Abstract
Background
Obesity can cause pathological changes in organs. We determined the effects of chronic high fat diet (HFD) and intermittent fasting, a paradigm providing organ protection, on mouse heart.
Methods
Seven-week old CD1 male mice were randomly assigned to control, HFD and intermittent fasting groups. Control mice had free access to regular diet (RD). RD was provided every other day to mice in the intermittent fasting group. Mice in HFD group had free access to HFD. Their left ventricles were harvested 11 months after they had been on these diet regimens.
Results
HFD increased cardiomyocyte cross-section area and fibrosis. HFD decreased active caspase 3, an apoptosis marker, and the ratio of microtubule-associated protein 1A/1B-light chain 3 (LC3) II/LC3 I, an autophagy marker. HFD increased the phospho-glycogen synthase kinase-3β (GSK-3β) at Ser9, a sign of GSK-3β inhibition. Nuclear GATA binding protein 4 and yes-associated protein, two GSK-3β targeting transcription factors that can induce hypertrophy-related gene expression, were increased in HFD-fed mice. Mice on intermittent fasting did not have these changes except for the increased active caspase 3 and decreased ratio of LC3II/LC3I.
Conclusions
These results suggest that chronic HFD induces myocardial hypertrophy and fibrosis, which may be mediated by GSK-3β inhibition.
Keywords: glycogen synthase kinase-3β, high fat diet, intermittent fasting, mice, myocardial hypertrophy
1. Introduction
Obesity is an increasing health threat in the world. About one third of adults and 20% teenagers in the U.S.A. are obese [1,2]. Intake of high fat diet (HFD) contributes to this increase of obesity in American [3]. Obesity is associated with significant metabolic disturbance including hyperlipidemia and may induce pathology in various organs [4–6]. For example, HFD feeding for 6 months induces myocardial hypertrophy in mice [7]. However, the mechanisms for this hypertrophy are not fully understood yet.
The role of glycogen synthase kinase-3β (GSK-3β) in aging- and pressure overload-induced myocardial hypertrophy has been proposed [8,9]. GSK-3β can phosphorylate β-catenin, which induces β-catenin for ubiquitination and degradation. Since β-catenin can induce the expression of genes for myocardial hypertrophy, the increased degradation of β-catenin by GSK-3β reduces the expression of these hypertrophy inducing genes, such as yes-associated protein (YAP) [10,11]. In addition, GSK-3β can negatively regulate GATA binding protein 4 (GATA4), a hypertrophic marker and transcription factor [12]. Thus, GSK-3β may play an important role in HFD-induced myocardial hypertrophy.
Apoptosis and autophagy occur under physiological conditions. Apoptosis is a way to eliminate excessive cells. Autophagy is a process to clean up damaged proteins and organelles. Apoptosis and autophagy play a critical role in maintaining physiological cellular environment [13,14]. It is not known whether HFD affects these two processes in the heart. Of note, GSK-3β can regulate apoptosis and autophagy [15,16].
In this study, we started to feed 7 week old mice with HFD for 11 months to simulate adolescent onset obesity. A group of mice with intermittent fasting were included in the study. This inclusion is because our previous study showed that intermittent fasting improved learning, memory and brain structure [6]. Intermittent fasting also provides cellular protection and improves exercise tolerance [6,17]. It is not known yet whether this feeding has an effect on myocardial hypertrophy or fibrosis. Thus, our study was designed to determine the effects of HFD and intermittent fasting on myocardial hypertrophy and fibrosis and the possible role of GSK-3β in these effects.
2. Materials and Methods
All experimental protocols were approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal and experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996.
2.1. Animal groups
As described before [6], seven week-old male CD-1 wild type mice were randomly assigned into control, intermittent fasting and HFD group. Control mice had free access to regular chow (4.5% calories supplied by fat; total energy provided: 4.14 kcal/g) (rodent diet #5010, Ralston-Purina Co., St. Louis, MO). Mice in the intermittent fasting group had free access to regular chow every other day and no food on the alternate days. Mice in HFD group had free access to high-fat food (45% calories supplied by fat; total energy provided: 4.73 kcal/g) (D12451, Research Diets, Inc., New Brunswick, NJ). All animals had free access to water all the time. We choose to use 45% fat diet because this composition represents a typical Western diet [18].
2.2. Heart harvesting
The mice were sacrificed under deep anesthesia with isoflurane (Sigma-Aldrich, St Louis, MI, USA). The hearts were rapidly excised by middle thoracotomy and rinsed in normal saline. The hearts were blotted briefly against filter paper to absorb surface water and then weighed. The atrial myocardium and right ventricular myocardium were removed. Some of the left ventricles were placed in 4% phosphate-buffered paraformaldehyde and the rest of left ventricular tissues were frozen in isopantane cooled with dry ice and stored at −80°C until they are used for Western analysis.
2.3. Histology and morphometric analysis
A 2-mm slice at the mid-ventricular level was harvested and embedded in paraffin. Serial sections at 5 µm were cut and stained with hematoxylin and eosin or Masson’s trichrome (Polysciences, Inc., Warrington, PA, USA). The cross-sectional area of myocytes and the ratio of fibrosis area in the total view area were assessed in 5 randomly chosen fields in each section. Twenty determinations (5 viewing fields × 4 sections) from each heart slice were averaged to represent the values for each mouse. All results were obtained by quantitative morphometry using National Institutes of Health Image 1.60 (NIH, Bethesda, MD, USA) as we described before[19].
2.4. Immunofluorescence
After deparaffinization and antigen retrieval, 5-µm thick sections were incubated with the rabbit monoclonal anti-β-catenin antibody (1:200 dilution; catalog number: 8480; Cell Signaling Technology Inc., Danvers, MA), the rabbit polyclonal anti-microtubule-associated protein 1A/1B-light chain 3 (LC3) A/B antibody (1:200 dilution; catalog number: 4108; Cell Signaling Technology Inc.), and the rabbit monoclonal anti-cleaved caspase-3 (Asp175) antibody (1:200 dilution; catalog number: 9664; Cell Signaling Technology Inc.) overnight at 4°C. After being washed thoroughly in phosphate-buffered saline (PBS), sections were incubated in fluorochrome-conjugated donkey anti-rabbit IgG Northern Lights 557 secondary antibodies (1:200 dilution; catalog number: NL004; R&D Systems, Inc., Minneapolis, MN) for 1 h at room temperature. Sections were then counterstained with Hoechst 33342 (1:5000 dilution; catalog number: 62249; Thermo Scientific, Brookfield, WI). They were washed in distilled water before being cover-slipped using Vectorshield mounting media (Vector Laboratories, Burlingame, CA). An Olympus BX51 epifluorescence microscope (model: U-LH100-3, Olympus Optical Co., LTD, Tokyo, Japan) equipped with mercury burner and filter sets for detection of Northern Lights 557 (red) and Hoechst 33342 (blue) fluorescence, respectively, was used to examine the immunostaining of the sections.
2.5. Western blot analysis
Mouse left ventricular tissues were homogenized in lysis buffers for Western analysis. For total cellular protein extracts, the left ventricles were homogenized in RIPA buffer (Cat. No. 89901; Thermo Scientific, Worcester, MA, USA) containing protease inhibitor cocktail (Cat. No. P2714; Sigma, St. Louis, MO, USA) and Phosphatase Inhibitor Cocktail Tablets (Cat. No. 04906845001; Roche Diagnostics Corporation, Mannheim, Germany). Homogenates were centrifuged at 13,000 g at 4°C for 15 min. The supernatant was saved and its protein concentration was determined by Bradford assay. For the nuclear extracts, left ventricular tissues were lysed for 10 min in ice-cold buffer A (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.05% NP-40, 0.1 mM EDTA, 0.5 mM DTT, pH 7.9) containing protease inhibitor cocktail and Phosphatase Inhibitor Cocktail Tablets. The lysates were centrifuged for 10 min at 1000 g at 4°C. The supernatants were centrifuged at 13,000 g at 4°C for 15 min. The resulted supernatants were reserved as cytoplasmic extracts. The nuclear pellet was resuspended in the buffer B [5 mM HEPES, 1.5 mM MgCl2, 300 mM NaCl, 0.2 mM EDTA, 26% glycerol (v/v), 0.5 mM DTT, pH 7.9] containing protease inhibitor cocktail (Cat. No. P2714; Sigma, St. Louis, MO, USA), homogenized with 20 full strokes in a glass homogenizer, and incubated at 4°C for 30 min. The homogenates were centrifuged at 24,000 g for 20 min at 4°C. The supernatants were reserved as nuclear extracts. The protein concentrations of samples were measured by Bradford assay.
Equal protein samples (50 µg per lane) were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gels and then electrotransferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). After being blocked with Protein-Free T20 Blocking Buffer (Cat. No. 37573, Thermo Scientific), membranes were incubated with the following primary antibodies: the rabbit monoclonal anti-GSK-3β antibody (1:1000 dilution; catalog number: 9315; Cell Signaling Technology Inc.), the rabbit polyclonal anti-p-GSK-3β (Ser9) antibody (1:500 dilution; catalog number: 9336; Cell Signaling Technology Inc.), the rabbit polyclonal anti-p-GSK-3β (Tyr216) antibody (1:500 dilution; catalog number: sc-135653; Santa Cruz Biotechnology, Santa Cruz, CA), the rabbit monoclonal anti-β-catenin antibody (1:500 dilution; catalog number: 8480; Cell Signaling Technology Inc.), the rabbit polyclonal anti-YAP antibody (1:500 dilution; catalog number: 4912; Cell Signaling Technology Inc., Danvers, MA), the rabbit polyclonal anti-p-YAP (Ser127) antibody (1:500 dilution; catalog number: 4911; Cell Signaling Technology Inc.), the rabbit polyclonal anti-LC3A/B antibody (1:500 dilution; catalog number: 4108; Cell Signaling Technology Inc.), the rabbit polyclonal anti-p62 antibody (1:500 dilution; catalog number: 5114; Cell Signaling Technology Inc.), the rabbit monoclonal anti-cleaved caspase-3 (Asp175) antibody (1:500 dilution; catalog number: 9664; Cell Signaling Technology Inc.), the goat polyclonal anti-GATA4 antibody (1:500 dilution; catalog number: sc-1237; Cell Signaling Technology Inc.), the rabbit polyclonal anti-glyceraldehydes 3-phosphate dehydrogenase (GAPDH) antibody (1:2000 dilution; Catalog number: G9545; Sigma-Aldrich), or the rabbit polyclonal anti-histone H3 antibody (1:3000 dilution; catalog number: 9715; Cell Signaling Technology Inc.). Appropriate secondary antibodies were used. Protein bands were visualized and quantified using a G:Box equipped with Gene tools analysis software (Syngene, Frederick, MD, USA). To control for errors in protein sample loading and transferring during Western blotting, the densities of GSK-3β, phospho-YAP, cleaved caspase-3, p62, and LC3 protein bands were normalized to those of GAPDH bands. The densities of β-catenin, YAP and GATA4 protein bands were normalized to those of Histone H3 bands. The phospho-specific proteins were normalized to their total protein. The results of mice in different groups were then normalized to the average values of control mice in the same Western blot.
2.6. Total RNA extraction and real-time PCR
As we described before [20], total RNA was extracted from left ventricles using an RNeasy micro kit (Qiagen, Valencia, CA), followed by DNase digestion to eliminate genomic DNA contamination. Primers for real-time PCR were designed using the Primer Express 3.0 software (Applied Biosystems, Carlsbad, CA). The primers were as follows: YAP: forward, 5′-GAAGGAGAGACTGCGGTTGAA-3′ and reverse, 5′-TGGCTGCGCAGAGCTAATTC-3′; β-catenin: forward, 5′-AACTTGCTCAGGACAAGGAGG-3′ and reverse, 5′-CGTATGTTGCCACGCCTTCA-3′.
Real-time PCR was performed by using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Carlsbad, CA). A single PCR product was determined by amplifying each transcript and analyzing dissociation curve through 7900HT Sequence Detection Software. The PCR of the housekeeping genes GAPDH and actin also was run for each cDNA sample. The relative amount of mRNA in each sample was verified with the comparative threshold cycle method and then normalized to those of the housekeeping genes.
2.7. Statistical analysis
Data are means ± S.D. (n ≥ 4 for each experimental condition). Data were analyzed by one-way analysis of variance followed by the Tukey test or by one-way analysis of variance on ranks followed by the Tukey test. A P value < 0.05 was considered significant.
3. Results
The lipid profile of the mice used in this study were reported in our previous study [6]. They have increased low density lipoproteins [6]. The body weights of animals fed HFD were heavier than control mice (Table 1). Thus, those mice on HFD were obese and had hyperlipidemia.
Table 1.
Body and heart weights of the animals.
| Control | High fat diet | intermittent fasting | |
|---|---|---|---|
| Body weight (g) | 47.5 ± 4.9 | 68.6 ± 4.2* | 43.9 ± 4.0 |
| Heart weight (mg) | 183 ± 19 | 211 ± 24* | 181 ± 25 |
| Heart weight/body weight (%) | 3.9 ± 0.4 | 3.1 ± 0.4* | 4.2 ± 0.8 |
Results are mean ± S.D. (n = 8).
P < 0.05 compared with control.
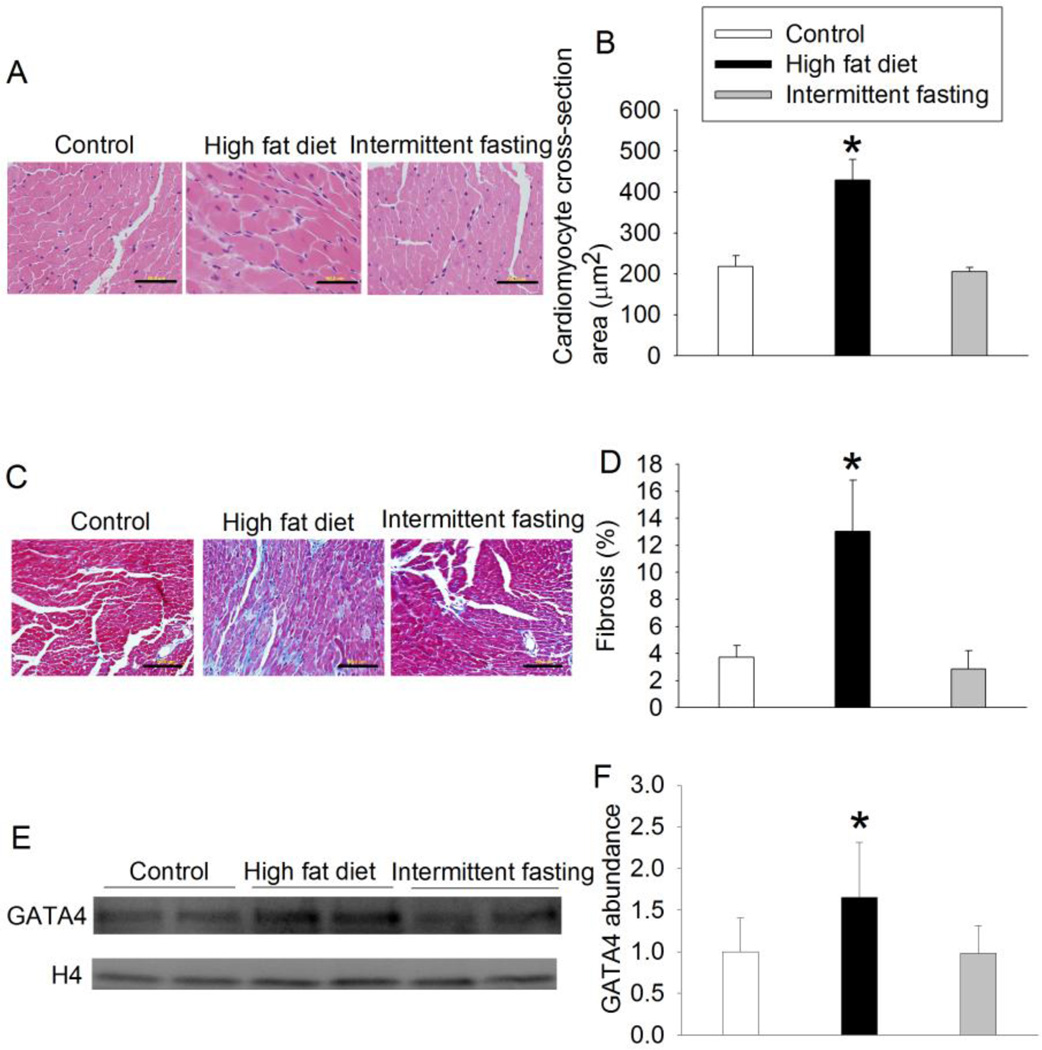
The heart weights of the mice fed HFD were heavier than those of control animals. However, the percentage of heart weights in total body weights of animals fed HFD was smaller than that of control mice (Table 1). Nevertheless, mice fed HFD had increased cardiomyocyte cross-section area and GATA4 expression when compared with control mice (Fig. 1). GATA4 is a hypertrophic marker [7], HFD also increased the amount of collagen fibers in the heart as stained by Masson’s trichrome. Intermittent fasting did not affect the cardiomyocyte cross-section area, GATA4 expression and fibrosis in the heart (Fig. 1). These results suggest that HFD but not intermittent fasting induces myocardial hypertrophy and fibrosis.
Fig. 1.
HFD-induced hypertrophy and fibrosis. A: representative cross sections of left ventricle stained with hematoxylin and eosin. Scale bar = 50 µm. B: quantification of cardiomyocyte cross-section area. C: representative cross sections of left ventricle stained by Masson’s trichrome. Collagen fibers are stained in blue. Scale bar = 50 µm. D: quantification of fibrosis. E: representative Western blotting images. F: quantification of GATA4 protein. Results are means ± S.D. (n = 4 for panels B and D, = 8 for panel F). * P < 0.05 compared with control.
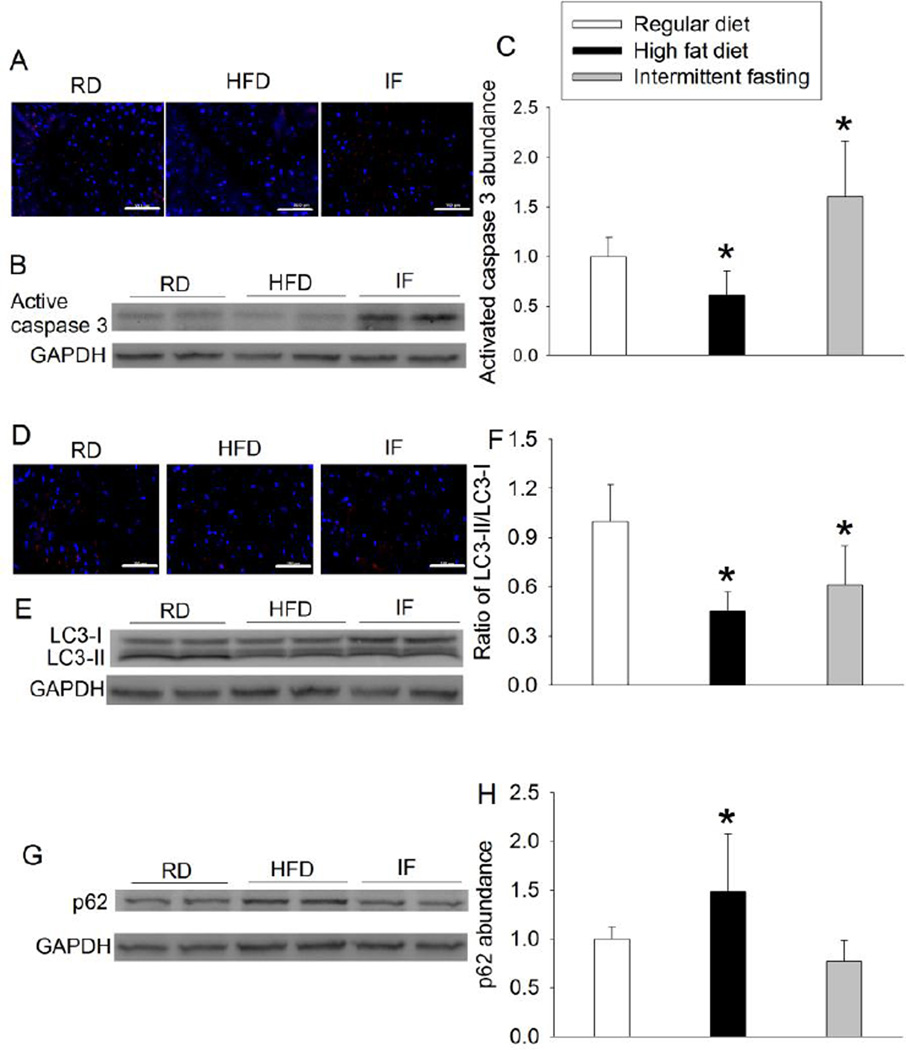
HFD decreased active caspase 3 and the ratio of LC3II/LC3I but increased p62, an autophagy adaptor (Fig. 2). These results suggest that HFD may decrease apoptosis and autophagy. Intermittent fasting increased active caspase 3 but decreased the ratio of LC3II/LC3I (Fig. 2), suggesting that intermittent fasting may enhance apoptosis and decreased autophagy in the heart.
Fig. 2.
Effects of HFD and intermittent fasting on the expression of active caspase 3, LC3 and p62 in the left ventricle. A: representative images of active caspase 3 immunostaining. Active caspase 3 is stained in red and Hoechst 33342 staining is in blue. Scale bar = 100 µm. B: Western blotting image of active caspase 3. C: quantification of active caspase 3 protein. D: representative images of LC3 immunostaining. LC3 is stained in red and blue color is Hoechst 33342 staining. Scale bar = 100 µm. E: Western blotting image of LC3. F: ratio of LC3-II/LC3-I proteins. G: Western blotting image of p62. H: quantification of p62 protein. Results are means ± S.D. (n = 6 for panels C and F, = 8 for panel H). * P < 0.05 compared with control. RD: regular diet, HFD: high fat diet, IF: intermittent fasting.
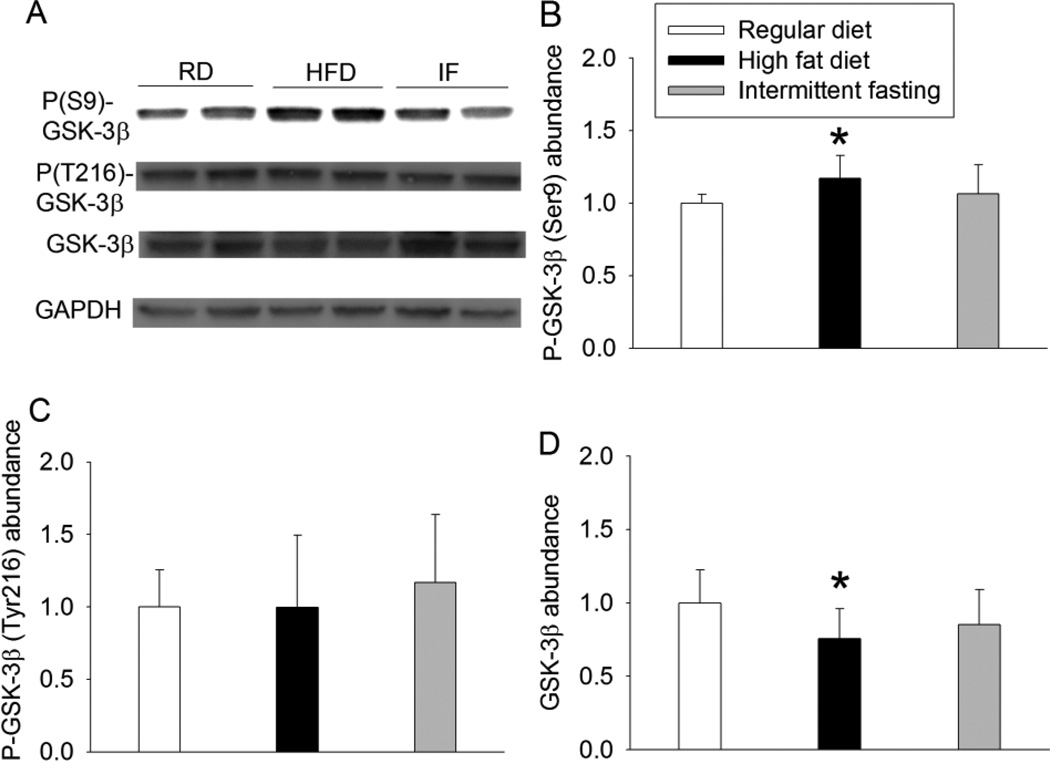
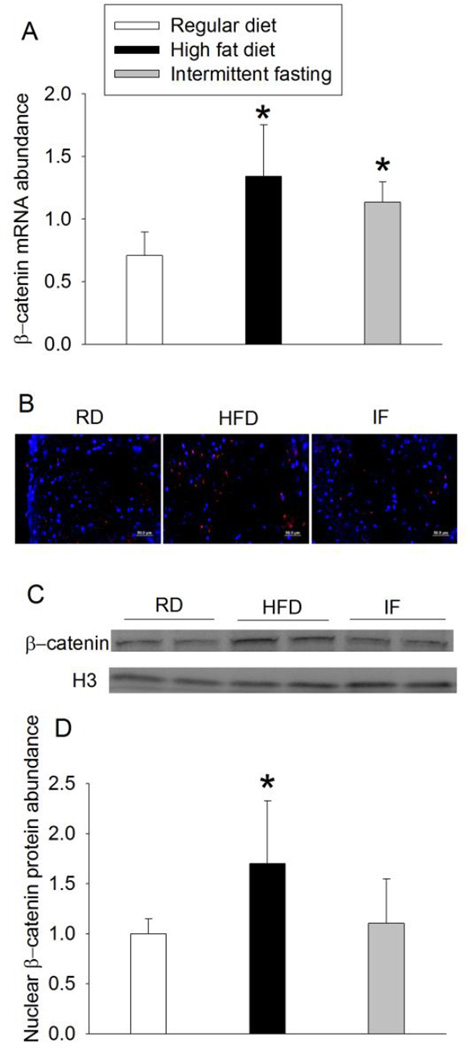
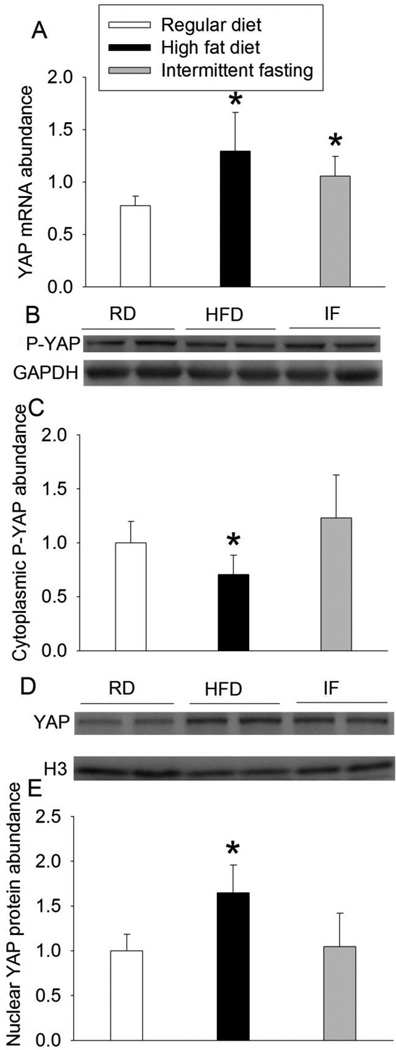
HFD increased the expression of phospho-GSK-3β at Ser9 but did not affect phospho-GSK-3β at Tyr216. HFD also decreased total GSK-3β expression. Intermittent fasting did not affect the expression of phospho-GSK-3β or total GSK-3β (Fig. 3). HFD and intermittent fasting increased β-catenin mRNA. However, only HFD increased the nuclear β-catenin expression (Fig. 4). Similarly, HFD and intermittent fasting increased YAP mRNA. Only HFD decreased the cytosolic phospho-YAP and increased nuclear YAP (Fig. 5), suggesting that HFD increases nuclear translocation of YAP.
Fig. 3.
Effects of HFD and intermittent fasting on the expression of GSK-3β in the left ventricle. A: representative Western blotting. B: quantification of phospho-GSK-3β (P-GSK-3β) at Ser9. C: quantification of phospho-GSK-3β (P-GSK-3β) at Tyr216. D: quantification of GSK-3β protein. Results are means ± S.D. (n = 6 for panels B and C, = 8 for panel D). * P < 0.05 compared with control. RD: regular diet, HFD: high fat diet, IF: intermittent fasting.
Fig. 4.
HFD-induced increase of β-catenin in the left ventricle. A: quantification of β-catenin mRNA. B: representative images of β-catenin immunostaining. β-catenin is stained in red and blue color is Hoechst 33342 staining. Scale bar = 50 µm. C: Western blotting image of nuclear β-catenin. D: quantification of nuclear β-catenin protein. Results are means ± S.D. (n = 6 for panels A and D). * P < 0.05 compared with control. RD: regular diet, HFD: high fat diet, IF: intermittent fasting.
Fig. 5.
Effects of HFD and intermittent fasting on the expression of YAP in the left ventricle. A: quantification of YAP mRNA. B: Western blotting image of cytosolic YAP. C: quantification of cytosolic YAP protein. D: Western blotting image of nuclear YAP. E: quantification of nuclear YAP protein. Results are means ± S.D. (n = 6 for panels A and C, 8 for panel E). * P < 0.05 compared with control. RD: regular diet, HFD: high fat diet, IF: intermittent fasting.
4. Discussion
We maintained the mice on HFD or intermittent fasting for 11 months. This prolonged HFD feeding clearly induced myocardial hypertrophy because the heart weights and especially the cardiomyocyte cross-section areas were increased in the HFD fed mice. Increased cardiomyocyte size has been proposed as the gold standard for diagnosing myocardial hypertrophy [21]. However, animals fed HFD had a decreased ratio of heart weight/body weight. This finding is similar to that in a recent study in which rats were fed HFD for 24 weeks [22] and suggests the inadequacy of correcting the heart weight by body weight, especially in the context of obesity, to reflect cardiac pathology as pointed out in a previous publication [23]. Also, consistent with our results, a previous study showed that HFD feeding to 4-month old BVD mice for 6 months induced myocardial hypertrophy [7], although the degree of hypertrophy was mild in the previous study. In addition, our results also showed cardiac fibrosis. These results suggest that HFD induces significant myocardial pathology. However, intermittent fasting did not change the heart weight, myocardial cross section area and fibrosis, suggesting that intermittent fasting may not have a significant effect on myocardial structure.
Our results may suggest a role of GSK-3β in the effects of HFD on myocardium. HFD increased the phospho-GSK-3β at Ser9 and decreased total GSK-3β but did not change the amount of phospho-GSK-3β at Tyr216. Since phosphorylation at Ser9 inhibits GSK-3β and phosphorylation at Tyr216 activates GSK-3β [24], our results suggest that HFD significantly inhibits GSK-3β. GSK-3β can phosphorylate β-catenin. Phosphorylation of β-catenin renders β-catenin for ubiquitination and degradation [10]. β-catenin can enhance nuclear translocation of YAP, which induces the expression of genes for myocardial hypertrophy [11]. Phospho-YAP stays in the cytosolic compartment and will not induce gene expression [11]. Our results showed that HFD increased β-catenin expression and YAP nuclear translocation. These results are consistent with the finding that HFD decreased active GSK-3β. Together, our results suggest a possible role of GSK-3β-β-catenin-YAP pathway in the chronic HFD-induced myocardial hypertrophy.
GSK-3β can phosphorylate GATA4. This process enhances nuclear export of GATA4 [12]. GATA4 is a transcription factor that regulates the expression of many cardiac-specific genes, such as atrial natriuretic factor, and cardiac hypertrophy-related genes, such as α- and β-myosin heavy chain [25,26]. Consistent with this knowledge, our results showed that HFD reduced active GSK-3β and increased nuclear GATA4. These results, together with the effects of HFD on GSK-3β-β-catenin-YAP pathway, suggest that GSK-3β inhibition may be a critical upstream event for HFD to induce cardiac hypertrophy. In supporting this suggestion, our results showed that intermittent fasting did not induce cardiac hypertrophy, GSK-3β inhibition and the nuclear expression of YAP and GATA4.
Based on our results, we propose the following mechanisms for HFD to induce cardiac hypertrophy. HFD induces GSK-3β inhibition, which reduces β-catenin degradation. β-catenin then facilitates nuclear translocation of YAP, leading to the expression of genes involved in cardiac hypertrophy. GSK-3β inhibition also results in maintaining GATA4 in the nuclei, further enhancing the expression of genes for cardiac hypertrophy. However, other mechanisms may play a role in HFD-induced cardiac hypertrophy. For example, mice with glucose transporter 4 knockout in the heart develop cardiac hypertrophy [27,28]. Glucose transporter 4 is an insulin sensitive protein [29]. It is possible that HFD induces insulin resistance [30], which then reduces glucose transporter 4 expression in the heart. Ultimately, these effects lead to cardiac hypertrophy.
It is not clear how HFD may lead to GSK-3β inhibition. Akt can phosphorylate Ser9 in GSK-3β [31–33]. HFD has been shown to activate Akt in mouse tissues [34], although the opposite effects of HFD on Akt activation have also been shown [32,35]. Multiple other protein kinases, such as protein kinase A and protein kinase C can inhibit GSK-3β [33]. HFD may work on those kinases to inhibit GSK-3β.
Our results showed that HFD and intermittent fasting increased the expression of β-catenin and YAP mRNA, suggesting that these two feeding methods increase the transcription of β-catenin and YAP. However, only HFD increased the nuclear expression of β-catenin and YAP. Nuclei are a functional location of these proteins. It is not known whether the increased β-catenin and YAP mRNAs lead to increased protein expression of β-catenin and YAP in mice with intermittent fasting. Nevertheless, studies to determine this effect may not be very significant because intermittent fasting did not increase the nuclear β-catenin and YAP protein and did not induce cardiac hypertrophy and fibrosis.
Our results showed that HFD reduced active caspase 3 and the ratio of LC3II/LC3I and increased p62, an adaptor protein for autophagy [36]. These results may suggest that HFD reduces apoptosis and autophagy. Consistent with our study, a recent study showed that HFD reduced autophagy [37]. On the other hand, intermittent fasting increased active caspase 3 and reduced the ratio of LC3II/LCI. The significance of these effects of HFD and intermittent fasting is not clear. Apoptosis and autophagy are two evolutionarily conserved processes to maintain intrinsic environment [13,14]. Interfering in these processes may affect the intrinsic environment in the heart. Interestingly, GSK-3β activation is known to increase apoptosis and decrease autophagy [15,16].
Our study has significant limitation. We subjected 7-week old mice to various feeding methods for 11 months. This design is to simulate adolescent onset obesity that lasts at least to middle age. Our results suggest a role of GSK-3β inhibition in the HFD-induced cardiac hypertrophy. However, we did not use any interventional methods to solidify this suggestion. Using pharmacological or molecular biology reagents to reverse HFD-induced GSK-3β inhibition for 11 months may be very difficult to achieve. It is also difficult to chronically inhibit GSK-3β to simulate the HFD effects.
In summary, we have shown that HFD induces cardiac hypertrophy and fibrosis. These effects may be mediated by GSK-3β inhibition. Intermittent fasting does not induce cardiac hypertrophy and fibrosis. A significant strength of our study is that animals were on HFD and intermittent fasting for a long time, simulating clinical situation. However, Interventions to reduce HFD-induced cardiac hypertrophy and fibrosis were not included in the study. Nevertheless, our results clearly indicate the harmful effects of HFD on the heart and the lack of these effects of intermittent fasting, even when these diets are given at young ages.
Acknowledgments
This study was supported by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia. The funding sources had no involvement in the experimental design, data analysis, manuscript writing and decision on submission of the manuscript for publication.
Abbreviations
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- GATA4
GATA binding protein 4
- GSK-3β
phospho-glycogen synthase kinase-3β
- HFD
high fat diet
- LC3
microtubule-associated protein 1A/1B-light chain 3
- PBS
phosphate-buffered saline
- RD
regular diet
- YAP
yes-associated protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest.
Authors’ contributions: ZZ, ZW and LL conceived the idea of the study. ZZ, ZW, LL and SP designed the study. ZW, LL and HZ carried out the experiments. ZW and ZZ analyzed the data. ZW drafted the methods and materials section. ZZ wrote the manuscript. All authors had final approval of the submitted manuscript.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S, et al. Lifestyle Risk Factors for Ischemic Stroke and Transient Ischemic Attack in Young Adults in the Stroke in Young Fabry Patients Study. Stroke. 2013;44(1):119–125. doi: 10.1161/STROKEAHA.112.665190. [DOI] [PubMed] [Google Scholar]
- 5.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann. Neurol. 2011;70(5):713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8(6):e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008;295(3):H1206–H1215. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res. Cardiol. 2011;106(6):1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi A, Matsushita M, Asai T, Masuda Z, Kuriyama M, Kanki K, et al. Effect of inhibition of glycogen synthase kinase-3 on cardiac hypertrophy during acute pressure overload. General thoracic and cardiovascular surgery. 2010;58(6):265–270. doi: 10.1007/s11748-009-0505-2. [DOI] [PubMed] [Google Scholar]
- 10.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc. Natl. Acad. Sci. U. S. A. 2003;100(8):4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. U. S. A. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J. Biol. Chem. 2001;276(30):28586–28597. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 15.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Wang H, Wang S, Xu M, Liu M, Liao M, et al. GSK3beta signaling is involved in ultraviolet B-induced activation of autophagy in epidermal cells. Int. J. Oncol. 2012;41(5):1782–1788. doi: 10.3892/ijo.2012.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. U. S. A. 2003;100(10):6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studzinski CM, Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM, et al. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP × PS1 knock-in mice. J. Neurochem. 2009;108(4):860–866. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neurosci. 2014;261(1–10) doi: 10.1016/j.neuroscience.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng C, Zuo Z. Regulatory factor X1-induced down-regulation of transforming growth factor beta2 transcription in human neuroblastoma cells. J. Biol. Chem. 2012;287(27):22730–22739. doi: 10.1074/jbc.M111.338590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorn GW, 2nd, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ. Res. 2003;92(11):1171–1175. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- 22.Leite RD, Durigan Rde C, de Souza Lino AD, de Souza Campos MV, Souza M, Selistrede-Araujo HS, et al. Resistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet rats. Metabolism. 2013;62(10):1477–1484. doi: 10.1016/j.metabol.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Dewey FE, Rosenthal D, Murphy DJ, Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117(17):2279–2287. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 24.Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004;377(Pt 1):249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Lee SJ, Jobe SM, Markham BE, Kitsis RN. cis-Acting sequences that mediate induction of beta-myosin heavy chain gene expression during left ventricular hypertrophy due to aortic constriction. Circulation. 1997;96(11):3943–3953. doi: 10.1161/01.cir.96.11.3943. [DOI] [PubMed] [Google Scholar]
- 26.Herzig TC, Jobe SM, Aoki H, Molkentin JD, Cowley AW, Jr, Izumo S, et al. Angiotensin II type1a receptor gene expression in the heart: AP-1 and GATA-4 participate in the response to pressure overload. Proc. Natl. Acad. Sci. U. S. A. 1997;94(14):7543–7548. doi: 10.1073/pnas.94.14.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J. Clin. Invest. 1999;104(12):1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domenighetti AA, Danes VR, Curl CL, Favaloro JM, Proietto J, Delbridge LM. Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca(2+) and proton flux dysregulation. J. Mol. Cell. Cardiol. 2010;48(4):663–672. doi: 10.1016/j.yjmcc.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 1980;255(10):4758–4762. [PubMed] [Google Scholar]
- 30.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54(12):3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 31.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11(3):297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Deng J, Zuo Z. High-fat diet reduces neuroprotection of isoflurane post-treatment: Role of carboxyl-terminal modulator protein-Akt signaling. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ. Res. 2002;90(10):1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 34.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2(62):ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, et al. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IkappaB kinase beta/nuclear factor-kappaB-mediated inflammatory pathways in mice. Brain. Behav. Immun. 2011;25(8):1658–1667. doi: 10.1016/j.bbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim. Biophys. Acta. 2013;1832(8):1136–1148. doi: 10.1016/j.bbadis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]