Abstract
We previously developed adenovirus serotype 5 (Ad5) vectors displaying the sigma 1 protein from reovirus as mucosal vaccines. Ad5-sigma retargets to JAM-1 and sialic acid, but had 40-fold reduced gene delivery when compared to Ad5. While weaker at transduction, Ad5-sigma generated stronger T cell responses than Ad5 when used for mucosal immunization. New Ad5-fiber-sigma vectors were generated here by varying the number of fiber β-spiral shaft repeats (R) fused between fiber tail and the sigma. Ad5 virions encoding R3, R14, and R20 chimeras were rescued. Increasing chimera length led to their decreasing encapsidation of these proteins in the virions. Ad5-R3 and R14 mediated JAM-1- retargeting in vitro. When used to immunize mice by the intranasal route, Ad5-R3-sigma produced similar luciferase activity to Ad5, but higher serum and vaginal antibody responses. These data suggest optimized Ad-Sigma vectors may be useful vectors for mucosal vaccination.
Keywords: Adenovirus, Reovirus, Sigma 1, Mucosal, Immunization
Introduction
Most pathogens enter the body at mucosal surfaces. Generating robust “barrier protection” at mucosal surfaces may therefore be an ideal strategy to block infections before they become systemic (reviewed in (Lycke, 2012)).
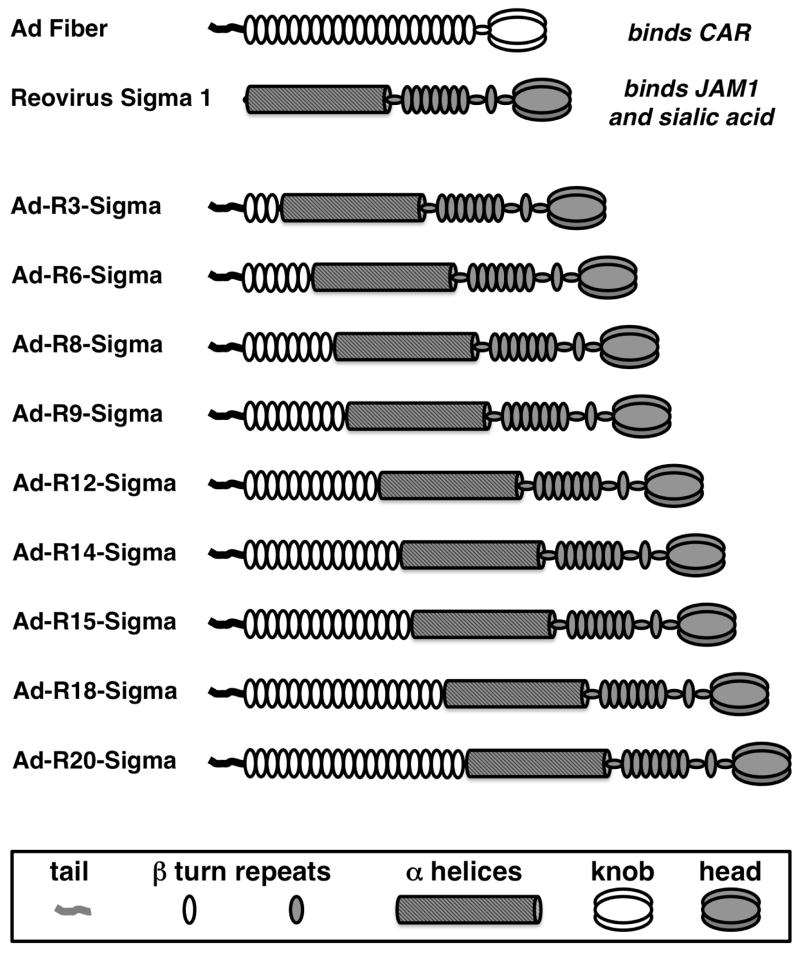
Adenoviruses (Ads) are non-enveloped DNA viruses (reviewed in (Campos and Barry, 2007)). Ads are potent vectors for gene-based vaccination (Lasaro and Ertl, 2009). Adenovirus serotype 5 (Ad5) binds to the coxsackie and adenovirus receptor (CAR) as its primary high affinity receptor using its fiber protein (Fig. 1 and reviewed in (Khare et al., 2011)). The trimeric Ad5 fiber contains a 44 amino acid n-terminal “tail” on its n-terminus that docks into the penton base of the viral icosahedron, 21 β-spiral repeats in its shaft domain, and its CAR-binding c-terminal “knob” domain (Fig. 1).
Figure 1.
Cartoon of Fiber-Sigma Chimeric Proteins.
Reoviruses are RNA viruses that are also non-enveloped viruses, but that have evolved separately from Ads (Kirchner et al., 2008). Reoviruses display a protein called sigma 1 protein to bind different receptors: junctional adhesion molecule 1 (JAM-1) and sialic acid. Despite having evolved separately and binding different receptors, sigma 1 it also has a shaft domain bearing β-spiral repeats that are remarkably similar to those in adenovirus fibers ((Forrest et al., 2003) and Fig. 1). Fiber and sigma 1 are both trimeric proteins with a shaft and knob (or head) type structure. Ad5 fiber is approximately 35 nm long. Sigma 1 is approximately 48 nm long (Kirchner et al., 2008). Fiber proteins from different Ad serotypes vary in length due to the number of β-spiral repeats. In contrast, sigma 1 has α-helical coiled-coil domain on its N-terminus followed by a shorter β-spiral repeat domain fused to its c-terminal head group.
Because Ads naturally cause a number of ocular, respiratory, and digestive infections, they can be one of the most robust vectors for vaccination at mucosal surfaces. While this is true, CAR-utilizing adenoviruses may not be optimal for mucosal vaccination because mucosal epithelial cells do not actually display CAR on their luminal surfaces (Grubb et al., 1994; Zabner et al., 1997). Instead, CAR is sequestered on the basolateral surface of mucosal cells making infection there less efficient. In contrast, the T3D reovirus sigma 1 protein binds to sialic acid that is expressed on nearly all mucosal epithelial cells. Sigma 1 also binds JAM-1, which is expressed on microfold cells (M cells) of Peyer’s patches in the lumen of the gut. JAM-1 is also expressed on dendritic cells (Mercier et al., 2004). In contrast, CAR is not expressed on these professional antigen-presenting cells.
Given the desire to improve mucosal vaccination, we previously generated a chimeric adenovirus that displays the sigma 1 protein ((Mercier et al., 2004) and Fig. 1). This was accomplished by replacing the virion docking motif of the sigma 1 protein from reovirus T3D with the 44 amino acid Ad5 fiber tail. When the Tail-Sigma 1 protein was engineered into Ad5 vectors in place of the fiber, sigma 1 was successfully displayed on Ad5 virions. When tested in vitro, Ad5-Sigma 1 was shown to no longer bind CAR, but instead bind to sialic acid and JAM-1 (Mercier et al., 2004). When Ad5-Sigma 1 was subsequently tested in vivo in mice, it was 40-fold less efficient at transducing muscle or nasal mucosa than Ad5 (Weaver et al., 2012). This weak transduction correlated to weak antibody production against its transgene product. This weak vector function could be due to defects in either end of the chimeric protein. The tail-sigma fusion might be inefficient at docking into the Ad5 penton base on the viral icosahedron. Alternately, this one fusion protein might not display sigma 1 in a fashion that allowed efficient use of its cognate receptors. Based on this, we have engineered a series of fiber-sigma fusion proteins and displayed them on Ad5 to test for in vivo transduction and antibody production.
Materials and Methods
Generation of Chimeric Fiber-Sigma T3D proteins
Fiber-sigma 1 fusion genes were generated by overlap PCR and standard cloning procedures in a manner similar to that used to produce the original fusion protein (Mercier et al., 2004). These were cloned in place of the Ad5 fiber protein in a CMV expression plasmid with the adenovirus tripartite leader to enhance expression and with a zeocin resistance gene between the chimera and the Ad E4 domain for recombination in bacteria (Campos and Barry, 2004). For expression testing, the plasmids were transfected into 293 cells with Polyfect (Qiagen, Hilden, Germany).
Western Blot Analysis for Fiber and Sigma Chimera Expression
Transfected cells were lysed in standard Laemli SDS-PAGE loading buffer or with trimerization loading buffer with reduced SDS that preserves fiber trimerization in SDS-PAGE (Parrott et al., 2003). Standard Laemli sample were heated for 5 minutes at 95°C prior to loading. Trimerization samples were not heated prior to loading. The samples were separated on SDS-PAGE gels, transfered to PVDF membranes, and chimeras were detected with mouse 4D2 antibody against the fiber tail (Abcam).
Generation of Sigma-modified Ad5
To generate sigma-modified Ad5 vectors, fiber-sigma-E4 cassettes were recombined into E1/E3-deleted Ad5 plasmids by co-transformation in recombinogenic bacteria as in (Campos and Barry, 2004). Fiber-sigma cassettes were recombined into pAd-GFPLuc expressing the Aequorea victoria jellyfish GFP fused in place of the start methionine of firefly luciferase. Once generated and confirmed by sequencing, these adenovirus genomes were linearized with Pac I and transfected into fiber expressing 633 cells (Von Seggern et al., 2000) in the presence of 0.3 μM dexamethasone as in (Mercier et al., 2004). The viruses were amplified by serial passage in 633 cells until the final round of amplification in 293 cells to generate viruses that display only the virally-encoded sigma 1 chimera. For their final round of amplification, viruses were purified from 633 cells by CsCl banding to avoid transfer of excess Ad5 fiber protein produced from these helper cells as these can contaminate subsequent virions. These CsCl-banded viruses from 633 cells were then used infect 10 plate CellStacks of 293 cells to produce virions displaying only the virally-encoded sigma chimera (Mercier et al., 2004). Viruses were purified twice by CsCl gradient centrifugation, desalted, quantitated by OD260, and frozen at −80°C in 50 mM Tris, 0.5 M sucrose pH 8.
Virion Protein Analysis
CsCl-purified viruses were separated on 7–15% Tris-glycine SDS-PAGE gels (Biorad). To detect total viral protein, the gels were stained with Sypro Rubytm (Life Technologies). Fiber and fiber tail-sigma chimeras were detected by western blot with custom rabbit antibody 1561 raised fiber tail peptide ARPSEDTFNPVY (Mercier et al., 2004).
Cell culture
293, A549, CHO, and HAK cells were purchased from ATCC and were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS; HyClone, Rockford, IL) and penicillin/streptomycin at 100 U/mL (Invitrogen). Chinese hamster ovary (CHO) and Syrian hamster kidney (HAK) cells were modified to express the Junctional Adhesion Molecule 1 (JAM-1) receptor for T3D reovirus by stable transfection with pCDNA-JAM1 as in (Mercier et al., 2004).
In Vitro Virus Transduction
Viruses were thawed and diluted to 2×109 virus particles (vp) per ml of DMEM tissue culture media (Life Technologies). Viruses were either untreated, were freeze-thawed a series of times, or were treated for varied times at 47.5°C. As the viruses were originally frozen at −80°C, all were by definition freeze/thawed a single time. Freeze/thaw 1 represents viruses that were only thawed this first time. 100 μl of untreated or treated virions were added to the indicated cells in 96 well dishes in 100 μl of DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin (Life Technologies). Cells were incubated for 48 hours and virus transduction was measured by luciferase expression using Bright Glow reagent (Promega) on a Beckman Coulter DTX 880 Multimode Detector.
Animals
6 week old female BALB/c mice were maintained under Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines in the Mayo Clinic Animal Facility after approval by the Mayo Clinic Institutional Animal Care and Use Committee. All animal experiments were performed in accordance with the provisions of the Animal Welfare Act, the PHS Animal Welfare Policy, and the principles of the NIH Guide for the Care and Use of Laboratory Animals. Mice were immunized intranasally (i.n.) with 1 × 1010 virus particles (vp)/mouse in a 20 μl total volume (10 μl per nare) and were imaged and samples taken for immune assays in the timelines described below.
In Vivo Luciferase Imaging
Mice were imaged at varied times on an In Vivo F Imaging System (Kodak) or a Lumazone Imaging System (Roper) as in (Weaver et al., 2012). Mice were anesthetized with ketamine/xylazine, injected intraperitonealy (i.p.) with 150 μl of 20 mg/ml d-luciferin, and were placed in the imager after 5 minutes to allow distribution of the substrate. Images were taken by a 10-minute exposure with 2×2 binning using no filters and no photo-multiplication. Each image was background subtracted and photons were quantitated with the Lumazone Imaging Software.
Enzyme Linked Immunosorbent Assay (ELISA)
ELISAs were performed as described in (Weaver et al., 2012). Briefly, Immulon 4 HBX plates were coated with 100 μl of recombinant luciferase or GFP protein at 1 μg/ml in PBS overnight at 4°C. The plates were blocked 2 mg/ml BSA and serial dilutions of serum or vaginal washes were added to the plate for 1 h at room temperature. The plates were washed 5 times with PBS and 100 μl of Goat anti-mouse HRP conjugated antibody diluted 1:2000 in PBS with BSA (1 mg/ml) was added for 1 h at room temperature. The plates were washed 5 times with PBS and 100 μl Ultra TMB-ELISA substrate was added for 1 h. Reactions were stopped with 50 μl of 2 M sulfuric acid and analyzed at 450 nm using a Beckman Coulter DTX 880 Multimode Detector.
Statistical Analyses
Data was graphed and analyzed with GraphPad Prism 6 software.
Results
Construction of Fiber-Sigma Chimeric Proteins
To allow the reovirus sigma protein to dock into the Ad penton base, sigma 1 was originally fused to just the minimal 44 amino acid tail of the fiber tail (Fig. 1 and (Mercier et al., 2004)). The Ad5 fiber has 21 β-turn repeats in its shaft that provides the bulk of the trimer’s length. The third β-turn, repeat 3 (R3), contains a four amino acid insertion that provides flexibility to the shaft of fiber (Nicklin et al., 2005; Wu and Nemerow, 2004). We hypothesized that including tail-R1-R2-R3 with this flexibility motif might enhance Ad-Sigma functionality. It was also possible that adding more fiber repeats to the fusions might optimize the protein for mucosal infection.
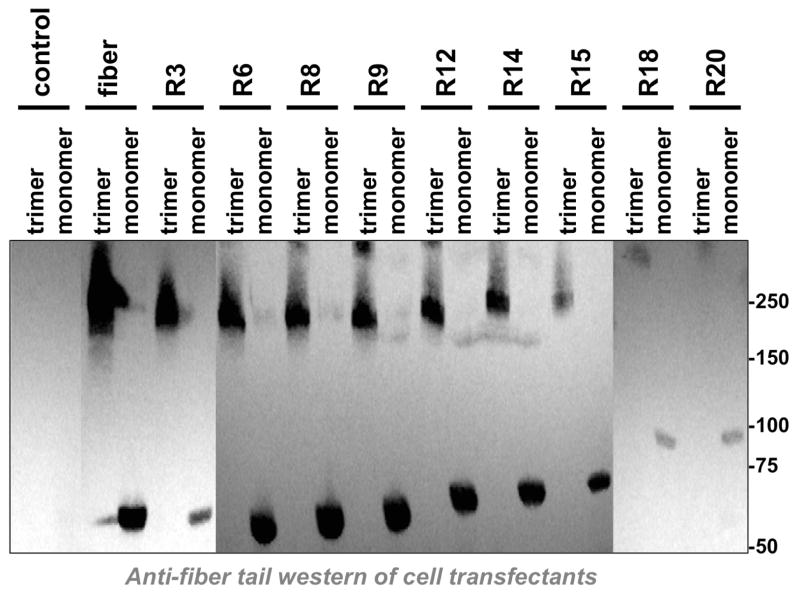
To test this, a series of fiber-sigma chimeras were generated and these were cloned into expression plasmids (Fig. 1). Each of the plasmids were transfected into 293 cells and cell lysates were evaluated by western blot using an antibody against the fiber tail (Fig. 2). Increasing the number of fiber shaft repeats from 3 (R3) to 20 (R20) led to the expression of increasing longer fiber-sigma chimeric proteins. Notably, all of the chimeras trimerized, suggesting that they had the potential to be incorporated into the penton base of Ad5 virions (Parrott et al., 2003).
Figure 2. Western Blot of Fiber-Sigma Chimeras.
The indicated fiber chimeras shown in Fig. 1 were used to transfect cells and cell lysates were boiled in standard Laemli loading buffer to observe fiber monomers. To observe trimers, samples were mixed with loading buffer with reduced SDS and were not boiled prior to loading. After SDS-PAGE and transfer to PVDF membranes, the fiber chimeras were detected by western blot with an antibody against the Ad5 fiber tail.
Sigma 1 Virus Generation
R3, R14, and R20 fiber-sigma chimeric proteins were selected for further testing. Each was used to replace the fiber gene in replication-defective E1/E3 deleted Ad5 vectors expressing the Aequorea victoria green fluorescent protein fused to firefly luciferase (GFPLuc) by homologous recombination in bacteria as in (Mercier et al., 2004). The recombinants were verified by sequencing and then rescued in fiber-expressing 633 cells (Von Seggern et al., 2000) as in (Mercier et al., 2004). In their final round of amplification, the viruses were produced in 293 cells for display only the virally-encoded sigma chimera (Mercier et al., 2004).
Virus Composition
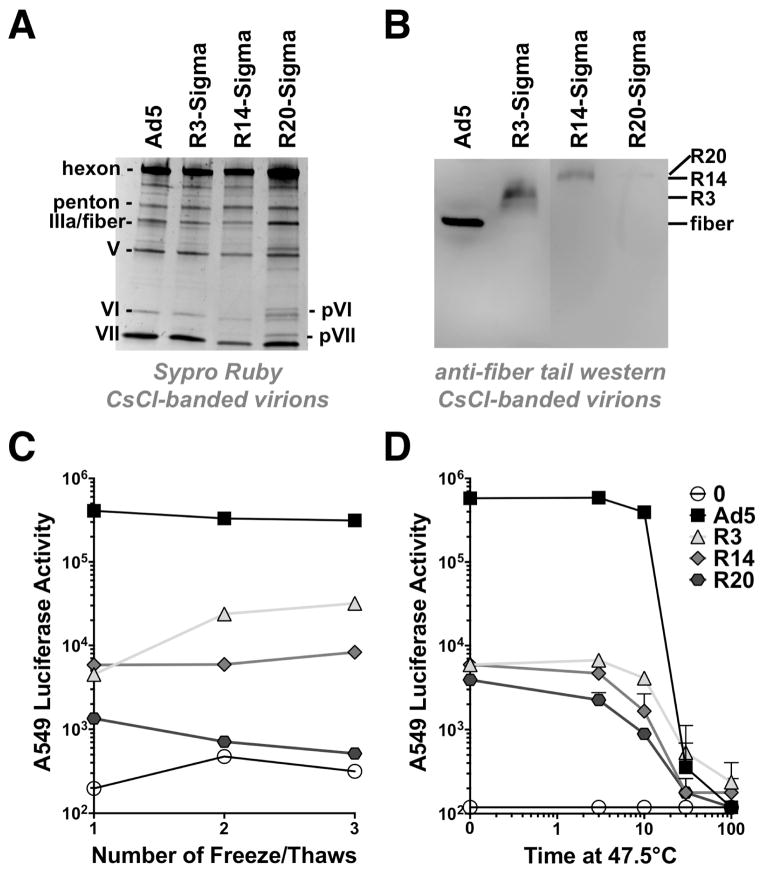
Ad5, R3, R14, and R20 fiber-sigma chimera viruses were purified by CsCl banding. These purified virions were separated by SDS-PAGE and stained with Sypro Rubytm to detect all virion proteins (Fig. 3A). All viruses had normal virion capsomers including hexon, penton, IIIa, V, VI, and VII. There are normally 36 copies of fiber per virion. Fiber and fiber-sigma chimeras are difficult to discern when staining for total protein, since the 60 copy IIIa and penton base proteins overlap these lower copy proteins. These were detected by western blot with an antibody directed against the fiber tail (Fig. 3B). Western blotting detected wild type fiber as well as the larger sigma chimeras in all the viruses. As chimera size increased, progressively less fiber-tail detected protein appeared to be incorporated. R3-Sigma intensity appeared to be similar to that of the fiber, but there was markedly less R14 protein on the purified virus and R20 was barely detectable. Adenoviruses lacking fiber fail to mature and proteolytically cleave precursor proteins in the virion including processing pVI and pVII to VI and VII (Legrand et al., 1999). While Ad5, R3, and R14 virions appeared fully mature, R20 virions had pVI and pVII bands indicated they were immature in this slightly overloaded lane (Fig. 3A). This as well the low R20 sigma encapsidation similar makes it likely that a high fraction of Ad5-R20 virus are essentially “fiberless” (Legrand et al., 1999).
Figure 3. Viral Composition and Stability.
A) Total protein composition of CsCl-purified viruses. 1010 vp of the indicated virus was separated on SDS-PAGE gels and stained with Sypro Ruby to detect proteins. B) Western blot with anti-Ad5 tail antibody on the same CsCl-purified virions from panel A. C) Effects of serial freeze/thaw on viral function. The indicated viruses were thawed from −80°C storage. This constituted freeze/thaw 1. The virions were diluted in media and freeze/thawed additional times and these solutions were used to infect A549 cells at a multiplicity of infection (MOI) of 10,000 vp/cell. Transduction was measured by luciferase assay. D) Effects of heat treatment on viral function. The indicated viruses were thawed from −80°C storage and were diluted in media. Each virus was treated for the indicated times at 47.5°C and immediately chilled on ice prior to infection of A549 cells and luciferase assay (n = 2).
Virus Stability
Ad5, R3, R14, and R20 fiber-sigma chimera viruses were tested for stability by exposing them to a series of freeze/thaw cycles and by treatment at 47.5°C. Following these treatments, the virions were used to infect human lung A549 cells and functionality was measured by luciferase assay (Fig. 3C and D). Since the viruses were stored at −80°C, thawing them for any use constitutes one freeze/thaw cycle.
Treatment of human lung A549 cells with freshly thawed viruses produced highest transduction by unmodified Ad5 (Fig. 3C and 4). R3 and R14 mediated 80 to 100-fold lower transduction than Ad5 on A549 cells. In contrast, R20 was 4-fold lower than R3 and R14 and 300-fold lower than Ad5 on these cells. Additional freeze/thaw cycles had little effect on virion transduction (Fig. 3C). Surprisingly, additional cycles appeared to slightly increase R3 activity.
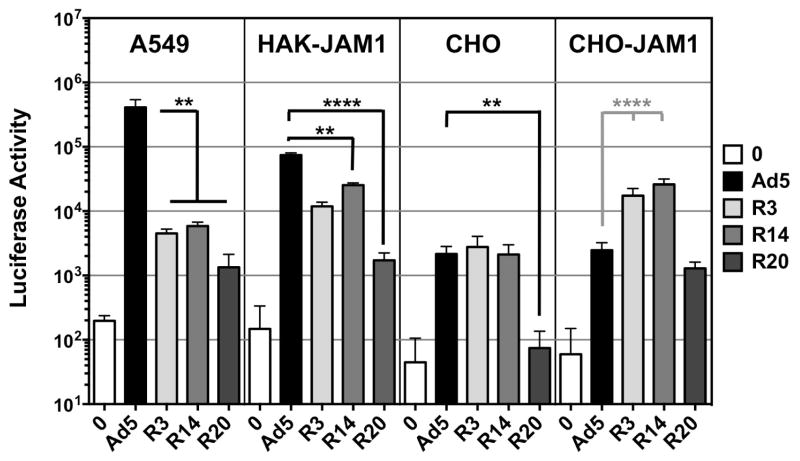
Figure 4. In Vitro Transduction on Different Cells.
The indicated cells were plated in 96 well plates and treated with the indicated viruses at an MOI of 10,000 vp/cell in replicates of 8 or 12. Transduction was assessed by luciferase assay. A549 cells are human lung cells. HAK-CD46 cells are hamsters cells that are permissive to species C Ad infection that are stably transfected with human CD46. CHO cells are Chinese hamster ovary cells that lack both CAR and JAM1, but that express αv integrins. CHO-CD46 cells are CHO cells that are stably transfected with human CD46. Error bars indicate standard error. ** indicates p < 0.01 and **** indicates p < 0.0001 by one way ANOVA.
Incubation of the viruses at 47.5°C for 10 or more minutes reduced transduction for all of the viruses relative to untreated viruses (Fig. 3D). 30 minute treatment of Ad5 reduced its activity 1,000-fold. The same treatment reduced R3 activity 10-fold. While their fold effects were quite different, 30 minute treatment essentially equalized the activity to all of the viruses. These data suggested that the sigma viruses were not particularly unstable relative to Ad5 at least by these interventions.
Virus Activity In Vitro
Previous work showed that the original fiber-tail-sigma virus infected via interactions with JAM1 (Mercier et al., 2004). However, subsequent comparisons of Ad5 with Tail-Sigma virus showed that this virus mediated markedly weaker in vitro and in vivo transduction, but better T cell responses in vivo (Weaver et al., 2012). To test this for the new viruses, Ad5, R3, R14, and R20 fiber-sigma chimera viruses were used to infect several cell lines with varied JAM1 expression and virus functionality was assessed by luciferase assay (Fig. 4). Chinese hamster ovary (CHO) cells lack both CAR and JAM1, but express αv integrins. On these cells, transduction was equal between Ad5, R3, and R14. R20 activity was 20-fold lower than the other viruses. On CHO-JAM1 cells that were modified to express the human protein, R3 and R14 activity was significantly higher than Ad5 demonstrating that the viruses use the sigma 1 cognate receptor. In contrast R20 was no better than Ad5. Syrian hamsters and their HAK kidney cancer cells are permissive for Ad5 infection presumably due to expression of the hamster CAR protein (Thomas et al., 2007). HAK cells were transfected with human JAM1 and tested with the viruses (Fig. 4). On these cells, Ad5 still mediated superior transduction when compared to the sigma viruses, but this was only 3 to 4-fold better than the R3 and R14. R20 transduction was again lower than the other three viruses.
Mucosal Transduction by Ad-Sigma Variants
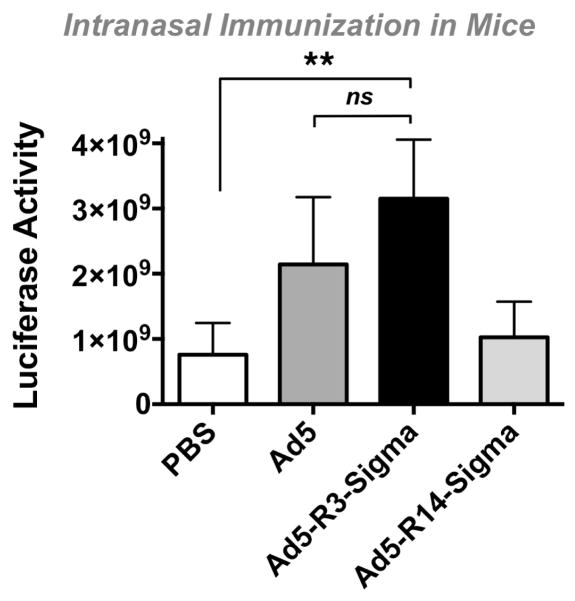
Groups of 10 female BALB/c mice were inoculated with 1010 virus particles (vp) of Ad5, Ad5-R3-Sigma, or Ad5-R14-Sigma by the intranasal route and luciferase expression was monitored (Fig. 5). Under these conditions, Ad5 and Ad-R3 mediated similar luciferase activity (not statistically different (ns) by one way ANOVA). In contrast, Ad-R14 generated luciferase levels that were similar to the background in the control animals.
Figure 5. In Vivo Transduction by Ad5 and Ad5 Displaying Sigma Chimeras.
Mice were immunized intranasally with the indicated vectors and were imaged 4 days later. Error bars indicate standard error. ** indicates p < 0.01 by one way ANOVA.
Antibody Responses Generated by Ad-Sigma Variants
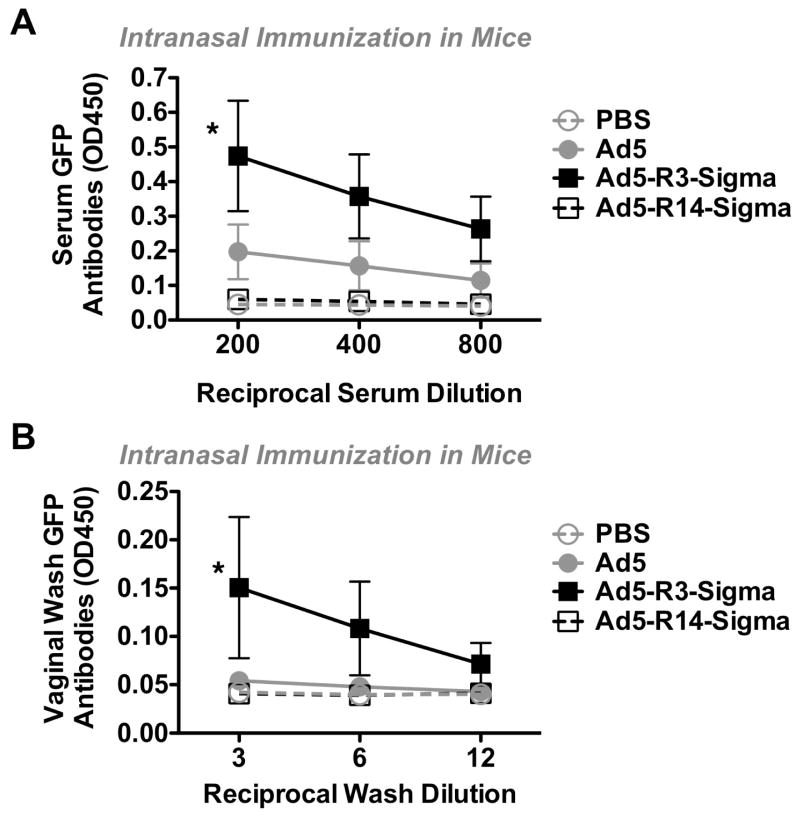
At the six week time point, anti-GFP antibody levels were also measured from the serum and from vaginal washes of the animals (Fig. 6A and B). R14 generated responses that were no better than controls. Ad5 produced detectable serum antibodies against GFP with this one immunization. In contrast, R3 generated stronger serum and vaginal wash antibodies than any of the other vectors (p < 0.05).
Figure 6. Systemic and Mucosal Antibody Responses Generated by Sigma Chimeras.
Mice were immunized intranasally as in Figure 5 and serum or vaginal washes were collected 6 weeks after single immunization. Antibodies against GFP were measured by serial dilution in ELISA. Error bars indicate standard error. * designates p < 0.05 by ANOVA.
Discussion
We previously replaced the Ad5 fiber with the sialic acid and JAM-1-binding reovirus T3D sigma 1 protein in an attempt to improve adenovirus vaccines for mucosal immunization. While this original Ad-Tail-Sigma vector was indeed retargeted to these new receptors, its overall in vivo transduction activity was markedly weaker than the original Ad5 vector. This weak raw transduction translated to a weak ability to generate antibodies against HIV-1 gag when compared to the benchmark Ad5 vector (Weaver et al., 2012). While Ad-Tail-Sigma had overall weak transduction potency, it surprisingly generated markedly better T cell responses than Ad5 in vivo (Weaver et al., 2012). When Ad-Tail-Sigma was combined with Ad5 as a vaccine, the two synergized to generate higher antibody responses than either alone (Weaver et al., 2012). This suggested that sigma 1 may indeed have benefits for mucosal vaccination, if the vectors ability to deliver genes could be improved.
In this work, we aimed to improve the functionality of Ad-Sigma by modifying the fusion between the Ad5 fiber tail and the reovirus sigma 1 protein. Any sigma chimera must have the fiber tail to dock into the penton base of the Ad5 icosahedron. The original Fiber Tail-Sigma chimera had only this tail domain. We therefore speculated that adding repeats from the Ad5 fiber might either improve penton base docking of the chimera or improve sigma’s ability to bind its receptors.
We show that including the first three β-turn repeats of the Ad5 fiber markedly improved vector function in vivo. We speculate that this improvement may be primarily due to the inclusion of the third repeat (R3) from the Ad5 fiber. This β spiral repeat is interrupted by a four amino acid insertion that is thought to provide flexibility to the shaft of fiber (Nicklin et al., 2005; Wu and Nemerow, 2004). For Ad5, this flexibility allows the fiber to bend nearly 90° allowing the CAR binding motifs on the sides of the knob domain to engage their receptor. This ability to bend may also move the large 35 nm fiber out of proximity with the penton base allowing its RGD motif to engage αV integrins (Nicklin et al., 2005; Wu and Nemerow, 2004). For Ad-Sigma, we hypothesize that including repeat 3 may improve interactions with sigma receptors. Alternately, addition of this flexibility motif may allow the chimera to dock more efficiently into the penton base during encapsidation.
The R3, R14, and R20 chimeras all contain the R3 flexibility domain. In vitro comparisons between the viruses demonstrated that R3 and R14 mediated similar transduction on several cell types whereas R20 was markedly weaker. Notably, both R3 and R14 were able to utilize JAM1 on target cells and that only these viruses utilized JAM-1 as a receptor. The R20 virus was largely ineffective on all cells. This difference in functionality may be due to the overall size of the chimeric proteins. Ad5 fiber is approximately 35 nm long (Fig. 1). In contrast, native sigma 1 on reovirus is nearly 48 nm in length, so it is already larger than the fiber. Adding fiber tail and the first three repeats to sigma should make this chimera slightly longer than native sigma 1. In contrast, R14 and R20 have 14 and 20 β spiral repeats from fiber which likely adds more than 20 to 30 nm of length to these chimeras. This would make these proteins nearly 68 to 78 nm in length or nearly twice as long as Ad5 fiber itself.
Analysis of the protein content of purified virions revealed that Ad5-R3 and R14 had essentially normal viral protein composition (with the exception of fiber). In contrast, R20 virions appeared immature as indicated by the presence of unprocessed pVI and pVII in the virions. This observation was concordant with the observation that very little R20 chimeric protein was present on CsCl-banded virions. This suggests that the very long R20 chimera was poorly packaged in virions during assembly. If R20 was packaged and subsequently lost from virions during CsCl banding, the virus should not have uncleaved pVI and pVII proteins.
With increasing chimera size, less of the fusion proteins were incorporated. R3 appeared to be incorporated to similar levels as the fiber, although this was not entirely clear as the fiber bands on western blot were sharp and the R3 band was broader. R14 encapsidation was lower than R3, but better than R20. This lower encapsidation did not seem to interfere overtly with virion maturation or in vitro transduction on cell lines. In contrast, R14 was ineffective in vivo for gene delivery or vaccination than Ad5 or R3. This suggests that R14 may be less stable in vivo, perhaps due to proteolysis or other effects. Stability testing by freeze thaw and heating did not reveal any fundamental instability associated with R3, R14, or R20 relative to Ad5 suggesting that R14 may not suffer simple stability problem in vivo. However, failure to fully occupy all penton base sites on the Ad5 virion may well make these viruses susceptible to proteolysis or nuclease attack in vivo. Finally, the exceptional length of R14 protein may reduce its interactions with JAM1 that is sequestered in junctional adhesions in mucosa in vivo.
The new R3-sigma vector mediated similar transduction to Ad5 after intranasal immunization of mice. In contrast, R3 generated markedly more robust systemic and mucosal antibody responses than the benchmark Ad5 vector. This effect was similar to the observation that mixing the original Ad5-Tail-sigma virus with Ad5 generates stronger antibodies than Ad5 alone or Tail-sigma alone. This suggests that the improved R3-sigma may now harness some of the unique functionality of this new receptor targeting protein for mucosal vaccination.
These studies were performed in the context of human Ad5 vectors using sigma 1 protein from T3D reovirus. Ad5 is arguably the worst serotype for use in humans due to pre-existing immunity in 27 to 100% of humans (Abbink et al., 2007). The choice of Ad5 as a platform is historical as this was the original vector that was modified with sigma 1 (Mercier et al., 2004). The improved functionality of R3 now justifies its use in lower seroprevalence Ad vectors. Reovirus is also a human pathogen that can have high seroprevalence (Tai et al., 2005). Children become increasingly seropositive to reovirus T3D over the first 5 years of life with approximately 50% having binding antibodies in their sera to the virus (Tai et al., 2005). Adults would seem to have similar or higher seroprevalence as indicated by the presence of maternally transmitted anti-T3D antibodies in 75% of the sera of tested infants (Tai et al., 2005). While these data suggest that anti-sigma antibodies may challenge an Ad-sigma vaccine in humans, these titers are based on reovirus binding antibodies rather than neutralizing antibodies, so it is unclear how well they would repel an Ad-sigma vaccine. Pilot studies showed that priming with the original Ad5-Tail-sigma did not prevent the ability of Ad5-Tail-sigma to mediate an effective boost in anti-HIV gag antibodies (data not shown). This suggests that anti-sigma antibodies may not disable the use of this retargeting platform as a vaccine. However, work is underway to identify sigma analogs that may evade theoretical neutralization in humans.
This work was initiated to improve mucosal vaccination with adenovirus gene-based vaccines. These results are notable, since mice were vaccinated only a single time with Ad-R3-sigma, yet provoked robust immune responses without any boosting. Ad-R3-sigma not only generated antibody responses in the serum, but also importantly generated antibodies in vaginal washes. This suggests that Ad-R3-sigma may be able to establish a barrier to infection at mucosal surfaces where most pathogens enter the body. This single vaccination with R3 also generated systemic antibodies in the blood. This suggests that R3 can establish systemic immunity to serve as an immunological back up should vaccine protection at the mucosal barrier protection fail and infections spread beyond the site of entry. Considering the burgeoning interest in mucosal vaccines, optimized Ad-sigma vectors may have utility against a number of mucosal pathogens including HIV-1, influenza, and emerging pathogens like Ebola virus and MERS.
Constructed adenoviruses (Ads) displaying different reovirus sigma 1 fusion proteins.
Progressively longer chimeras were more poorly encapsidated onto Ad virions.
Ad5-R3-sigma mediated better systemic and mucosal immune responses than Ad5.
Acknowledgments
We would like to thank Mary Barry for excellent technical assistance. This work was supported by NIH/NIAID Grant R01 AI096967 and the Walter & Lucille Rubin Fund in Infectious Diseases Honoring Michael Camilleri, M.D. at Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O’Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos SK, Barry MA. Rapid construction of capsid-modified adenoviral vectors through bacteriophage lambda red recombination. Human gene therapy. 2004;15:1125–1130. doi: 10.1089/hum.2004.15.1125. [DOI] [PubMed] [Google Scholar]
- Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Current gene therapy. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest JC, Campbell JA, Schelling P, Stehle T, Dermody TS. Structure-function analysis of reovirus binding to junctional adhesion molecule 1: Implications for the mechanism of reovirus attachment. The Journal of biological chemistry. 2003 doi: 10.1074/jbc.M305649200. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Pickles RJ, Ye H, Yankaskas JR, Vick RN, Engelhardt JF, Wilson JM, Johnson LG, Boucher RC. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- Khare R, Chen CY, Weaver EA, Barry MA. Advances and future challenges in adenoviral vector pharmacology and targeting. Current gene therapy. 2011;11:241–258. doi: 10.2174/156652311796150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner E, Guglielmi KM, Strauss HM, Dermody TS, Stehle T. Structure of reovirus sigma1 in complex with its receptor junctional adhesion molecule-A. PLoS pathogens. 2008;4:e1000235. doi: 10.1371/journal.ppat.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand V, Spehner D, Schlesinger Y, Settelen N, Pavirani A, Mehtali M. Fiberless recombinant adenoviruses: Virus maturation and infectivity in the absence of fiber. J Virol. 1999;73:907–919. doi: 10.1128/jvi.73.2.907-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nature reviews Immunology. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS, Barry MA. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6188–6193. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005;12:384–393. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically Biotinylated Adenovirus for Cell-targeting, Ligand Screening, and Vector Purification. Molecular Therapy. 2003;8:689–702. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE, Jr, Dermody TS. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. The Journal of infectious diseases. 2005;191:1221–1224. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, Wold WS. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med. 2007;130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
- Von Seggern DJ, Huang S, Fleck SK, Stevenson SC, Nemerow GR. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. Journal of virology. 2000;74:354–362. doi: 10.1128/jvi.74.1.354-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver EA, Mercier GT, Gottschalk S, Barry MA. T-cell-biased immune responses generated by a mucosally targeted adenovirus-sigma1 vaccine. Mucosal immunology. 2012;5:311–319. doi: 10.1038/mi.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Nemerow GR. Virus yoga: the role of flexibility in virus host cell recognition. Trends in microbiology. 2004;12:162–169. doi: 10.1016/j.tim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Zabner J, Freimuth P, Puga A, Fabrega A, Welsh MJ. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]