberculosis. Am J Respir Crit Care Med. 2009;179:75–9. doi: 10.1164/rccm.200711-1736OC. [DOI] [PubMed] [Google Scholar]
- 88.Ashtekar DR, Costa-Perira R, Nagrajan K, Vishvanathan N, Bhatt AD, Rittel W. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1993;37:183–6. doi: 10.1128/aac.37.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stover CK, Warrener P, VanDevanter DR, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–6. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 91.Singh R, Manjunatha U, Boshoff HI, et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008;322:1392–5. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.OPC-67683. Tuberculosis (Edinb) 2008;88:132–3. doi: 10.1016/S1472-9792(08)70017-9. [DOI] [PubMed] [Google Scholar]
- 93.Manjunatha UH, Boshoff H, Dowd CS, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006;103:431–6. doi: 10.1073/pnas.0508392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi KP, Kendrick N, Daniels L. Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F(420) and FO biosynthesis. J Bacteriol. 2002;184:2420–8. doi: 10.1128/JB.184.9.2420-2428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi KP, Bair TB, Bae YM, Daniels L. Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F(420) biosynthesis by Mycobacterium bovis BCG. J Bacteriol. 2001;183:7058–66. doi: 10.1128/JB.183.24.7058-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lenaerts AJ, Gruppo V, Marietta KS, et al. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob Agents Chemother. 2005;49:2294–301. doi: 10.1128/AAC.49.6.2294-2301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu Y, Coates AR, Mitchison DA. Comparison of the sterilising activities of the nitroimidazopyran PA-824 and moxifloxacin against persisting Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2008;12:69–73. [PubMed] [Google Scholar]
- 98.Tyagi S, Nuermberger E, Yoshimatsu T, et al. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother. 2005;49:2289–93. doi: 10.1128/AAC.49.6.2289-2293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nuermberger E, Rosenthal I, Tyagi S, et al. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2006;50:2621–5. doi: 10.1128/AAC.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tasneen R, Tyagi S, Williams K, Grosset J, Nuermberger E. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother. 2008;52:3664–8. doi: 10.1128/AAC.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nuermberger E, Tyagi S, Tasneen R, et al. Powerful Bactericidal and Sterilizing Activity of a Regimen Containing PA-824, Moxifloxacin, and Pyrazinamide in a Murine Model of Tuberculosis. Antimicrob Agents Chemother. 2008;52:1522–4. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother. 2009;53:3720–5. doi: 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ginsberg A, Diacon A, Dawson R, Whitney K, Rouse D, Laurenzi M, Van Niekerk C, Maritz JS, Venter A, Donald P, Spigelman M. Extended early bactericidal activity (EBA) of PA-824, a novel drug for tuberculosis treatment. Final Program of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the Infectious Diseases Society of America (IDSA) 46th Annual Meeting; 2008. [Google Scholar]
- 104.Ahmad Z, Peloquin C, Tyagi S, Grosset JH, Nuermberger EL. PA-824 exhibits time-dependent bactericidal activity in a murine tuberculosis model. Abstracts of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Protopopova M, Hanrahan C, Nikonenko B, et al. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J Antimicrob Chemother. 2005;56:968–74. doi: 10.1093/jac/dki319. [DOI] [PubMed] [Google Scholar]
- 106.Chen P, Gearhart J, Protopopova M, Einck L, Nacy CA. Synergistic interactions of SQ109, a new ethylene diamine, with front-line antitubercular drugs in vitro. J Antimicrob Chemother. 2006;58:332–7. doi: 10.1093/jac/dkl227. [DOI] [PubMed] [Google Scholar]
- 107.Reddy VM, Einck L, Andries K, Nacy CA. The new antitubercular drugs SQ109 and TMC207 act synergistically in vitro to kill Mycobacterium tuberculosis. Abstracts of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the Infectious Diseases Society of America (IDSA) 46th Annual Meeting; 2008. pp. 145–146. [Google Scholar]
- 108.Jia L, Tomaszewski JE, Hanrahan C, et al. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br J Pharmacol. 2005;144:80–7. doi: 10.1038/sj.bjp.0705984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nikonenko BV, Protopopova M, Samala R, Einck L, Nacy CA. Drug therapy of experimental tuberculosis (TB): improved outcome by combining SQ109, a new diamine antibiotic, with existing TB drugs. Antimicrob Agents Chemother. 2007;51:1563–5. doi: 10.1128/AAC.01326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jia L, Noker PE, Coward L, Gorman GS, Protopopova M, Tomaszewski JE. Interspecies pharmacokinetics and in vitro metabolism of SQ109. Br J Pharmacol. 2006;147:476–85. doi: 10.1038/sj.bjp.0706650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alcala L, Ruiz-Serrano MJ, Perez-Fernandez TC, et al. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother. 2003;47:416–7. doi: 10.1128/AAC.47.1.416-417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43:1189–91. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fattorini L, Tan D, Iona E, et al. Activities of moxifloxacin alone and in combination with other antimicrobial agents against multidrug-resistant Mycobacterium tuberculosis infection in BALB/c mice. Antimicrob Agents Chemother. 2003;47:360–2. doi: 10.1128/AAC.47.1.360-362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest. 2008;134:187–92. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 115.Fortun J, Martin-Davila P, Navas E, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56:180–5. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 116.Nam HS, Koh WJ, Kwon OJ, Cho SN, Shim TS. Daily half-dose linezolid for the treatment of intractable multidrug-resistant tuberculosis. Int J Antimicrob Agents. 2009;33:92–3. doi: 10.1016/j.ijantimicag.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 117.Park IN, Hong SB, Oh YM, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother. 2006;58:701–4. doi: 10.1093/jac/dkl298. [DOI] [PubMed] [Google Scholar]
- 118.von der LB, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB)--a report of ten cases. J Infect. 2006;52:92–6. doi: 10.1016/j.jinf.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Yew WW, Chau CH, Wen KH. Linezolid in the treatment of ‘difficult’ multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12:345–6. [PubMed] [Google Scholar]
- 120.Migliori GB, Eker B, Richardson MD, et al. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur Respir J. 2009;34:387–93. doi: 10.1183/09031936.00009509. [DOI] [PubMed] [Google Scholar]
- 121.Koh WJ, Kwon OJ, Gwak H, et al. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J Antimicrob Chemother. 2009;64:388–91. doi: 10.1093/jac/dkp171. [DOI] [PubMed] [Google Scholar]
- 122.Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50:49–55. doi: 10.1086/648675. [DOI] [PubMed] [Google Scholar]
- 123.Yew WW, Chang KC, Chau CH. Comment on: Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J Antimicrob Chemother. 2009;64:1119–20. doi: 10.1093/jac/dkp291. [DOI] [PubMed] [Google Scholar]
- 124.Yew WW, Chang KC, Chau CH. What is the optimal dosage of linezolid in treatment of complicated multidrug-resistant tuberculosis? Eur Respir J. 2009;34:1492–4. doi: 10.1183/09031936.00111009. [DOI] [PubMed] [Google Scholar]
- 125.Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178:1180–5. doi: 10.1164/rccm.200806-892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barbachyn MR, Hutchinson DK, Brickner SJ, et al. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J Med Chem. 1996;39:680–5. doi: 10.1021/jm950956y. [DOI] [PubMed] [Google Scholar]
- 127.Sood R, Rao M, Singhal S, Rattan A. Activity of RBx 7644 and RBx 8700, new investigational oxazolidinones, against Mycobacterium tuberculosis infected murine macrophages. Int J Antimicrob Agents. 2005;25:464–8. doi: 10.1016/j.ijantimicag.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 128.Vera-Cabrera L, Castro-Garza J, Rendon A, et al. In vitro susceptibility of Mycobacterium tuberculosis clinical isolates to garenoxacin and DA-7867. Antimicrob Agents Chemother. 2005;49:4351–3. doi: 10.1128/AAC.49.10.4351-4353.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williams KN, Stover CK, Zhu T, et al. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother. 2009;53:1314–9. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams KN, Brickner SJ, Stover CK, et al. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med. 2009;180:371–6. doi: 10.1164/rccm.200904-0611OC. [DOI] [PubMed] [Google Scholar]
- 131.Kumar V, Jakubiec W, Li W, Silvia A, Dimitrova D, Campbell S, Mitton-Fry M, Wallis RS. Safety, tolerability, PK and whole blood bactericidal activity (WBA) against Mycobacterium tuberculosis of single ascending doses of PNU-100480. Final Program of the 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2009. [Google Scholar]
- 132.Kasik JE. The nature of mycobacterial penicillinase. Am Rev Respir Dis. 1965;91:117–9. doi: 10.1164/arrd.1965.91.1.117. [DOI] [PubMed] [Google Scholar]
- 133.Flores AR, Parsons LM, Pavelka MS., Jr Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology. 2005;151:521–32. doi: 10.1099/mic.0.27629-0. [DOI] [PubMed] [Google Scholar]
- 134.Hugonnet JE, Blanchard JS. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry. 2007;46:11998–2004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chambers HF, Moreau D, Yajko D, et al. Can penicillins and other beta-lactam antibiotics be used to treat tuberculosis? Antimicrob Agents Chemother. 1995;39:2620–4. doi: 10.1128/aac.39.12.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, III, Blanchard JS. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215–8. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cynamon MH, Palmer GS. In vitro activity of amoxicillin in combination with clavulanic acid against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1983;24:429–31. doi: 10.1128/aac.24.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yew WW, Wong CF, Lee J, Wong PC, Chau CH. Do beta-lactam-beta-lactamase inhibitor combinations have a place in the treatment of multidrug-resistant pulmonary tuberculosis? Tuber Lung Dis. 1995;76:90–2. doi: 10.1016/0962-8479(95)90588-x. [DOI] [PubMed] [Google Scholar]
- 139.Nadler JP, Berger J, Nord JA, Cofsky R, Saxena M. Amoxicillin-clavulanic acid for treating drug-resistant Mycobacterium tuberculosis. Chest. 1991;99:1025–6. doi: 10.1378/chest.99.4.1025. [DOI] [PubMed] [Google Scholar]
- 140.Chambers HF, Kocagoz T, Sipit T, Turner J, Hopewell PC. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis. 1998;26:874–7. doi: 10.1086/513945. [DOI] [PubMed] [Google Scholar]
- 141.Donald PR, Sirgel FA, Venter A, et al. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand J Infect Dis. 2001;33:466–9. doi: 10.1080/00365540152029954. [DOI] [PubMed] [Google Scholar]
- 142.Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob Agents Chemother. 2005;49:2816–21. doi: 10.1128/AAC.49.7.2816-2821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Watt B, Edwards JR, Rayner A, Grindey AJ, Harris G. In vitro activity of meropenem and imipenem against mycobateria: development of a daily anitbiotic dosing schedule. Tuberc Lung Dis. 1992;73:134–6. doi: 10.1016/0962-8479(92)90145-A. [DOI] [PubMed] [Google Scholar]
- 144.Conte JE, Jr, Golden JA, Kelley MG, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of meropenem. Int J Antimicrob Agents. 2005;26:449–56. doi: 10.1016/j.ijantimicag.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 145.Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47(Suppl 1):S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 146.Anonymous. Science Daily. Feb 27, 2009. Antibiotic combination defeats extensively drug-resistant TB. 1-6-0010. [Google Scholar]
- 147.Deidda D, Lampis G, Fioravanti R, et al. Bactericidal activities of the pyrrole derivative BM212 against multidrug-resistant and intramacrophagic Mycobacterium tuberculosis strains. Antimicrob Agents Chemother. 1998;42:3035–7. doi: 10.1128/aac.42.11.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arora S. Eradication of Mycobacterium tuberculosis infection in 2 months with LL-3858: a preclinical study. Int J Tuberc Lung Dis. 2004;8(11 Suppl 1):S29. [Google Scholar]
- 149.du Toit LC, Pillay V, Danckwerts MP. Tuberculosis chemotherapy: current drug delivery approaches. Respir Res. 2006;7:118. doi: 10.1186/1465-9921-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pandey R, Zahoor A, Sharma S, Khuller GK. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis (Edinb) 2003;83:373–8. doi: 10.1016/j.tube.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 151.Pandey R, Khuller GK. Subcutaneous nanoparticle-based antitubercular chemotherapy in an experimental model. J Antimicrob Chemother. 2004;54:266–8. doi: 10.1093/jac/dkh260. [DOI] [PubMed] [Google Scholar]
- 152.Anisimova YV, Gelperina S, Peloquin C, Heifets LB. Nanoparticles as antituberculosis drug carriers: effect on activity against M. tuberculosis in human monocyte-derived macrophages. J Nanoparticle Res. 2000;2:165–71. [Google Scholar]
- 153.Barrow EL, Winchester GA, Staas JK, Quenelle DC, Barrow WW. Use of microsphere technology for targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob Agents Chemother. 1998;42:2682–9. doi: 10.1128/aac.42.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fawaz F, Bonini F, Maugein J, Lagueny AM. Ciprofloxacin-loaded polyisobutylcyanoacrylate nanoparticles: pharmacokinetics and in vitro anti-microbial activity. Int J Pharm. 1998;168:255–9. [Google Scholar]
- 155.Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob Agents Chemother. 2003;47:833–6. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pandey R, Khuller GK. Antitubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother. 2005;55:430–5. doi: 10.1093/jac/dki027. [DOI] [PubMed] [Google Scholar]
- 157.Fiegel J, Garcia-Contreras L, Thomas M, et al. Preparation and in vivo evaluation of a dry powder for inhalation of capreomycin. Pharm Res. 2008;25:805–11. doi: 10.1007/s11095-007-9381-6. [DOI] [PubMed] [Google Scholar]
- 158.Garcia-Contreras L, Fiegel J, Telko MJ, et al. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob Agents Chemother. 2007;51:2830–6. doi: 10.1128/AAC.01164-06. [DOI] [PMC free article] [PubMed] [Google Scholar]