Abstract
Few studies characterize longitudinal female plasma and genital antiretroviral pharmacokinetics and pharmacodynamics. Among 20 regimen-naïve HIV-infected adult women initiating atazanavir- (n=9) or efavirenz-based therapy (n=11), we measured blood CD4+ T-lymphocytes, and paired plasma and genital HIV RNA and atazanavir or efavirenz 2 days before starting therapy, and 2, 4, 7, 10, 21, 28, 60, 120, and 180 days after. The mean (range) log10 baseline plasma viral load was 4.89 (2.64 – 6.09) copies/mL and genital was 3.30 (1.60 – 5.00). In the atazanavir and efavirenz groups, mean (SD) days to 50% decrease in plasma viral load was 8.2 (4.9) vs. 9.3 (7.4), P=0.7, and in the genital tract was 7.3 (3.5) vs. 9.3 (7.7), P=0.5. The median (interquartile range) plasma:genital concentration ratio for atazanavir was 0.11 (0.001 to 0.46), vs. 0.34 (0.05 to 1.30) for efavirenz, P=0.5. Average plasma efavirenz or atazanavir concentrations over time did not affect virologic response. Blood CD4+ percentages increased by +2.3 (P=0.06) and +3.0 (P=0.003) for every 1 mg/L increase in average plasma and genital drug concentration, respectively. Plasma and genital viral pharmacodynamics were similar between the groups and independent of average concentrations, but blood CD4+ response was related in particular to genital extravascular drug concentrations.
Keywords: HIV, female, genital, pharmacokinetics, pharmacodynamics
Introduction
Globally, 52% of HIV-infected individuals are women.1 We previously showed that cervical HIV RNA can be detected on cross-sectional sampling in 15% of women with <500 RNA copies/mL in the plasma, and that receipt of highly active antiretroviral therapy (HAART) based on non-nucleoside reverse transcriptase inhibitors (NNRTIs) was an independent risk factor associated with genital shedding vs. HAART based on protease inhibitors (PIs).2
Much work has been done to characterize the penetration of antiretroviral drugs into the female genital tract, which has been comprehensively summarized.3 Genital concentrations are variable and usually lower than in plasma. NRTIs tend to have the highest ratios. Among the NNRTIs, efavirenz has the lowest, while etravirine and rilpivirine are better, but also have fewer studies to truly assess the variability. Among the PIs, indinavir and possibly darunavir appear to be the best.
Few studies have simultaneously measured female genital antiretroviral pharmacokinetics and pharmacodynamics,4,5 and only one has measured these outcomes longitudinally.6 That study sampled over a period of only 3 weeks, and in women with stable therapy and suppressed plasma virus. To better understand early and chronic pharmacokinetic-pharmacodynamic relationships between antiretroviral drug concentrations and compartmental suppression of virologic replication and sanctuary site shedding, we conducted a prospective study in HIV-infected women beginning HAART based on atazanavir or efavirenz, antiretroviral drugs currently in common use, and measured drug concentrations and quantified HIV RNA in both plasma and genital secretions. We hypothesized that genital viral dynamics would differ among women on NNRTI vs. PI-based regimens.
Methods
Study design and patient population
We conducted an open-label, non-randomized cohort study of HIV-infected women 16 years or older at the Maternal Child and Adolescent Center for Virology and Infectious Diseases at the Los Angeles County + University of Southern California (LAC+USC) Medical Center, which was approved by the IRB, and all patients consented in writing prior to undergoing any study procedures. Eligible patients were starting a new antiretroviral regimen containing either atazanavir with or without ritonavir, or efavirenz, but not both, and were naïve to the study drug. The two drugs were the most commonly prescribed PI and NNRTI, respectively, in the clinic. We controlled for background NRTI by only including those on tenofovir and emtricitabine.
Study schedule and samples
The baseline study visit was 1-2 days prior to starting the new antiretroviral regimen. Because we anticipated a rapid drop in plasma and genital viral load after initiating therapy,7 we scheduled visits more intensely early in the study, balancing the feasibility of repeated vaginal sampling. Therefore, visits after baseline occurred 2, 4, 7, 10, 21 and 28 days after starting therapy. To collect longer term data, three visits were scheduled for 2, 4, and 6 months after enrollment, although the timing was not strictly enforced.
At baseline, prior to initiation of study medications, we measured virologic, immunologic, pharmacologic and safety laboratories, including plasma and genital viral loads by HIV RNA PCR, complete blood cell count with T-cell subsets, plasma and genital study medication concentrations, bilirubin, liver enzymes and creatinine. All participants began their HAART with morning dosing.
At all subsequent visits, we queried participants about the times of their previous three doses. We calculated adherence as the percentage of the three doses reported as taken, regardless of time. We asked them to report any adverse effects of their medications. On all visits except the day 4 or day 7 visit, we collected one pair of blood and genital samples for quantification of study drug and viral load in both samples. For either the day 4 or day 7 visit, based on convenience, we drew a blood sample for drug and viral load measurements, followed by witnessed dosing of the study drug, and additional blood samples 0.5, 1, 2, and 4 hours after the dose, with one genital sample. This visit was early in the study to minimize the impact of missed doses and adherence on either pharmacokinetics or pharmacodynamics, and the main purpose was to characterize absorption.
Study treatments
Doses for the study medications were according to the package inserts, which was efavirenz 600 mg once daily, atazanavir 300 mg once daily in combination with ritonavir 100 mg once daily, or atazanavir 400 mg once daily without ritonavir in subject 1.
Collection of samples
A study nurse or research clinic phlebotomist collected blood samples using universal precautions. Within 30 minutes, we transported the samples to the lab for centrifugation, division of plasma into aliquots of 0.25 – 0.5 mL each, and freezing at -80°C until analysis. An experienced female nurse practitioner collected all genital samples during a speculum exam. We scheduled visits to avoid menses when possible, particularly up until day 10. After day 10, women who were menstruating rescheduled their study visit. Upon inserting the speculum and visualizing the cervix, the nurse practitioner inserted a Puritan Sterile Dacron Polyester Tip Applicator 25-806-1PD Swab into the os, rotated it twice, placed the swab in a vial containing 0.5 mL of guanidine isothiocyanate solution containing fresh 2-mercaptoethanol, and sealed the vial 8. Within 30 minutes, we froze the buffer and swab at -80°C until analysis for HIV RNA copies.
The nurse practitioner next injected 2 mL of sterile saline into the posterior fornix, waited one minute and withdrew the sample. Within 30 minutes we also froze this sample at -80°C until analysis of cervicovovginal lavage evaluations for ARV concentration.
Measurement of plasma and cervical viral load
HIV-1 RNA plasma and cervical quantification was performed in our laboratory (University of Southern California, Los Angeles, CA), which is CLIA and NIH certified by Virology Quality Assessment Program (VQA). We used quantitative real-time PCR (Abbott RealTime HIV-1 assay) with manual extraction method.
After vortexing each sample vial, we removed the swab and adjusted the volume of the buffer to 1.2 mL with additional guanidine isothiocyanate 2-mercaptoethanol solution. We then centrifuged these vials for 10 minutes at 14000 × g to eliminate fiber contamination. From each, we added 600 μL of the swab solution into Lysis Buffer. An RNA sequence different from HIV was added to the Lysis Buffer-sample mixture and served as internal control. RNA was extracted with magnetic particle technology to capture nucleic acid and wash out unbound components. The RNA was amplified by real-time reverse transcriptase (RT)-PCR, and the concentration quantified by calibrated fluorescent-labeled probe on the Abbott m2000rt instrument. The limit of detection was 40 copies/mL.
Measurement of urea in plasma and vaginal samples
Since urea is a small, non-polar molecule that rapidly diffuses across biologic membranes to equilibrium, concentrations are very similar in differing body compartments. The technique of simultaneously measuring urea in plasma and sampled bronchoalveolar lavage fluid is a well-established method to correct the sample volume for dilution with lavage fluid and estimate drug concentrations in the epithelial lining fluid.9 To our knowledge, the method of urea dilution has not been applied previously to measure vaginal drug concentrations. Direct aspiration of vaginal secretions can be technically difficult and takes longer than lavage because pooling of vaginal secretions must occur while the woman is recumbent, prior to sampling. Since the concentrations of urea in plasma10 and vaginal fluid11 are also almost identical (∼13 mg/dL), to correct for dilution of vaginal fluid with saline, we simultaneously measured plasma and vaginal lavage urea concentrations, following the manufacturer's recommended instructions (Urea Nitrogen Procedure No. 640; Sigma Diagnostics; St. Louis, MO). We calculated drug concentration in vaginal fluid by multiplying the measured concentration in lavage fluid by the plasma:lavage urea ratio.
Atazanavir and Efavirenz Vaginal and Plasma Concentration Determination
To 100 μL of each plasma and vaginal lavage sample obtained from the subjects, we added 100 μL of internal standard solution containing 1000 ng/mL Saquinavir (SQV) and 100 ng/mL nevirapine (NVP). Samples were then extracted by solid phase extraction (SPE) using 4 mL Burdick-Jackson C18 columns that were pre-conditioned with 3 mL MeOH, followed by 3 mL of distilled water. Samples were applied to the cartridges and then washed with 3 mL of distilled water. The analytes eluted using 1 mL of MeOH, and evaporated to dryness using a speed vacuum dryer overnight. Samples were reconstituted using 100 μL running buffer, which was 80:20 (v/v) of MeOH and 20 mM ammonium acetate, pH adjusted to 3.5 with formic acid. An aliquot of 50 μL of reconstituted sample was injected into an Agilent 1100 linked onto a Sciex API 3000 tandem MS system. The analytes were separated using a Hypurity C18 50 × 2.1 mm column with a particle size of 5 μ and eluted using a mobile phase consisting of MeOH and 20 mM running buffer as above, with the flow rate set at 350 μL/min. ATV concentration was determined with the program set in the positive ion mode and the MRM monitoring for mass transition ions 671.7 → 570.5 and 705.6 → 168.3, for SQV and ATV, respectively. The retention times for SQV and ATV were 4 and 4.5 minutes, respectively. To determine the concentration of EFV, the MS was set in the negative ion mode, and the MRM monitored for mass transition ions 267.2 → 226.2 and 314.2 → 244.0 for NVP and EFV, respectively. The retention times for NVP and EFV were 3 and 3.5 minutes, respectively.
The lowest level of detection was established at 2.5 ng/mL for vaginal and 25 ng/mL for plasma ATV and EFV.
CD4+ T-lymphocytes
CD4 cells were measured using 4-color flow cytometry (FC500 with TetraCXP System, Beckman Coulter) in the LAC+USC CLIA-certified special hematology laboratory, according to the manufacturer's instructions.
Statistical analysis
We used R12 in Rstudio (available at http://www.rstudio.com) for all analyses and plots. We compared continuous normally distributed variables with the Student's T-test, non-normal continuous variables with the Mann-Whitney U test, and categorical variables with Fisher's Exact test. For longitudinal comparisons, we used generalized estimating equations with independent correlation matrices to account for repeated samples from the same individual. To model viral decay rates, we used the Perelson model13 and fitted the data with Pmetrics, our non-parametric population modeling package for R.14 Our sample size of 10 women in each group was calculated based on the ability to detect a 30% difference in any pharmacokinetic or pharmacodynamic parameter (e.g. average drug concentration or time to 1 log10 drop in viral load from baseline) with a power of 80% and an alpha of 0.05, assuming a coefficient of variation for the parameter in both groups of 85%.
Results
Study population
For a planned final sample size of 20, there were 22 enrolled women, with mean age of 33.7 years (range 21-58). There were 13 Caucasians (9 Hispanics), 8 African Americans, and 1 Asian. Of the 22 women who enrolled, 2 withdrew before or at the first visit, leaving 9 in the atazanavir arm and 11 in the efavirenz arm. Only four of the women were fully antiretroviral naïve at the time of study entry – two in the efavirenz group, and two receiving atazanavir. One woman in the atazanavir arm was changing therapy due to failure of her current regimen at the time (zidovudine, lamivudine and nevirapine), and the rest had been off therapy for a mean of 44.1 months (range 8 – 119) at the time their study regimen started. Among the women in the efavirenz group, 7 (64%) had been exposed to nevirapine during a pregnancy in the past, but had not had a documented NNRTI mutation. One actually had also been exposed to efavirenz in the past, which was not recognized at the time of enrollment, but she also did not have a documented NNRTI mutation. Three were naïve to all NNRTIs. Among the women in the PI group, 4 (44%) were naïve to all PIs, 1 (11%) had been exposed to one PI (nelfinavir), and 3 (33%) were exposed to two PIs in the past, nelfinavir and either lopinavir/ritonavir (1) or indinvavir (2). One woman each in both treatment groups had been treated at another clinic, and we did not have their complete histories, but they denied prior study drug exposure and did not have documented study drug resistance.
All but one participant of the atazanavir arm also received ritonavir 100 mg once daily. Mean self-reported adherence across all treatment visits 2 through 10, a maximum 27 doses queried per woman, was 77%, ranging from 56% to 97%.
Viral dynamics
Of 187 samples, a total of 34 (18.1%) plasma and 98 (52.4%) genital viral loads were undetectable and set to the assay limit of 1.6 log10 copies/mL for analysis. The mean (range) log10 baseline viral load in plasma was 4.89 (2.64 – 6.09) copies/mL. The two women with <3 log10 copies/mL at baseline had been over this threshold at screening prior to study entry. Seventeen (85%) of the women had a measureable genital viral load at baseline. The baseline genital viral load was 3.30 (1.60 – 5.00) copies/mL. The geometric mean ratio of genital to plasma viral load was 0.15 (interquartile range 0.04 – 0.85), with a Pearson's correlation between genital and plasma viral load of 0.50 (95% CI 0.09 – 0.76, P=0.02). Baseline viral load by regimen is shown in Table 1.
Table 1.
Data are number (%) or Mean (SD) for plasma and genital viral loads (VL). Rebound is detectable virus after achieving < 50 copies/mL.
| Plasma | Genital | |||||
|---|---|---|---|---|---|---|
| ATV n=9 | EFV n=11 | P-value | ATV n=9 | EFV n=11 | P-value | |
| Baseline log10 VL | 4.39 (1.02) | 4.66 (0.95) | 0.545 | 3.25 (1.27) | 3.23 (0.86) | 0.9 |
| VL <50 copies/mL | 5 (56%) | 9 (82%) | 0.336 | 8 (89%) | 11 (100%) | 0.5 |
| Rebound | 1 (20%) | 1 (11%) | 0.604 | 1 (20%) | 1 (11%) | 0.6 |
| Days to VL < 50 | 109.6 (71.5) | 77.1 (5.7) | 0.394 | 23.3 (9.5) | 25.8 (14.5) | 0.9 |
| Days to 50% decline in VL | 8.2 (4.9) | 9.3 (7.4) | 0.707 | 7.3 (3.5) | 9.3 (7.7) | 0.5 |
| Days to >1 log10 drop in VL | 56.3 (119.1) | 12.7 (10.1) | 0.305 | 62.9 (136.0) | 25.0 (43.4) | 0.5 |
| End of study log10 VL | 1.98 (0.61) | 2.79 (1.68) | 0.129 | 1.93 (0.50) | 2.54 (1.25) | 0.1 |
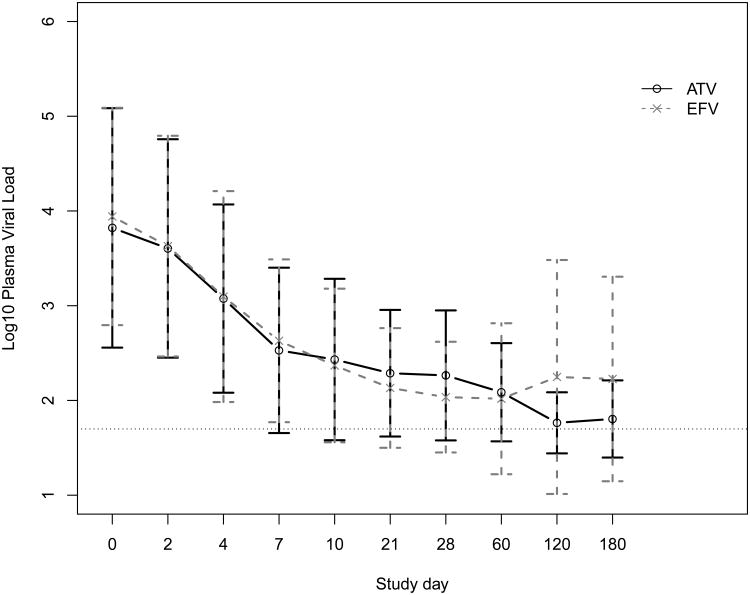
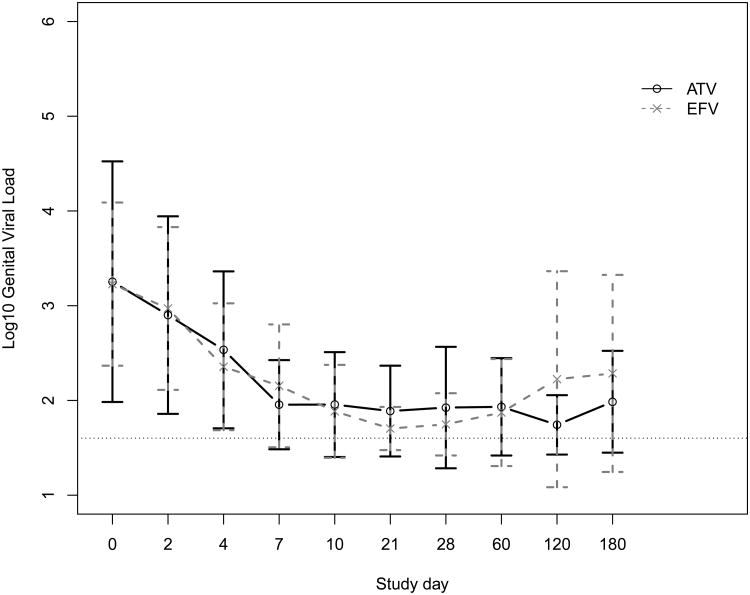
Fitting the data for the first 30 days to the Perelson model, we found a strong biphasic decay in both plasma and genital viral RNA (Figure 1), as have other researchers.7 Our median estimated initial half-life of decay was 7.1 days in plasma and 5.1 days in genital secretions (P=0.4). Terminal decay was biased by many values below the limit of detection, which led to a fitted slope near 0, i.e. a very long half-life. There was no difference in rates of initial viral decay in either plasma or genital secretions according to treatment with atazanavir or efavirenz.
Figure 1.
Mean and standard deviation (A) plasma and (B) genital viral loads at each study day for women taking atazanavir (ATV) or efavirenz (EFV) plus tenofovir and emtricitabine. The dashed horizontal line in each plot indicates the limit of detection.
As shown in Table 1 and Figure 1, there were no significant differences in baseline log10 viral load, the proportion of participants achieving a plasma viral load <50 copies/mL or the time to this threshold, the time to at least a 10-fold drop in viral load, or the proportion of participants who rebounded, defined as detectable virus after achieving <50 copies/mL. There was no virologic rebound in the genital secretions that preceded plasma rebound. Four (20%) women, on seven (3.8%) of 183 total study person-visits, had undetectable plasma virus but detectable genital virus on at least one occasion. Three of these four were receiving efavirenz. Altogether, four (28.5%) of the 14 women in both treatment groups who achieved undetectable plasma virus were shedding HIV RNA on at least one occasion during the study. The mean blood CD4+ T-lymphocytes (CD4) percent was higher on these occasions (34.1 vs. 23.7, P=0.04) than visits with concordant plasma and genital viral loads, but the absolute CD4 numbers were not significantly different (599 vs. 398, P=0.1). Overall, as seen in Table 1 and Figure 1, for both treatment groups, there was a rapid decline in both plasma and genital viral loads, roughly in parallel, after starting therapy.
Immunologic response
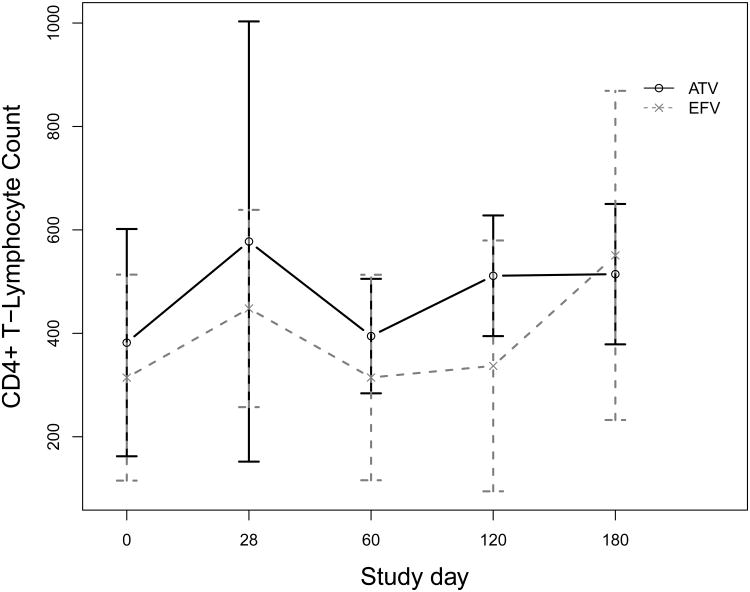
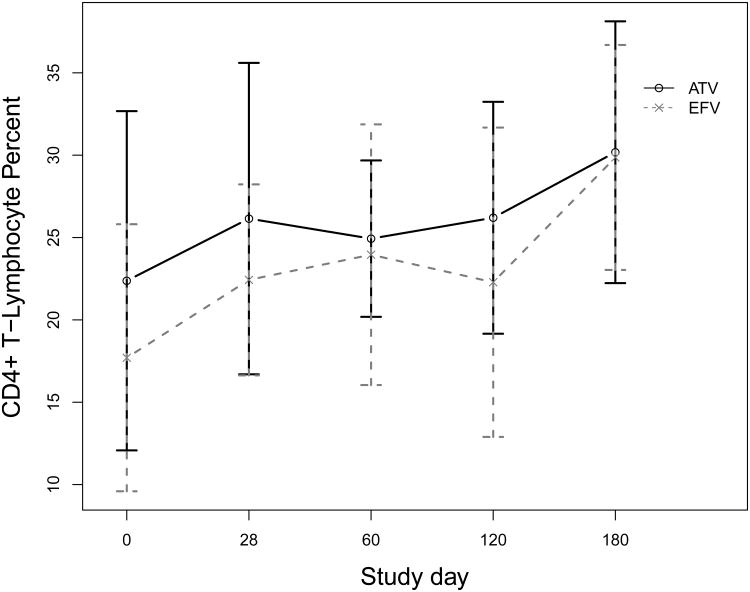
Figure 2 depicts the immunologic response during the study period. The mean overall change in CD4 count from baseline to last study visit was +161 cells/mm3, ranging from -73 to +463 cells/mm3 (P<0.01). There was no significant difference in CD4 count change between women on atazanavir or efavirenz (+185 vs. +146, P=0.6). Similarly, the mean overall change in the percentage of CD4 (CD4%) over the study period was +6.7 percentage points, ranging from -4.4 to +26.0 (P<0.01). Mean change in CD4% percentage points was very similar between women on atazanavir and efavirenz (+6.9 vs. +6.6, P=0.9).
Figure 2.
Mean and standard deviation CD4+ T-lymphocyte percent at each study day for women taking atazanavir (ATV) or efavirenz (EFV) plus tenofovir and emtricitabine.
Atazanavir and efavirenz concentrations
A total of 18 (6.5%) of 274 plasma samples and 56 (30.7%) of 182 vaginal samples were below 25 ng/mL and 2.5 ng/mL, respectively and set to 0 for the analysis. There was no correlation between each subject's mean plasma concentration and self-reported average adherence, adjusting for drug (P=0.41). The mean plasma atazanavir concentration over all visits for the single woman on unboosted atazanavir was 1.7 mg/L, compared to 2.0 mg/L for the other women receiving boosted atazanavir. Plasma and genital drug concentrations were highly variable between patients under the controlled circumstances of the multi-sampling visit (Figure 3) and even more variable within subjects over time (Figure 4), with overall population plasma and genital median (interquartile) atazanavir at all time points 1.32 (0.16 – 3.10) mg/L and 0.091 (0 – 0.53) mg/L. For efavirenz it was 0.65 (0.16 – 1.52) mg/L and 0.16 (0 – 1.10) mg/L. The median of the individual genital:plasma ratios for each woman at each sample time for atazanavir was 0.11 (interquartile range 0.001 to 0.46) and for efavirenz it was 0.34 (0.05 to 1.30), P=0.5.
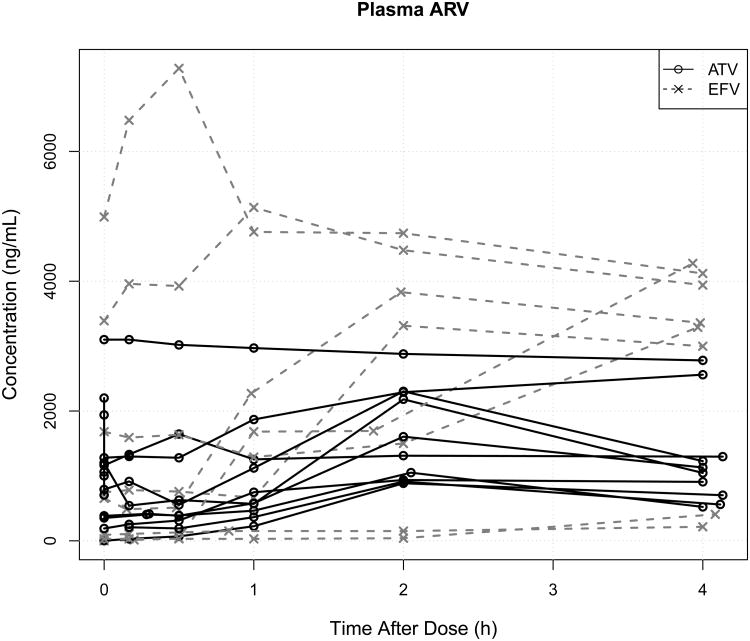
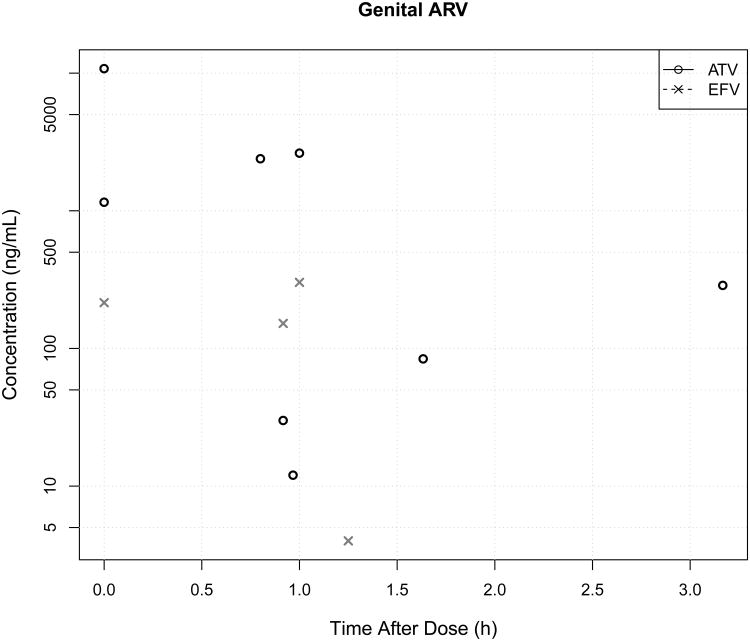
Figure 3.
Plasma (A) and genital (B) atazanavir (ATV) or efavirenz (EFV) concentrations on the multi-sampling day, showing >100-fold inter-subject variability in plasma EFV exposure and >10-fold inter-subject variability in plasma ATV exposure. In contrast, genital ATV variability was higher than EFV variability. Women with undetectable drug are not plotted and one woman each in the EFV and ATV groups missed her multi-day sampling visit.
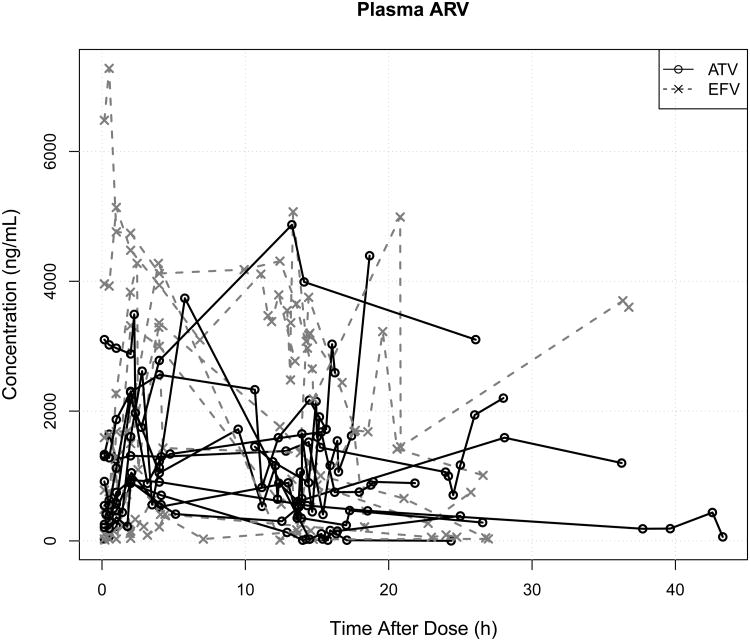
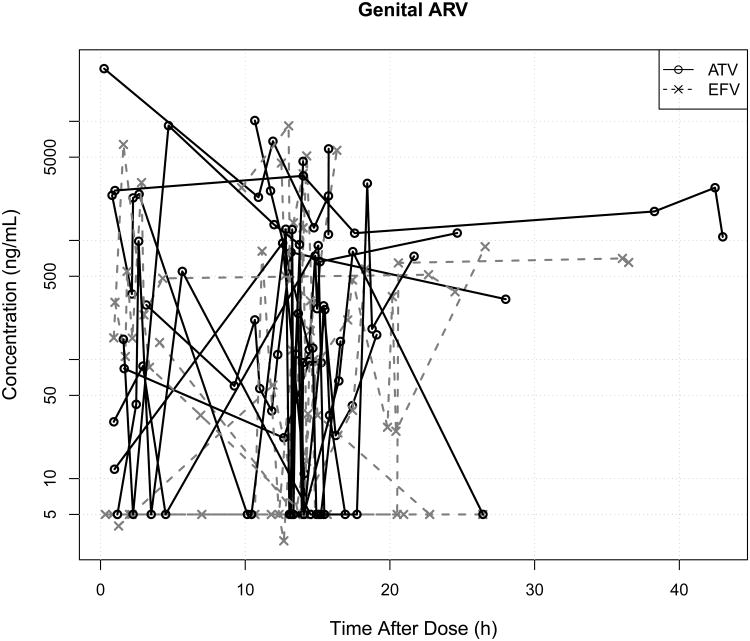
Figure 4.
Plasma (A) and genital (B) atazanavir (ATV) or efavirenz (EFV) concentrations vs. time after dose. Line segments join samples from the same woman, and concentrations after differing doses have been superimposed according to the time after the previous dose. There is tremendous longitudinal within-subject and between-subject variability in both plasma and genital drug concentrations for ATV and EFV.
Hepatic and renal toxicity
At baseline, total bilirubin was normal, with a mean of 0.4 (range 0.2 to 1.1) mg/dL. During the study, as expected, the mean total bilirubin in the women receiving atazanavir increased to 1.58 (range 0.2 to 4.5) mg/dL, compared to 0.28 (0.1 to 1.1) mg/dL in the efavirenz group (P<0.01). Of the nine women receiving atazanavir, 7 had at least one bilirubin measured after baseline, and of these seven, five (71%) experienced a rise. There were no significant changes in ALT or serum creatinine during the study period overall or by drug. Therefore, apart from asymptomatic hyperbilirubinemia associated with atazanavir, no participants suffered hepatic or renal toxicity during the study.
Pharmacokinetic-pharmacodynamic relationships
The average times to a 1 log10 decline in plasma or genital viral load from baseline were shorter by 0.9 (P=0.7) or 11.1 (P=0.3) days, respectively, for every increase in average study drug concentration of 1 mg/L in the respective compartment. We cannot rule out that these differences were due to chance. There was also no significant difference in the odds of achieving undetectable viral RNA in plasma or genital secretions per unit increase in average study drug compartmental concentrations.
Pre-dose trough concentrations >0.15 mg/L for atazanavir or >1.0 mg/L for efavirenz have been proposed as targets associated with improved chances of virologic suppression.15 Based on extrapolation from the package inserts, these targets correspond roughly to average concentrations of 0.5 mg/L for atazanavir and 1.4 mg/L for efavirenz. Women who had average plasma atazanavir or efavirenz concentrations above these thresholds had an average time to 50% decrease in plasma viral load of 6.1 (P=0.1) or 7.1 (P=0.1) days faster, respectively, than women with average drug concentrations below these thresholds. The same 50% decrease in genital viral load was observed 10.3 (P=0.3) and 3.4 (P=0.1) days faster for the atazanavir- and efavirenz-treated women, respectively, with average plasma concentrations above these thresholds.
Each increase in average plasma atazanavir or efavirenz concentration of 1 mg/L did not change the CD4+ response, but did increase the CD4% percentage point response by an average of 2.4 (P=0.06), controlling for drug. The mean rise in CD4% percentage points was 9.1 among women whose average plasma drug concentration was above the drug-specific thresholds in the preceding paragraph, vs. 4.7 in those with average concentrations below the thresholds (P=0.2).
Since the majority of lymphocytes in the body are not in blood, but rather in tissues,16 we also examined the relationship of average genital drug concentrations as a sample of tissue drug distribution, and blood CD4+ cells, likewise considering them to be a sample of the extravascular immunologic pool. Again, there was no significant relationship between genital atazanavir and efavirenz concentrations and blood CD4+ count, but for every increase in average genital atazanavir or efavirenz of 1 mg/L, blood CD4% increased by 3.0 percentage points (P=0.003), controlling for drug.
Among the five women receiving atazanavir who had a rise in bilirubin, each increase in average atazanavir of 1 mg/L was associated with an increase in total bilirubin of 0.5 mg/dL (P=0.1).
Discussion
In this unique, prospective, longitudinal study of simultaneous plasma and genital HIV viral dynamics in women starting atazanavir or efavirenz, genital HIV RNA shedding was closely related to the plasma viral load and was on the order of 1 log10 lower, which is very consistent with previous data.5 A very interesting finding was that higher genital drug concentrations were associated with better plasma immunologic gains in terms of CD4% (P=0.003). This suggests that the genital drug concentrations may have been reflective of other extravascular drug concentrations where the majority of CD4+ T-lymphocytes reside. Although higher plasma average antiretroviral drug concentrations also improved immunologic gains in terms of plasma CD4%, the P-value of 0.08 did not quite meet the customary threshold.
Despite our intensive early sampling schedule within the first two weeks of therapy, we did not find significant differences in the viral dynamics in either plasma or genital compartments according to treatment with atazanavir or efavirenz. Nevertheless, as we and others have described previously,2,7,17,18 genital RNA shedding is not completely determined by plasma viral load, since approximately 30% of women in this cohort who achieved undetectable plasma RNA had detectable genital RNA on at least one occasion. This is consistent with a recent large longitudinal study by others in our group who found that proportion to be 20%.19 However, the clinical significance of genital HIV RNA, particularly in the absence of detectable plasma replication, is unknown.20 It may be related to the low level of residual HIV sexual transmission in the setting of effective HAART.21
Drug plasma concentrations and penetration into the genital secretions from plasma were highly variable between patients and occasions, with penetration ratios not significantly different between atazanavir and efavirenz. Our median plasma:genital ratio of atazanavir (0.11) was somewhat lower compared to other reports (0.16 – 0.33) as summarized by Else et al,22 but our ratio of efavirenz genital penetration (0.34) was somewhat higher (<0.01 – 0.25). These observations may be related to our novel use of urea dilution with vaginal lavage, as other studies used a direct aspiration technique, although one might expect the ratios to be similarly affected if true. However, we believe that our use of the urea dilution method for the first time to correct for vaginal lavage volume was valid based on previously and independently documented similar concentrations of urea in plasma and undiluted vaginal fluid,10,11 and extensive use of the method for bronchoalveolar lavage volume correction.9 Furthermore, allowing for slight differences, our results are not much different compared to measured concentrations in samples obtained by the direct aspiration method. If not methodological, then this cohort of women may be different with respect to antiretroviral drug penetration than previously published cohorts. One intriguing and obvious difference is race/ethnicity, as our women were primarily Latinas, but we did not have sufficient diversity or numbers to test this hypothesis.
The lack of association between viral RNA load and drug concentrations, whether in plasma or genital secretions is most certainly due in part to the highly variable inter-occasion (within patient) variability in both endpoints, particularly drug concentrations. Adherence is a factor that confounds interpretation of drug concentrations throughout the body, and it was sub-optimal in our study. Furthermore, according to their package inserts, atazanavir is 86% protein bound and efavirenz is >99% protein bound, which will make their day-to-day distribution into the genital compartment highly susceptible to changes in vaginal protein content and fluid volume associated with the menstrual cycle23 or other factors such as inflammation. These two considerations – adherence and unmeasured physiological variables – will complicate and confound any longitudinal study of the relationship of drug concentrations to virologic response, regardless of the compartment.
There are several limitations to our study. The sample size was too small given the greater than anticipated variability in measured outcomes and the study ended up underpowered. We did not formally assess the virologic genotypic resistance in this study, although we had clinically obtained results available on some of the women. However, all women but one were naïve to their study drug, most had been off antiretrovirals for more than a year when this study was initiated, and we did not have any documented evidence of baseline or prior resistance to the study drugs. Based on the similar virologic response in plasma and genital secretions between atazanavir- and efavirenz-treated women, it is unlikely that there were major differences in baseline viral susceptibility to the antiretroviral drugs used in this study. We did not measure tenofovir or emtricitabine concentrations. Although nucleoside reverse transcriptase inhibitors (NRTIs) distribute more freely into genital secretions,4,6 all participants were receiving the same NRTIs as a control, because the aim of the study was to determine if there were differences in virologic and immunologic responses between atazanavir- or efavirenz-based highly-active antiretroviral therapy. Finally, we were not able to fully control factors that might affect penetration of drug into genital secretions, such as by sampling only at the same point in the menstrual cycle.
The significance of our findings is that clinicians can be reasonably confident that whether antiretroviral treatment is based on atazanavir or efavirenz, virologic response in the genital secretions of HIV-infected women is, on average, similar in trajectory to the response in their plasma, with about 10-fold lower HIV RNA copies/mL in genital secretions than in plasma. Moreover, higher average plasma drug concentrations may be associated with faster declines in plasma and genital HIV RNA and better gains in CD4%, especially if the drugs distribute well into tissues outside blood.
Acknowledgments
The authors gratefully acknowledge the assistance of Tricia de Leon, R.N., N.P. Sara Villanueva, L.V.N. Yvonne Lozano, L.V.N., and Ana Melendrez, R.N. for assistance in enrolling patients and completing study procedures, Keyla Whitenhill for assistance with data management, and all the patients who participated in the research study.
Support: Training for Dr. Neely and conduct of the study was supported by NIH-NIAID K23AI076106 (PI Neely). Additional data analysis was supported by NIH-NIGMS GM068968 (PI Neely) and NIH-NICHD HD070996 (PI Neely).
Footnotes
Conflicts: The authors declare no conflicts of interest.
References
- 1.HIV AIDS JUNPO. [Accessed February 8, 2014];Global Report. 2013 Available at: http://www.unaids.org/en/dataanalysis/knowyourepidemic/epidemiologypublications/
- 2.Neely MN, Benning L, Xu J, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(1):38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson CG, Cohen MS, Kashuba ADM. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S240–7. doi: 10.1097/QAI.0b013e3182986ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwara A, Delong A, Rezk N, et al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis. 2008;46(5):719–725. doi: 10.1086/527387. [DOI] [PubMed] [Google Scholar]
- 5.Launay O, Tod M, Tschöpe I, et al. Residual HIV-1 RNA and HIV-1 DNA production in the genital tract reservoir of women treated with HAART: the prospective ANRS EP24 GYNODYN study. Antivir Ther. 2011;16(6):843–852. doi: 10.3851/IMP1856. [DOI] [PubMed] [Google Scholar]
- 6.Sheth AN, Evans-Strickfaden T, Haaland R, et al. HIV-1 Genital Shedding is Suppressed in the Setting of High Genital Antiretroviral Drug Concentrations Throughout the Menstrual Cycle. J Infect Dis. 2014;210(5):736–744. doi: 10.1093/infdis/jiu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham SM, Holte SE, Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21(4):501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 8.Bremer J, Nowicki M, Beckner S, et al. Comparison of two amplification technologies for detection and quantitation of human immunodeficiency virus type 1 RNA in the female genital tract. Division of AIDS Treatment Research Initiative 009 Study Team. J Clin Microbiol. 2000;38(7):2665–2669. doi: 10.1128/jcm.38.7.2665-2669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodvold KA, George JM, Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet. 2011;50(10):637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Rennard SI, Basset G, Lecossier D, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 11.Kariman N, Afrakhte M, Hedayati M, Fallahian M, Majd HA. Diagnosis of premature rupture of membranes by assessment of urea and creatinine in vaginal washing fluid. Iran J Reprod Med. 2013;11(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team. R: A language and environment for statistical computing. R project. 2013 Available at: http://www.R-project.org/
- 13.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387(6629):188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 14.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.aidsinfonihgov. Department of Health and Human Services; [Accessed March 21, 2013]. Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 16.Storek J, Lalovic BB, Rupert K, Dawson MA, Shen DD, Maloney DG. Kinetics of B, CD4 T, and CD8 T Cells Infused into Humans: Estimates of Intravascular:Extravascular Ratios and Total Body Counts. Clinical Immunology. 2002;102(3):249–257. doi: 10.1006/clim.2001.5174. [DOI] [PubMed] [Google Scholar]
- 17.Tanton C, Weiss HA, Le Goff J, et al. Correlates of HIV-1 genital shedding in Tanzanian women. PLoS ONE. 2011;6(3):e17480. doi: 10.1371/journal.pone.0017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis. 2010;202(10):1543–1552. doi: 10.1086/656720. [DOI] [PubMed] [Google Scholar]
- 19.Homans J, Christensen S, Stiller T, et al. Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acquir Immune Defic Syndr. 2012;60(1):99–110. doi: 10.1097/QAI.0b013e31824aeaaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukura LR, Ghosh M, Fahey JV, Cu-Uvin S, Wira CR. Genital Tract Viral Load in HIV Type 1-Positive Women Correlates with Specific Cytokine Levels in Cervical-Vaginal Secretions But Is Not a Determinant of Infectious Virus or Anti-HIV Activity. AIDS Res Hum Retroviruses. 2012;28(11):1533–1539. doi: 10.1089/AID.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther. 2011;16(8):1149–1167. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 23.Eschenbach DA, Thwin SS, Patton DL, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30(6):901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]