Abstract
AMD3100 (plerixafor), is a specific CXCR4 antagonist approved by the FDA for mobilizing hematopoietic stem cells from bone marrow to blood for transplantation in cancer. AMD3100 also mobilizes most mature leukocyte subsets to blood; however, their source and trafficking potential have not been fully delineated. Here, we show that a single injection of AMD3100 10 mg/kg into C57Bl/6 mice rapidly mobilizes (peak ~ 2.5 hours) the same leukocyte subsets to blood as in humans. Using this model, we found that AMD3100 mobilization of neutrophils, lymphocytes and monocytes to blood isn’t reduced by splenectomy or by blockade of lymphocyte egress from lymph node with FTY720, but is coupled to i) reduced content of each of these cell types in the bone marrow; ii) reduced T-cell numbers in thymuses; iii) increased lymphocytes in lymph nodes; and iv) increased neutrophil and monocyte content in the lung. Direct intrathymic labeling showed that AMD3100 selectively mobilizes naïve thymic CD4+ and CD8+ T cells to blood. Finally, AMD3100-induced neutrophil mobilization to blood did not reduce neutrophil trafficking to thioglycollate-inflamed peritoneum. Thus, AMD3100 redistributes lymphocytes, monocytes and neutrophils from primary immune organs to secondary immune organs, peripheral tissues and blood, without compromising neutrophil trafficking to inflamed sites.
Keywords: AMD3100, mouse, CXCR4, lymphoid organs, leukocytes, cell trafficking
Introduction
AMD3100 (Mozobil, plerixafor) is a specific small molecule antagonist at CXCR4, a homeostatic chemokine receptor expressed on hematopoietic stem cells (HSC) [1] and most mature leukocytes [2, 3], as well as on many non-hematopoietic cell types [4–7]. CXCR4 signaling directly promotes HSC retention in bone marrow niches, whereas, conversely, blockade of CXCR4 by AMD3100 promotes HSC egress to blood [8]. As a result, AMD3100 has been approved by the FDA in conjunction with G-CSF to mobilize HSCs to peripheral blood for certain cancer patients requiring bone marrow transplantation [9]. In the immune system, CXCR4 regulates mature lymphocyte trafficking within secondary lymphoid tissue [2, 10], controls the blood distribution of the myeloid and lymphoid lineages by regulating homing to and egress from bone marrow [8, 9, 11–13], and causes retention of mature neutrophils in bone marrow [14]. Consistent with this, AMD3100 rapidly mobilizes most leukocyte subsets to blood in healthy subjects [15]. Moreover, in patients with WHIM syndrome, a primary immunodeficiency disease characterized by panleukopenia and myelokathexis (retention of neutrophils in bone marrow) that is caused by hyperfunctional CXCR4 mutations [16], low dose plerixafor was able to durably increase blood levels of lymphocytes, monocytes and neutrophils while reducing infection frequency and wart burden [17]. Despite these advances, the sources of cells mobilized to blood by AMD3100 have remained controversial, in part due to limitations related to the specific cell type and organ studied as well as differences in methodology [10, 14, 18–20]. In particular, Devi et al have challenged the dogma that AMD3100 mobilizes neutrophils from bone marrow [18]. Instead, using direct imaging techniques, they found that the neutrophilia induced by AMD3100 is due to neutrophil mobilization from the marginated pool in lung and not from bone marrow. Blockade of neutrophil homing to bone marrow from blood was also found to contribute to AMD3100-induced neutrophilia.
Here we present an expanded systematic investigation of the distribution of leukocytes after high dose AMD3100 injection in a mouse model, including lymph node, spleen, thymus, lung, peritoneal cavity and bone marrow, as well as neutrophils, monocytes and the major subsets of lymphocytes. We have also tested the trafficking potential of AMD3100-mobilized neutrophils under an inflammatory condition in vivo.
Results
AMD3100 redistributes leukocyte subsets to blood in mice
AMD3100 has been reported to mobilize leukocytes to blood in mice [21], healthy humans [15] and patients with WHIM syndrome [22]; however, the source(s) of the mobilized cells have not been fully defined systemically and simultaneously. To address this, we have analyzed mice, which like humans respond rapidly to AMD3100 with a peak WBC increase in the blood occurring at ~2.5 hours post-injection [23]. As shown in Table 1, compared with PBS, AMD3100 markedly increased the peak absolute number of all leukocyte subsets, including absolute neutrophil counts (ANC); absolute lymphocyte counts (ALC); B220+ B cells (CD27− non-memory > CD27+ memory); CD3+ T cells (CD8+ > CD4+) including CD44low/neg naïve T cells and CD44high memory T cells (memory T cells > naïve T cells); and absolute monocyte counts (AMC), including CD11b+Ly6Chi inflammatory and CD11b+ Ly6Clow resident monocytes [24]. These results conform to previous reports in humans [22], suggesting that mice can faithfully model humans with regard to the hematologic response to AMD3100 in the blood.
Table I.
AMD3100 mobilizes most leukocyte subsets to blood in mice.
| Cell Type | Treatment | |||
|---|---|---|---|---|
| PBS (Mean±SEM) | AMD3100 (Mean±SEM) | Fold Increase | P value | |
| WBCa) | 1700±151 | 8100±1022 | 4.8 | .0001 |
| ANC | 256±96 | 2313±294 | 9.0 | < .0001 |
| ALC | 1154±141 | 5210±460 | 4.5 | < .0001 |
| AMC | 158±22 | 556±87 | 3.5 | .0012 |
| CD3+ T cells | 406±36 | 1522±138 | 3.7 | < .0001 |
| CD3+ CD44low/neg cells | 373±33 | 1275±93 | 3.4 | < .0001 |
| CD3+ CD44high cells | 33±11 | 272±55 | 8.2 | .0012 |
| CD4+ T cells | 222±19 | 605±55 | 2.7 | < .0001 |
| CD4+CD44low/neg T cells | 202±17 | 515±40 | 2.5 | < .0001 |
| CD4+CD44high T cells | 13±4 | 91±17 | 7 | .0011 |
| CD8+ T cells | 136±15 | 718±104 | 5.3 | .0002 |
| CD8+CD44low/neg T cells | 130±14 | 620±76 | 4.8 | < .0001 |
| CD8+CD44high T cells | 5±1 | 88±19 | 17.6 | .0006 |
| B220+ B cells | 278±53 | 1546±245 | 5.6 | .0008 |
| B220+CD27+ Memory | 72±22 | 218±54 | 3 | .0453 |
| B220+CD27− | 251±27 | 1329±195 | 5.3 | .0016 |
| CD11b+Ly6Chigh Inflammory Monocytes | 30±10 | 159 ±28 | 5.3 | .0016 |
| CD11b+Ly6Clow Resident Monocytes | 129±14 | 397±69 | 3 | .0034 |
The numbers of cells/μl blood in 5–6 mice 2.5 hours after treatment are shown.
SEM, standard error of the mean; CD, cluster of differentiation; ANC, absolute neutrophil count; AMC, absolute monocyte count; ALC, absolute lymphocyte count.
The spleen is dispensable for AMD3100-induced leukocyte redistribution
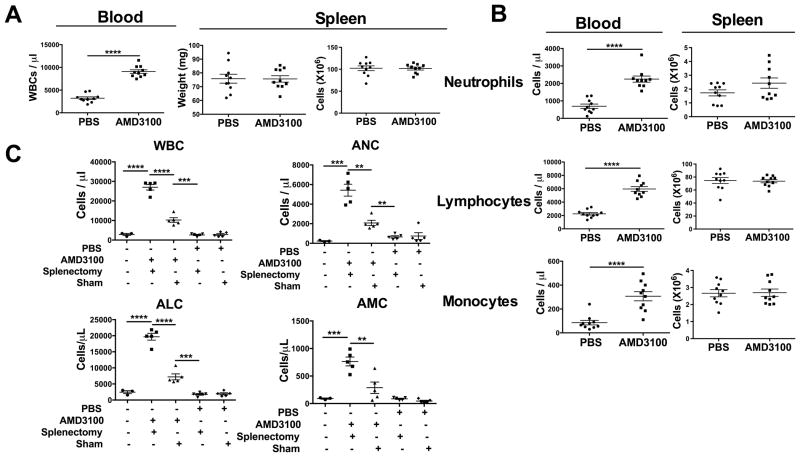
The spleen is composed of white pulp (mainly T cells and B cells) and red pulp, a reservoir for monocytes [25] and a major clearance site for neutrophils [26]. To test whether spleen might be a source for leukocytes redistributed by AMD3100, first we checked the total WBC count in the blood in splenectomized and sham-operated mice before AMD3100 treatment and found no difference (data not shown). Then, we compared three parameters in unoperated PBS- and AMD3100-treated mice: 1) peak WBC count in the blood; 2) spleen weight; and 3) total spleen leukocyte content. As shown in Fig. 1, although the WBC was significantly increased in blood from AMD3100-treated mice compared to PBS-treated mice, there was no difference between the groups in spleen weight, or spleen content of total leukocytes, neutrophils, lymphocytes or monocytes (Fig. 1A and B). Splenectomy did not affect baseline WBC and differential in the blood and did not reduce leukocyte redistribution in the blood in response to AMD3100 (Fig. 1C). Instead, it unexpectedly potentiated AMD3100-induced leukocyte redistribution in blood when compared to the sham-operated mice given AMD3100.
Figure 1. Splenectomy enhances mobilization of neutrophils, monocytes and lymphocytes to the blood in response to AMD3100.
(A, B) WT mice (n=4–5/group) were injected with PBS or AMD3100, then bled 2.5 h later for determination of (A) total WBC left and (B) differential leukocyte distribution (left) on stained blood smears. Mice were sacrificed 2.5 h p.i. and spleens were harvested for determination of weight (A, middle), total leukocyte content (A, right) and leukocyte subset content (B, right). Data were combined from 2 independent experiments, plotted for individual mice and summarized as the mean ± SEM. (C) WT mice (n=3–5 mice/group) received splenectomy or sham surgery. Four weeks later mice were injected with PBS or AMD3100, then bled 2.5 h later for enumeration of whole blood cells (WBC), absolute neutrophil counts (ANC), absolute leukocyte counts (ALC) and absolute monocyte counts (AMC). Contemporary untreated and unoperated mice were included as a control group. Results are from a single experiment representative of two independent experiments. Data were plotted for individual mice and summarized as the mean ± SEM. **p<0.01, ***p<0.005, ****p<0.0001, two-tailed unpaired parametric t-test
The lymph node is not a source for AMD3100-induced lymphocyte redistribution
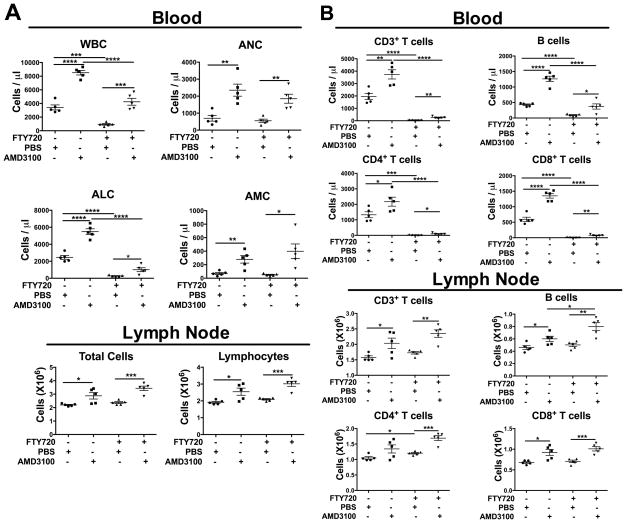
Lymph nodes contain CXCR4+ T and B lymphocytes [21]. Thus, we studied the effect of AMD3100 on the distribution of these cells in lymph node. After AMD3100 treatment, total lymphocytes, B220+ B cells and CD3+ T cells (including CD4+ and CD8+ subsets) were increased in both blood (data not shown) and lymph node (Fig. 2). To interrogate this further, we used the S1P1 agonist FTY720 to block lymphocyte egress from lymph nodes. In control groups, AMD3100 markedly increased the ANC, ALC and AMC in the circulation, whereas FTY720 reduced the WBC and ALC, as expected, without affecting the ANC or AMC [27]. AMD3100 plus FTY720 treatment markedly elevated blood ANC and AMC to the levels seen in mice given AMD3100 alone (Fig. 3A). Blood ALC was also significantly increased, but to levels markedly lower than those in mice given AMD3100 alone (Fig. 3A). Thus, AMD3100 partially reversed FTY720-mediated lymphocyte retention in secondary lymphoid organs, possibly due to the finding that FTY720–induced T cell homing partly depends on CXCR4 [28]. In lymph node, the increase of leukocytes induced by AMD3100 was not affected by FTY720 treatment (Fig. 3A).
Figure 2. AMD3100 induces B and T lymphocyte accumulation in lymph node.
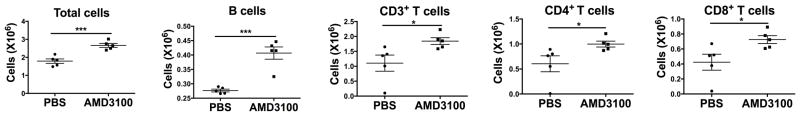
WT mice (n=5/group) were injected with PBS or AMD3100, then bled and sacrificed at 2.5 h p.i. Six peripheral lymph nodes were collected and processed into single cell suspensions, and the leukocyte subsets indicated at the top of each panel were enumerated by flow cytometry. Cells were gated on lymphocyte population (supporting information 1). Results shown are from a single experiment representative of three independent experiments. Data are plotted for individual mice and summarized as the mean ± SEM. *p<0.05, ***p<0.005, two tailed unpaired parametric t-test.
Figure 3. Blockade of lymphocyte egress from lymph node does not prevent AMD3100-induced redistribution of lymphocytes to blood or lymph node.
(A, B) WT mice (n=5/group) were given FTY720. One day later untreated mice or FTY720-treated mice were injected with PBS or AMD3100. At 2.5 h p.i. the mice were bled and sacrificed for enumeration of (A) WBC, ANC, ALC and AMC and (B) specific leukocyte populations CD3+ T cells, B220+ B cells, CD4+ T cells and CD8+ T cells in the blood (top) and lymph nodes (bottom) by flow cytometry. Cells were gated on lymphocytes (supporting information 1). Results shown are from a single experiment representative of two independent experiments. Data are plotted for individual mice and summarized as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001, two-tailed unpaired parametric t-test.
When lymphocyte subsets were analyzed (Fig. 3B) in blood, AMD3100 increased T cells (both CD4+ and CD8+ subsets) and B cells, and FTY720 reduced baseline CD3+, CD4+, CD8+ and B220+ cell counts to levels lower than those in PBS-treated mice, consistent with selective blockade of lymphocyte egress from secondary lymphoid organs. Coadministration of AMD3100 partially reversed the reduction of CD3+, CD4+, CD8+ and B220+ cells in FTY720-treated mice (Fig. 3B). In lymph node, AMD3100 alone dramatically increased CD3+ T cells (mainly CD8+) and B220+ B cells when compared to PBS-treated mice; FTY720 increased CD4+ T cells compared with PBS-treated mice. However, AMD3100 + FTY720 still markedly increased CD3+, CD4+, CD8+ and B220+ cells when compared to FTY720 + PBS treated mice (Fig. 3B). Taken together, lymph node does not appear to be a major source of T and B cells redistributed by AMD3100 to blood.
The lung is not a major source of leukocytes redistributed by AMD3100 to blood
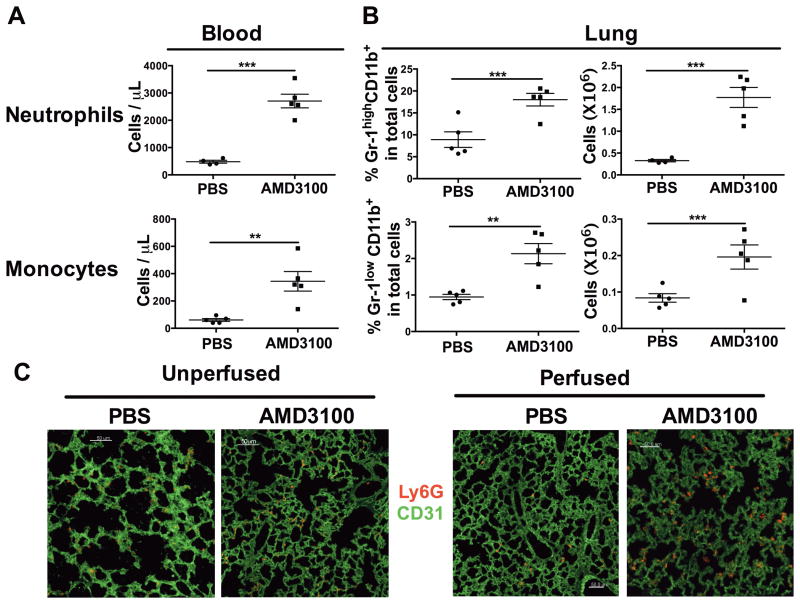
Lung is well-known to contain a marginated pool of neutrophils [29] and monocytes [30], and could therefore be a source of these cells redistributed by AMD3100 to blood. Nevertheless, it cannot be the only source, nor even the major source of these cells, since 2.5 hours after AMD3100 injection, we observed markedly increased frequency and total number of both CD11b+ Gr-1high neutrophils and CD11b+ Gr-1low monocytes extracted from lung (Fig. 4).
Figure 4. Neutrophils and monocytes rapidly accumulate in lung in response to AMD3100 treatment.
(A, B) WT mice (n=4–5/group) were injected with PBS or AMD3100, then 2.5 h later were (A) bled for determination of ANC and AMC and (B) sacrificed for analysis of lung leukocyte content. Left lungs were processed into single cell suspensions which were stained with fluorescently-labeled mAbs and analyzed by flow cytometry. (A) The number of CD11b+ Gr-1high neutrophils and CD11b+ Gr-1low monocytes in blood and (B) the percentage and total number of neutrophils and monocytes in left lung from PBS- or AMD3100-treated mice are shown. Data are plotted for individual mice and summarized as the mean ± SEM. Results are from a single experiment representative of two independent experiments. (C, D) WT mice were treated with PBS or AMD3100, then anesthetized 2.5 h later. (C) In some mice, lungs were inflated and collected for frozen section. (D) In other mice, lungs were perfused first and then inflated and collected for frozen section. In both cases, the lung sections were then stained with Alexa Fluor-CD31 (green) and PE-Ly6G [25]. Images were collected by Leica SP5 confocal microscopy under 400X magnification. Scale bar represents 50 μm. Data are from a single experiment representative of 2 experiments with 2–5 mice tested in each group. **p<0.01, ***p<0.005, two-tailed unpaired parametric t-test.
To confirm this in situ, we performed immunofluorescent staining of neutrophils and endothelial cells in frozen sections of inflated lungs. Neutrophils (Ly6G+) were associated with capillaries (CD31+) in the lungs of PBS-treated mice, consistent with a marginated pool (Fig. 4C). In the lungs from AMD3100-treated animals, there was a marked increase in neutrophils associated with capillaries that was not affected by perfusion (Fig. 4D). We also checked the absolute number of neutrophils in the images of the lung stained with Ly6G mAb at 1 and 2 hours after injection of mice with 5 mg/kg, a dose used in many other papers testing the effects of AMD3100 in mice. The lower dose also increased the lung neutrophil content (n=2), with a magnitude that was only ~15% and 39% lower than the higher dose at 1 and 2 hours p.i., respectively. At 2 hours, the lower dose was ~4-fold increased over baseline, whereas the higher dose was ~7-fold increased over baseline (data not shown).
The thymus is a source of AMD3100-mobilized naïve T cells in blood
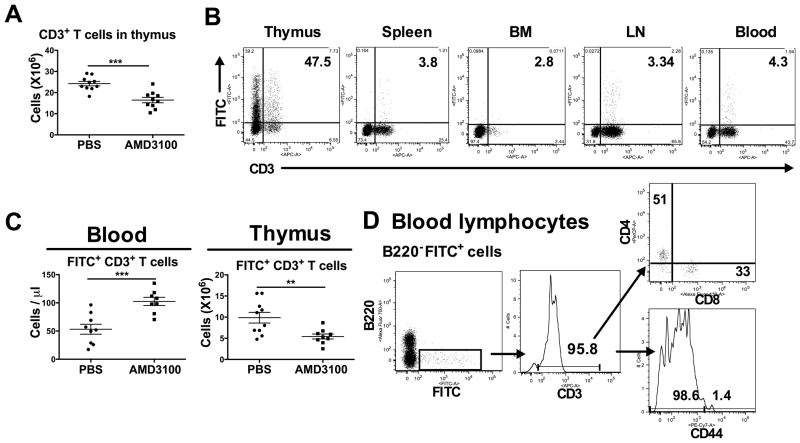
Thymus is the primary immune organ devoted to T cell development, and CXCR4 plays a role in this process [31]. To test the role of AMD3100 in thymocyte egress to blood, we injected AMD3100 into wild type mice, and measured the number of CD3+ T cells within blood and thymus by flow cytometry. After AMD3100 injection, the total WBC, lymphocytes and T cells increased in the blood (data not shown) while the total number of CD3+ T cells was reduced in thymus (Fig. 5A). To further test this, we directly labeled thymocytes in vivo by intrathymic injection of FITC, and monitored FITC expression on CD3+ T cells from thymus, spleen, bone marrow, lymph nodes and blood two days after FITC injection. In thymus, 47.5% of CD3+ T cells and 46.8% of CD3− cells (mainly immature T cells) were FITC+ (Fig. 5B). In contrast, at most 4.3% of the CD3+ population in spleen, bone marrow, lymph node and blood were FITC+, and almost no CD3− cells were FITC+ in these compartments.
Figure 5. AMD3100 mobilizes naïve T cells from thymus to blood.
(A) WT mice (n=5/group) were injected with PBS or AMD3100, then 2.5 h later were bled and sacrificed to harvest thymi. CD3+ thymocytes in thymus were enumerated by flow cytometry. Data are combined from two independent experiments. (B) Two untreated mice were injected with FITC intrathymically and sacrificed two days later. Cells extracted from the indicated organs were analyzed for CD3 and FITC expression by flow cytometry. (C) The absolute number of FITC+ CD3+ cells in blood and thymus were quantitated by flow cytometry after PBS or AMD3100 treatment. Data are combined from two independent experiments. (D) Blood lymphocytes from FITC-labeled mice after AMD3100 treatment were stained with anti-B220, CD3, CD4, CD8 and CD44 mAbs for flow cytometry. The cells were gated on the FITC+ B220− population and CD3 expression determined. The cells were then gated on the CD3+ population and expression of CD4, CD8 and CD44 was determined. Data are from a single experiment representative of 2 experiments with 5 replicates in each group. (A, C) Data are plotted for individual mice and summarized as the mean ± SEM. **p<0.01, ***p<0.005, two-tailed unpaired parametric t-test.
Two days after intrathymic FITC injection, we challenged mice with PBS or AMD3100. Compared to PBS-treated mice, AMD3100 markedly increased the absolute number of FITC+CD3+ T cells in the blood while reducing the number of these cells from the thymus (Fig. 5C). To address whether AMD3100 mainly mobilized naïve T cells from thymus, we stained blood lymphocytes from AMD3100-treated and intrathymic FITC-labeled mice for B220, then gated on B220−FITC+ cells, and examined CD3, CD4, CD8 and CD44 expression. B220−FITC+ blood lymphocytes were mainly CD3+ T cells (95.8%) expressing either CD4 (51%) or CD8 (33%) (Fig. 5D). Cells in this gate were also mainly CD44neg/low (98.6%). Thus, AMD3100 mobilizes naïve single positive T cells from thymus to the blood.
Bone marrow is a major source for AMD3100-redistributed B cells, neutrophils, monocytes and T cells
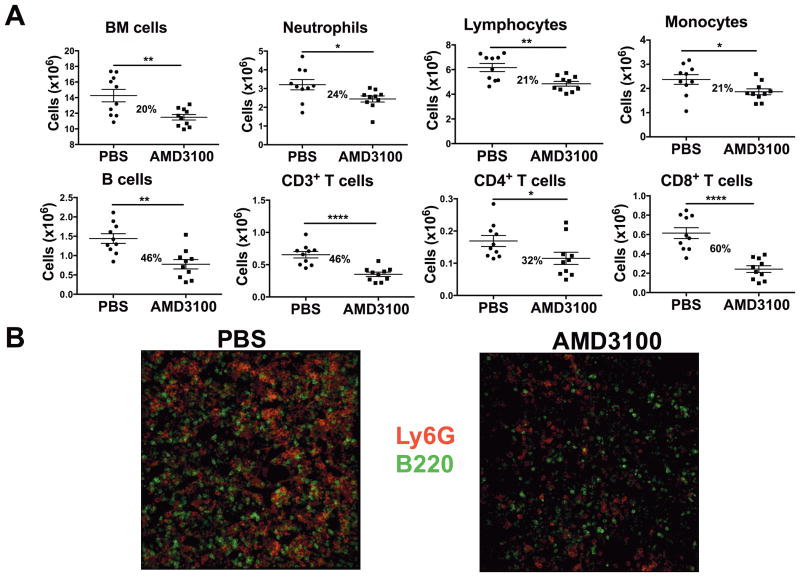
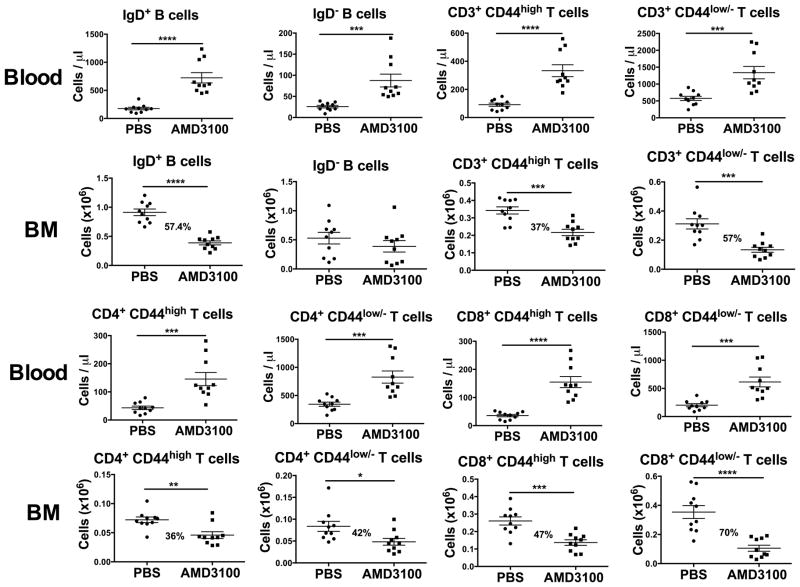
Bone marrow is a development site for all subsets of leukocytes except T cells, and CXCR4 plays a role in leukocyte homing and retention [2, 9, 11, 12, 20, 32], and in neutrophil clearance in bone marrow [26]. The source of leukocytes redistributed by AMD3100 was next tested by counting bone marrow cell content from flushed femurs. In blood, AMD3100 increased the WBC, ANC, ALC (including CD4+ and CD8+ T cells and B220+ B cells) and AMC compared with PBS-injected mice (data not shown). In bone marrow, the numbers of cells in each of these subsets were all significantly reduced after AMD3100 compared with PBS (Fig. 6A), confirming and extending previous findings [2, 19, 21, 32–34]. In Fig. 6B, compared to PBS-treated mice, the numbers of Ly6G+ neutrophils [25] and B220+ B cells (green) in bone marrow were markedly reduced in AMD3100-treated mice. CD44low/− naïve and CD44high memory T cells were both markedly reduced in bone marrow (Figure 7). Furthermore, while both naïve and memory CD4+ and CD8+ T cells were markedly increased in blood, they were dramatically reduced in bone marrow after AMD3100. Although AMD3100 increased both IgD+ and IgD− B cells in blood, it primarily reduced IgD+ B cells in bone marrow. Thus, AMD3100 redistributes T cells and B cells as well as neutrophils and monocytes from bone marrow to the circulation.
Figure 6. AMD3100 treatment rapidly reduces both lymphoid and myeloid cells from bone marrow.
(A, B) WT mice (n=5/group) were injected with PBS or AMD3100, and sacrificed 2.5 h later. (A) Peripheral blood and bone marrow cells flushed from femurs were processed into single cell suspensions that were stained with fluorescently-labeled mAbs for flow cytometry. The femoral bone marrow content of the indicated cell types after PBS or AMD3100 treatment are shown. The percent values represent the reduction in each cell type in response to AMD3100. Results are from a single experiment representative of three independent experiments. Data are plotted for individual mice and summarized as the mean ± SEM. *p<0.05, **p<0.01, ****p<0.0001, two-tailed unpaired parametric t-test. (B) Bone marrow sections were stained with Ly6G-PE and B220-Alexa-Fluor 647 mAb. Images were collected by Leica SP5 confocal microscopy under 630X magnification. Data are from a single experiment with 2–5 mice tested in each group.
Figure 7. AMD3100 depletes most but not all B-cell and T-cell subsets from bone marrow.
WT mice (n=5/group) were injected with PBS or AMD3100, sacrificed 2.5 hrs later and analyzed as in Figure 6 with mAbs to the indicated lymphocyte subsets. Each panel depicts the peripheral blood or femoral bone marrow (BM) content for the cell type after PBS or AMD3100 treatment. The percent reduction in content after AMD3100 treatment is given in the bone marrow panels where there is a significant reduction. Data are plotted for individual mice and summarized as the mean ±SEM, pooled from 2 experiments representative of 3 experiments performed. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001, two-tailed unpaired parametric t-test.
AMD3100 does not block neutrophil migration to sites of inflammation
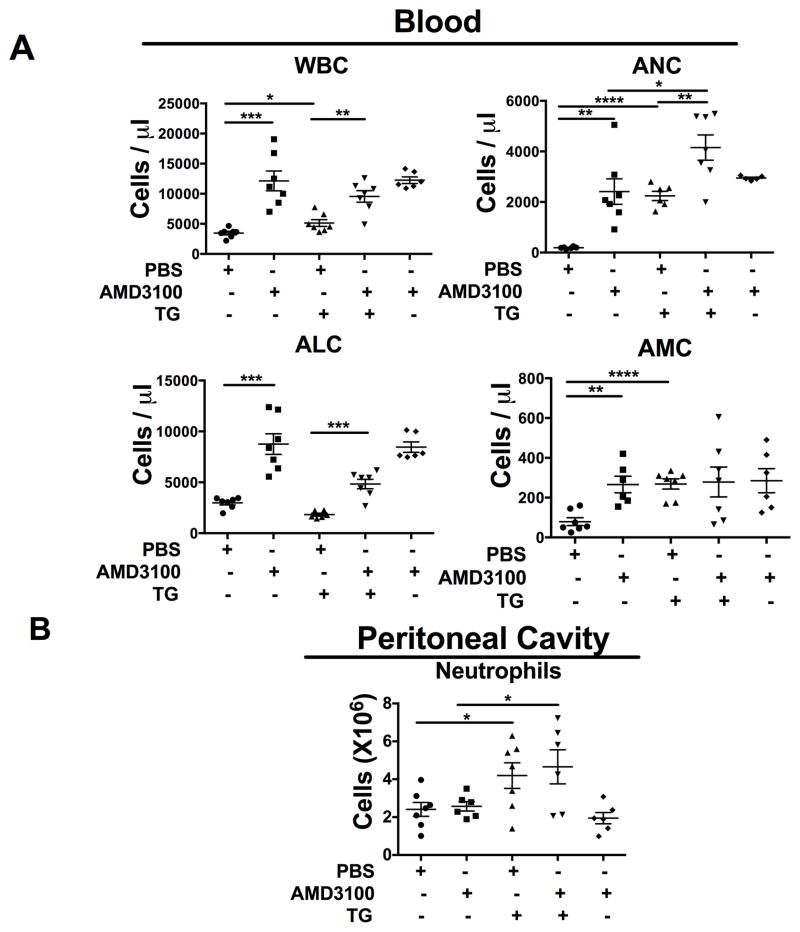
To study whether redistribution of neutrophils to the blood by AMD3100 might affect neutrophil trafficking from blood to an inflamed site, we adapted the thioglycollate-induced acute peritonitis model which mainly recruits neutrophils at 2–4 hours [35]. We injected mice with PBS or AMD3100. One hour later some of the AMD3100-treated mice were bled and sacrificed as a control group, while others were injected i.p. with thioglycollate. Three hours later, these mice were bled and sacrificed. Cells recovered from the peritoneal cavity were analyzed. As shown in Fig. 8A, one hour after injection of AMD3100 total WBC, ANC, ALC and AMC increased in the blood, compared to PBS-treated mice. This pattern persisted at 4 hours after AMD3100 and PBS injection. In PBS-treated mice, WBC, ANC and AMC in the blood markedly increased at 3 hours after thioglycollate injection. In mice injected with both AMD3100 and thioglycollate, WBC and ALC in the blood rose to the same level as in mice injected with AMD3100 alone. Compared to PBS and thioglycollate-treated mice, the ANC of mice receiving both AMD3100 and thioglycollate was markedly increased in the blood, to a level higher than in mice receiving AMD3100 alone. Thus, AMD3100 and thioglycollate synergized in mobilizing neutrophils to blood. Unlike its effect in blood, bone marrow, lymph node and lung, AMD3100 treatment by itself did not decrease the number of neutrophils that could be recovered from the peritoneal cavity in mice (Fig. 8B). Moreover, there was no difference in the number of neutrophils recovered from the peritoneal cavity after thioglycollate injection between AMD3100- and PBS control-injected mice. This suggests that systemic high dose AMD3100 treatment does not interfere with neutrophil trafficking to a site of inflammation.
Figure 8. Systemic AMD3100 treatment does not prevent neutrophil accumulation at inflamed peritoneum.
(A, B) WT mice (n=3–4/group) were injected with PBS or AMD3100. One hour later, some of the mice were bled and sacrificed for peritoneal lavage with PBS. Also one hour after PBS or AMD3100 injection, half of the remaining mice were injected with thioglycolate ip to induce an acute peritonitis. Three hours later, all remaining mice were bled and sacrificed for peritoneal lavage with PBS. (A) The WBC and differential leukocyte population counts were determined for the blood of all animals and (B) the number of neutrophils harvested from the peritoneal cavity was enumerated for the relevant groups. Data are plotted for individual mice and summarized as the mean ±SEM of data pooled from 2 independent experiments. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001, two-tailed unpaired parametric t-test.
Discussion
In this study, we used a combination of immunological, pharmacological and surgical methods to identify the sources of leukocytes mobilized to the blood in wild type C57Bl/6 mice by the CXCR4 antagonist AMD3100. This question is of basic relevance and importance since CXCR4 is known to be a key regulator of hematopoiesis; it is also clinically relevant since AMD3100 is already marketed for HSC mobilization in cancer, is under development for the treatment of patients with WHIM syndrome, and has been considered for other indications that would require long-term dosing in the setting of chronic disease. Our report is the first to address this question broadly in one study by analyzing the effects of AMD3100 on the distribution of all three major leukocyte subsets—lymphocytes, monocytes and neutrophils—in both primary and secondary immune organs, blood and peripheral tissue, as well as its effect on neutrophil accumulation at an inflamed tissue site. Together, our results confirm some previously reported results [14, 19, 21, 32, 33, 36] in this field while providing novel insights.
We found that by 2.5 hours after injection AMD3100 had induced a major redistribution of both lymphoid and myeloid cells, with a decrease in primary immune organs and accumulation in lymph node, blood and lung. In particular, T cell content was markedly reduced in both thymus and bone marrow, whereas B cell content was selectively reduced in bone marrow. Monocyte and neutrophil content was reduced in bone marrow, but markedly increased in blood and lung. A unifying interpretation of these results is that AMD3100 mobilizes leukocytes from primary immune organs to blood, which then redistributes lymphocytes to lymph nodes and myeloid cells to peripheral tissues such as lung. We validated this interpretation directly for thymus by labeling thymocytes in situ in vivo, finding that both naïve CD4 and CD8 single positive T cells were rapidly mobilized to the blood in response to AMD3100. A previous study has shown that CXCR4 plays a critical role in thymocyte localization and development in thymus [31], but the role of CXCR4 in egress of mature T cells from thymus had not been clear. Our findings in thymus disagree with a previously published study [37]; however, this study involved a very different system, a modified fetal thymus organ culture system ex vivo.
We directly excluded spleen as a major source of leukocytes mobilized by AMD3100 by testing the effects of the drug in splenectomized mice. Surprisingly, splenectomy actually increased AMD3100-induced mobilization to the blood for lymphocytes, monocytes and neutrophils. Since AMD3100 treatment did not increase splenic leukocyte content in non-splenectomized animals, and splenectomy did not affect either the baseline WBC in PBS-treated mice or the AMD3100-induced decrease in bone marrow leukocyte content (data not shown), the most likely explanation for splenectomy enhancement of the AMD3100 mobilization response in blood is that the spleen serves as a reservoir for some of the mobilized cells. In particular, the excess of leukocytes in the blood that could be attributable to splenectomy is ~22 million assuming a 1.3 ml total blood volume (Figure 1C). Since this number is small compared to the total leukocyte content of a spleen (>100 million cells), the spleen weight and total cell content after AMD3100 treatment would change relatively little. In fact, we measured no change in these splenic parameters after AMD3100 treatment (Figure 1A). In contrast, since 22 million is a large number compared to the total leukocyte content of the blood (~4.0 million [Figure 1C]), the increase in the magnitude of AMD3100-induced leukocyte mobilization to blood conferred by loss of the splenic reservoir is predicted to be and is in fact large. Of course, other factors could also play a role, and may account for the reason why we did not see an AMD3100-induced difference in weight or leukocyte content in spleen. Our results do not establish that AMD3100 cannot induce mobilization of any leukocytes from spleen, just that the absence of a spleen does not reduce mobilization of leukocytes to blood. Thus, even though CXCL12 is broadly expressed in secondary lymphoid organs, where it may direct positioning of leukocytes within microenvironments, according to our data, and those of others [38], it probably plays at most a small role in driving leukocyte egress from spleen.
We also directly excluded a major role for lymph node as a source for AMD3100-induced lymphocyte mobilization by blocking lymphocyte egress from lymph node with the S1P agonist FTY720. In the presence of this agent, AMD3100-induced leukocyte mobilization to blood was not reduced. Of the three major remaining sources of lymphocyte mobilization by AMD3100-- skin, gut and bone marrow--we tested bone marrow indirectly and found that as lymphocytes were mobilized to the blood, they were reduced in number in bone marrow, consistent with bone marrow as the source and confirming a previous report [33].
Our analysis of myeloid cell redistribution by AMD3100 is also indirect, based on quantification of neutrophils and monocytes in primary and secondary immune organs as well as in lung 2.5 hours after injection of the drug. The patterns in bone marrow and blood that we found are consistent with those reported by others [14, 19], which have been interpreted until recently as mobilization from bone marrow to blood by the drug. Devi et al have challenged this interpretation based on direct live imaging studies of the effect of AMD3100 on neutrophils in situ in bone marrow and lung [18]. Unlike G-CSF, they found that AMD3100 was unable to directly mobilize marked, adoptively transferred neutrophils from bone marrow niches. Instead they claimed that the mechanism of neutrophilia induced by the drug is a combination of neutrophil mobilization to blood from the marginated pool of neutrophils in lung [29, 30] and impaired homing of blood neutrophils to bone marrow. In this regard, our data suggest that lung is unlikely to be a major source of AMD3100-mobilized neutrophils, since the drug induced a major increase, not a decrease, of neutrophil (and monocyte) content in this organ. The major point is not that the drug does not mobilize any neutrophils from lung, which our data do not address, but that there are insufficient data available to define the portion of total drug-induced neutrophilia accounted for by the lung. Moreover, the interpretation of Devi et al implies that CXCL12/CXCR4 signaling is not involved in neutrophil retention in the bone marrow [18], which is not consistent with all previous studies of this question in mouse [14, 19] or with the hematologic picture of myelokathexis in patients with WHIM syndrome [16]. Most leukocytes express CXCR4, and bone marrow contains the highest levels of the CXCR4 ligand CXCL12, which was originally isolated from bone marrow stromal cells and named stromal cell-derived factor-1 [39]. CXCL12-CXCR4 signaling has been reported to be involved in bone marrow retention of B cells [2, 36], neutrophils [14, 34], monocytes [32], naïve and memory T cells [33] [21] and Tregs [33]. The mechanisms by which CXCL12-CXCR4 signaling regulates retention of leukocytes, especially neutrophils in bone marrow, have been addressed by previous studies and include: 1) Src-mediated c-kit phosphorylation [40], 2) the VLA4/VAM-1 pathway [41], and 3) JNK activation [42].
AMD3100 can reverse panleukopenia in WHIM patients and may decrease the risk of infection and progression of warts [17]. Moreover, neutrophils mobilized to the blood in WHIM patients by AMD3100 are able to generate normal levels of reactive oxygen species (ROS) and kill bacteria normally in vitro [22]. Our present study extends these results by demonstrating that AMD3100 does not block the ability of neutrophils to traffic to a site of inflammation. This is consistent with a previous report [43]. Thus, the evidence to date supports the notion that neutrophils mobilized by AMD3100 treatment have normal function. Whether this is the case for T and B cells as well as monocytes is not yet known and will be the subject of future investigation.
Our study was designed to broadly define the redistribution of the major leukocyte subsets throughout the immune system and in tissue in response to CXCR4 blockade with AMD3100, as well as to define the sources of the redistributed cells. Additional work will be needed to define quantitatively the contribution of each source to the redistribution response, as well as the relative roles of CXCR4-dependent leukocyte proliferation, maturation, retention and apoptosis. Nevertheless, since our observations took place only 2.5 hours after treatment, the contribution of cell proliferation, maturation and even apoptosis can be considered to provide a negligible contribution to leukocyte content in each compartment. Another caveat is that the sources of mobilized cells could differ in an AMD3100 dose-dependent manner [44]. It is also worth noting that AMD3100 has been reported to function as a weak agonist at the related receptor CXCR7 to cause β-arrestin recruitment (EC50 140 μM) [45]. However, the dose of AMD3100 we have used is low compared to this value, therefore it is most likely that all of the effects we have observed are mediated by blockade of CXCR4.
In summary, our data confirm and extend previous reports of the effects of AMD3100 on leukocyte distribution [14, 19]. We have provided three direct tests of the source (splenectomy, FTY720 blockade and in situ labeling of thymocytes), and we provide quantitative data indicating a marked and rapid increase in neutrophil and monocyte content of the lung after AMD3100 treatment. Our approach, which included analysis of all three major leukocyte subsets in all primary and secondary immune organs, blood and lung, is comprehensive relative to previous studies which have tended to focus on one cell type, blood and bone marrow [21, 14, 18, 19]. In addition, the high dose of drug that we tested is relevant to clinical applications where complete receptor blockade may be desirable. Importantly, we and others have found that the mouse responds similarly to humans with regard to the types of leukocytes mobilized to the blood by AMD3100 (Table I and references [15, 17, 21, 22, 46, 47]). Therefore, the sources and depots of these cells may be the same in humans, including patients with WHIM syndrome, as in mice.
Materials and Methods
Animals
Four- to ten-week-old female C57BL/6 mice from Taconic (Derwood, MD) were maintained in a specific pathogen-free NIH facility; all studies were reviewed and approved by the Animal Care and Use Committee of the NIAID, NIH.
Drug treatments
Mice received 5 or 10 mg/kg AMD3100 subcutaneously (s.c.) (Sigma-Aldrich, St. Louis, MO) or PBS, and complete blood counts (CBC) were determined 1, 2 or 2.5 hours later. Some mice received 2 μg/ml FTY720 (Cayman Chemical, Ann Arbor, Michigan) in drinking water starting one day before AMD3100 treatment [48]. Chemical peritonitis was induced by 3% thioglycollate intraperitoneal injection (i.p.) one hour after injecting AMD3100; 3 hours later, mice were bled and the peritoneal cavity was lavaged with 10 ml of precooled PBS.
Thymocyte Labeling
Four-week old mice were anaesthetized with ketamine, 60 mg/kg and xylazine, 8 mg/kg, i.p. Thymi were injected in vivo with fluorescein isothiocyanate (FITC, 2 mg/ml; Sigma-Aldrich, St. Louis, MO) 10 microliters/lobe, as previously described [49].
Splenectomy
General anesthesia was established i.p. with the ketamine/xylazine cocktail in 6 week old mice. Spleens were exteriorized, the splenic artery branches were tied off using 4–0 sutures, and the spleen was removed as previously described [50]. After splenectomy, mice were allowed to convalesce for 4 weeks before receiving PBS or AMD3100.
Cell preparation
EDTA-anti-coagulated peripheral blood was obtained before sacrifice and CBCs were determined. Primary leukocyte suspensions were prepared from peripheral blood, lymph nodes and spleens and thymi as previously described [51]. Femurs were collected and flushed by PBS to harvest bone marrow cells, which were washed and filtered to obtain single cell suspensions. Left lung was fragmented then incubated with 100 U/ml liberase TL and 250 μg/ml DNAse I (Roche Diagnostics, Indianapolis, IN) for 45 minutes at 37°C before being minced and filtered, washed, then resuspended in FACS buffer.
Flow cytometry
Single cell suspensions were Fc-blocked with rat anti-mouse CD16/32 at 4°C for 20 minutes. Cells were stained for 30 min at 4°C with the following mouse-specific antibodies (BioLegend San Diego, CA): CD3-allophycocyanin, CD4-PerCP, CD8-Pacific blue, CD44-PE-Cy7, B220-Alexa 700, CD27-PE or PE-Cy7, IgD-allophycocyanin-Cy7, CD11c-FITC or PECy7, CD11b-PerCP-Cy5.5, CD19-PerCP-Cy5.5, CD25-PerCP-Cy5.5, Ly6G-Pacific blue, F4/80-allophycocyanin-Cy7, I-Ab-allophycocyanin, Gr-1-PE, Ly6C-Alexa Fluor 700. For T and B cells, we gated cells on the lymphocyte population. For monocytes and neutrophils, we stained monocytes with Ly6C and CD11b after excluding the Ly6G+ neutrophils and CD11c+ I-Ab+ dendritic cells as well as F4/80+ macrophages. Cells were gated on total live cell population. Data were acquired on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (version 9.4.2; Treestar, Ashland, OR).
Immunofluorescent staining
Mice were injected with PBS or AMD3100, and 1, 2 or 2.5 hours later anaesthetized with a ketamine/xylazine cocktail. The lungs were injected intratracheally with 2.5 ml of a 1:1 ratio of OCT compound and 20% sucrose. Tracheas were ligated and lungs stored in 20% sucrose overnight, immersed in OCT compound for frozen sectioning. Some mice were perfused until the lungs were cleared of blood, then inflated and collected for frozen section. Femurs were collected, fixed with 4% PFA for 6 hours before transfer into 20% sucrose overnight. Bones were decalcified and immersed in OCT for frozen sectioning. For lung immunofluorescent staining, sections were fixed with acetone, stained with CD31− Alexa Fluor-488, and/or Ly6G- PE mAbs (Biolegend, San Diego, CA). For bone marrow, sections were stained with Ly6G-PE and Alexa-647-B220 (Biolegend, San Diego, CA). Images were acquired using a Leica DMI6000 SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a 40X, NA1.25 Oil Immersion Objective and processed using IMARIS Image analysis software (Bitplane, Switzerland). To quantify the number of neutrophils in an image of a lung, Ly6G+ neutrophils were counted in 50 pictures as tiles in a 400X magnification.
Statistics
Data were analyzed using unpaired parametric t-tests (two-tailed) with Prism 6 (GraphPad Software, La Jolla, CA) and are presented as the mean ± SEM of summary data (the data were approximately normally distributed). The cut-off for statistical significance was defined as P < 0.05 (****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; ns, P ≥ 0.05).
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases.
We thank Dr. Michail S. Lionakis and Dr. Estefania Claudio for providing us the lung tissue digestion and perfusion protocol.
Abbreviations used in this article
- CBC
complete blood counts
- ANC
absolute neutrophil count
- ALC
absolute lymphocyte count
- WBC
white blood cell
- p.i
post injection
Footnotes
Conflicts of Interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Rosu-Myles M, Gallacher L, Murdoch B, Hess DA, Keeney M, Kelvin D, Dale L, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci U S A. 2000;97:14626–14631. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung SH, Seki K, Choi BI, Kimura KB, Ito A, Fujikado N, Saijo S, et al. CXC chemokine receptor 4 expressed in T cells plays an important role in the development of collagen-induced arthritis. Arthritis Res Ther. 2010;12:R188. doi: 10.1186/ar3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 5.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 6.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, Matsushima K, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger JA, Stewart DJ, Wald O, Peled A. Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev Anticancer Ther. 2011;11:621–630. doi: 10.1586/era.11.11. [DOI] [PubMed] [Google Scholar]
- 8.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 9.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 10.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 12.Bernardini G, Sciume G, Bosisio D, Morrone S, Sozzani S, Santoni A. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood. 2008;111:3626–3634. doi: 10.1182/blood-2007-08-106203. [DOI] [PubMed] [Google Scholar]
- 13.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 15.Hubel K, Liles WC, Broxmeyer HE, Rodger E, Wood B, Cooper S, Hangoc G, et al. Leukocytosis and Mobilization of CD34+ Hematopoietic Progenitor Cells by AMD3100, a CXCR4 Antagonist. Support Cancer Ther. 2004;1:165–172. doi: 10.3816/SCT.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009;16:20–26. doi: 10.1097/MOH.0b013e32831ac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O’Brien S, Ulrick J, Kwatemaa N, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014 doi: 10.1182/blood-2013-09-527226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devi S, Wang Y, Chew WK, Lima R, N AG, Mattar CN, Chong SZ, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013 doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 21.Kean LS, Sen S, Onabajo O, Singh K, Robertson J, Stempora L, Bonifacino AC, et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood. 2011;118:6580–6590. doi: 10.1182/blood-2011-06-359331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O’Brien S, Penzak SR, Filho JO, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118:4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DA, Noel P, Takemoto CM, et al. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010;116:2793–2802. doi: 10.1182/blood-2010-01-265942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol. 2010;88:241–251. doi: 10.1189/jlb.0210112. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 28.Yopp AC, Fu S, Honig SM, Randolph GJ, Ding Y, Krieger NR, Bromberg JS. FTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol. 2004;173:855–865. doi: 10.4049/jimmunol.173.2.855. [DOI] [PubMed] [Google Scholar]
- 29.Gee MH, Albertine KH. Neutrophil-endothelial cell interactions in the lung. Annu Rev Physiol. 1993;55:227–248. doi: 10.1146/annurev.ph.55.030193.001303. [DOI] [PubMed] [Google Scholar]
- 30.Wakabayashi K, Wilson MR, Tatham KC, O’Dea KP, Takata M. Volutrauma, but not atelectrauma, induces systemic cytokine production by lung-marginated monocytes. Crit Care Med. 2014;42:e49–57. doi: 10.1097/CCM.0b013e31829a822a. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Cui L, Gonsiorek W, Min SH, Anilkumar G, Rosenblum S, Kozlowski J, et al. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm (Lond) 2009;6:32. doi: 10.1186/1476-9255-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng Q, Nie Y, Zou Y, Chen J. Elevated CXCL12 expression in the bone marrow of NOD mice is associated with altered T cell and stem cell trafficking and diabetes development. BMC Immunol. 2008;9:51. doi: 10.1186/1471-2172-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 36.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vianello F, Kraft P, Mok YT, Hart WK, White N, Poznansky MC. A CXCR4-dependent chemorepellent signal contributes to the emigration of mature single-positive CD4 cells from the fetal thymus. J Immunol. 2005;175:5115–5125. doi: 10.4049/jimmunol.175.8.5115. [DOI] [PubMed] [Google Scholar]
- 38.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 39.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 40.Cheng M, Zhou J, Wu M, Boriboun C, Thorne T, Liu T, Xiang Z, et al. CXCR4-mediated bone marrow progenitor cell maintenance and mobilization are modulated by c-kit activity. Circ Res. 2010;107:1083–1093. doi: 10.1161/CIRCRESAHA.110.220970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petty JM, Lenox CC, Weiss DJ, Poynter ME, Suratt BT. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J Immunol. 2009;182:604–612. doi: 10.4049/jimmunol.182.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–1296. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theiss HD, Vallaster M, Rischpler C, Krieg L, Zaruba MM, Brunner S, Vanchev Y, et al. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res. 2011;7:244–255. doi: 10.1016/j.scr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Gravel S, Malouf C, Boulais PE, Berchiche YA, Oishi S, Fujii N, Leduc R, et al. The peptidomimetic CXCR4 antagonist TC14012 recruits beta-arrestin to CXCR7: roles of receptor domains. J Biol Chem. 2010;285:37939–37943. doi: 10.1074/jbc.C110.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balabanian K, Brotin E, Biajoux V, Bouchet-Delbos L, Lainey E, Fenneteau O, Bonnet D, et al. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood. 2012;119:5722–5730. doi: 10.1182/blood-2012-01-403378. [DOI] [PubMed] [Google Scholar]
- 48.Sugito K, Koshinaga T, Inoue M, Ikeda T, Hagiwara N, Fukuzawa M. The effect of a novel immunosuppressant, FTY720, in mice without secondary lymphoid organs. Surg Today. 2005;35:662–667. doi: 10.1007/s00595-005-3011-x. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Issazadeh S, Sayegh MH, Khoury SJ. In vivo mechanisms of acquired thymic tolerance. Cell Immunol. 1997;179:165–173. doi: 10.1006/cimm.1997.1165. [DOI] [PubMed] [Google Scholar]
- 50.Kamer AR, Galoyan SM, Haile M, Kline R, Boutajangout A, Li YS, Bekker A. Meloxicam improves object recognition memory and modulates glial activation after splenectomy in mice. Eur J Anaesthesiol. 2012;29:332–337. doi: 10.1097/EJA.0b013e3283534f56. [DOI] [PubMed] [Google Scholar]
- 51.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, et al. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog. 2012;8:e1002865. doi: 10.1371/journal.ppat.1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.