Abstract
Arginase has roots in early life forms. It converts L-arginine to urea and ornithine. The former provides protection against NH3; the later serves to stimulate cell growth and other physiological functions. Excessive arginase activity in mammals has been associated with cardiovascular and nervous system dysfunction and diseases. Two relevant aspects of this elevated activity may be involved in these disease states. First, excessive arginase activity reduces the supply of L-arginine needed by nitric oxide (NO) synthase to produce NO. Second, excessive production of ornithine leads to vascular structural problems and neural toxicity. Recent research has identified inflammatory agents and reactive oxygen species (ROS) as drivers of this pathologic elevation of arginase activity and expression. Here we review involvement of arginase in cardiovascular and nervous system dysfunction and discuss potential therapeutic interventions targeting excess arginase.
Keywords: arginase, vascular dysfunction, neurodegeneration, polyamine, oxidative stress, nitric oxide, peroxynitrite, superoxide
Arginase, a ubiquitous enzyme
Arginase is a manganese metalloenzyme that catalyzes the conversion of L-arginine to L-ornithine and urea. It is found in bacteria, yeasts, plants, invertebrates and vertebrates, and is thought to have appeared first in bacteria [1]. The subsequent transfer of arginase to a eukaryotic cell has been suggested to have occurred through mitochondria. Most, invertebrates plants, bacteria and yeasts have only one form of arginase that is localized in mitochondria. Vertebrates and other animals that metabolize excess nitrogen as urea express a second cytosolic isoform. The cytosolic and mitochondrial arginase isoenzymes are named A1 and A2, respectively. The mitochondrial A2 isoform is thought to be derived from the ancestral arginase, because A1 is restricted to a subset of more recently evolved species.
Human A1 consists of 322 amino acids [2] and A2 has 354 amino acids [3]. The two isoforms are encoded by distinct genes on separate chromosomes, but share more than 50% of their amino acid residues, with 100% homology in areas critical to enzymatic function [4]. High-resolution crystallography has shown that both consist of 3 identical subunits with an active site at the bottom of a 15 Å deep cleft. Manganese ions, essential for enzyme activity, are located at the bottom of the cleft. The overall fold of each subunit belongs to the α/β family and consists of a parallel, 8 stranded β-sheet flanked on both sides by numerous α-helices [5].
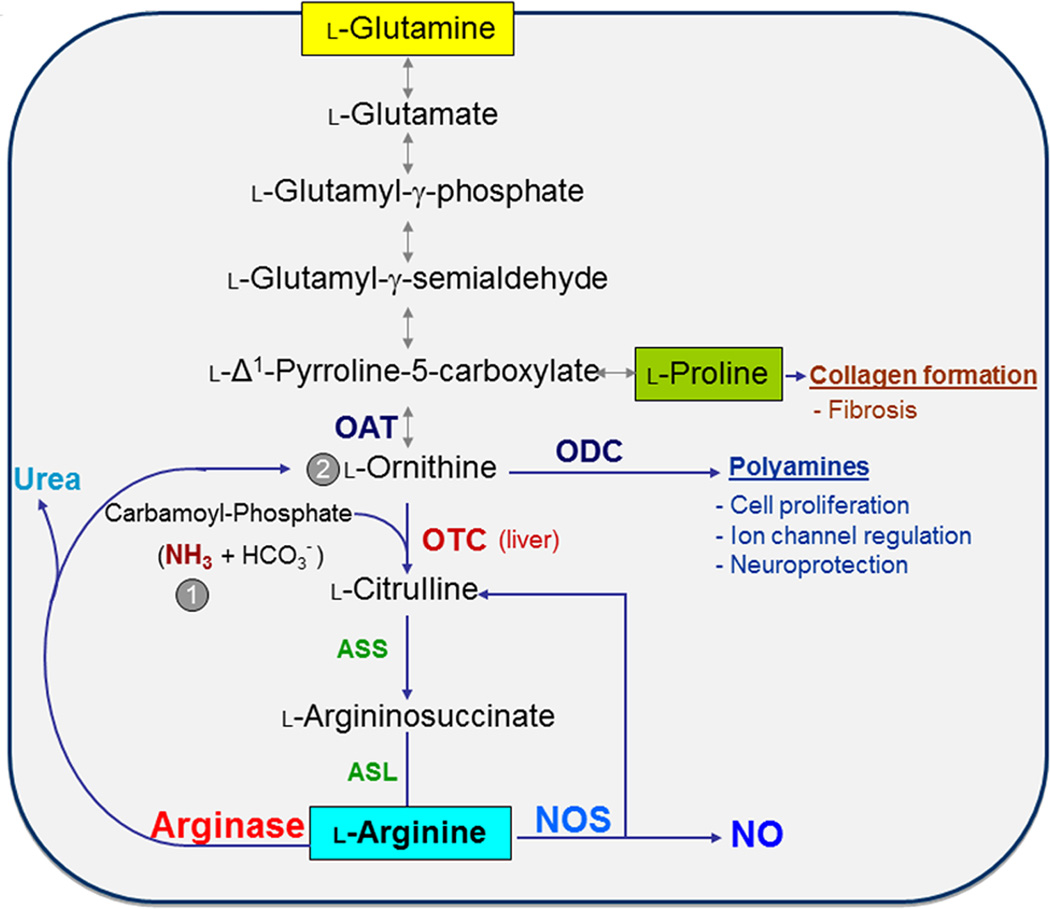
The two arginase isoforms have similar mechanisms, requirement of manganese as a co-factor and identical metabolites. A1 is cytoplasmic and mainly expressed in the liver. A2 is mainly located within mitochondria and highly expressed in kidney. Arginases can be expressed in many different cell types and can be induced by a wide variety of agents and conditions, depending on tissue and species. Both isoforms are found in the endothelium of the vasculature. Arginase activity has two major homeostatic purposes: first, to rid the body of ammonia through urea synthesis, and second, to produce ornithine, the precursor for polyamines and prolines (Box 1, Figure 1) [6]. Polyamines produced through ornithine decarboxylase (ODC) are necessary for cell proliferation and regulation of several ion channels. Proline produced through ornithine aminotransferase (OAT) is necessary for production of collagen [7, 8]. Although there is functional redundancy of the arginase isozymes, inherited defects in A1 can lead to severe and even lethal health problems.
Box 1. Arginase-ornithine pathway in health and disease.
In the liver, A1 catalyzes the last step in the urea cycle, whereby the body disposes of ammonia produced by protein catabolism (Figure 1). Ornithine is converted to L-citrulline by activity of ornithine transcarbamylase (OTC) and carbamoyl phosphate synthase-1 (CPS-1) and to polyamines (putrescine, spermidine, and spermine) and proline by ornithine decarboxylase (ODC) and ornithine amino transferase (OAT), respectively. Polyamines play an important role in cell growth and proliferation and are involved in wound healing, tissue repair and neural development [101, 102]. Proline is required for collagen formation [103]. In most cell types L-citrulline is recycled back to L-arginine by argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL). ASS and ASL are a portion of the urea cycle [104]. However, most non-hepatic tissues lack CPS-1 or OTC and therefore do not have the complete urea cycle. Dysregulation of urea cycle enzymes can result in hyperammonemia, and if left untreated, will cause seizures, mental disorders and early morbidity [105]. Treatment of patients with A1 deficiency involves decreasing protein intake, dietary supplementation with essential amino acids, and in severe cases orthotropic liver transplant [105]. The adverse effects of A2 mutations are not well studied, but A2 knockout mice have been found to develop hypertension [106] and the presence of a rare 2 allele has been linked to an increase in the risk of Alzheimer’s disease in men and with an earlier age at onset for both genders [72].
The acute phase of wound repair involves oxidative insult through activation of resident macrophages which express high levels of NOX2 NADPH oxidase and inducible nitric oxide synthase (iNOS) and produce cytotoxic levels of superoxide and NO. Both are important for eradicating pathogens [101]. The acute phase is followed within 3–5 days by a repair phase in which arginase is upregulated [107]. As explained above, arginase converts L-arginine to L-ornithine which is metabolized by OAT to form proline needed for collagen synthesis and by ODC to form polyamines which enhance cell proliferation. The balance between rate of consumption of L-arginine by iNOS (for NO production) and arginase (for synthesis of collagen and polyamines) determines the course of wound repair.
Polyamine production by arginase is also important for neural growth, development, and regeneration [102, 108, 109]. Elevated arginase activity can promote axon regeneration. Following spinal cord transection, treatment of nerve grafts with acidic fibroblast growth factor has been reported to improve locomotor function by a mechanism involving increases in A1 and spermine within motor neurons and macrophages [110]. However, excessive function of the arginase-ornithine pathway can be damaging in other contexts. For example, arginase-dependent increases in formation of polyamines and proline can lead to thickened, fibrotic and stiff blood vessels and airways, hypertrophied and fibrotic hearts and kidneys and growth of cancers [9, 10, 111–113]. These effects of excessive arginase activity are pathologically significant in diseases like diabetes, hypertension, aging and may play a role in tumor growth.
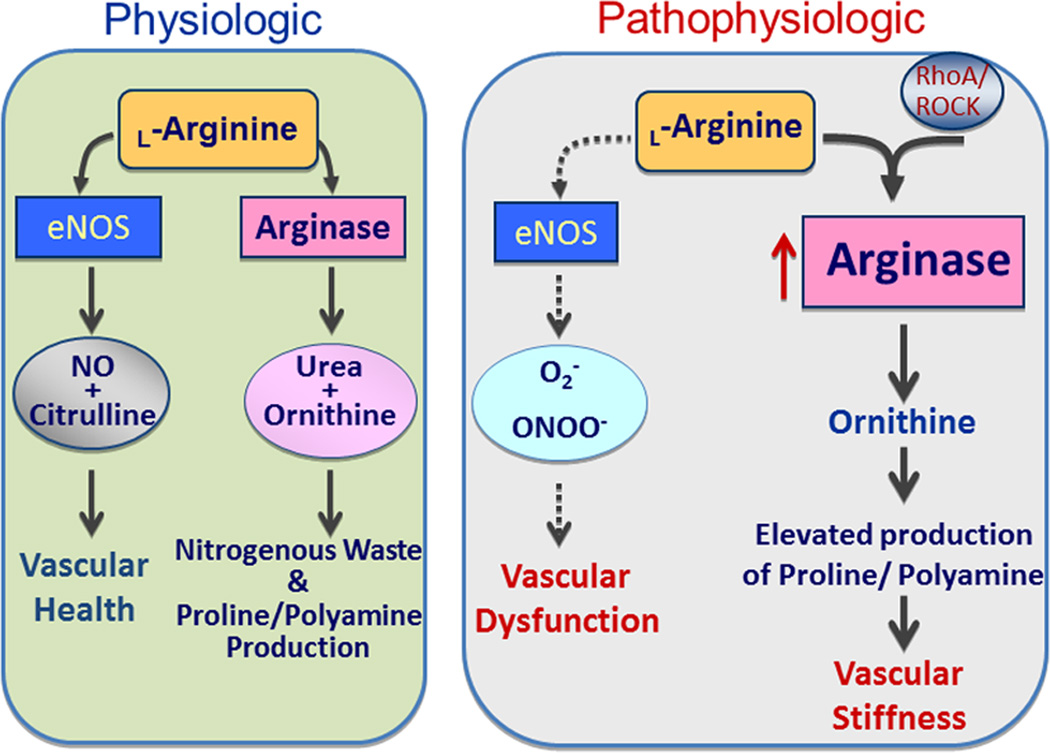
Many research studies have concentrated on the role of arginase in altering NO production and levels because both arginase and NOS utilize L-arginine as their common substrate. Overactive arginase could lead to a deficiency in L-arginine available to NOS. This could cause uncoupling of NOS, decreased NO production, and increased production of the oxidants, superoxide and peroxynitrite (Box 2, Figs. 2, 3), resulting in vascular dysfunction. Accordingly many studies have correlated vascular endothelial dysfunction with increased levels of arginase activity and expression [12, 24, 49, 114–116]. Increased levels of ornithine resulting from enhanced arginase activity can lead to vascular hypertrophy, fibrosis and stiffness – important aspects of vascular disease [112]. Arginase also plays important roles in reducing NO production by iNOS [10].
Polyamine metabolism has also been shown to be involved in the pathogenesis of ischemic neuronal injury [117–119]. Amino aldehydes, acrolein and hydrogen peroxide, which are generated as byproducts during the oxidation of spermine and spermidine by polyamine oxidases (Fig. 4) are toxic and have been implicated in brain and retinal injury [120]. Arginase activity and expression are increased by inflammatory processes and reactive oxygen species associated with disease states [8, 47, 121].
Figure 1.
Scheme for arginase catabolism of L-arginine to L-ornithine/urea or L-citrulline/NO, production of polyamines and anabolism and catabolism of proline. Also shown are the pathway for synthesis of L-arginine from L-glutamine, the reversible pathway between L-ornithine and L-glutamine, and the recycling of L-citrulline into L-arginine. Abbreviations: ASL, aminosuccinate lyase; ASS, aminosuccinate synthase; NOS, nitric oxide synthase; OAT; ornithine aminotransferase; ODC, ornithine decarboxylase; OTC, ornithine transcarbamylase. Arginase (bottom, left) is the final enzyme in the urea cycle within the liver, which restarts the cycle through the synthesis of L-citrulline from carbamoyl-phosphate (1) and L-ornithine (2) by OTC (center). It should be noted that that these reactions do not all occur within any given cell. In particular, the urea cycle is independent of the other reactions; i.e., L-arginine produced within the urea cycle is not a substrate for NOS and L-ornithine produced within the urea cycle is not a substrate for OAT or ODC
L-arginine is a semi-essential amino acid because it is normally provided thru protein turnover, but in certain cases it is required from the diet. Acute administration of supplemental L-arginine is reported to prevent or reverse endothelial dysfunction and restore endothelial-dependent vasodilation in diabetes, hypertension, and heart failure. However, a number of studies in animals and humans have found no benefit or worsening of adverse outcomes with prolonged administration of supplemental L-arginine [9]. These negative outcomes may be related to the ability of l-arginine to induce expression/activity of arginase and reduce plasma L-arginine levels.
Enhanced arginase activity and the resultant decreases in L-arginine levels can also impair T cell-mediated immune function and allow tumor growth by limiting the supply of L-arginine needed for formation of cytotoxic levels of NO by iNOS [10]. Increased arginase expression/activity may also limit iNOS expression through reducing L-arginine required for iNOS translation [11].
L-arginine is also the substrate for nitric oxide synthase (NOS) (Box 2, Figure 2). When arginase activity is excessive, it can compete with NOS for their common substrate, L-arginine. When the supply of L-arginine required for NO production is insufficient, NOS will become uncoupled [12, 13] and will produce less NO and use more molecular oxygen to form superoxide. The superoxide will react rapidly with any available NO to form peroxynitrite, further decreasing NO and further uncoupling NOS by oxidizing the co-factor BH4 [14] (Figure 3).
Box 2. Nitric oxide synthase.
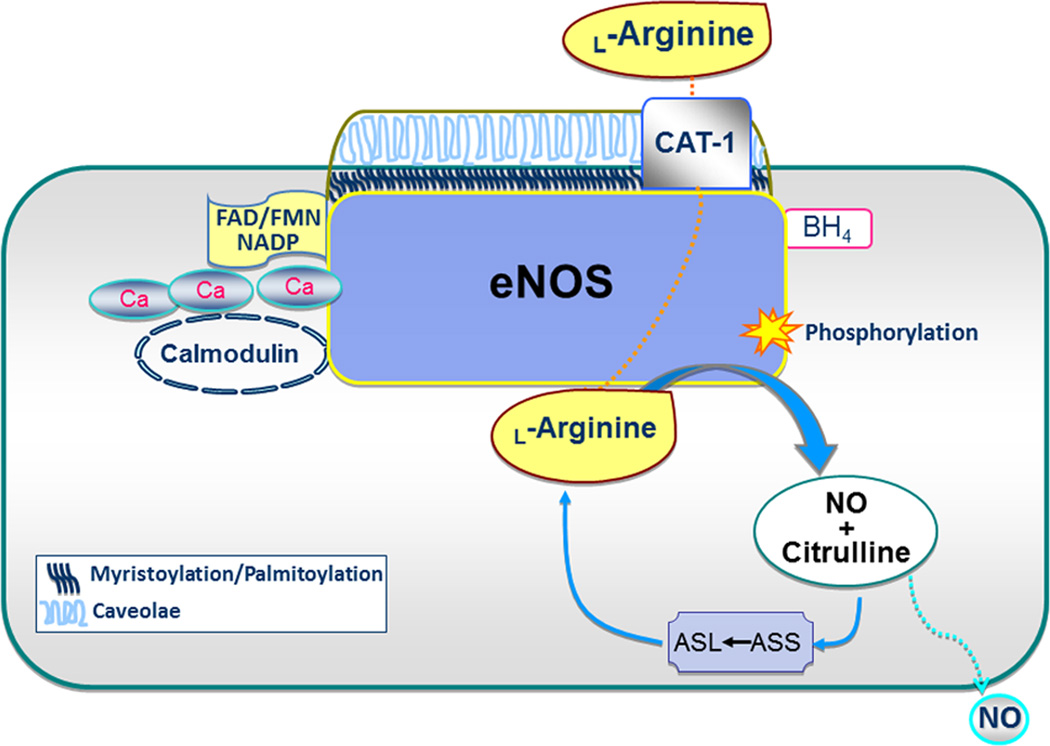
Three isoforms of NOS exist: eNOS, nNOS and iNOS. The eNOS and nNOS isoforms are constitutively expressed and iNOS is constitutively active. In vascular endothelial cells, normal function of eNOS plays a critical role in vascular health, catalyzing the conversion of L-arginine into L-citrulline, and NO plays a critical role in vascular health, catalyzing the conversion of L-arginine into L-citrulline and NO. eNOS binds tetrahydrobyopterin (BH4) and heme at the N-terminal region and calcium/calmodulin, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN) and nicotinamide adenine dinucleotide phosphate (NADP) at the C-terminal region [122–124] (Figure 2). These co-factors are required for NO production. eNOS is post-translationally modified for specific subcellular localization to the plasma membrane. Myristoylation and subsequent palmitoylation of eNOS direct it to membrane caveolae, but these modifications are not necessary for its activity. Location of eNOS at the caveolae is believed to be important due to its proximity to the L-arginine transporter [cationic amino acid transporter-1 (CAT-1)] and L-arginine recycling enzymes that support NO production [125]. In contrast, caveolin-1 operates to inhibit eNOS at caveolae. eNOS is activated by a number protein-protein interactions, phosphorylation events as well as chemical, hormonal stimuli and physical stimuli such as sheer stress.
NO is produced by eNOS or nNOS at low-to-moderate rates. In contrast, iNOS produces NO at greater rates for alternative signaling mechanisms and microbial defense. eNOS-derived NO regulates vascular tone by relaxing vascular smooth muscle cells (SMCs) and also by preventing leukocyte adhesion and platelet aggregation [126–128]. NO diffuses to SMCs where it binds to and activates soluble guanylate cyclase (sGC). This results in production of cGMP, efflux of K+ and relaxation of SMCs.
There are other physiologic and pathophysiologic roles of NO. It is involved in macrophage-mediated host defense, neuronal signaling and modulation of synaptic plasticity as well as protein nitrosylation [129]. NO and superoxide (O2․−) react rapidly to produce peroxynitrite (ONOO−) at a faster rate than NO can react with sGC or O2․− can interact with superoxide dismutase. This reduces levels of NO and increases ONOO−, a potent oxidant. Both O2․− and ONOO− can oxidize BH4, leading to further increases in oxidative stress due to uncoupling of eNOS [14]. Reduced NO bioavailability and increased levels of O2․− and ONOO− can lead to vascular endothelial dysfunction [130].
Figure 2.
Scheme for endothelial nitric oxide synthase (eNOS) catabolism of L-arginine to NO and L-citrulline, which can be recycled back to L-arginine. Abbreviations: ASL, aminosuccinate lyase; ASS, aminosuccinate synthase; BH4, tetrahydrobiopterin; CAT-1, cationic amino acid transporter-1; FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; NADP, nicotinamide adenine dinucleotide phosphate.
Figure 3.
Physiologic and pathophysiologic activities of endothelial nitric oxide synthase (eNOS) and arginase. Under physiologic conditions eNOS maintains a healthy vasculature with production of NO and arginase produces ornithine, polyamines and proline, for tissue growth and collagen formation, respectively. Under pathologic stimulation by RhoA/ROCK, increased arginase expression/activity putatively depletes eNOS of its substrate, L-arginine. When eNOS does not have sufficient substrate it can be uncoupled, becoming more a producer of superoxide (O2․−) rather than NO. Increased production of polyamines and proline can also lead to pathologic vascular remodeling and stiffness.
We outline here the role played by arginase in health and disease with a particular emphasis on its involvement in disease and injury conditions that affect the peripheral cardiovascular system and the central nervous system (CNS). Upregulation of arginase expression and activity has been demonstrated in many diseases characterized by cardiovascular dysfunction, but is only recently been recognized as a potential mediator of neurovascular disease and injury in the CNS. The sections that follow summarize recent research on the role of arginase in cardiovascular and neurovascular disease. New pharmacological tools are emerging to modulate the activities or expression levels of arginase and will be discussed in the last section.
Arginase in cardiovascular disease
In the time since NO was named “The Molecule of the Year” in Science [15], many cardiovascular disease states have been linked to impaired vascular endothelial cell production of NO. Additionally, reduced availability of L-arginine has been implicated in vascular dysfunctions. Realization that enhanced arginase activity might compete with NOS for L-arginine and reduce NO levels fueled a number of studies on its involvement in states of vascular endothelial dysfunction. Elevated levels of L-ornithine, the product of arginase, also have been shown to be a key factor in vascular smooth muscle hyperplasia, fibrosis and stiffening. We review below some of the recent evidence for involvement of these arginase pathways in cardiovascular disease and injury conditions.
Hypertension
Hypertension is a major risk factor in cardiovascular disease. It involves reduced NO levels, increased superoxide production, diminished levels of the eNOS substrate L-arginine, co-factor BH4 and increased expression and activity of arginase. Studies in animal models have shown that elevated arginase activity and A1 expression in the aorta are associated with increased blood pressure [16]. Pulmonary hypertension is also associated with increased arginase activity. In contrast with systemic hypertension, the A2 isoform seems to more important in pulmonary hypertension [17–19]. Elevation of arginase expression/activity is associated with decreased NO production [20]. Additionally, increases in A2 limit endothelium-dependent vasodilation of pulmonary segments in experimental pulmonary embolism. Treatment with an arginase inhibitor was found to preserve L-arginine and reduce pulmonary resistance [21]. Thus, upregulation of arginase seems to play a detrimental role in increasing blood pressure and causing endothelial dysfunction during both systemic and pulmonary hypertension. The mechanisms underlying the differential involvement of A1 and A2 in these systemic vs pulmonary hypertension are unclear, but likely involve the differences in the cellular and subcellular distribution. Further study is needed to clarify this issue.
Diabetic Vascular Disease
Diabetes mellitus is strongly associated with cardiovascular disease, accounting for significant morbidity and mortality in diabetic patients. Type 1 and 2 diabetes are both associated with signs of vascular dysfunction and injury, including impaired endothelial-dependent relaxation, pathological remodeling of SMCs and decreased vascular compliance. Decreases in L-arginine have been reported in plasma of diabetic patients [22, 23] and vascular tissue of diabetic rats. Increased arginase activity seems to be involved. Studies have shown that increases in arginase activity and A1 expression are involved in diabetes and high glucose-induced dysfunction of aorta, coronary and retinal arteries [12, 24–26].
Coronary artery disease (CAD) with impaired blood flow is a key manifestation of diabetes-associated vascular dysfunction. Increased A1 expression has been observed in coronary arteries from diabetic patients [27]. Arginase inhibition with L-NOHA restored endothelium-dependent vasodilation in coronary arteries from diabetic patients [28]. Also, studies in a Type 2 diabetes animal model revealed that nor-NOHA restores coronary microvascular function by a mechanism involving increased L-arginine supply and improved NO bioavailability [29]. Furthermore, a study of forearm blood flow in CAD patients treated with local infusion of the arginase inhibitor nor-NOHA demonstrated improved endothelium-dependent vasodilatation [30]. The beneficial effects were particularly prominent in patients with Type 2 diabetes, suggesting that increased arginase activity is involved in Type 2 diabetes-associated CAD.
Atherosclerosis
Inflammation, vasoconstriction and thrombus formation are critically involved in the pathogenesis of atherosclerosis. Impaired vascular endothelial function is considered an early and critical event in atherosclerosis, causing abnormalities in the arterial wall and plaque formation. Accumulating evidence indicates that oxidized low-density lipoprotein (OxLDL) is involved in atherosclerosis [31–33]. Increased arginase activity and expression are observed in atherosclerosis and OxLDL seems to mediate this elevation through oxidized low density lipoprotein receptor-1 (LOX-1) and Rho kinase (ROCK) activation. A2 activation through LOX-1 causes eNOS uncoupling and reduced NO generation [33]. Furthermore, pharmacological inhibition of LOX-1 and ROCK attenuated arginase activity in endothelial cells. Moreover, studies in atherosclerotic apolipoprotein E-deficient mice crossed with A2 knockout mice demonstrated reduced atheromatous plaque burden, decreased oxidative stress, and increased NO [31]. OxLDL stimulates A2 activation by inducing translocation from the mitochondria to the cytosol through processes that are dependent on mitochondrial processing peptidases (MPP). A2 has a putative MPP cleavage site in its N-terminal that may influence its translocation. Importantly, knockdown of MPP prevented OxLDL-induced arginase translocation, blocked eNOS uncoupling and improved vascular function [34]. In contrast with the suggested detrimental effects of macrophage A2 expression on the development of atherosclerotic plaques, elevated expression of A1 in macrophages has been reported to be beneficial in regressing atherosclerotic plaques [35, 36]. Thus, selective targeting of the arginases could offer an attractive strategy for the management of vascular dysfunction and injury associated with atherosclerosis [37, 38].
Regarding the selective involvement of A1 vs A2 in atherosclerosis and other forms of vascular inflammation, it should be noted that there is considerable controversy about whether or not A1 is expressed in human macrophage cells. A similar controversy exists for iNOS. Studies using human monocyte-derived macrophages or monocyte-macrophage cell lines have shown these cells cannot be readily induced to produce iNOS or NO [39]. However, the source of the macrophages used for the analyses varies. Many studies of human cells have used blood-derived monocytes, which has led some researchers to conclude there are major species differences in macrophages and that humans macrophages are unable to produce iNOS/NO or arginase/ornithine [40]]. However, in studies where human tissue macrophages have been examined, they have been found to be fundamentally similar to those of other vertebrate species [41].
Myocardial-ischemia-reperfusion (I/R) injury
Myocardial ischemic injury, a pathology caused by occlusion of a coronary artery, is a major cause of disability and death and is particularly common in patients with diabetes and atherosclerosis. Following ischemic insult, reperfusion is necessary to minimize tissue damage. However, reperfusion can cause induction of reactive oxygen species, pro-inflammatory factors, and death of cardiomyocytes. Decreased endothelial dependent vasorelaxation due to decreased NO bioavailability is a central mechanism behind I/R injury. Studies have revealed the involvement of increased arginase expression and activity in myocardial I/R injury in different animal models [42–45]. Pharmacological inhibition of arginase with nor-NOHA before ischemia, during late ischemia and early reperfusion markedly decreased cardiac infarct size while restoring NO availability. However arginase function, particularly in “healing” macrophages, is important for tissue repair. Studies have shown that intracellular signaling leading to arginase activation includes Rho kinase, mitogen-activated protein kinase, protein kinase A, cytokines, reactive oxygen and nitrogen species and hypoxia [37, 46–48].
Aging and cellular senescence
Aging-induced endothelial cell senescence has been linked to excessive arginase activity in humans and animals [49]. Studies in aged rats have shown higher levels of arginase activity, lower NO and higher superoxide production as compared with young rats [50]. Furthermore, acute inhibition of NOS and arginase was found to prevent uncoupling of eNOS and reduce superoxide production in old rats. Acceleration of endothelial cell senescence along with decreased generation of NO has also been shown to occur with chronic L-arginine supplementation [51]. The L-arginine-induced endothelial cell senescence was reported to involve upregulation of A2 expression [52]. Interestingly, a recent study by Xiong and colleagues has shown that A2 can induce senescence of vascular SMCs by a mechanism involving activation of p66Shc and p53 independently of its L-arginine ureahydrolase activity [53].
Erectile Dysfunction
Strong evidence supports NO as the principal mediator of penile erection [54]. Both eNOS and nNOS serve as sources of NO to cause relaxation of corpora cavernosal (CC) smooth muscle [54, 55]. Both A1 and A2 are expressed in CC. Studies in humans and animals have suggested a link between increased cavernosal arginase activity and erectile dysfunction [16, 56, 57]. Inhibition of arginase enhances relaxation of cavernosal smooth muscle cells [58, 59]. Whereas NO production from the endothelium and nitrergic nerves regulates intrinsic tone, arginase activity in CC modulates tone by inhibiting NO production, presumably by competiting with NOS for L-arginine. Diabetic patients and animal models with erectile dysfunction exhibit elevated CC arginase activity and expression, diminished NO production, and reduced cavernosal relaxation [16, 56, 57]. Additionally, diabetes-induced impaired endothelium- and nitrergic nerve-dependent CC relaxation responses are abrogated by deletion of the A2 gene [60].
Arginase and CNS disease/injury
Arginase function in increasing polyamine formation is known to have a positive role in neuroprotection and neural regeneration [61, 62]. However, upregulation of arginase has also been linked to CNS disease conditions, including stroke, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, traumatic brain injury and several retinal diseases. Immunolocalization studies have shown the presence of both A1 and A2 in brain, especially in hippocampal neurons [63]. Both isoforms are also expressed in retina. Prominent immunoreactivity for A1 is evident in retinal glia [64, 65] and A2 is abundant in horizontal cells, photoreceptor inner segments and cellular processes throughout the neural retina, consistent with its mitochondrial localization [66, 67].
NO release is a critical component of neurovascular signaling pathways and is particularly important for maintaining cerebral blood flow (CBF). Disruption of NO pathways is a critical feature of brain injury. NO derived from eNOS, nNOS and iNOS has been shown to influence the evolution of brain damage in different ways [68]. Uncontrolled NO production through iNOS during inflammation or NO formed by nNOS promotes nitrative stress, leading to neurodegeneration and apoptosis. However, NO from eNOS maintains blood flow and limits platelet aggregation and leukocyte attachment to the vessel wall, thereby dampening oxidative stress and inflammation. On the other hand, arginase can be both a target and source of oxidative stress and inflammation. Expression of A1 has been shown to be increased by oxidative stress and inflammatory mediators [8, 35, 36] and overactive arginase can contribute to further increases in oxidative stress and inflammation by causing NOS uncoupling. The role of arginase activity in inflammation, oxidative stress and CNS injury has been considered only recently. We explore the current evidence for involvement of the arginase pathways in neurovascular injury in the sections that follow.
Ischemic stroke
Alterations in NO signaling during ischemic stroke are well documented [69]. Given that arginase and NOS compete for L-arginine, it is likely that arginase has an important role in this dysfunction. Increases in A1 expression and arginase activity have been reported in a rat model of ischemic stroke [70]. No changes were observed in A2 expression. The increases in A1 were localized to the lesion area and were associated with an early and long-lasting up-regulation of A1 in activated macrophages and astrocytes. The increases in A1 and were associated with increases in glial fibrillary acidic protein, a marker of activated astrocytes. Delayed down-regulation of A1 was noted in neurons near the lesion. Moreover, the expression pattern of A1 was similar to that of brain-derived neurotrophic factor (BDNF), suggesting a role for A1 in neuroplasticity. However, the specific role of arginase activity in the ischemia-induced injury and subsequent repair is as yet unknown. Given the suggested involvement of A1 in macrophage-mediated repair processes, it is possible that the A1 increase represents a healing mechanism. Further studies using genetic strategies are needed to address this issue.
Alzheimer’s disease
Alterations in L-arginine metabolism may also play a role in the pathogenesis of Alzheimer’s disease (AD). A number of studies have investigated arginase expression in AD brains, with conflicting results. One group found increased A1 mRNA with no change in A2 in the frontal cortex of AD patients [71], whereas another found increased A2 mRNA levels with no change in A1 [72]. Additionally, the latter authors reported that the presence of a rare A2 allele was associated with an increased risk of early onset AD. A recent study has also demonstrated an association between age and region-specific decreases in activity and expression of NOS and increases in arginase activity during AD [73]. These studies demonstrate that increased arginase activity is correlated with the pathogenesis of AD. However, whether AD is mediated by alterations in A1 or A2 activity is an open question. Our studies using models of retinal neurodegeneration and work of others in brain injury models suggest that A2 is the major isoform involved in neuronal damage. Further investigations are needed to fully understand arginase function in AD brain and to demonstrate its potential value as a biomarker for disease progression.
Traumatic Brain Injury
Traumatic brain injury (TBI) has been shown to cause vascular changes that lead to decreased CBF. The dysfunction is thought to involve alterations in NOS function and NO formation [69]. Within minutes after trauma, levels of NO in the injured brain transiently increase and then decrease and remain below baseline values for periods ranging from hours to days. The NO decrease is closely correlated with decreases in constitutive NOS activity and CBF [74]. Studies showing that treatment with supplemental L-arginine increases CBF and reduces signs of injury suggest L-arginine depletion due to excessive arginase activity could be involved [75]. An analysis using arterial spin-labeling magnetic resonance imaging in a mouse model of TBI showed that deletion of A2 improved CBF after TBI, suggesting involvement of A2 in hemodynamic processes [76]. Effects of the A2 deletion on tissue damage and CNS function were not reported. However, enhanced polyamine catabolism has been reported in a rat model of TBI [77]. Moreover, inhibition of the ornithine/polyamine pathway was found to reduce cognitive impairment associated with TBI [78]. Given that A2 deletion improves CBF after TBI and that arginase can regulate polyamine levels, it is likely that excessive activity of the arginase/polyamine pathway plays a role in TBI.
Multiple sclerosis
Visual dysfunction is a common clinical manifestation of multiple sclerosis (MS). Studies have shown that MS patients develop optic neuritis characterized by thinning of the nerve fiber layer, loss of retinal ganglion cells (RGCs) and impaired retinal function [79, 80] A1 expression and activity were shown to be increased in brain and spinal cord samples in a rat model of MS induced by EAE (experimental autoimmune encephalomyelitis). Increased production of reactive oxygen species and elevated levels of iNOS were also observed [81, 82]. The data showed that A1 was significantly increased at the peak stage of EAE and was maintained until the recovery stage, whereas iNOS was increased at early stages and remained high until the recovery stage. The same group also found elevated arginase levels in the cerebrospinal fluid of MS patients [82]. These results support the involvement of NO and arginase in the pathogenesis of acute neuroinflammation, and support their potential use as surrogate markers for disease.
Retinal neurovascular injury
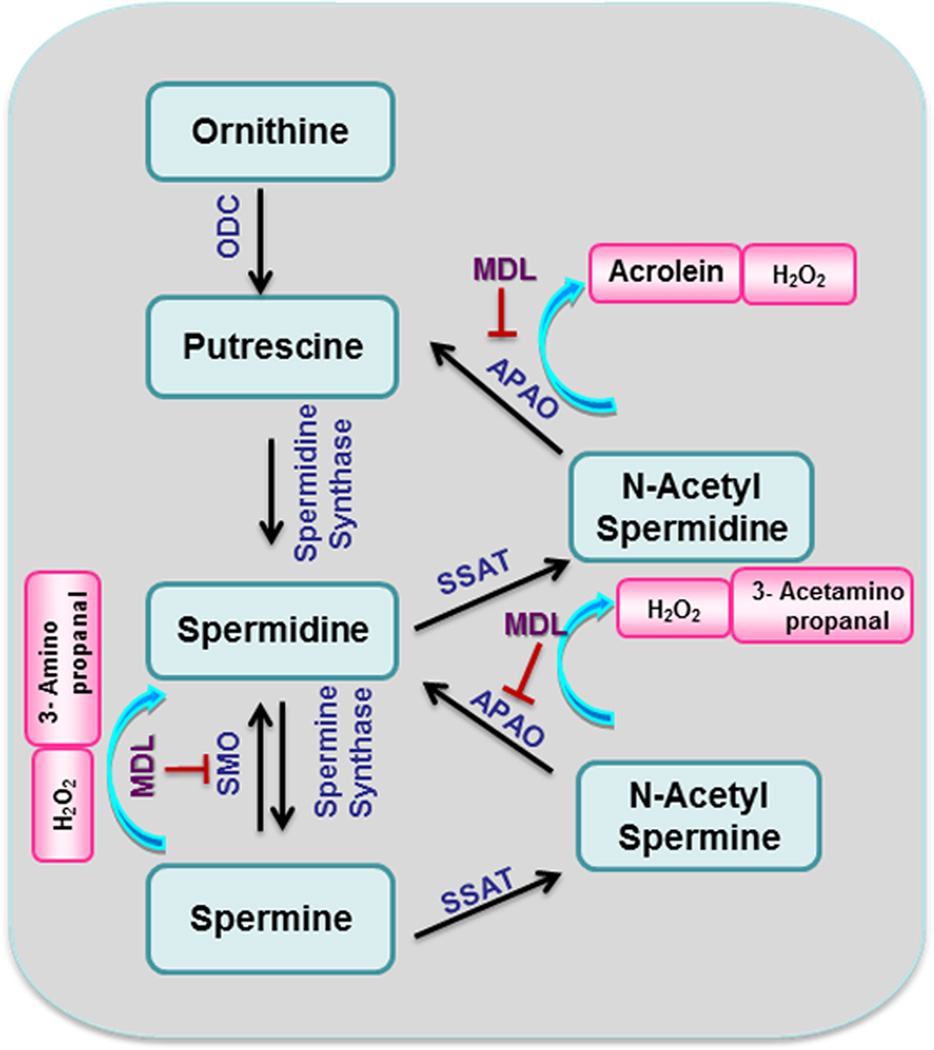
Retinal neurodegeneration is associated with a variety of disease conditions, including glaucoma, diabetic retinopathy, optic neuritis, TBI and retinopathy of prematurity (ROP). We and others have used ROP models to elucidate the mechanisms of retinal neurovascular injury associated with activity of NOS and arginase pathways. ROP is a complex condition affecting the developing retina and is characterized by neuronal and vascular degeneration followed by pathological neovascularization. Recent studies have demonstrated a role for A2 in the neuronal injury [66]. Studies using a mouse model for oxygen induced retinopathy (OIR) to mimic neuronal injury during ROP showed that deletion of A2 significantly reduced neurodegeneration and improved retinal function. Increased expression of A2 was observed in retinal horizontal cells of hyperoxia-treated mice [66]. Previous work had suggested that arginase might have a beneficial role in limiting retinal injury in this model [83]. In that study, the authors demonstrated reduced retinal cell death and increased astrocyte survival in TNFα-deficient mice. The TNFα-deficient OIR retinas had lower activity of iNOS, increased A2 mRNA and higher arginase activity relative to the wild type OIR retinas. In contrast with those results, our studies using A2 deficient mice demonstrated involvement of A2 in retinal neurodegeneration [66]. The data showed that A2 deletion significantly reduced neuronal degeneration during OIR. Electroretinographic recordings showed that the protective effects of A2 deletion were accompanied by significant preservation of retinal neuronal function. Partial deletion of A1 along with A2 did not confer any additional protection, suggesting that A2 is the more important isoform involved in the neurodegeneration. Further studies demonstrated increased expression of spermine oxidase together with decreases in spermine, increases in spermidine and increases in hydrogen peroxide formation in the OIR retina compared to normoxic controls, suggesting an increase in backward polyamine catabolism (Figure 4) [67]. These alterations were significantly reduced in the A2-deficient OIR retina. Furthermore, treatment with the polyamine oxidase inhibitor MDL-72527 [N,N'-bis(2,3-butadienyl)-1,4-butanediamine] significantly reduced the hyperoxia-induced neuronal death, indicating that polyamine catabolism is involved in neurodegeneration during OIR.
Figure 4.
Flow chart of the ornithine pathway showing formation of polyamines and polyamine oxidation. Abbreviations: MDL, MDL-72,527 [N,N'-bis(2,3-butadienyl)-1,4-butanediamine]; ODC, ornithine decarboxylase; OAT, ornithine aminotransferase; SMO, spermine oxidase; APAO, N (1)-acetyl polyamine oxidase; SSAT, spermine spermidine acetyl transferase.
Studies with the same OIR model have demonstrated the involvement of eNOS, peroxynitrite and A2 in hyperoxia mediated degeneration of the vascular endothelial cells [84, 85]. Upon hyperoxia treatment for two days, obliteration of the developing retinal microvessels was observed in wild type mice. This damage was significantly blunted in eNOS deficient mice as well as in wild type mice treated with the NOS inhibitor Nω nitro L-arginine (L-NNA). Further, levels of the peroxynitrite biomarker nitrotyrosine were also reduced in the eNOS deficient OIR retina as compared to the WT retinas [84], indicating that NO is an important factor in the vascular injury. Work by others using iNOS knockout mice and the iNOS inhibitor 1400W showed that iNOS is highly increased in the ischemic retina and that blockade improved physiological vascular repair [86]. While the role of NO in vascular injury is well discussed, involvement of arginase in the vascular injury has only recently been appreciated. Our laboratory has found that A2 plays a key role in the hyperoxia-mediated vascular injury through a mechanism involving superoxide and peroxynitrite formation [87]. Studies using A2 deficient mice in the OIR model showed significant decreases in vaso-obliteration compared to the wild type OIR retina, demonstrating involvement of A2 in OIR-induced vascular injury. Deletion of A2 in combination with haploid deficiency of A1 did not show any additional protection compared with wild type mice. Levels of superoxide and peroxynitrite were also reduced in the A2 deficient OIR retina relative to the wild type OIR mice. This suggests that the observed vascular protection in the A2-knockout retina is mediated by reduction in nitrative stress. Restoration of normal NO levels and decreases in eNOS derived superoxide were also found in the A2 knockout retina, suggesting that the A2-induced injury is mediated by a mechanism involving NOS uncoupling. Studies using eNOS knockout mice have demonstrated a role for eNOS in this pathology [84]. However, neuronal NOS (nNOS) is highly expressed in retinal cells and could also be involved. Further study is needed to address the impact of A2 on nNOS function.
Diabetic retinopathy
Studies in models of retinal inflammation and diabetes have demonstrated a link between elevation of arginase activity and oxidative stress, inflammation and decreased NO bioavailability [26, 64, 65]. Mice with endotoxin-induced acute retinal inflammation had increases in arginase activity and A1 expression that were accompanied by increased formation of superoxide, upregulation of inflammatory genes and leukocyte attachment to the retinal vessels. These alterations were prevented in double knockout mice lacking one copy of A1 and both copies of A2. Furthermore the endotoxin-induced increase in A1 expression was blunted by NADPH oxidase blockade or NOX2 deletion, suggesting that endotoxin induced retinal inflammation is mediated by NOX2-dependent increases in arginase. Studies in streptozotocin-induced diabetic mice and high glucose-treated retinal endothelial cells showed similar associations [64]. Moreover, the diabetes-induced increases in oxidative stress and retinal inflammation were associated with a significant drop in bioavailable NO. Further studies using a high-resolution fundus microscope system to image the primary branches of the central retinal artery (CRA) in living mice demonstrated involvement of arginase in diabetes-induced impairment of endothelium-dependent vasodilation because the defect was largely absent in mice lacking one copy of the A1 gene [26]. Additionally, treatment of the mice with a specific arginase inhibitor prevented diabetes-induced endothelial dysfunction. Similar beneficial effects of inhibiting arginase were observed in ex vivo experiments using a pressure myograph system to measure endothelial dependent vasorelaxation in CRAs isolated from diabetic rats and pretreated with an arginase inhibitor [26]. Taken together these results indicate a prominent role for arginase in diabetes- and endotoxin-induced increases in oxidative stress and inflammation as well as in diabetes-induced impairment of retinal endothelial-dependent vasodilation responses.
Arginase inhibitors
The first arginase inhibitors to be developed had non-specific actions and many side effects because of the high concentrations required [8]. For example, norvaline is a substrate for amidotransferases [88]. Similarly, while Nω-hydroxy-L-arginine (NOHA) is a potent inhibitor for arginase, it is also an intermediate precursor in the production NO from L-arginine by NOS. An analog, Nor-NOHA is also a potent arginase inhibitor, but has a much longer half-life than NOHA. Neither inhibits NOS. α-Difluoromethylornithine (DFMO) is a non-specific weak inhibitor of arginase, but is a potent inhibitor for ornithine decarboxylase [8]. Thus, the effects of DFMO in increasing NO production in models of elevated arginase activity are likely due to increased accumulation of ornithine which is known to inhibit arginase [89]. Besides producing urea, arginase is also involved in synthesis of polyamines and amino acids such as ornithine, proline and glutamate. In fact, ornithine, leucine, valine, lysine, isoleucine and nor-valine inhibit arginase with ornithine being the most potent [90]. Also, L-citrulline is an allosteric inhibitor of arginase [91], but it also increases NO formation.
Recently, competitive inhibitors of arginase have been developed which have greater specificity for the enzyme. Initial development of these compounds involved determination of the crystal structure of arginase. Christianson and colleagues found that a binuclear manganese cluster was required for catalytic activity [92]. They also identified the full structures of human A1 and A2 [93]. Boronic acid analogs of L-arginine [S-(2-boronoethyl)-L-cysteine (BEC) and 2(S)-amino-6-boronohexanoic acid (ABH)] are highly selective arginase inhibitors. Both contain a boronic acid or N-hydroxyguanidinium head which binds the manganese cluster, the active catalytic site in arginase [49]. Several inhibitors of arginase are commercially available, including nor-NOHA, BEC and ABH.
Although several specific arginase inhibitors have been developed, none of those currently available are isoform selective. This is a significant limitation of the field in light of the growing evidence that arginase activity can be damaging in some contexts and protective in others. For example, activity of A2-positive macrophages has been implicated in the development and progression of atherosclerosis, whereas A1-positive macrophages may promote plaque resolution [33–36, 94]. A1-positive macrophages are clearly involved in repair functions after tissue injury, whereas excessive A2 activity appears to be damaging in a number of CNS disease/injury conditions. In the absence of isoform selective inhibitors, RNA interference has been used to specifically inhibit expression of A1 or 2. For example, administration of short hairpin RNA (shRNA) against A1 greatly reduced IL-13 induced airway hyper-responsiveness with a concomitant decrease in A1 mRNA and protein levels in the lungs of mice [95]. Direct delivery of anti-sense A1 using adeno-virus vector in corpus cavernosum improved erectile function in aged mice [96]. Anti-sense A1 also increased NO production in endothelial cells exposed to high glucose [12]. The search for more potent and isoform selective inhibitors is ongoing and involves structure-based design and plant extracts [38].
So far studies using arginase inhibitors in humans with cardiovascular disease has been limited to small-scale clinical “proof-of-concept” studies involving local administration of arginase inhibitors by cutaneous microdialysis or intra-arterial infusion. However, promising results have been reported in patients with coronary artery disease and type 2 diabetes [30, 97], heart failure [98], hypertension [99] and following resuscitation after cardiac arrest [100]. These observations suggest that inhibiting arginase activity could be beneficial in the treatment of cardiovascular disease. Systemic treatment with arginase inhibitors is used in the treatment of parasitic disease without significant adverse effects. However further study is needed to demonstrate the safety of long-term treatment in patients with cardiovascular disease. Given the important role that A1 plays in detoxification of ammonia in the urea cycle, it will be important to make sure that the treatment does not suppress hepatic arginase activity enough to adversely impact the urea cycle. Another potential limitation is that suppression of A1 may further limit tissue repair function which is already suppressed in many cardiovascular diseases.
Concluding remarks
The role of excessive expression and activity of arginase and its downstream targets in cardiovascular dysfunction and injury has been well established by studies in both animal models and human disease conditions. Studies also have clearly demonstrated the involvement of these pathways in CNS disease and injury. Targeting specific components of the arginase/ornithine pathway holds great promise as a therapy for both cardiovascular and CNS diseases. Recent proof-of-concept studies in cardiovascular disease patients have shown beneficial effects of local delivery of arginase inhibtors. As has been noted above, the two arginase isoforms share 100% homology in areas critical for enzyme function. Moreover, both have been shown to be fundamentally involved in dysregulation of NOS function. However the two isoforms are encoded by different genes, localized to different intracellular compartments, expressed in different cell types and tissues and involved in different cellular functions. Analyses using knockout mice and specific gene knockdown have been informative about the differential involvement of increases in A1 and A2 in various disease conditions. However, there is also considerable evidence to support a positive impact of arginase on cell growth, collagen synthesis and neuronal development during physiological conditions as well as tissue repair following injury. Thus, there is some risk associated with global inhibition of arginase activity. Studies are beginning to examine the subcellular and molecular regulation of arginase activity and this information should facilitate design of new approaches to specifically limit its pathological effects. However, there are many questions that need to be answered to better understand the specific role of the two arginase isoforms and their downstream targets in both health and disease and to identify better therapies (Box 3).
Box 3. Outstanding Questions.
For the future of targeting arginase activity as therapy for cardiovascular and neurovascular disease, some important questions and areas for further study include:
What are the mechanistic differences between the functions of A1 and A2? Both enzymes metabolize L-arginine to produce ornithine and urea, yet their intracellular localization and tissue distribution are very different. Is their function in producing downstream products of ornithine metabolism different? While, these questions can be addressed using genetic strategies for deletion/inactivation of each isoform, development of isoform-specific inhibitors would greatly facilitate research in this area.
What are the specific roles of A1 and A2 in macrophage inflammatory responses? There is strong support for the healing role of macrophage expression of A1 in the repair phase of wound healing, but recent work has implicated macrophage expression of A2 in chronic inflammatory disease conditions [113]. Further study is required to define the specific roles of A1 and A2 in macrophage inflammatory responses and elucidate the underlying mechanisms.
Are arginase activities of A1 regulated at the post-translational level as has been reported for A2? A2 in SMCs has been found to increase its activity upon translocation from the mitochondria into the cytosol [34]. Does A1 activity depend on its cytosolic localization? Is activity of either isoform modified by phosphorylation events?
Is ureahydrolase enzyme activity required for arginase functions? Experiments using a catalytically inactive A2 mutant in models of atherosclerosis found that A2 induces SMC senescence and apoptosis independently of its L-arginine ureahydrolase activity [53]. Does this phenomenon occur in other cell types and disease models?
Inhibitors and blockers of the arginase/ornithine pathway are already in use clinically for treatment of patients with parasitic infections and some cancers. Can these agents be used effectively for treatment of patients with cardiovascular or neurovascular disease?
Can circulating levels of arginase and/or its metabolic products serve as useful biomarkers for development or progression of disease?
Highlights.
Arginase is the enzyme that converts L-arginine to urea and L-ornithine, functions important for protection against NH3 toxicity and cell growth and repair
Excessive arginase activity has been linked to cardiovascular diseases due to actions in reducing the supply of L-arginine needed by nitric oxide (NO) synthase to produce NO
Excessive activity of the arginase/ornithine pathway can also contribute to vascular structural problems and neural toxicity.
Recent research has identified inflammatory agents and reactive oxygen species as drivers of the pathologic elevation of arginase activity and expression.
Acknowledgements
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, office of Research and Development and by research grants from the Vision Discovery Institute at Georgia Regents University (RBC), VA Research Career Scientist Award (RBC), VA Merit Review Award (RBC); PHS grants EY011766 (RBC & RWC), HL070215 (RWC), AHA (HAT, SPN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
RBC, HAT, SPN, RWC prepared and edited the manuscript. HAT and SPN prepared the figures.
None of the authors have any competing interests.
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work, no relationships or activities that could appear to have influenced the submitted work.
No ethical or Institutional Review Board (IRB) approval was required for this study.
Contributor Information
Ruth B. Caldwell, Email: rcaldwel@gru.edu.
Haroldo A. Toque, Email: hflorestoque@gru.edu.
S. Priya Narayanan, Email: pnarayanan@gru.edu.
R. William Caldwell, Email: wcaldwel@gru.edu.
References
- 1.Dzik JM. Evolutionary roots of arginase expression and regulation. Front Immunol. 2014;5:544. doi: 10.3389/fimmu.2014.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dizikes GJ, et al. Isolation of human liver arginase cDNA and demonstration of nonhomology between the two human arginase genes. Biochem Biophys Res Commun. 1986;141:53–59. doi: 10.1016/s0006-291x(86)80333-3. [DOI] [PubMed] [Google Scholar]
- 3.Gotoh T, et al. Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett. 1996;395:119–122. doi: 10.1016/0014-5793(96)01015-0. [DOI] [PubMed] [Google Scholar]
- 4.Vockley JG, et al. Cloning and characterization of the human type II arginase gene. Genomics. 1996;38:118–123. doi: 10.1006/geno.1996.0606. [DOI] [PubMed] [Google Scholar]
- 5.Ash DE. Structure and function of arginases. J Nutr. 2004;134:2760S–2764S. doi: 10.1093/jn/134.10.2760S. discussion 2765S–2767S. [DOI] [PubMed] [Google Scholar]
- 6.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegg AE. The function of spermine. IUBMB Life. 2014;66:8–18. doi: 10.1002/iub.1237. [DOI] [PubMed] [Google Scholar]
- 8.Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas R, et al. Arginase in the vascular endothelium: friend or foe? Front Immunol. 2014;5:589. doi: 10.3389/fimmu.2014.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popovic PJ, et al. Arginine and immunity. J Nutr. 2007;137:1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, et al. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci U S A. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero MJ, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White AR, et al. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 14.Katusic ZS, et al. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koshland DE., Jr The molecule of the year. Science. 1992;258:1861. doi: 10.1126/science.1470903. [DOI] [PubMed] [Google Scholar]
- 16.Toque HA, Caldwell RW. New approaches to the design and discovery of therapies to prevent erectile dysfunction. Expert Opin Drug Discov. 2014;9:1447–1469. doi: 10.1517/17460441.2014.949234. [DOI] [PubMed] [Google Scholar]
- 17.Cho WK, et al. IL-13 receptor alpha2-arginase 2 pathway mediates IL-13-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013;304:L112–L124. doi: 10.1152/ajplung.00101.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B, et al. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1151–1159. doi: 10.1152/ajplung.00183.2009. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, et al. Mice deficient in Mkp-1 develop more severe pulmonary hypertension and greater lung protein levels of arginase in response to chronic hypoxia. Am J Physiol Heart Circ Physiol. 2010;298:H1518–H1528. doi: 10.1152/ajpheart.00813.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu W, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. Faseb J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 21.Watts JA, et al. Arginase depletes plasma l-arginine and decreases pulmonary vascular reserve during experimental pulmonary embolism. Pulm Pharmacol Ther. 2012;25:48–54. doi: 10.1016/j.pupt.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Pieper GM, Dondlinger LA. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J Pharmacol Exp Ther. 1997;283:684–691. [PubMed] [Google Scholar]
- 23.Hagenfeldt L, et al. Plasma amino acids in relation to metabolic control in insulin-dependent diabetic children. Acta Paediatr Scand. 1989;78:278–282. doi: 10.1111/j.1651-2227.1989.tb11070.x. [DOI] [PubMed] [Google Scholar]
- 24.Romero MJ, et al. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol. 2012;302:H159–H166. doi: 10.1152/ajpheart.00774.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tawfik HE, et al. Simvastatin improves diabetes-induced coronary endothelial dysfunction. J Pharmacol Exp Ther. 2006;319:386–395. doi: 10.1124/jpet.106.106823. [DOI] [PubMed] [Google Scholar]
- 26.Elms SC, et al. The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes. Diabetologia. 2013;56:654–662. doi: 10.1007/s00125-012-2789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagi Z, et al. Selective up-regulation of arginase-1 in coronary arteries of diabetic patients. Front Immunol. 2013;4:293. doi: 10.3389/fimmu.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beleznai T, et al. Arginase 1 contributes to diminished coronary arteriolar dilation in patients with diabetes. Am J Physiol Heart Circ Physiol. 2011;300:H777–H783. doi: 10.1152/ajpheart.00831.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronros J, et al. Arginase inhibition restores in vivo coronary microvascular function in type 2 diabetic rats. Am J Physiol Heart Circ Physiol. 2011;300:H1174–H1181. doi: 10.1152/ajpheart.00560.2010. [DOI] [PubMed] [Google Scholar]
- 30.Shemyakin A, et al. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation. 2012;126:2943–2950. doi: 10.1161/CIRCULATIONAHA.112.140335. [DOI] [PubMed] [Google Scholar]
- 31.Ryoo S, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 32.Ming XF, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 33.Ryoo S, et al. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214:279–287. doi: 10.1016/j.atherosclerosis.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey D, et al. OxLDL triggers retrograde translocation of arginase2 in aortic endothelial cells via ROCK and mitochondrial processing peptidase. Circ Res. 2014;115:450–459. doi: 10.1161/CIRCRESAHA.115.304262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khallou-Laschet J, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feig JE, et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98:334–343. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 38.Steppan J, et al. Development of novel arginase inhibitors for therapy of endothelial dysfunction. Front Immunol. 2013;4:278. doi: 10.3389/fimmu.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneemann M, Schoeden G. Macrophage biology and immunology: man is not a mouse. J Leukoc Biol. 2007;81:579. doi: 10.1189/jlb.1106702. discussion 580. [DOI] [PubMed] [Google Scholar]
- 40.Mills CD, et al. Macrophage: SHIP of Immunity. Front Immunol. 2014;5:620. doi: 10.3389/fimmu.2014.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas AC, Mattila JT. "Of mice and men": arginine metabolism in macrophages. Front Immunol. 2014;5:479. doi: 10.3389/fimmu.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung C, et al. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res. 2010;85:147–154. doi: 10.1093/cvr/cvp303. [DOI] [PubMed] [Google Scholar]
- 43.Gonon AT, et al. Local arginase inhibition during early reperfusion mediates cardioprotection via increased nitric oxide production. PLoS One. 2012;7:e42038. doi: 10.1371/journal.pone.0042038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gronros J, et al. Arginase inhibition improves coronary microvascular function and reduces infarct size following ischaemia-reperfusion in a rat model. Acta Physiol (Oxf) 2013;208:172–179. doi: 10.1111/apha.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tratsiakovich Y, et al. Arginase as a target for treatment of myocardial ischemia-reperfusion injury. Eur J Pharmacol. 2013;720:121–123. doi: 10.1016/j.ejphar.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Shatanawi A, et al. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol. 2011;300:C1181–C1192. doi: 10.1152/ajpcell.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandra S, et al. Oxidative Species Increase Arginase Activity in Endothelial Cells through RhoA/Rho Kinase Pathway. Br J Pharmacol. 2012;165(2):506–519. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, et al. Arginase inhibition enhances angiogenesis in endothelial cells exposed to hypoxia. Microvasc Res. 2014;98C:1–8. doi: 10.1016/j.mvr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berkowitz DE, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scalera F, et al. Paradoxical effect of L-arginine: acceleration of endothelial cell senescence. Biochem Biophys Res Commun. 2009;386:650–655. doi: 10.1016/j.bbrc.2009.06.091. [DOI] [PubMed] [Google Scholar]
- 52.Yepuri G, et al. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. 2012;11:1005–1016. doi: 10.1111/acel.12001. [DOI] [PubMed] [Google Scholar]
- 53.Xiong Y, et al. Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its l-arginine ureahydrolase activity: implications for atherosclerotic plaque vulnerability. J Am Heart Assoc. 2013;2:e000096. doi: 10.1161/JAHA.113.000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–395. doi: 10.1016/j.ucl.2005.08.007. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toda N, et al. Nitric oxide and penile erectile function. Pharmacol Ther. 2005;106:233–266. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Bivalacqua TJ, et al. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 57.Cox JD, et al. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat Struct Biol. 1999;6:1043–1047. doi: 10.1038/14929. [DOI] [PubMed] [Google Scholar]
- 58.Fraga-Silva RA, et al. An increased arginase activity is associated with corpus cavernosum impairment induced by hypercholesterolemia. J Sex Med. 2014;11:1173–1181. doi: 10.1111/jsm.12482. [DOI] [PubMed] [Google Scholar]
- 59.Segal R, et al. Chronic oral administration of the arginase inhibitor 2(S)-amino-6-boronohexanoic acid (ABH) improves erectile function in aged rats. J Androl. 2012;33:1169–1175. doi: 10.2164/jandrol.111.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toque HA, et al. Arginase II deletion increases corpora cavernosa relaxation in diabetic mice. J Sex Med. 2011;8:722–733. doi: 10.1111/j.1743-6109.2010.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma TC, et al. A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J Neurosci. 2010;30:739–748. doi: 10.1523/JNEUROSCI.5266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng K, et al. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J Neurosci. 2009;29:9545–9552. doi: 10.1523/JNEUROSCI.1175-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters D, et al. Arginase and Arginine Decarboxylase - Where Do the Putative Gate Keepers of Polyamine Synthesis Reside in Rat Brain? PLoS One. 2013;8:e66735. doi: 10.1371/journal.pone.0066735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel C, et al. Arginase as a mediator of diabetic retinopathy. Front Immunol. 2013;4:173. doi: 10.3389/fimmu.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W, et al. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol. 2009;175:891–902. doi: 10.2353/ajpath.2009.081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narayanan SP, et al. Arginase 2 deletion reduces neuro-glial injury and improves retinal function in a model of retinopathy of prematurity. PLoS One. 2011;6:e22460. doi: 10.1371/journal.pone.0022460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narayanan SP, et al. Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism. Cell Death Dis. 2014;5:e1075. doi: 10.1038/cddis.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toda N, et al. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61:62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- 69.Garry PS, et al. The role of the nitric oxide pathway in brain injury and its treatment - From bench to bedside. Exp Neurol. 2015;263C:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Quirie A, et al. Effect of stroke on arginase expression and localization in the rat brain. Eur J Neurosci. 2013;37:1193–1202. doi: 10.1111/ejn.12111. [DOI] [PubMed] [Google Scholar]
- 71.Colton CA, et al. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansmannel F, et al. Is the urea cycle involved in Alzheimer's disease? J Alzheimers Dis. 2010;21:1013–1021. doi: 10.3233/JAD-2010-100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu P, et al. Altered arginine metabolism in Alzheimer's disease brains. Neurobiol Aging. 2014;35:1992–2003. doi: 10.1016/j.neurobiolaging.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Cherian L, et al. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cherian L, Robertson CS. L-arginine and free radical scavengers increase cerebral blood flow and brain tissue nitric oxide concentrations after controlled cortical impact injury in rats. J Neurotrauma. 2003;20:77–85. doi: 10.1089/08977150360517209. [DOI] [PubMed] [Google Scholar]
- 76.Bitner BR, et al. Impact of arginase II on CBF in experimental cortical impact injury in mice using MRI. J Cereb Blood Flow Metab. 2010;30:1105–1109. doi: 10.1038/jcbfm.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zahedi K, et al. Polyamine catabolism is enhanced after traumatic brain injury. J Neurotrauma. 2010;27:515–525. doi: 10.1089/neu.2009.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosi S, et al. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PLoS One. 2012;7:e31094. doi: 10.1371/journal.pone.0031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walter SD, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakai RE, et al. Vision in multiple sclerosis: the story, structure-function correlations, and models for neuroprotection. J Neuroophthalmol. 2011;31:362–373. doi: 10.1097/WNO.0b013e318238937f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ljubisavljevic S, et al. Modulation of nitric oxide synthase by arginase and methylated arginines during the acute phase of experimental multiple sclerosis. J Neurol Sci. 2012;318:106–111. doi: 10.1016/j.jns.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Ljubisavljevic S, et al. The importance of nitric oxide and arginase in the pathogenesis of acute neuroinflammation: are those contra players with the same direction? Neurotox Res. 2014;26:392–399. doi: 10.1007/s12640-014-9470-3. [DOI] [PubMed] [Google Scholar]
- 83.Stevenson L, et al. Reduced nitro-oxidative stress and neural cell death suggests a protective role for microglial cells in TNFalpha−/− mice in ischemic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:3291–3299. doi: 10.1167/iovs.09-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brooks SE, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228. [PubMed] [Google Scholar]
- 85.Gu X, et al. Effects of sustained hyperoxia on revascularization in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2002;43:496–502. [PubMed] [Google Scholar]
- 86.Sennlaub F, et al. Inducible nitric oxide synthase mediates the change from retinal to vitreal neovascularization in ischemic retinopathy. J Clin Invest. 2001;107:717–725. doi: 10.1172/JCI10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suwanpradid J, et al. Arginase 2 deficiency prevents oxidative stress and limits hyperoxia-induced retinal vascular degeneration. PLoS One. 2014;9:e110604. doi: 10.1371/journal.pone.0110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davoodi J, et al. Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases. J Biol Chem. 1998;273:4982–4989. doi: 10.1074/jbc.273.9.4982. [DOI] [PubMed] [Google Scholar]
- 89.Huynh NN, et al. The vascular effects of different arginase inhibitors in rat isolated aorta and mesenteric arteries. Br J Pharmacol. 2009;156:84–93. doi: 10.1111/j.1476-5381.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hunter A, Downs D. The inhibition of arginase by amino acids. J Biol Chem. 1945;157:427–446. [Google Scholar]
- 91.Shearer JD, et al. Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am J Physiol. 1997;272:E181–E190. doi: 10.1152/ajpendo.1997.272.2.E181. [DOI] [PubMed] [Google Scholar]
- 92.Kanyo ZF, et al. Structure of a unique binuclear manganese cluster in arginase. Nature. 1996;383:554–557. doi: 10.1038/383554a0. [DOI] [PubMed] [Google Scholar]
- 93.Di Costanzo L, et al. Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response. Proc Natl Acad Sci U S A. 2005;102:13058–13063. doi: 10.1073/pnas.0504027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ryoo S, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 95.Yang M, et al. Inhibition of arginase I activity by RNA interference attenuates IL-13-induced airways hyperresponsiveness. J Immunol. 2006;177:5595–5603. doi: 10.4049/jimmunol.177.8.5595. [DOI] [PubMed] [Google Scholar]
- 96.Bivalacqua TJ, et al. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340–H1351. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 97.Kovamees O, et al. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PLoS One. 2014;9:e103260. doi: 10.1371/journal.pone.0103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quitter F, et al. Increased arginase levels in heart failure represent a therapeutic target to rescue microvascular perfusion. Clin Hemorheol Microcirc. 2013;54:75–85. doi: 10.3233/CH-2012-1617. [DOI] [PubMed] [Google Scholar]
- 99.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–872. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung C, et al. Increased arginase levels contribute to impaired perfusion after cardiopulmonary resuscitation. Eur J Clin Invest. 2014;44:965–971. doi: 10.1111/eci.12330. [DOI] [PubMed] [Google Scholar]
- 101.Satriano J. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids. 2004;26:321–329. doi: 10.1007/s00726-004-0078-4. [DOI] [PubMed] [Google Scholar]
- 102.Lange PS, et al. Novel roles for arginase in cell survival, regeneration, and translation in the central nervous system. J Nutr. 2004;134:2812S–2817S. doi: 10.1093/jn/134.10.2812S. discussion 2818S–2819S. [DOI] [PubMed] [Google Scholar]
- 103.Witte MB, Barbul A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003;11:419–423. doi: 10.1046/j.1524-475x.2003.11605.x. [DOI] [PubMed] [Google Scholar]
- 104.Osowska S, et al. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut. 2004;53:1781–1786. doi: 10.1136/gut.2004.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deignan JL, et al. Contrasting features of urea cycle disorders in human patients and knockout mouse models. Mol Genet Metab. 2008;93:7–14. doi: 10.1016/j.ymgme.2007.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huynh NN, et al. Arginase II knockout mouse displays a hypertensive phenotype despite a decreased vasoconstrictory profile. Hypertension. 2009;54:294–301. doi: 10.1161/HYPERTENSIONAHA.108.121731. [DOI] [PubMed] [Google Scholar]
- 107.Kampfer H, et al. Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair. J Invest Dermatol. 2003;121:1544–1551. doi: 10.1046/j.1523-1747.2003.12610.x. [DOI] [PubMed] [Google Scholar]
- 108.Esch F, et al. Purification of a multipotent antideath activity from bovine liver and its identification as arginase: nitric oxide-independent inhibition of neuronal apoptosis. J Neurosci. 1998;18:4083–4095. doi: 10.1523/JNEUROSCI.18-11-04083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Filbin MT. The Muddle with MAG. Mol Cell Neurosci. 1996;8:84–92. doi: 10.1006/mcne.1996.0047. [DOI] [PubMed] [Google Scholar]
- 110.Kuo HS, et al. The combination of peripheral nerve grafts and acidic fibroblast growth factor enhances arginase I and polyamine spermine expression in transected rat spinal cords. Biochem Biophys Res Commun. 2007;357:1–7. doi: 10.1016/j.bbrc.2007.02.167. [DOI] [PubMed] [Google Scholar]
- 111.Narayanan SP, et al. Arginase in retinopathy. Prog Retin Eye Res. 2013;36:260–280. doi: 10.1016/j.preteyeres.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Durante W. Role of arginase in vessel wall remodeling. Front Immunol. 2013;4:111. doi: 10.3389/fimmu.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z, Ming XF. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol. 2014;5:533. doi: 10.3389/fimmu.2014.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hein TW, et al. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. Faseb J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 115.Johnson FK, et al. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- 116.Zhang C, et al. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]
- 117.Wood PL, et al. Neurotoxicity of reactive aldehydes: the concept of "aldehyde load" as demonstrated by neuroprotection with hydroxylamines. Brain Res. 2006;1095:190–199. doi: 10.1016/j.brainres.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 118.Takano K, et al. Neuronal and glial responses to polyamines in the ischemic brain. Curr Neurovasc Res. 2005;2:213–223. doi: 10.2174/1567202054368335. [DOI] [PubMed] [Google Scholar]
- 119.Ivanova S, et al. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc Natl Acad Sci U S A. 2002;99:5579–5584. doi: 10.1073/pnas.082609299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seiler N. Oxidation of polyamines and brain injury. Neurochem Res. 2000;25:471–490. doi: 10.1023/a:1007508008731. [DOI] [PubMed] [Google Scholar]
- 121.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 123.Bryan NS, et al. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci (Landmark Ed) 2009;14:1–18. doi: 10.2741/3228. [DOI] [PubMed] [Google Scholar]
- 124.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McDonald KK, et al. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the "arginine paradox". J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 126.De Caterina R, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. The Journal of clinical investigation. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kubes P, et al. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Radomski MW, et al. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 129.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci Rep. 1999;19:133–154. doi: 10.1023/a:1020265417394. [DOI] [PubMed] [Google Scholar]
- 130.Pacher P, et al. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]