Abstract
Maintenance of proper biomechanics of the eye lens is important for its structural integrity and for the process of accommodation to focus near and far objects. Several studies have shown specialized cytoskeletal systems such as the beaded filament (BF) and spectrin-actin networks contribute to mammalian lens biomechanics; mutations or deletion in these proteins alters lens biomechanics. Aquaporin 0 (AQP0), which constitutes ~45% of the total membrane proteins of lens fiber cells, has been shown to function as a water channel and a structural cell-to-cell adhesion (CTCA) protein. Our recent ex vivo study on AQP0 knockout (AQP0 KO) mouse lenses showed the CTCA function of AQP0 could be crucial for establishing the refractive index gradient. However, biomechanical studies on the role of AQP0 are lacking. The present investigation used wild type (WT), AQP5 KO (AQP5−/−), AQP0 KO (heterozygous KO: AQP0+/−; homozygous KO: AQP0−/−; all in C57BL/6J) and WT-FVB/N mouse lenses to learn more about the role of fiber cell AQPs in lens biomechanics. Electron microscopic images exhibited decreases in lens fiber cell compaction and increases in extracellular space due to deletion of even one copy of AQP0. Biomechanical assay revealed that loss of one or both copies of AQP0 caused a significant reduction in compressive load-bearing capacity of the lenses compared to WT lenses. Conversely, loss of AQP5 did not alter the lens load-bearing ability. Compressive load-bearing at the suture area of AQP0+/− lenses showed easy separation while WT lens remained intact. These data from KO mouse lenses in conjunction with previous studies on lens-specific BF proteins (CP49 and filensin) suggest that AQP0 and BF proteins could act co-operatively in establishing normal lens biomechanics. We hypothesize that AQP0, with its prolific expression at the fiber cell membrane, could provide anchorage for cytoskeletal structures like BFs and together they help to confer fiber cell shape, architecture and integrity. To our knowledge, this is the first report identifying the involvement of an aquaporin in lens biomechanics. Since accommodation is required in human lenses for proper focusing, alteration in the adhesion and/or water channel functions of AQP0 could contribute to presbyopia.
1. Introduction
The mammalian ocular lens consists of two types of cells, epithelial and fiber cells. Epithelial cells express Aquaporin 1 (AQP1) and AQP5, high permeability water channels, while fiber cells express AQP0, a much less efficient water channel, and AQP5.
AQP0 is the most abundant protein in the fiber cell membrane. Mutation and knockout of AQP0 causes lens cataract. It plays various roles in lens biology. First, AQP0 functions as a water channel [1,2]. AQP water channels, gap junction channels and solute transporters play significant roles in creating a microcirculation within the avascular lens. The circulation provides nourishment to central fiber cells and disposes of their metabolic wastes, thus helping to maintain transparency and homeostasis [3,4,5]. C-terminal phosphorylation affects calmodulin binding and regulation of AQP0 [6,7]. AQP0 also functions as a structural fiber cell-to-fiber cell adhesion (CTCA) protein [8,9,10,11,12]. A genetically-engineered transgenic mouse model expressing AQP1 in fiber cells of AQP0 KO mice showed only partial recovery from cataract. In this model, AQP1 more than compensated for the reduced water permeability due to KO of AQP0, implying an additional unique function for AQP0 [11,12]. Ultrastructural studies revealed loss of integrity of the characteristic fiber cell hexagonal arrangement in AQP0 null or mutant lenses. This is consistent with a role for AQP0 in maintaining the cellular architecture of the fiber cells [12,13,14,15]. A role for AQP0 in establishing and maintaining the lens refractive index gradient has also been recently suggested [16].
Does AQP0 have a role in maintaining lens biomechanics? The lens has to maintain its biomechanical properties for sharp focusing on the retina. Presbyopia generally develops with age as the ability of the lens to accommodate is compromised [17]. Two cytoskeletal structures, as the BFs and the actin-spectrin network, participate in maintaining lens biomechanics [18,19]. Lens BFs have been shown to interact and colocalize with AQP0 [20], suggesting there could be a biomechanical role for AQP0.
The present investigation was undertaken to determine the role of AQP0 in establishing and/or maintaining the biomechanical properties of the lens. For these studies we compared lenses obtained from WT, AQP5 KO, and AQP0 KO mice. Our data strongly suggest that AQP0 may impart appropriate stiffness and elasticity in different parts of the lens, probably through its water channel and CTCA functions.
2. Materials and methods
2.1. Animals
Wild type (WT), AQP5 KO (AQP5−/−), AQP0 KO heterozygous (AQP0+/−) and homozygous KO (AQP0−/−) mouse in C57BL/6J background, and WT-FVB/N mouse were used. All procedures were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by Stony Brook University Animal Care and Use Committee.
2.2. Genotyping
Genotyping by PCR using primers described by Alizadeh et al.[21] and competitive PCR using primers described by Simirskii et al.[22] were performed to confirm the absence or presence of CP49 deletion mutation in the mouse strains.
2.3. Analysis of lens AQP0, CP49 and filensin protein expression
To analyze the expression of AQP0 in WT-C57BL/6J, AQP5−/−, AQP0+/− and WT-FVB/N mouse, whole lens proteins were extracted using NuPAGE LDS sample buffer (Invitrogen). Samples were resolved in 4–12% gradient NuPAGE gel (Invitrogen). Western blotting was done as described[3,5,21] using AQP0 antibody. Antibody binding was detected using alkaline phosphatase kit (Vector Laboratories, CA).
Lens outer cortex (OC) membrane preparations (4M urea-washed) of WT-C57BL/6J, AQP5−/−, AQP0+/− and WT-FVB/N mouse were done as described previously [16]. Membrane pellets were extracted using LDS sample buffer. Equal amounts were used for Western blotting using CP49 or filensin antibody as described above.
2.4. Light microscopic and ultrastructural analyses of lenses
Light microscopic analysis was performed as described [16]. Scanning electron microscopic studies of WT and AQP0+/− lenses were performed as in Kumari et al. [12] (see Supplement 1).
2.5. Lens morphometric analysis
Lens was placed on a milled circular dimple (~50μm diameter) of a testing chamber filled with physiological saline. Sagittal images were captured and axial (ax) and equatorial (eq) diameters were calculated using a micrometer and ImageJ software (http://imagej.nih.gov/ij/). Lens volume (V) and aspect ratio (AR) were calculated: V = (4/3)πreq2rax; AR = req/rax ; rax and req are corresponding axial and equatorial radii (see Supplement 1).
2.6. Compression stress test to evaluate lens biomechanics
Compression stress testing was conducted to study biomechanical stiffness of 1-month or 2.5-month-old WT and experimental lenses as described previously[19,23], with slight modifications (Details given in Supplement 1). Each lens was subjected to gentle unrestricted compressive stress using weighed glass coverslips (18×9mm: 72mg or 18×18mm: 144mg). Sagittal images of lens shape alterations were digitized. ImageJ software (NIH) was used to measure lens ax and eq diameters which were converted to compression-stress (T): T =((d-d0)/d0), where d is the ax or eq diameter at a given load, and d0 is the corresponding ax or eq diameter at zero load. ‘T’ was plotted against load (mg).
2.7. Effect of load on lens suture integrity
Anterior side of one-month-old WT-C57BL/6J or AQP0+/− lens showing ‘Y’ suture was oriented to face the objective of an inverted confocal microscope and imaged with or without coverslip load under low and high magnifications; images were analyzed using Photoshop 9.
2.8. Statistical Analysis
SigmaPlot 10 was used for Student’s t-tests. P values ≤0.05 was considered significant.
3. Results and Discussion
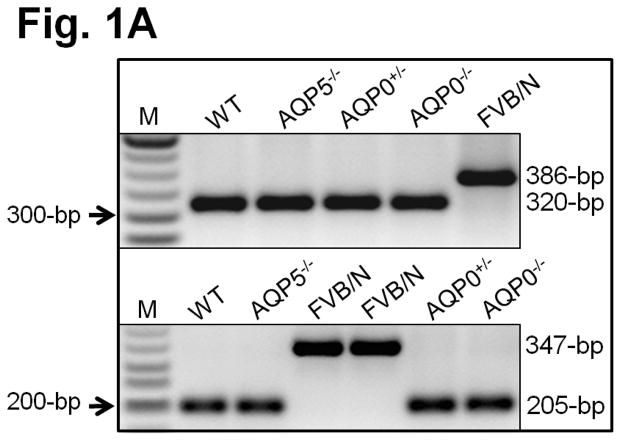
Except where otherwise indicated, lenses from mice of C57BL/6J genetic background were used in these studies. The exception is some control studies of WT lenses in FVB/N background. Initially AQP0 KO was developed in a mixed background of 129S5 and C57BL/6J-albino[2]. Mouse strains 129/SvJ [21] and FVB/N [22] do not express CP49 protein, a member of lens-specific BFs, due to a deletion mutation. CP49 participates in establishing lens biomechanics [18,19]. To study the involvement of AQP0 in lens biomechanics, the mixed strain AQP0 KO was first backcrossed with C57BL/6J for 20 generations. To ensure the data we collected on biomechanics would reflect the loss of AQP0 and not the absence of CP49 (BFs), pups were genotyped (Fig. 1A). PCR using the primers described by Alizadeh et al.[21] or Simirskii et al.[22] (competitive PCR) showed a 320-bp (Fig. 1A, top panel) or 205-bp product (Fig. 1A, bottom panel), respectively, in WT, AQP5−/−, AQP0+/− and AQP0−/− mice indicating the presence of WT CP49 allele, and a 386-bp or 347-bp product in FVB-N indicating presence of the mutant CP49 allele.
Fig. 1.
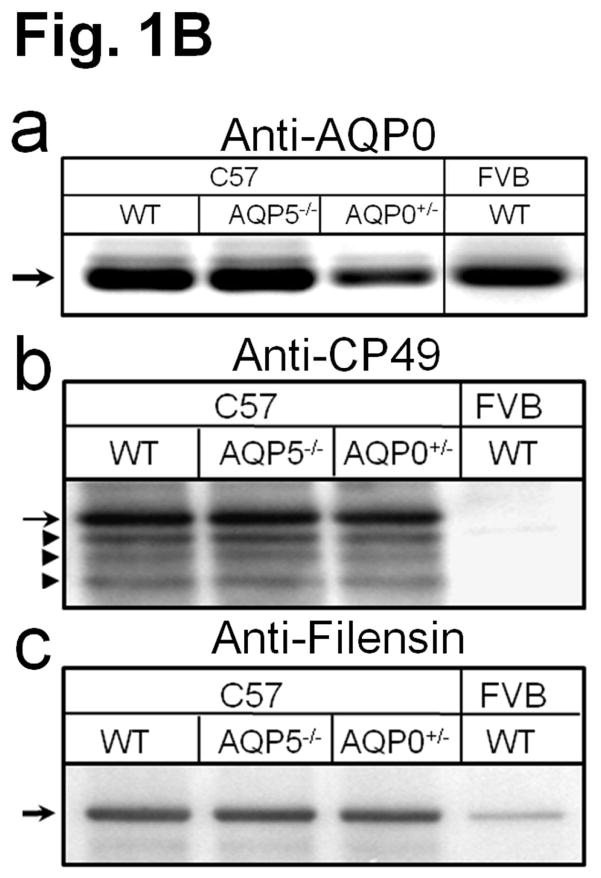
(A) Genotyping. Top panel: PCR using primers as in Alizadeh et al [21]; 320 bp, indicates presence of intact CP49 allele; 386 bp, indicates presence of mutant CP49 allele. M-Marker (50-bp Ladder). Bottom panel: Competitive PCR [22]. 205-bp indicates presence of intact CP49 allele; 347-bp indicates presence of mutant CP49 allele. (B) Western blotting of lens total membrane proteins using (a) AQP0 antibody (arrow, AQP0 : 28 kDa) and cortex total membrane proteins using (b) CP49 antibody (arrow, CP49 : ~49 kDa) or (c) filensin antibody (arrow, filensin : ~95 kDa). Arrowheads: lower immunoreactive bands to anti-CP49.
Western blotting of lens total proteins extracted from lenses of WT, AQP5−/−, and AQP0+/− mice or FVB/N mice using AQP0 antibody recognized a band of 28kDa corresponding to AQP0 (Fig. 1Ba). AQP0+/− expressed about half the quantity of AQP0. Western blotting of lens cortex membrane proteins using protein-specific antibodies for CP49 and filensin showed the presence of CP49 (~49kDa (arrow); Fig. 1Bb), and filensin (~95kDa; Fig. 1Bc) in WT, AQP5−/−, AQP0+/− mouse lenses. Similarly tested FVB/N proteins showed lack of immunoreaction with anti CP49 due to the absence of CP49 or weak binding to anti-filensin due to poor expression of filensin in the absence of CP49. CP49 and filensin are stable as BFs; loss of CP49 protein in 129/SvJ [21,24] and FVB/N [22] mice causes loss of BFs even though they express filensin transcripts.
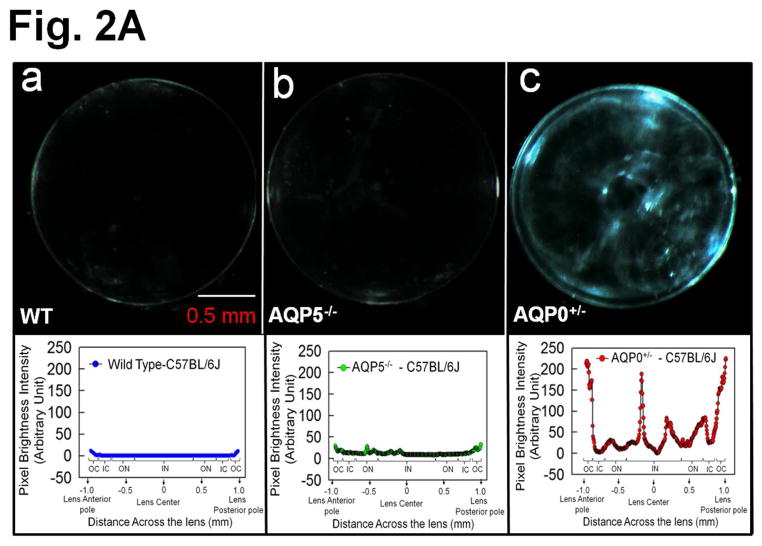
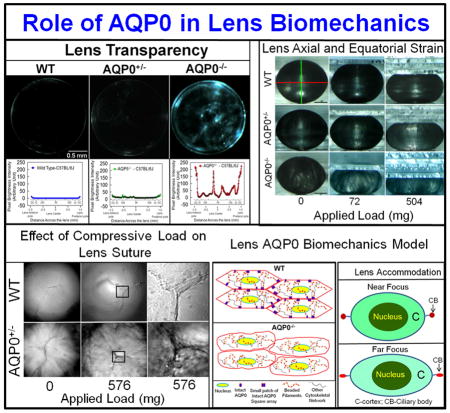
After ensuring we were using the desired genotypes, 2.5 month-old lenses were imaged for transparency. WT, and AQP5−/− lenses (Fig. 2Aa,b) were relatively transparent, but AQP0+/− lens (Fig. 2Ac) had significant opacities. Lens transparency quantification graph below each respective lens showed pixel brightness intensity values of WT at base line ~0 (Fig. 2Aa) due to near zero light scatter. Pixel brightness data indicating light scatter fluctuated at different regions of AQP0+/− lens (Fig. 2Ac) probably reflecting alterations in cellular architecture due to fiber cell swelling, reduced CTCA and lack of compact packing.
Fig. 2.
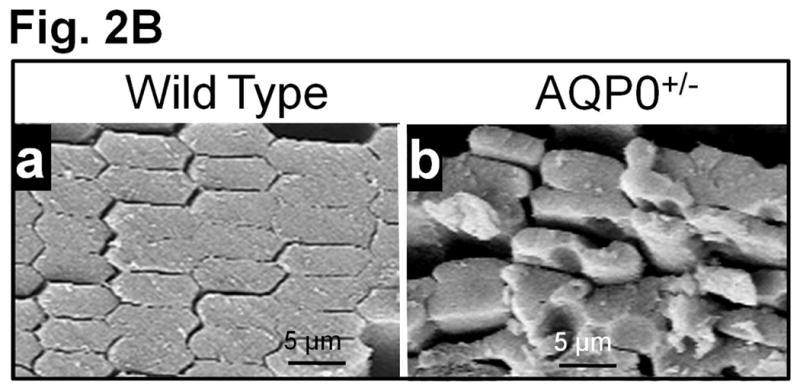
(A) Lens Transparency. (a) WT, (b) AQP5 KO (AQP5−/−). (c) AQP0 KO (heterozygous; AQP0+/−). Graph - quantification of lens transparency. Pixel brightness increases as light scatter increases due to opacity/cataract. (B) Scanning electron microscopy of 2.5-month-old (a) WT and (b) AQP0+/− lenses sectioned along polar axis.
When compared to outer cortical (OC) fiber cells of WT lenses (Fig. 2Ba), AQP0+/− lenses (Fig. 2Bb) had significantly disrupted fiber cell architecture, packing and CTCA, which caused increases in extracellular spaces. Lens OC expresses intact AQP0 [14,16] and lack of AQP0 significantly alters fiber cell architecture [12]. Intact AQP0 promotes CTCA [12,14,26] and compact arrangement of fiber cells [12,13]; it helps to reduce extracellular space and possibly, water content also from extracellular space and cytosol [12,14,16]. All these functions of AQP0 probably help to establish the required optical quality and biomechanics of the normal lens.
Lens morphometric parameters such as axial diameter, equatorial diameter, lens volume, and aspect ratio were determined as shown in Figure 3a. Lenses were imaged and the respective parameters were calculated. Comparison of one-month-old and 2.5-month-old WT and AQP0+/− (Fig. 3) lenses showed that loss of 50% of AQP0 in the AQP0+/− lens did not cause any significant (P>0.05) alterations in the axial or equatorial diameter, lens volume or aspect ratio. However, these values were significantly (P<0.001) affected in 1-month old AQP0−/− lenses (Fig. 3b–e). For the present study, we did not include 2.5 month-old AQP0−/− since the lenses develop severe cataract. As in Figure 2A, data gathered for AQP5−/− were similar to the WT (data not shown) suggesting AQP5 may not have a significant role in lens growth and morphology. AQP5 appears to act as an osmoregulator under hyperglycemic conditions [27]. Overall, the data suggest that AQP0 is critical for fiber cell structural integrity; at early stages (1 and 2.5 month-old), lens growth was not affected by 50% loss of AQP0 (AQP0+/−) protein in contrast to AQP0−/− lenses. Therefore, we selected mainly AQP0+/− lenses along with WT and AQP5−/− for testing lens biomechanics.
Fig. 3.
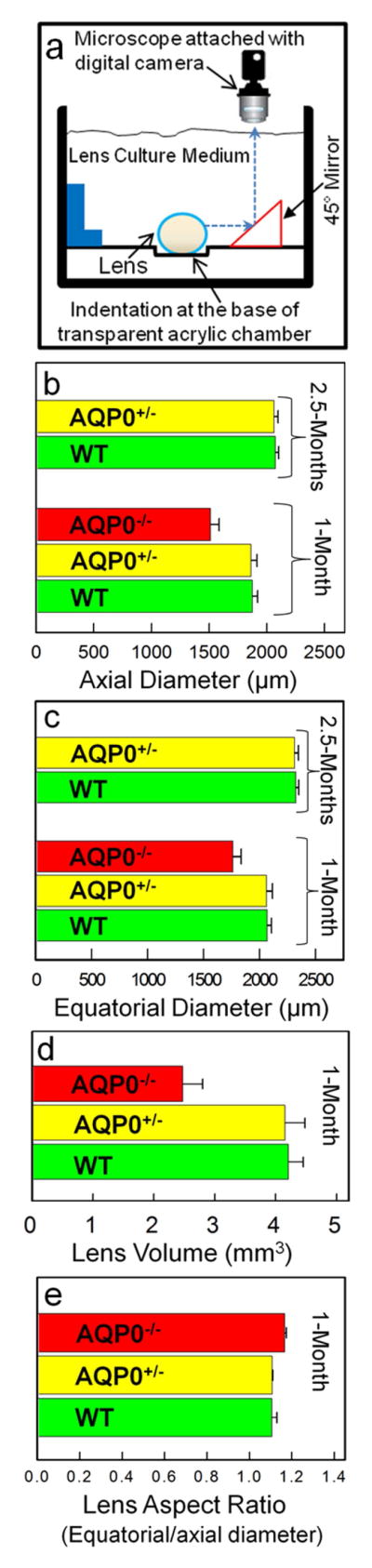
(a). Schematic of experimental set-up; a mouse lens is placed in the testing chamber with lens physiological saline and imaged. (b) Axial diameter, (c) equatorial diameter, (d) lens volume, (e) aspect ratio.
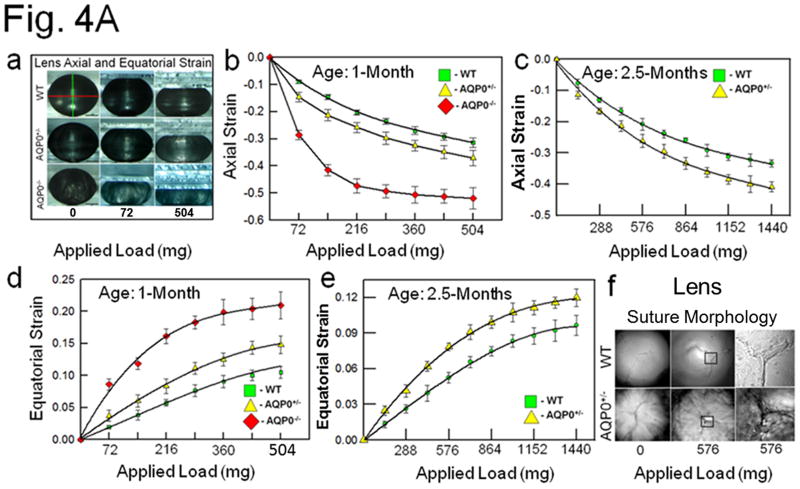
To investigate the role of AQP0 in lens biomechanics, the axial and equatorial stress techniques utilizing glass coverslip-based lens compression were conducted [19,23], with slight modifications (Fig. 4A). Lenses were imaged (as in Fig. 3a) and initial axial and equatorial diameters of one-month-old (young) and 2.5 month-old (adult) lenses of WT, AQP5−/−, AQP0+/− and AQP0−/− (only used 1 month-old lenses) mice were calculated. Biomechanical stiffness was assessed from the axial compression or equatorial expansion of the lenses due to the stress/strain exerted by the coverslip load (Fig. 4Aa). As the strain increases the stiffness decreases. Data suggest that loss of one copy of AQP0 or both significantly affects the load-bearing ability of lens, presumably, due to impairment of biomechanical stiffness (Fig. 4Aa-e). Similar observations were previously made on lenses that lacked cytoskeletal network proteins, CP49 [18] or tropomodulin 1 [19]. One month-old AQP0+/− and AQP0−/− lenses showed significant (P<0.001) loss of axial and equatorial stiffness compared to those of WT (Fig. 4Ab,d). A similar trend was observed for 2.5 month-old adult AQP0+/− lenses (Fig. 4Ac,e). Comparison of the axial (Fig. 4Ab) and equatorial (Fig. 4Ad) strains between AQP0+/− and AQP0−/− lenses showed significantly greater stiffness (or resistance to the applied load; P<0.001) in AQP0+/− than AQP0−/− suggesting the amount of AQP0 protein in the fiber cell membrane could influence the load-bearing capacity of the lens. Comparing axial (Fig. 4Ab,c) and equatorial strain (Fig. 4Ad,e) as a function of age (excluding AQP0−/−) revealed that 2.5-month-old adult lenses have significantly (P <0.001) more tolerance than one-month-old lenses, suggesting that the number of fiber cells and total amount of AQP0 protein could influence load-bearing capacity of the lens. Comparison of the effect of coverslip load (576 mg) on lens fiber cell anterior suture morphology (Fig. 4Af) showed that the suture region of AQP0+/− lens was unable to resist the load and began to separate while similar region in the WT lens remained intact. These data suggest that loss of 50% of intact AQP0 protein and reduction in CTCA [12] in the outermost cortex could severely affect lens biomechanics.
Fig. 4.
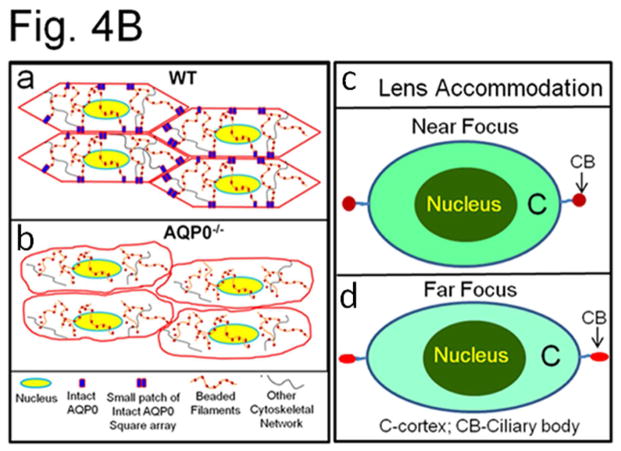
(A) (a) Compression stress test. Images of mouse lenses compressed by coverslips. Red line on WT - equatorial plane; green line - axial plane. Axial strain-load curves of: (b) 1-month-old WT, AQP0+/− and AQP0−/− mice and (c) 2.5 month-old lenses of WT and AQP0+/− mice. Equatorial strain-load curves of: (d) 1-month old WT, AQP0+/− and AQP0−/− mice and (e) 2.5 month-old lenses of WT and AQP0+/− mice. (f) Confocal imaging of lens suture morphology of WT and AQP0+/− mouse without (0 mg) or with (576 mg) coverslip squeezing. Small square areas denoted in the lenses are magnified to the right to show splitted suture area in AQP0+/− (bottom) and intact suture in WT (top). B. (a) Schematic model illustrating the role of AQP0 in lens biomechanics. Packed closer, outer cortical fiber cells of WT show characteristic hexagonal architecture with least extracellular space. Cytoskeletal proteins are tethered to AQP0 at the membrane. (b) Loss of fiber cell AQP0 causes loss of cellular architecture possibly due to loss of fiber to fiber adhesion and interaction with cytoskeletal networks. (c,d). Schematic of the possible mechanism of mammalian lens accommodation. While focusing near objects, ciliary muscles contract, the suspensory ligaments relax and the lens appears round (4Bc). While focusing far objects, the opposite events happen (4Bd). Note, the shape of the lens nucleus does not change. AQP0 may facilitate lens accommodation by establishing appropriate spatial biomechanics at the cortex and nucleus.
Data presented in this report show that AQP0 could be an important component in establishing and/or maintaining the overall lens biomechanics. Mouse lens fiber cells express AQP0 which constitutes ~45% of the total fiber cell plasma membrane proteins. AQP0 performs water channel and CTCA functions [12,14]. It has been demonstrated both in vitro [10,11,14] and in vivo [12] that both intact and cleaved forms of AQP0 conduct water and provide CTCA in the lens.
Biomechanical properties of fiber cells are probably maintained by the cytoskeletal proteins which anchor to fiber cell membrane proteins. The specialized intermediate BF cytoskeletal network is tethered to the fiber cell plasma membranes [28]. Targeted deletion of BF protein CP49 or filensin caused a dramatic loss of the highly ordered architecture of the lens [29–32]. Despite this loss of structural order, the lenses remained remarkably transparent, showing only mild light scatter [29–32]. In addition, these lenses also showed reduced load-bearing ability [18,19]. The BF networks may interact with fiber cell membranes through AQP0 [20,25], and other transmembrane proteins. CP49-KO lenses showed haphazard fiber cell arrangement, some light scatter, decreased stiffness and increased resilience compared to those of WT lenses [18,19]. Tropomodulin 1 null lenses showed reduced stiffness and load-bearing ability compared to WT lenses [19] indicating the involvement of spectrin-actin membrane skeleton in regulating lens fiber cell architecture and biomechanical properties [19].
AQP0 and BF proteins colocalize at the fiber cell membrane [20,25]. BFs (CP49 and filensin) are present primarily at the cortex; post-translationally cleaved forms are present in the cytoplasm of differentiated fiber cells in the inner cortex (IC) and absent in the nucleus [33]. The majority of AQP0 proteins become post-translationally modified by N- and/or C-terminal end cleavages. These events and the unique structural properties of AQP0 promote lens transparency and reduce light scatter, and may aid in establishing a refractive index gradient [14,16]. The lens fiber cells in the OC contain membrane protrusions with ball-and-socket conformations. During fiber cell maturation in the IC, the membranes are remodeled to large paddle-like structures. Mature fiber cell plasma membranes in the lens nucleus are remodeled to a smoother contour [12,13]. These events of fiber cell differentiation and membrane remodeling from outer to inner, at varying depths in the lens, could be critical for developing the biomechanical properties required for lens focusing and accommodation. Mutations and knockout of one or both copies AQP0 result in mild to severe cataract in an age-dependent manner. AQP0 may therefore play a significant role in biomechanics, transparency and homeostasis during lens development and aging.
Considering our data and the information available from the literature we have sketched a model to illustrate how AQP0 could be involved in biomechanical stability (Fig. 4Ba,b) and allow accommodation of the lens (Fig. 4Bc,d). In WT OC, BFs and other cytoskeletal proteins (actin, spectrin etc.) may be anchored by the abundantly expressed AQP0 (Fig. 4Ba). Lens accommodation may be facilitated in the cortex by CTCA of AQP0, by small patches of thin junctions and by the presence of cytoskeletal protein networks (Fig. 4Ba). With loss of AQP0 in the fiber cells of knockout animals, there is loss of CTCA and loss of tethering of cytoskeletal network proteins. Consequently, extracellular spaces widen and fiber cells become loose, lose their architecture, biomechanics, and structural integrity (Fig. 4Bb). Figure 4Bc,d illustrates lens accommodation by a normal eye. The cortex changes shape to accommodate while the lens nucleus does not. Normally, fiber cells undergo compaction in the IC and nucleus [34,35] to adjust the refractive index gradient to avoid spherical aberration while focusing [14,16]. Compaction, is probably achieved through dehydration which causes the fiber cells to become stiffer in a gradient-dependent manner, possibly due to gradual loss of water from the cytosol and extracellular spaces of IC fibers, and due to the absence of cytoskeletal proteins [14,16,33–36]. In addition, CTCA is favored by the formation of large patches of AQP0 thin junctions [14,16,35–37].
Why does the eye lens need a sturdy nucleus and an elastic cortex? In a biomechanical sense, stiffness at the IC and nucleus makes the inner core less flexible which probably serves as a scaffold for allowing increased flexibility for the OC during accommodation. Flexibility at the OC could also be due to high water content in the fiber cells, presence of cytoskeletal proteins in the cytosol, fewer and smaller patches of AQP0 thin junctions (~11–13 nm) and greater numbers of thick junctions formed by N-cadherin (~20–40 nm) [14,35,36]. Therefore, AQP0-aided differential biomechanics at the cortex and nucleus of the lens could be playing an important role in accommodation. Alterations in the functions of AQP0 due to natural mutations or age-onset oxidative modifications could affect lens biomechanics and manifest as presbyopia.
Supplementary Material
Highlights.
AQP0 aids in lens biomechanics
AQP0 provides lens stiffness
AQP0 is critical for lens transparency
AQP0 could play a significant role in lens accommodation in human
Alteration in the function(s) of lens AQP0 could lead to presbyopia
Acknowledgments
This research work was supported by NIH - NEI grants R01: EY20506 (K. Varadaraj), EY-012284 (A. Shiels), DE13825 (A.G. Menon), EY08747 (P.G. FitzGerald), EY06391 (R.T. Mathias).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varadaraj K, Kushmerick C, Baldo GJ, et al. The role of MIP in lens fiber cell membrane transport. J Membr Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]
- 2.Shiels A, Bassnett S, Varadaraj K, et al. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- 3.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;7:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Cheng C, Xia CH, White TW, Fletcher DA, Gong X. Connexin mediated cataract prevention in mice. PLoS One. 2010;5:e12624. doi: 10.1371/journal.pone.0012624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J, Wang H, Sun X, Varadaraj K, Li L, White TW, Mathias RT. The effects of age on lens transport. Invest Ophthalmol Vis Sci. 2003;54:7174–7187. doi: 10.1167/iovs.13-12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalman K, Németh-Cahalan KL, Froger A, Hall JE. AQP0-LTR of the CatFr mouse alters water permeability and calcium regulation of wild type AQP0. Biochim Biophys Acta. 2006;1758:1094–1099. doi: 10.1016/j.bbamem.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Maddala R, Nagendran T, de Ridder GG, Schey KL, Rao PV. L-type calcium channels play a critical role in maintaining lens transparency by regulating phosphorylation of aquaporin-0 and myosin light chain and expression of connexins. PLoS One. 2013;8:e64676. doi: 10.1371/journal.pone.0064676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costello MJ, McIntosh TJ, Robertson JD. Membrane specializations in mammalian lens fiber cells: distribution of square arrays. Curr Eye Res. 1985;4:1183–1201. doi: 10.3109/02713688509003364. [DOI] [PubMed] [Google Scholar]
- 9.Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994;33:7663–7669. doi: 10.1021/bi00190a021. [DOI] [PubMed] [Google Scholar]
- 10.Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochem Biophys Res Commun. 2009;39:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varadaraj K, Kumari S, Mathias RT. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp Eye Res. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumari SS, Eswaramoorthy S, Mathias RT, Varadaraj K. Unique and analogous functions of aquaporin 0 for fiber cell architecture and ocular lens transparency. Biochim Biophys Acta. 2011;1812:1089–1097. doi: 10.1016/j.bbadis.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Ghoul KJ, Kirk T, Kuszak AJ, et al. Lens structure in MIP-deficient mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:714–730. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- 14.Kumari SS, Varadaraj K. Intact and N- or C-terminal end truncated AQP0 function as open water channels and cell-to-cell adhesion proteins: End truncation could be a prelude for adjusting the refractive index of the lens to prevent spherical aberration. Biochim Biophys Acta. 2014;1840:2862–2877. doi: 10.1016/j.bbagen.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo WK, Biswas SK, Brako L, Shiels A, Gu S, Jiang JX. Aquaporin-0 targets interlocking domains to control the integrity and transparency of the eye lens. Invest Ophthalmol Vis Sci. 2014;55:1202–1212. doi: 10.1167/iovs.13-13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari SS, Varadaraj K. Aquaporin 0 plays a pivotal role in refractive index gradient development in mammalian eye lens to prevent spherical aberration. Biochem Biophys Res Commun. 2014;452:986–991. doi: 10.1016/j.bbrc.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013;54:ORSF54–59. doi: 10.1167/iovs.13-12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fudge DS, McCuaig JV, Van Stralen S, et al. Intermediate filaments regulate tissue size and stiffness in the murine lens. Invest Ophthalmol Vis Sci. 2011;52:3860–3867. doi: 10.1167/iovs.10-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gokhin DS, Nowak RB, Kim NE, et al. Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens. PLoS One. 2012;7:e48734. doi: 10.1371/journal.pone.0048734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsey Rose KM, Gourdie RG, et al. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- 21.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest Ophthalmol Vis Sci. 2004;45:884–891. doi: 10.1167/iovs.03-0677. [DOI] [PubMed] [Google Scholar]
- 22.Simirskii VN, Lee RS, Wawrousek EF, Duncan MK. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006;47:4931–4934. doi: 10.1167/iovs.06-0423. [DOI] [PubMed] [Google Scholar]
- 23.Baradia H, Nikahd N, Glasser A. Mouse lens stiffness measurements. Exp Eye Res. 2010;91:300–307. doi: 10.1016/j.exer.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Sandilands A, Wang X, Hutcheson AM, James J, Prescott AR, Wegener A, Pekny M, Gong X, Quinlan RA. Bfsp2 mutation found in mouse 129 strains causes the loss of CP49 and induces vimentin-dependent changes in the lens fiber cell cytoskeleton. Exp Eye Res. 2004;78:875–889. doi: 10.1016/j.exer.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Varadaraj K, Kumari SS. Involvement of beaded filament proteins in the regulation of Aquaporin 0, which is critical for lens transparency. Invest Ophthalmol Vis Sci. 2015;56:ARVO E-Abstract 3564. [Google Scholar]
- 26.Liu J, Xu J, Gu S, Nicholson BJ, Jiang JX. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J Cell Sci. 2011;124:198–206. doi: 10.1242/jcs.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumari SS, Varadaraj K. Aquaporin 5 knockout mouse lens develops hyperglycemic cataract. Biochem Biophys Res Commun. 2013;441:333–338. doi: 10.1016/j.bbrc.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FitzGerald PG. Lens intermediate filaments. Exp Eye Res. 2009;88:165–172. doi: 10.1016/j.exer.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–3727. [PubMed] [Google Scholar]
- 30.Yoon KH, Blankenship T, Shibata B, Fitzgerald PG. Resisting the effects of aging: a function for the fiber cell beaded filament. Invest Ophthalmol Vis Sci. 2008;49:1030–1036. doi: 10.1167/iovs.07-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandilands A, Prescott AR, Wegener A, Zoltoski, Hutcheson AM, Masaki S, Kuszak JR, Quinlan RA. Knockout of the intermediate filament protein CP49 destabilises the lens fibre cell cytoskeleton and decreases lens optical quality, but does not induce cataract. Exp Eye Res. 2003;76:385–391. doi: 10.1016/s0014-4835(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 32.Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44:5252–5258. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- 33.Alcala J, Maisel H. Biochemistry of lens plasma membrane and cytoskeleton. In: Maisel H, editor. The Ocular Lens: Structure, Function and Pathology. Marcel Dekker; NY: 1985. pp. 169–222. [Google Scholar]
- 34.Taylor VL, al-Ghoul KJ, Lane CW, et al. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37:1396–1410. [PubMed] [Google Scholar]
- 35.Costello MJ, Mohamed A, Gilliland KO, et al. Ultrastructural analysis of the human lens fiber cell remodeling zone and the initiation of cellular compaction. Exp Eye Res. 2013;116:411–418. doi: 10.1016/j.exer.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampighi GA, Eskandari S, Hall JE, et al. Micro-domains of AQP0 in lens equatorial fibers. Exp Eye Res. 2002;75:505–519. doi: 10.1006/exer.2002.2041. [DOI] [PubMed] [Google Scholar]
- 37.Scheuring S, Buzhynskyy N, Jaroslawski S, et al. Structural models of the supramolecular organization of AQP0 and connexons in junctional microdomains. J Struct Biol. 2007;160:385–394. doi: 10.1016/j.jsb.2007.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.