Abstract
Vaccinia virus mutants in which expression of the virion core protein gene E6R is repressed are defective in virion morphogenesis. E6 deficient infections fail to properly package viroplasm into viral membranes, resulting in an accumulation of empty immature virions and large aggregates of viroplasm. We have used immunogold electron microscopy and immunofluorescence confocal microscopy to assess the intracellular localization of several virion structural proteins and enzymes during E6R mutant infections. We find that during E6R mutant infections virion membrane proteins and virion transcription enzymes maintain a normal localization within viral factories while several major core and lateral body proteins accumulate in aggregated virosomes. The results support a model in which vaccinia virions are assembled from at least three substructures, the membrane, the viroplasm and a “pre-nucleocapsid”, and that the E6 protein is essential for maintaining proper localization of the seven-protein complex and the viroplasm during assembly.
Keywords: Poxvirus, vaccinia, structure, assembly, maturation, membrane, localization
Introduction
Poxviruses are large (130–375 kb) dsDNA containing viruses that replicate in the cell cytoplasm. The most notorious human pathogen within the family Poxviridae (genus Orthopoxvirus) is variola virus, the causative agent of smallpox disease, which was responsible for millions of deaths throughout history prior to its eradication in the late 1970’s. Vaccinia virus is a related orthopoxvirus originally isolated from nature that was used as a live vaccine for eradication of smallpox and has served as the prototypical poxvirus for laboratory study (Moss, 2013; Damon, 2013).
The vaccinia virion structure is both unique and complex (Condit et al., 2006). The virion exists in two infectious forms which differ by the number of membranes surrounding the particle. The simplest form, called “mature virus” (MV), is a 360×270×250 nm brick shaped particle which comprises four substructures: 1) a central folded tubular nucleocapsid structure surrounded by 2) a biconcave, proteinaceous core wall flanked by 3) proteinaceous lateral bodies, all of which is surrounded by 4) a single proteolipid membrane bilayer. A slightly more complex infectious form of the virus, called “extracellular virus” (EV), is essentially MV which acquires a second early endosome or Golgi-derived, virus protein-containing membrane during exocytosis from infected cells (Smith et al., 2002). Importantly, because the virus must launch infection in the cytoplasm using a DNA genome, the poxvirus virion contains a full complement of virus coded enzymes required for synthesis and modification of early virus mRNAs.
Each of the four MV substructures can be thought of as carrying out a specific function during the initial stages of infection. The MV proteolipid membrane is responsible for virus attachment and ultimately fusion either at the plasma membrane or with an endocytic vesicle, resulting in primary uncoating and release of the virion core and lateral bodies into the cell cytoplasm (Moss, 2006). The lateral bodies probably contain proteins for immediate use as countermeasures against the cellular innate antiviral response, analogous to the herpesvirus tegument (Kelly et al., 2009; Schmidt et al., 2013). The core wall provides a compartment for early virus transcription and possibly for export of mRNA into the cytoplasm, analogous to the reovirus infectious subvirus particle (Dryden et al., 1993; Mallardo et al., 2001; Mallardo et al., 2002). The nucleocapsid is likely the primary site of early virus gene transcription (McFadden et al., 2012).
MV contain approximately 70 proteins total, some of which can be assigned to specific virion substructures (Condit et al., 2006). The MV membrane contains two major structural proteins, A14 and A17, which form a disulfide bonded network surrounding the core, plus an additional 20 membrane proteins involved in virus attachment, fusion and entry (Krijnse-Locker et al., 1996; Wolffe et al., 1996; Rodriguez et al., 1997; Betakova & Moss, 2000; Moss, 2006; Unger et al., 2013). The virion core, including the lateral bodies, the core wall and the nucleocapsid, contains approximately 50 proteins, only a few of which have been definitively assigned to core substructures. The inner nucleocapsid contains the viral genome and transcription enzymes required for transcription of early viral genes during the initial stages of infection (McFadden et al., 2012). The nucleocapsid may contain major structural virus proteins as well; the virus coded L4 protein is a prime candidate for a nucleocapsid structural protein (Moussatche & Condit, 2014; Jesus et al., 2014). The core wall comprises at least three major virion structural proteins, A3, A10 and A4 (Ichihashi et al., 1984; Wilton et al., 1995; Roos et al., 1996; Pedersen et al., 2000; Moussatche & Condit, 2014). A3 has been localized to the inner surface of the core wall, while A10 and A4 have been localized to the outer surface of the core wall. Three proteins, including F17, have been definitively assigned to the lateral bodies, though several others, including both enzymes and structural proteins are candidates for lateral body proteins (Schmidt et al., 2013).
Assembly of pox virions is a fascinating, complex and poorly understood process (Condit et al., 2006). During the mid and late stages of virus infection, virus “factories” are established in the cell cytoplasm. Virus factories are distinguishable by fluorescence microscopy as DNA-containing foci and by electron microscopy as areas of uniform, relatively low electron density devoid of cellular organelles. As observed by electron microscopy, the first evidence of virion morphogenesis is the appearance within factories of crescent structures (cupules in 3 dimensions) representing the precursors to the MV membrane (Dales & Siminovitch, 1961). As the crescents grow to become circles (spheres in three dimensions), they encapsidate “viroplasm”, visible as material of uniform intermediate density. As the circles approach closure they encapsidate DNA, visible as material of high electron density spanning the membrane (Morgan, 1976). Closed circles (spheres) are called immature virions (IV). Probably all IV contain DNA condensed in a spherical “nucleoid” structure, however because the nucleoid is acentric it is visible in only some sections of the IV, called IVN (IV with nucleoid) (Morgan et al., 1955). Immature virions contain the majority of the MV membrane proteins and all of the core proteins, and they are coated with a honeycomb matrix of the viral D13 protein, which serves as a membrane scaffold during assembly of IV (Heuser, 2005; Szajner et al., 2005). IV undergo an apparently rapid transformation to become MV. This transformation requires proteolysis of several virion structural proteins catalyzed by one or two virion proteases (Ansarah-Sobrinho & Moss, 2004a; Ansarah-Sobrinho & Moss, 2004b). During transformation into MV, D13 dissociates from the membrane outer surface, additional membrane proteins become associated with the virion outer surface and the internal viroplasm and nucleoid are reorganized into lateral bodies, core wall and nucleocapsid (Bisht et al., 2009). MV are transported away from viral factories on cellular microtubules (Sanderson et al., 2000; Ward, 2005).
Insight into the pox virion assembly process can be acquired by using virus mutants defective in virion assembly. One class of assembly mutants is characterized by an apparent defect in association of viroplasm with growing crescents. Mutants in this class, comprising virus genes encoding eight different virion core proteins, F10, A30, G7, A15, D2, D3, J1, and E6, display an electron microscopic phenotype characterized by the presence of partial or complete virion membrane structures devoid of viroplasm, plus large aggregates of material of uniform intermediate electron density presumed to be unpackaged viroplasm. Products of seven of these eight genes, F10, A30, G7, A15, D2, D3, and J1, form a physical complex in which all the proteins are mutually interdependent on each other for stability, that is, repression of synthesis of any member of the complex results in destabilization of all other members of the complex (Szajner et al., 2004). Despite the similar phenotype of E6 mutants, the E6 protein is apparently not associated with the seven-protein complex, and repression of E6 synthesis does not destabilize the other seven proteins (Boyd et al., 2010b).
The E6R gene is transcribed late during infection and the E6 protein is packaged into virion cores (Resch et al., 2009; Boyd et al., 2010a; Boyd et al., 2010b). The sublocalization of E6 within cores, specifically lateral bodies, core wall or nucleocapsid, is unknown. Two different types mutants exist affecting gene E6R, temperature sensitive and inducible. One temperature sensitive mutant in E6R, Cts52, has been characterized in detail (Boyd et al., 2010a). Under nonpermissive conditions, Cts52 produces E6 protein which is packaged into virions that are indistinguishable from wild type virions using conventional preparation techniques for electron microscopy, however the virions produced are transcriptionally inactive and non-infectious. In the inducible mutants the E6 protein is under lac operator-repressor control in the virus genome such that under nonpermissive conditions the E6 protein is not synthesized (Resch et al., 2009; Boyd et al., 2010b). Under these conditions, viroplasm does not associate with growing membrane crescents and “empty IV” are produced along with large aggregates of material resembling viroplasm, termed “aggregated virosomes”.
In order to gain additional insight into the role of E6 in vaccinia virus assembly, we have assessed the effect of repression of E6 synthesis on the intracellular localization of representatives of each of the vaccinia virion sub structures, and on the distribution of the seven-protein complex. Our results show that in the absence of the E6 protein, localization of proteins associated with some virion substructures is significantly changed, while localization of proteins associated with other substructures is not noticeably perturbed. The results support a model in which vaccinia virions are assembled from at least three substructures, and that the E6 protein is essential for maintaining proper localization of the seven-protein complex and at least one of the three substructures during assembly.
Results
In the course of this work we have used a selection of antibodies to detect proteins associated with each of the MV substructures: membrane, lateral body, core wall and nucleocapsid. The antibodies used are listed in Table 1 and a graphical representation of the localization of the target proteins in mature virions is presented in Fig. 1.
Table 1.
Antibodies
| Protein | Function/Location | Type | Source | IF dilution | EM dilution |
|---|---|---|---|---|---|
| A14 | membrane structural | mouse monoclonal | Yan Xiang | 1:250 | |
| A14 | membrane structural | rabbit polyclonal | Paula Traktman | 1:200 | |
| A17 | membrane structural | rabbit polyclonal | Paula Traktman | 1:500 | |
| D13 | membrane scaffold | rabbit polyclonal | Bernard Moss | 1:500 | 1:2000 |
| A3 | core wall | rabbit polyclonal | Bernard Moss Mariano | 1:500 | 1:2000 |
| A4 | core wall | rabbit polyclonal | Esteban | 1:900 | 1:1000 |
| A10 | core wall | mouse monoclonal | Yan Xiang | 1:250 | |
| A10 | core wall | rabbit polyclonal | Bernard Moss Mariano | 1:250 | |
| A10 | core wall | rabbit polyclonal | Esteban | 1:2000 | |
| L4 | nucleocapsid | rabbit polyclonal | Paula Traktman | 1:1000 | |
| L4 | nucleocapsid | mouse monoclonal | Richard Condit | 1:250 | |
| F17 | lateral body early RNA polymerase | rabbit polyclonal | Paula Traktman | 1:900 | 1:2000 |
| H4 | subunit | rabbit polyclonal | Bernard Moss | 1:500 | 1:50 |
| D6 | early transcription factor | rabbit polyclonal | Edward Niles | 1:500 | 1:50 |
| D12 | capping enzyme core RNA polymerase | rabbit polyclonal | Edward Niles | 1:500 | |
| E4 | subunit | rabbit polyclonal | Stephen Broyles | 1:500 | |
| A30 | seven protein complex | rabbit polyclonal | Bernard Moss | 1:250 | |
| A30 | seven protein complex | rabbit polyclonal | Paula Traktman | 1:100 |
Fig. 1. Localization of proteins in mature virions.
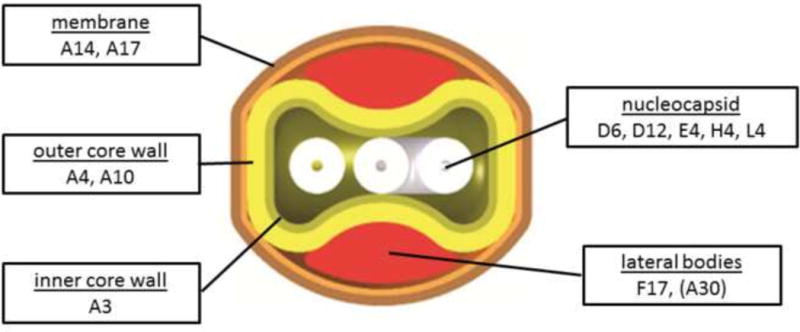
A cross section of MV is shown and known locations of proteins probed in this study are indicated. Assignment of A30 to lateral bodies is preliminary but consistent with published immunogold labeling and with a role in binding viroplasm to viral membranes (Szajner et al., 2001).
Strategy for tracking virion structural proteins using immunofluorescence confocal microscopy and immunogold electron microscopy
The catalog of antibodies used in this work comprises both rabbit polyclonal and mouse monoclonal antibodies, permitting simultaneous detection of two virus proteins using immunofluorescence confocal microscopy. To determine the distribution of virion structural proteins during infection in the absence of E6 protein, we tested most pairwise combinations of these antibodies (Table S1). A subset of these tests selected to demonstrate the major findings is presented in the body of the results below, while numerous other combinations are presented as supplementary data (Fig. S1). In all cases we compared wt infections with vE6i infections done in the absence of inducer. Infections were done at a moi of 1–2 so that uninfected cells could be observed simultaneously with infected cells. Infected cells were sampled at 9 hpi, midway into the late phase of virus infection. A control experiment demonstrates that when a vE6i infection is done in the presence of inducer, the phenotype is indistinguishable from a wt infection, demonstrating that the phenotypes observed for vE6i infections done in the absence of inducer are attributable to repression of E6R gene expression (Fig. S2).
Immunogold electron microscopy was used as a complement to immunofluorescence confocal microscopy. In this case we infected cells with vE6i in the absence of inducer at moi=5 and prepared samples for immunogold labeling and electron microscopy 21 h post infection.
Localization of virion core and membrane proteins in the absence of E6
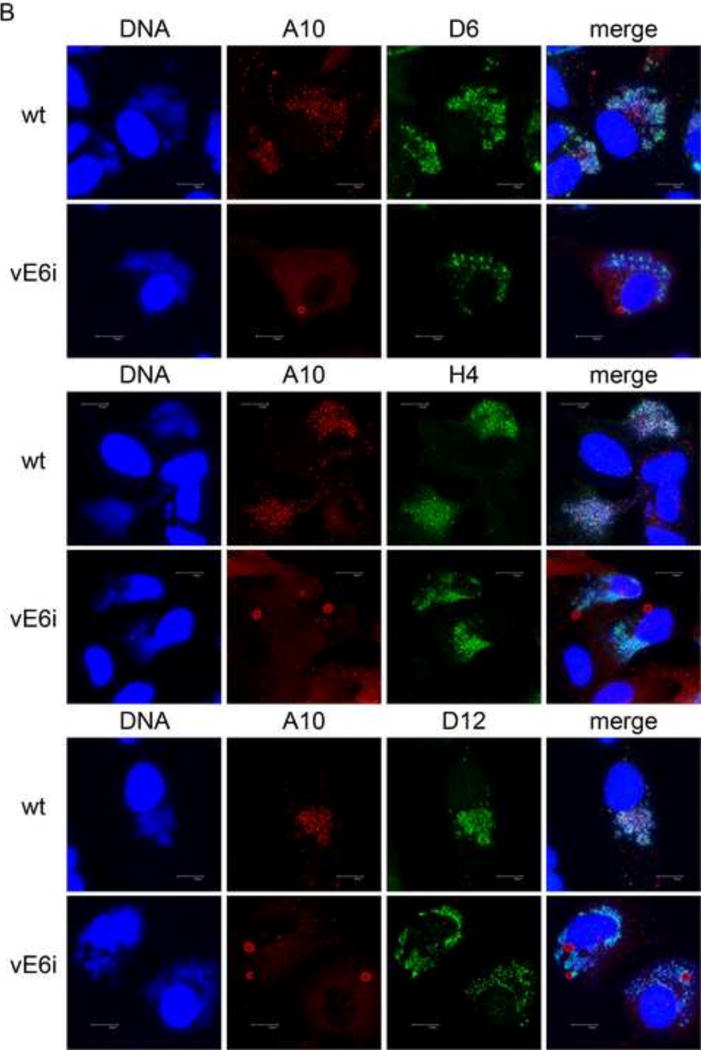
To determine the fate of the major virion membrane and core wall structural proteins in the absence of E6 protein, wt and vE6i infections were done in the absence of inducer and stained for the membrane proteins A17 and A14 and the core wall proteins A3, A4 and A10 (Fig. 2). Electron micrographs recapitulate the previously reported vE6i phenotype, namely large aggregated virosomes and empty membrane crescents and IVs (Fig. 2A). Immunogold labeled electron micrographs of vE6i infections show that aggregated virosomes label heavily with antibody to the core wall proteins A3, A4 and A10, but not with the virion membrane protein, A14. Antibody to A14 labels viral membranes in empty crescents and empty IV. In confocal micrographs (Fig. 2B), viral factories are detected by cytoplasmic DAPI staining. In wt infections the bulk of both core wall proteins and membrane proteins were localized in puncta in viral factories. Wild type infections also show numerous mature virions appearing as small cytoplasmic puncta distant from the factories that stain with antibody against either A10 or A17. Note that many more mature virion particles stain with anti-A17 than with anti-A10, and none stain with both. We presume that the A17 positive particles retain the virion membrane which occludes A10 from staining, while the A10 positive particles have lost the virion membrane during detergent treatment, thus losing A17 staining and revealing A10. Very few mature virions stain with anti-A14; we presume that the A14-reactive epitope is occluded in mature virions. In vE6i infections the virion membrane proteins A14 and A17 maintain a wt staining pattern, namely puncta localized to virus factories. In vE6i infections, the membrane scaffold protein D13 also maintains a relatively normal punctate staining pattern in viral factories, however some aggregation of D13 within and outside of factories is also observed (Fig. S1D). By contrast, in vE6i infections, all three virion core wall proteins, A10, A3 and A4, stain numerous large and small spherical structures, many but not all of which are localized to virus factories. Note that staining for A10 and A4 is precisely overlapping (Fig. 2B) as is staining for A10 and A3 (Fig. S1A). Comparing these results to electron micrographs shown in Fig. 2A, we conclude that the large spherical structures visualized by immunofluorescence microscopy are identical to the aggregated virosomes identified by electron microscopy, and that the aggregated virosomes contain the major core wall structural proteins A10, A3 and A4. Furthermore maintenance of a wt membrane protein staining pattern in vE6i infections is consistent with the formation of normal, A14-positive viral membrane crescents observed by electron microscopy.
Fig. 2. Localization of virion core and membrane proteins in the absence of E6.
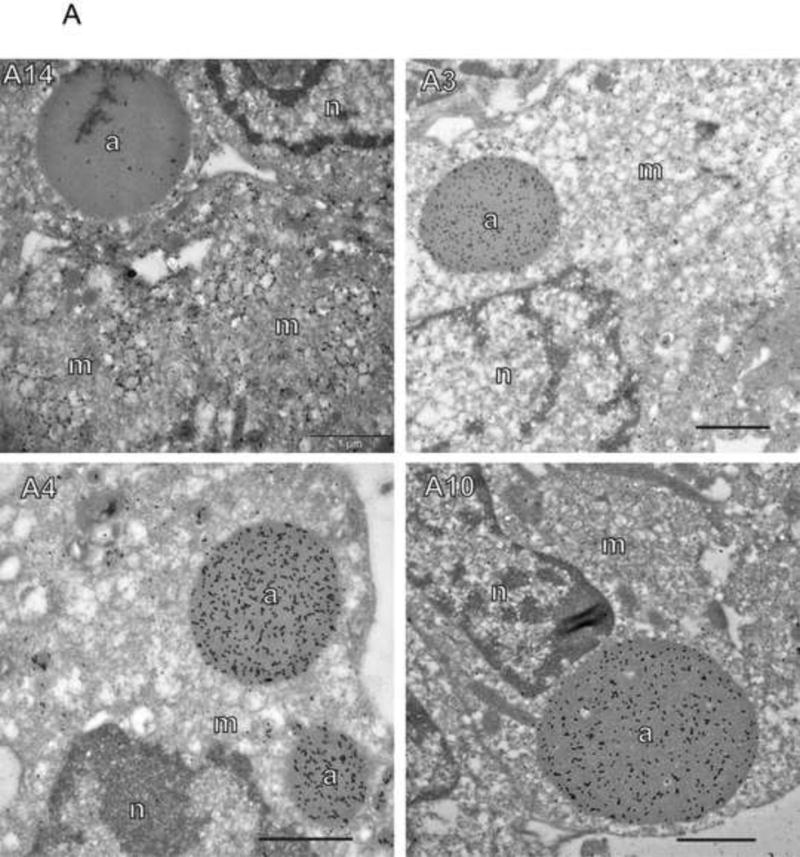

A) Immunogold labeling of cells infected with vE6i. Primary antibodies used are indicated in each panel. a=aggregated virosome; m=viral membranes; n=nucleus. Scale bars=l uM. B) Immunofluorescence confocal microscopy of cells infected with wt or vE6i. Primary antibodies used are indicated in each panel.
Virus transcription proteins remain localized to factories in the absence of E6
To determine the fate of the virus transcription apparatus in the absence of E6, vE6i infections were stained with antibodies against D6, a subunit of the viral early transcription factor (Gershon & Moss, 1990); H4, an early gene-specific subunit of the viral RNA polymerase (Ahn et al., 1994); D12, a subunit of the viral mRNA capping enzyme (Niles et al., 1989); and E4, a core RNA polymerase subunit (Ahn et al., 1990) (Figs. 3 and S1B, C). Importantly, whereas the D12 capping enzyme subunit and the E4 core RNA polymerase subunit are expressed early and function throughout infection, the D6 early transcription factor and H4 early gene-specific RNA polymerase subunit are expressed at intermediate and late times after infection when early gene expression has ceased, and are presumably made specifically for incorporation into virions (Yang et al., 2011). Samples for immunofluorescence confocal microscopy were also counterstained with antibody against the A10 core wall protein. Electron micrographs immunogold labeled with antibody to D6 show clusters of D6 protein within factories containing numerous empty viral membrane crescents and IV (Fig. 3A). Labeling of aggregated virosomes with anti-D6 antibody was sparse or at background levels. Using confocal immunofluorescence microscopy, in control wt infections, the bulk of A10, D6, H4, D12 and E4 staining is localized in puncta in virus factories (Fig. 3B and S1B, C). In vE6i infections, A10 staining is redistributed to aggregated virosomes as before, however all of the transcription proteins maintain punctate staining within virus factories. We conclude from these experiments that whereas virion core wall proteins are mislocalized in the absence of E6, transcription enzymes destined for packaging into virions maintain their normal localization.
Fig. 3. Virus transcription proteins remain localized to factories in the absence of E6.
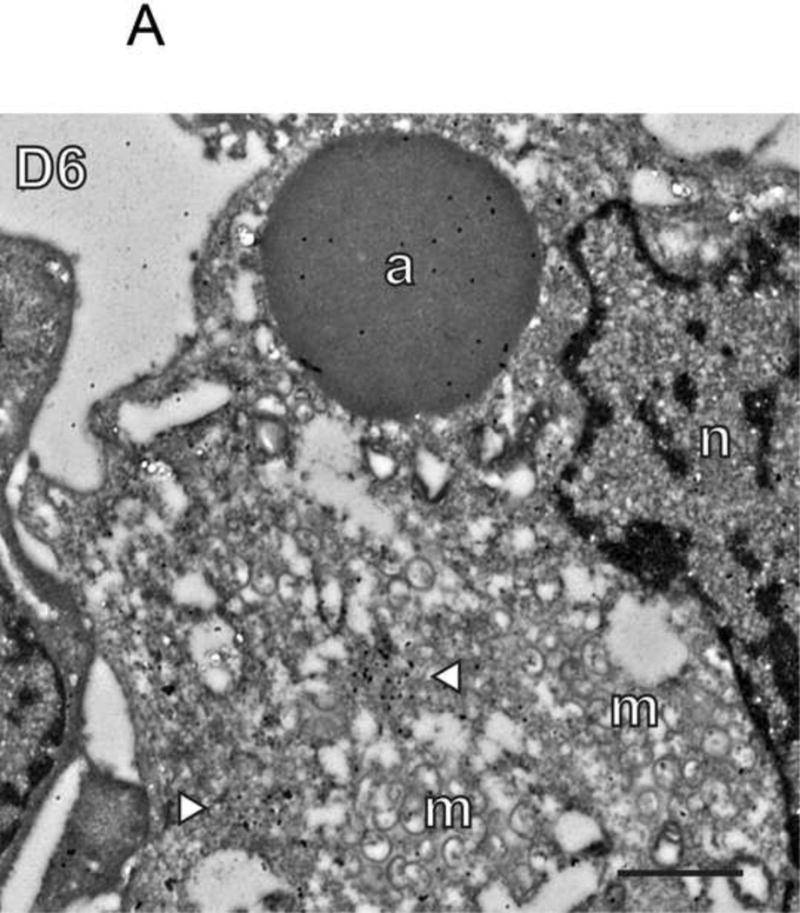
A) Immunogold labeling of cells infected with vE6i. Primary antibody to D6 was used as indicated in the panel. a=aggregated virosome; m=viral membranes; n=nucleus. White arrowheads indicate clusters of gold particles within factories. Scale bar=l uM. B) Immunofluorescence confocal microscopy of cells infected with wt or vE6i. Primary antibodies used are indicated in each panel.
Localization of nucleocapsid and lateral body proteins in the absence of E6
To determine the fate of other core structural components in the absence of E6, vE6i infections were stained with antibodies against L4, a nucleocapsid protein and F17, a lateral body protein (Fig. 4). Samples for immunofluorescence were counterstained with antibody against either the A3 or the A10 core wall proteins. Immunogold labeling of electron micrographs demonstrates dense labeling of aggregated virosomes with both anti-F17 and anti-L4 in vE6i infections (Fig. 4A). Using confocal immunofluorescence microscopy, in control wt infections, L4 and F17 display punctate staining in viral factories combined with diffuse staining throughout the cytoplasm, while the A3 and A10 core wall proteins reveal predominantly punctate staining in viral factories as observed previously (Figs. 4B and S1D, E). (The diffuse staining with L4 and F17 antibodies is not observed in uninfected cells and therefore represents the authentic distribution of these proteins rather than background. Uninfected cells staining only with DAPI but not L4 are present in the vE6i infection in Fig. 4B and the wt infection in Fig. 5B. Data demonstrating a lack of F17 staining in uninfected cells is not shown.) In vE6i infections, the A3 and A10 core wall proteins are relocalized into aggregated virosomes as expected. In vE6i infections, neither L4 nor F17 maintain the normal punctate staining in viral factories, but rather assume a predominantly diffuse cytoplasmic staining. Both L4 and F17 seem to localize to some extent to aggregated virosomes as revealed by the orange appearance of the aggregated virosomes in the merged images. A typical feature of vE6i infections is the apparent condensation of DNA in puncta in factories as revealed by DAPI staining. Interestingly, in vE6i infections, L4 colocalizes with these areas of DNA condensation, consistent with the DNA binding properties of the L4 protein. Combining the immunogold EM and immunofluourscence confocal microscopy results, we conclude that both L4 and F17 are mislocalized during a vE6i infection, losing their normal punctate staining in viral factories and localizing both to aggregated virosomes and diffusely throughout the cytoplasm.
Fig. 4. Localization of nucleocapsid and lateral body proteins in the absence of E6.
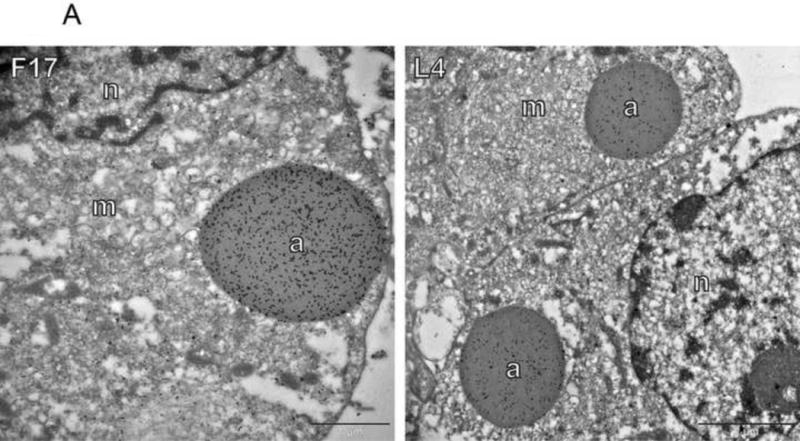

A) Immunogold labeling of cells infected with vE6i. Primary antibodies used are indicated in each panel. a=aggregated virosome; m=viral membranes; n=nucleus. Scale bars=l or 2 uM as indicated. B) Immunofluorescence confocal microscopy of cells infected with wt or vE6i. Primary antibodies used are indicated in each panel.
Fig. 5. Localization of the seven-protein complex in the absence of E6.

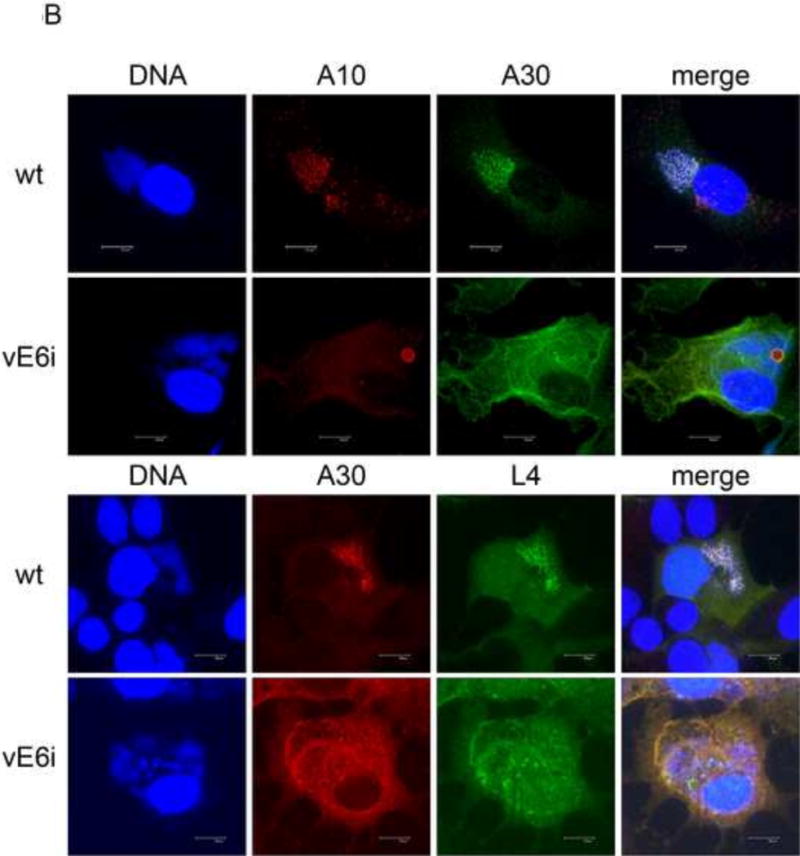
A) Immunogold labeling of cells infected with vE6i. Primary antibody to A30 was used as indicated in the panel. a=aggregated virosome; m=viral membranes; n=nucleus. Scale bar=2 uM. B) Immunofluorescence confocal microscopy of cells infected with wt or vE6i. Primary antibodies used are indicated in each panel.
Localization of the seven-protein complex in the absence of E6
The so-called “seven-protein complex” is comprised of F10, A30, G7, A15, D2, D3, and J1. Mutants in most of the genes encoding these proteins results in a phenotype similar to the E6R mutant phenotype, specifically the presence of empty immature virions and aggregated virosomes (Szajner et al., 2004). To determine the fate of the seven-protein complex in the absence of E6, we stained vE6i infections with antibody to A30 (Figs. 5 and S2E). For immunofluorescence confocal microscopy, to determine the relative localization of A30 with two other core proteins, we counter stained with antibody to either the A10 core wall protein or the L4 nucleocapsid protein. In immunogold labeled electron micrographs, aggregated virosomes were not labeled with anti-A30 at levels above background (Fig. 5A). Instead, A30 was detected throughout the cell cytoplasm. Interestingly, in areas containing accumulations of viral crescent membranes, anti-A30 specifically decorated the membranes. Using immunofluorescence microscopy, in wt infections, A30 displayed punctate staining in viral factories (Fig. 5B). In wt infections as previously described A10 showed punctate staining in viral factories while L4 showed punctate staining in viral factories superimposed on a more diffuse cytoplasmic staining. In vE6i infections, A10 is localized to aggregated virosomes as described previously. Also as described previously, in vE6i infections L4 lost its normal punctate staining in viral factories and instead assumed a predominantly diffuse cytoplasmic staining with some concentration in aggregated virosomes and in condensed factory DNA. In vE6i infections, A30 lost its punctate staining in viral factories and assumed a cytoplasmic staining that is both diffuse and reticular. While there is some concentration of A30 at the periphery of aggregated virosomes, compared to other core proteins A30 seems to be excluded from aggregated virosomes. Note in particular in the merged image counterstained with L4 that the center of the larger aggregated virosomes are green whereas the diffuse cytoplasmic staining is orange, suggesting an exclusion of A30 from aggregated virosomes. We conclude that in the absence of E6 protein, A30 loses its normal localization to viral factories and becomes distributed throughout the cytoplasm, with some association with viral membranes and no accumulation within aggregated virosomes.
Discussion
To gain insight into the role of the vaccinia virus E6 protein during infection, we have investigated the intracellular distribution of numerous virion proteins during infection with an E6 mutant, vE6i, in which expression of the E6R gene is repressed. We observed that during E6 mutant infections, some proteins maintain their normal intracellular localization while others are mislocalized (Fig 6). Proteins that maintain an apparently normal localization in viral factories include viral membrane proteins (A14, A17) and a membrane associated protein (D13) and viral transcription enzymes destined to be packaged in virions including the early viral transcription factor (D6 subunit), the early gene specific form of the viral RNA polymerase (H4 subunit), the core RNA polymerase (E4 subunit) and the mRNA capping enzyme (D12 subunit). By contrast, all other virus structural proteins tested are mislocalized during a vE6i infection. These include three core wall proteins (A3, A4 and A10), a nucleocapsid structural protein (L4), a lateral body protein (F17) and a representative of the seven-protein complex (A30). The mislocalization is characterized by the disappearance of the normal punctate staining of viral factories and the appearance of aggregated virosomes. Aggregated virosomes contain core wall proteins, a nucleocapsid structural protein and a lateral body protein. The seven-protein complex protein A30 assumes a diffuse/reticular cytoplasmic localization and is excluded from aggregated virosomes. These results demonstrate that the E6 protein exerts a selective influence over localization of virion proteins during infection, affecting most structural components of the virion core but not affecting either virion membrane proteins or transcription enzymes.
Fig. 6. An illustration summarizing the phenotype of infection in the absence of E6R gene expression.
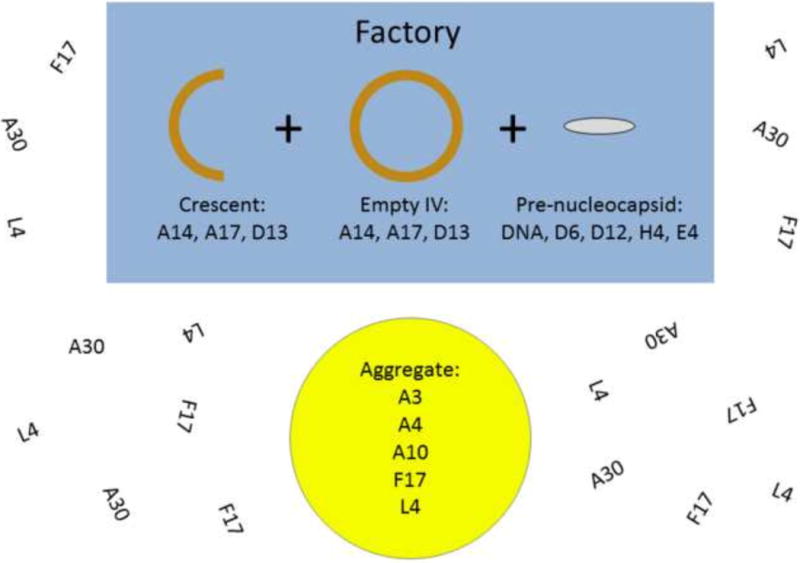
Factories contain empty viral membrane crescents and IV. Factories also contain viral transcription enzymes and DNA normally destined for packaging. Aggregated virosomes accumulate in factories of randomly in cytoplasm. The protein composition of aggregated virosomes is shown. F17 and L4 show some diffuse cytoplasmic localization in addition to localizing to virosomes. A30 is diffuse throughout the cytoplasm.
The mechanism by which E6 affects protein localization during vaccinia infection remains to be determined. Given the similarity between the E6 mutant phenotype and the phenotypes of mutants in the seven-protein complex, it seems likely that E6 functions on the same pathway as the seven-protein complex in attaching viroplasm to viral membranes. It is noteworthy that in the experiments reported here, one component of the seven-protein complex, A30, assumes an apparently random cytoplasmic distribution when E6 is absent during infection, as opposed to its normal punctate localization to viral factories. Electron microscopic analysis of wild type infections shows that A30 localizes both to unpackaged viroplasm and viroplasm packaged in IV (Szajner et al., 2001). A parsimonious interpretation of these observations is that E6 directs the localization of the seven-protein complex, and that when in the absence of E6 the seven-protein complex is mislocalized, attachment of viroplasm to crescent viral membranes is abrogated. There have been no reports of interactions between E6 and any other virus or cellular proteins, including members of the seven-protein complex, which could shed light on E6 function. Future investigations into the fine localization of E6 and members of the seven-protein complex in IVs and MVs, coupled with refined studies of interactions of E6 and seven-protein complex members with each other and with both membrane and viroplasm proteins should provide a clearer understanding of the role of these proteins in virus assembly.
Based on the results reported here we reason that the vaccinia virion is assembled from three substructures, the membrane, the viroplasm and a structure we call “pre-nucleocapsid” (Fig. 7). The membrane contains the full complement of membrane proteins scaffolded on the D13 protein as previously described (Heuser, 2005; Szajner et al., 2005). Viroplasm comprises most if not all of the remaining virion structural proteins, including both core wall proteins (e.g. A3, A4, A10), lateral body proteins (e.g. F17) and nucleocapsid structural proteins (e.g. L4). In this regard we perceive the aggregated virosomes formed in vE6i infections not as completely aberrant structures but rather as relatively normal viroplasm that accumulates to abnormally large aggregates when unbounded by viral membranes. We envision the pre-nucleocapsid to contain a complex of viral DNA with transcription enzymes including the viral early transcription factor, RNA polymerase, mRNA capping enzyme, and likely other transcription and/or RNA modification enzymes as well. We presume that the pre-nucleocapsid is formed and enters maturing IVs at a late stage, independently of the action of E6 or the seven-protein complex. This is consistent with previous studies suggesting that the transcription apparatus enters IV complexed with viral DNA (Zhang et al., 1994; McFadden et al., 2012). Interestingly, L4, which we believe is a major structural component of the nucleocapsid (Jesus et al., 2014; Moussatche & Condit, 2014), does not appear to co-localize with the pre-nucleocapsid but rather seems to be a component of viroplasm. This suggests that the final nucleocapsid structure is assembled during the IV to MV transition using components that enter IVs separately as viroplasm or pre-nucleocapsid.
Fig. 7. A model for vaccinia virion morphogenesis.
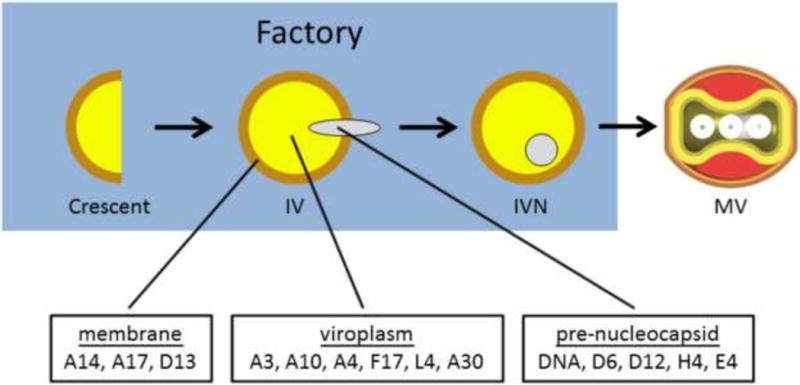
Immature virions are assembled from three subcomponents: the membrane, the core and the pre-nucleocapsid.
Detailing further the assembly of poxvirus immature virions and the remarkable morphogenesis of immature virions into mature virions remains a fascinating and rewarding challenge for the future.
Materials and Methods
Cells and viruses
Viruses were grown in BSC-40 cells cultured in Dulbecco’s Modified Eagle’s medium (Life Technologies – cat 12100-061) containing 10% fetal bovine serum, 0.12 mg/ml penicillin (Sigma), 0.2 mg/ml streptomycin (Sigma) and 250 μg/ml fungizone (Sigma). Wild-type vaccinia virus strain WR and vE6i have been described (Condit & Motyczka, 1981; Boyd et al., 2010b). When IPTG was included in vE6i infections, it was used at a final concentration of 100 uM.
Immunogold electron microscopy
The procedure for immunogold labeling of virus infected cells has been described previously (Jesus et al., 2014). Briefly, confluent monolayers of BSC-40 cells in 35 mm dishes were infected at moi= 5 with vE6i in the absence of the inducer for 21 h at 37°C. Infected cells were then washed with PHEM buffer (60 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 6.9) and fixed with a solution containing 0.6% glutaraldehyde, 4% paraformaldehyde, 5 mM CaCl2, and 0.5% sucrose in PHEM buffer for 1 h at 4°C. The monolayer was washed with 0.1 M glycine in PHEM buffer and cells were harvested by low speed centrifugation. The cell pellets were dehydrated in a dilution series of ice-cold ethanol, infiltrated with Lowicryl HM20 resin, and polymerized with 100% Lowicryl at −20°C with UV light for 2 days. Ultrathin sections were collected on nickel grids and prepared for immunolabeling by incubating the grids with 200 mM NH4Cl in PBS (20.8mM Na2HP04, 6.4mM KH2P04, 300mM NaCl, 40mM NaN3, pH 7.2) for 20 minutes, then in blocking solution (0.5g BSA,0.5g fish skin gelatin 0.01% Tween 20, 0.1% NP40, in PBS) for 1 h. After this period the grids were incubated overnight at 4°C in antibodies diluted in blocking solution as indicated in Table 1. After this time the grids were washed twice in PBS for 20 min each, once in in blocking solution for 5 min, and in the gold conjugated goat-anti-rabbit (1:50) (BBl Solutions, Cardiff, United Kingdom) secondary antibody for 2 hours. After this period the grids were washed twice in PBS for 20 min each, fixed in McDowell and Trumps fixative (Electronic Microscopy Science) diluted 1:4 for 5 min, washed once in H2O for 5 min, incubated in 1% uranyl acetate for 1 minute, dried and examined with an H-7000 transmission electron microscope (Hitachi High Technologies America, Inc., Schaumburg, IL).
Immunofluorescence confocal microscopy
BSC-40 cell monolayers were grown in 8 well culture slides (BD Falcon cat # 354108) and infected with 100 ul per well of virus in phosphate buffered saline (PBS; 0.17 M NaCl, 3.3 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.2) at an moi of 1–2. (The low MOI infection provides control uninfected cells in each sample.) After 30 minutes adsorption, the inoculum was removed, cells were washed twice with 400 ul per well PBS and 0.4 ml medium with or without IPTG was added per well. After 9 hours of incubation at 37°C, infected cells were processed for confocal microscopy. All the steps were done at room temperature. Medium was removed, cells were washed once with 400 ul per well PBS and fixed with 200 ul per well 4% paraformaldehyde in PBS for 10 min. The fixative was removed, cells were washed once with 400 ul per well PBS and then incubated for 5 min with 400 ul PBS containing 20 mM glycine per well to quench any remaining fixative. Cells were washed once with 400 ul PBS per well and permeabilized by addition of 200 ul per well of 0.2% Triton X-100 in PBS for 10 minutes. Cells were washed twice with 400 ul per well PBS, then blocked with 400 ul per well PBS containing 10% fetal bovine serum (FBS) for 10 min. Cells were then incubated with 100 ul per well primary antibody in PBS containing 0.1% Triton X-100 and 10% FBS for 60 min. Cells were washed three times with 400 ul per well PBS followed by incubation with 100 ul per well secondary antibody in PBS containing 0.1% Triton X-100 and 10% FBS for 30 min. Cells were washed twice with 400 ul per well PBS and stained with 200 ul per well 0.5 μg/ml DAPI (Invitrogen) in PBS for 15 min. Cells were washed three times with 400 ul per well PBS, plastic culture chambers were removed from the slides and coverslips were affixed using 7 ul per well vectashield (Vector Laboratories, Burlingame, CA). Samples were visualized with a LEICA TCS SP2 AOBS Spectral Confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL). Sources and dilutions of primary antibodies are listed in Table 1. Secondary antibodies Invitrogen, Carlsbad, CA) Alexafluor 488 goat anti-mouse IgG (cat #A11001), Alexafluor 488 goat anti-rabbit IgG (cat #A11008), Alexafluor 594 goat anti-mouse IgG (cat #A11005), and Alexafluor 594 goat anti-rabbit IgG (cat #A11012) were used at l00× dilution.
Supplementary Material
Mutation of E6 disrupts association of viral membranes with viral core proteins
Mutation of E6 does not perturb viral membrane biosynthesis
Mutation of E6 does not perturb localization of viral transcription enzymes
Mutation of E6 causes mislocalization and aggregation of viral core proteins
Vaccinia assembly uses three subassemblies: membranes, viroplasm, prenucleocapsid
Acknowledgments
We thank Stephen Broyles, Mariano Esteban, Bernard Moss, Edward Niles, Paula Traktman, and Yan Xiang for generous gifts of antibodies. We thank Doug Smith in the University of Florida Cell and Tissue Analysis Core for instruction on confocal microscopy. This work was supported by NIH grant R01 AI055560 to RCC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahn BY, Gershon PD, Jones EV, Moss B. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol Cell Biol. 1990;10:5433–5441. doi: 10.1128/mcb.10.10.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn BY, Gershon PD, Moss B. RNA polymerase-associated protein Rap94 confers promoter specificity for initiating transcription of vaccinia virus early stage genes. J Biol Chem. 1994;269:7552–7557. [PubMed] [Google Scholar]
- Ansarah-Sobrinho C, Moss B. Role of the 17 protein in proteolytic processing of vaccinia virus membrane and core components. J Virol. 2004a;78:6335–6343. doi: 10.1128/JVI.78.12.6335-6343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansarah-Sobrinho C, Moss B. Vaccinia virus G1 protein, a predicted metalloprotease, is essential for morphogenesis of infectious virions but not for cleavage of major core proteins. J Virol. 2004b;78:6855–6863. doi: 10.1128/JVI.78.13.6855-6863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betakova T, Moss B. Disulfide bonds and membrane topology of the vaccinia virus A17L envelope protein. J Virol. 2000;74:2438–2442. doi: 10.1128/jvi.74.5.2438-2442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H, Weisberg AS, Szajner P, Moss B. Assembly and disassembly of the capsid-like external scaffold of immature virions during vaccinia virus morphogenesis. J Virol. 2009;83:9140–9150. doi: 10.1128/JVI.00875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd O, Strahl AL, Rodeffer C, Condit RC, Moussatche N. Temperature-sensitive mutant in the vaccinia virus E6 protein produce virions that are transcriptionally inactive. Virology. 2010a;399:221–230. doi: 10.1016/j.virol.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd O, Turner PC, Moyer RW, Condit RC, Moussatche N. The E6 protein from vaccinia virus is required for the formation of immature virions. Virology. 2010b;399:201–211. doi: 10.1016/j.virol.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit RC, Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981;113:224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Dales S, Siminovitch L. The development of vaccinia virus in Earle’s L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon IK. Poxviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Wolters Kluwer-Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 2160–2184. [Google Scholar]
- Dryden KA, Wang G, Yeager M, Nibert ML, Coombs KM, Furlong DB, Fields BN, Baker TS. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J Cell Biol. 1993;122:1023–1041. doi: 10.1083/jcb.122.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon PD, Moss B. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc Natl Acad Sci USA. 1990;87:4401–4405. doi: 10.1073/pnas.87.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Deep-etch EM reveals that the early poxvirus envelope is a single membrane bilayer stabilized by a geodetic “honeycomb” surface coat. J Cell Biol. 2005;169:269–283. doi: 10.1083/jcb.200412169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y, Oie M, Tsuruhara T. Location of DNA-binding proteins and disulfide-linked proteins in vaccinia virus structural elements. J Virol. 1984;50:929–938. doi: 10.1128/jvi.50.3.929-938.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus DM, Moussatche N, Condit RC. Vaccinia Virus Mutations in the L4R Gene Encoding a Virion Structural Protein Produce Abnormal Mature Particles Lacking a Nucleocapsid. J Virol. 2014;88:14017–14029. doi: 10.1128/JVI.02126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BJ, Fraefel C, Cunningham AL, Diefenbach RJ. Functional roles of the tegument proteins of herpes simplex virus type 1. Virus Res. 2009;145:173–186. doi: 10.1016/j.virusres.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J, Schleich S, Rodriguez D, Goud B, Snijder EJ, Griffiths G. The role of a 21-kDa viral membrane protein in the assembly of vaccinia virus from the intermediate compartment. J Biol Chem. 1996;271:14950–14958. doi: 10.1074/jbc.271.25.14950. [DOI] [PubMed] [Google Scholar]
- Mallardo M, Leithe E, Schleich S, Roos N, Doglio L, Krijnse LJ. Relationship between vaccinia virus intracellular cores, early mRNAs, and DNA replication sites. J Virol. 2002;76:5167–5183. doi: 10.1128/JVI.76.10.5167-5183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallardo M, Schleich S, Krijnse LJ. Microtubule-dependent organization of vaccinia virus core-derived early mRNAs into distinct cytoplasmic structures. Mol Biol Cell. 2001;12:3875–3891. doi: 10.1091/mbc.12.12.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden BD, Moussatche N, Kelley K, Kang BH, Condit RC. Vaccinia virions deficient in transcription enzymes lack a nucleocapsid. Virology. 2012;434:50–58. doi: 10.1016/j.virol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. The insertion of DNA into vaccinia virus. Science. 1976;193:591–592. doi: 10.1126/science.959819. [DOI] [PubMed] [Google Scholar]
- Morgan C, Ellison SA, Rose HM, Moore DH. Serial sections of vaccinia virus examined at one stage of development in the electron microscope. Exp Cell Res. 1955;9:572–578. doi: 10.1016/0014-4827(55)90086-0. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Wolters Kluwer-Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 2129–2159. [Google Scholar]
- Moussatche N, Condit RC. Fine structure of the vaccinia virion determined by controlled degradation and immunolocalization. Virology. 2014;475C:204–218. doi: 10.1016/j.virol.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles EG, Lee-Chen GJ, Shuman S, Moss B, Broyles SS. Vaccinia virus gene D12L encodes the small subunit of the viral mRNA capping enzyme. Virology. 1989;172:513–522. doi: 10.1016/0042-6822(89)90194-3. [DOI] [PubMed] [Google Scholar]
- Pedersen K, Snijder EJ, Schleich S, Roos N, Griffiths G, Locker JK. Characterization of vaccinia virus intracellular cores: implications for viral uncoating and core structure. J Virol. 2000;74:3525–3536. doi: 10.1128/jvi.74.8.3525-3536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch W, Weisberg AS, Moss B. Expression of the highly conserved vaccinia virus E6 protein is required for virion morphogenesis. Virology. 2009;386:478–485. doi: 10.1016/j.virol.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JR, Risco C, Carrascosa JL, Esteban M, Rodriguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15- kilodalton envelope protein. J Virol. 1997;71:1821–1833. doi: 10.1128/jvi.71.3.1821-1833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos N, Cyrklaff M, Cudmore S, Blasco R, Krijnse-Locker J, Griffiths G. A novel immunogold cryoelectron microscopic approach to investigate the structure of the intracellular and extracellular forms of vaccinia virus. EMBO J. 1996;15:2343–2355. [PMC free article] [PubMed] [Google Scholar]
- Sanderson CM, Hollinshead M, Smith GL. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J Gen Virol. 2000;81(Pt 1):47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- Schmidt FI, Bleck CK, Reh L, Novy K, Wollscheid B, Helenius A, Stahlberg H, Mercer J. Vaccinia virus entry is followed by core activation and proteasome-mediated release of the immunomodulatory effector VH1 from lateral bodies. Cell Rep. 2013;4:464–476. doi: 10.1016/j.celrep.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Szajner P, Jaffe H, Weisberg AS, Moss B. A complex of seven vaccinia virus proteins conserved in all chordopoxviruses is required for the association of membranes and viroplasm to form immature virions. Virology. 2004;330:447–459. doi: 10.1016/j.virol.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Szajner P, Weisberg AS, Lebowitz J, Heuser J, Moss B. External scaffold of spherical immature poxvirus particles is made of protein trimers forming a honeycomb lattice. J Cell Biol. 2005 doi: 10.1083/jcb.200504026. RefType: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajner P, Weisberg AS, Wolffe EJ, Moss B. Vaccinia virus a30l protein is required for association of viral membranes with dense viroplasm to form immature virions. J Virol. 2001;75:5752–5761. doi: 10.1128/JVI.75.13.5752-5761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger B, Mercer J, Boyle KA, Traktman P. Biogenesis of the vaccinia virus membrane: genetic and ultrastructural analysis of the contributions of the A14 and A17 proteins. J Virol. 2013;87:1083–1097. doi: 10.1128/JVI.02529-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM. Visualization and characterization of the intracellular movement of vaccinia virus intracellular mature virions. J Virol. 2005;79:4755–4763. doi: 10.1128/JVI.79.8.4755-4763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton S, Mohandas AR, Dales S. Organization of the vaccinia envelope and relationship to the structure of intracellular mature virions. Virology. 1995;214:503–511. doi: 10.1006/viro.1995.0061. [DOI] [PubMed] [Google Scholar]
- Wolffe EJ, Moore DM, Peters PJ, Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J Virol. 1996;70:2797–2808. doi: 10.1128/jvi.70.5.2797-2808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Reynolds SE, Martens CA, Bruno DP, Porcella SF, Moss B. Expression profiling of the intermediate and late stages of poxvirus replication. J Virol. 2011;85:9899–9908. doi: 10.1128/JVI.05446-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ahn BY, Moss B. Targeting of a multicomponent transcription apparatus into assembling vaccinia virus particles requires RAP94, an RNA polymerase-associated protein. J Virol. 1994;68:1360–1370. doi: 10.1128/jvi.68.3.1360-1370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


