Abstract
Previous work shows altered methylation patterns in inorganic arsenic (iAs)-or cadmium (Cd)-transformed epithelial cells. Here, the methylation status near the transcriptional start site was assessed in the normal human prostate epithelial cell line (RWPE-1) that was malignantly transformed by 10 μM Cd for 11 weeks (CTPE) or 5 μM iAs for 29 weeks (CAsE-PE), at which time cells showed multiple markers of acquired cancer phenotype. Next generation sequencing of the transcriptome of CAsE-PE cells identified multiple dysregulated genes. Of the most highly dysregulated genes, five genes that can be relevant to the carcinogenic process (S100P, HYAL1, NTM, NES, ALDH1A1) were chosen for in depth analysis of the DNA methylation profile. DNA was isolated, bisulfite converted, and combined bisulfite restriction analysis was used to identify differentially methylated CpG sites, which was confirmed with bisulfite sequencing. Four of the five genes showed differential methylation in transformants relative to control cells that was inversely related to altered gene expression. Increased expression of HYAL1 (>25-fold) and S100P (>40-fold) in transformants was correlated with hypomethylation near the transcription start site. Decreased expression of NES (>15-fold) and NTM (>1000-fold) in transformants was correlated with hypermethylation near the transcription start site. ALDH1A1 expression was differentially expressed in transformed cells but was not differentially methylated relative to control. In conclusion, altered gene expression observed in Cd and iAs transformed cells may result from altered DNA methylation status.
Keywords: Inorganic Arsenic, Cadmium, DNA methylation, Prostate cancer, Gene expression
Introduction
Cadmium (Cd) and inorganic arsenic (iAs) are known human carcinogens that occur naturally in the environment (2012b; 2012a). Human exposure to Cd in the general population occurs primarily through consumption of contaminated food, although an additional source can be cigarette smoke (2012b). Cd is a human lung carcinogen and is possibly carcinogenic in the kidney, prostate, and breast (2012b; Strumylaite et al., 2014). Human exposure to environmental iAs occurs primarily by consumption of contaminated water (2012a). In humans iAs is carcinogenic in the lung, skin, and urinary bladder, and probably in the kidney, liver, and prostate (2012a).
Despite extensive research, it is unclear how either Cd or iAs cause cancer. Neither element appears to be directly mutagenic (2012b; 2012a). Several other mechanisms have been proposed for these inorganics including induction of oxidative stress, secondary genotoxic damage, and chromosomal abnormalities (Waalkes, 2003; 2012a; 2012b). Recent evidence suggests that epigenetic modifications play a role in both Cd- and iAs-induced cancer (Ren et al., 2011; Rossman and Klein, 2011). Epigenetic modifications are heritable changes in gene transcription that are not caused by changes in the DNA sequence. For example altered DNA methylation, histone modification, or microRNA expression can promote oncogene expression or silence tumor suppressor gene expression thereby creating a more favorable environment for cancer (Ren et al., 2011).1
The prostate is a potential target of both Cd and iAs carcinogenesis (2012b; 2012a). The non-tumorigenic immortalized human prostate epithelial cell line RWPE-1 is a well-characterized model for exploring the mechanism of malignant transformation by carcinogenic inorganics (Bello et al., 1997; Achanzar et al., 2001; Webber et al., 2001; Achanzar et al., 2002). Long-term exposure of RWPE-1 cells to environmentally relevant levels of Cd or iAs induces the acquisition of a malignant phenotype with both physical (e.g. invasion, colony formation) and genetic (e.g. loss of tumor suppressor gene expression) characteristics of cancer cells that form aggressive carcinoma upon inoculation into nude mice (Achanzar et al., 2001; Achanzar et al., 2002).
The cancer phenotype induced by Cd or iAs in these cells appears independent of mutagenesis or DNA damage events (Achanzar et al., 2001; Kojima et al., 2009). In this regard, Cd is not redox active so it cannot directly cause oxidative DNA damage (ODD), but given at very high concentrations it could displace redox active endogenous metals from their cellular binding sites. However, ODD was not observed during Cd-induced malignant transformation of RWPE-1 cells or the human lung epithelial cell line HPL-1D (Achanzar et al., 2001; Person et al., 2013). In fact, oncogene over-expression appears more important than oxidant stress in Cd-induced malignant transformation of liver epithelial cells (Qu et al., 2005). Furthermore, an analysis of four different rodent cell lines found no evidence of direct genotoxicity for Cd (Misra et al., 1998). These data indicate non-genotoxic or epigenetic mechanisms of transformation for Cd.
On the other hand, ODD with iAs exposure can occur in cells that biomethylate the metalloid (Kojima et al., 2009). RWPE-1 cells, however, are not able to biomethylate iAs and show no evidence of ODD during iAs-induced malignant transformation (Kojima et al., 2009). In contrast, liver cells, which will biomethylate iAs, show more rapid transformation and ODD when exposed to iAs suggesting that the ability of the target cell to methylate iAs dictates DNA damage (Kojima et al., 2009). Taken together, this lends further support for the potential role of epigenetic mechanisms in the malignant transformation by both iAs and Cd in these prostate epithelial cells.
Based on emerging evidence that epigenetic factors may be of etiologic significance in both Cd and iAs induced oncogenic phenotype, we sought to study such events in the current work. We used combined bisulfite restriction analysis (COBRA) and bisulfite sequencing to evaluate changes in DNA methylation patterns of five highly dysregulated genes in human prostate epithelial (RWPE-1) cells transformed either by chronic exposure to Cd or iAs, called CTPE or CAsE-PE cells, respectively (Achanzar et al., 2001; Achanzar et al., 2002; Merrick et al., 2013). The results were also compared to prostate epithelial cells that had previously been malignantly transformed with the direct mutagen N-Nitroso-N-methylurea (MNU) called B26 cells.
Materials & Methods
Cell Culture/ Inorganic Transformation
Cells were cultured in K-SFM (Life Technologies, Grand Island, NY) containing 50 mg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, and a 1× antibiotic/antimycotic solution. Cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 and passed weekly. Non-tumorigenic human prostate epithelium cells (RWPE-1; control) (Bello et al., 1997) were malignantly transformed following chronic exposure to Cd (termed CTPE) (Achanzar et al., 2001) or iAs (termed CAsE-PE) (Achanzar et al., 2002) as described previously. Transformed cells showed multiple molecular and cellular markers of acquired cancer cell phenotype and have the ability to form carcinoma upon inoculation into nude mice (Achanzar et al., 2001; Achanzar et al., 2002). For comparison, control RWPE-1 cells were transformed with the mutagenic organic carcinogen, MNU resulting in the WPE1-NB26 cell line (herein referred to as B26 cells), which also produces carcinoma after injection in nude mice (Webber et al., 2001).
Gene Expression
Preliminary work in this laboratory using next generation sequencing of the transcriptome identified multiple dysregulated genes in CAsE-PE cells (presented as a meeting abstract (Merrick et al., 2013)). Of the most highly dysregulated genes, five genes that were associated with stem cell function (ALHD1A1 and NES), extracellular matrix (HYAL1), cell adhesion (NTM), or aggressive cancers (S100P) were chosen for in depth analysis of expression and promoter region DNA methylation patterns across the four cell types evaluated in the current work.
Sub-confluent cells (1×106) from three flasks per cell type were collected in 1 mL Trizol (Life Technologies, Grand Island, NY) each and frozen at −80°C. Samples were warmed to room temperature, chloroform was added, and after centrifugation, the aqueous phase was removed, mixed with an equal volume of 75% ethanol, applied to an RNeasy mini-spin column (Qiagen,Valencia, CA), and purified following the manufacturer's recommendation. RNA was treated on-column with DNAse I, ethanol precipitated, resuspended in nuclease free water, and quantified on a Nanodrop 2000 spectrophotometer (Wilmington, DE). RNA quality was assessed by examination of the 18S and 28S bands following separation on an ethidium bromide stained 1% agarose gel. All agarose gels were visualized on a Kodak Gel Logic 2200 Imaging System using CareStream Molecular Imagine Software version 5.0 (Rochester, NY).
Two hundred nanograms of total RNA from three separate samples per cell type was reverse transcribed using the GeneAmp RNA PCR kit (Applied Biosystems, Grand Island, NY). The 20 μL cDNA reaction containing MuLV reverse transcriptase, random hexamers, and oligo d(T)16 primers and was carried out on a GeneAmp PCR 9700 system with incubation at 25°C for 10 minutes, 48°C for 60 minutes, and 95°C for 5 minutes. As a negative control (noRT), an identical reaction was set up for each sample except that no reverse transcriptase was added. cDNA was diluted 2-fold with nuclease free water prior to use in real time RT-PCR.
Real time RT-PCR was performed with SYBR green technology on a BioRad MyiQ 2 PCR machine (BioRad, Hercules, CA) using primers designed with Primer-BLAST (NCBI, Bethesda, MD) as previously described (Pelch et al., 2010) and synthesized by Sigma (St. Louis, MO) (Supplementary Table 1). Real time RT-PCR reactions were performed in duplicate in 25 μL with Absolute qPCR master mix containing Rox (Thermo, Lafayette, CO) according to the manufacturer's recommendations with primers at 70-100 nM each (Supplementary Table 1). The mRNA processing gene NONO was found to be constitutively expressed in the cell types investigated and was used to normalize the expression of the other mRNAs (Livak and Schmittgen, 2001). Fold changes (2ΔΔCT) relative to control RWPE-1 cells are plotted as the geometric mean +95% confidence interval (+95%CI) in Graphpad Prism version 6.00 for Mac (La Jolla, California). Fold changes were transformed logarithmically and compared by one way analysis of variance (ANOVA) and Dunnett's post-hoc test to compare transformed cells to control (RWPE-1).
Analysis of Differential DNA Methylation Profiles by Combined Bisulfite Restriction Analysis (COBRA) and Bisulfite Sequencing
Sub-confluent cells (1×106) from three flasks per cell type were lifted with trypsin, pelleted, and snap frozen in liquid nitrogen in triplicate. At processing, cell pellets were warmed to room temperature and DNA was isolated on DNEasy spin columns (Qiagen). DNA was treated with RNAse A, ethanol precipitated, and quantified on a Nanodrop 2000 spectrophotometer. DNA was bisulfite treated using the EZ DNA Methylation Direct Kit (Zymo, Irvine, CA) according to the manufacturer's directions except that the samples were passed through the column four times during processing. During bisulfite treatment unmethylated cytosines were converted to uracil whereas methylated cytosines occurring in a CpG context were retained.
PCR primers specific for bisulfite converted DNA (Table 1) were designed using MethPrimer software (Li and Dahiya, 2002) based on DNA sequences obtained from BLAT utilizing build hg19 from February 2009, of the human reference sequence (GRCh37). Primers targeted methylated or unmethylated regions within 1000 bases upstream or downstream of the transcription start site. When possible, multiple assays within this region were designed for each gene.
Table 1.
COBRA/bisulfite sequencing primers
| Primer # | Primer Sequence | Genomic Location | Amplicon Size (bp) | [Primer] nM | CpG sites | |
|---|---|---|---|---|---|---|
| ALDH1A1 | 1 | F: TGGTTTTAGTGGTTAGAGTAGTTGT | chr9(−): 75,567,973-75,568,256 | 284 | 200 | 6 |
| R: ACACTTAACTTTATTTATTCCTTTTT | ||||||
| HYAL1 | 1 | F: TTTTTTAAAGTGTTAGGATTATAGG | chr3(−): 50,341,463-50,341,302 | 162 | 500 | 4 |
| R: TACTCAAAAAATATTCTTTATCCAC | ||||||
| 2 | F: GGGTTTATTAATGTTAGATGTAGTTA | chr3(−): 50,341,353-50,340,980 | 374 | 150 | 7 | |
| R: CTACACCAAAAACTCCTAAAAAAAA | ||||||
| NES | 1 | F: GTTTGATTTATTGAGGATGGATAGA | chr1(−): 156,647,064-156,647,284 | 221 | 300 | 18 |
| R: TCTAAAAAATTAAAACCTAAATCCC | ||||||
| 2 | F: ATTAGGTTTTTGAGAATTGGTTTTT | chr1(−): 156,646,139-156,646,387 | 250 | 300 | 7 | |
| R: AAACTACAACAACTCCAAACTAAAC | ||||||
| NTM | 1 | F: AAATGGTTAAATTTTTGGTTTGG | chr11(+): 131,780,281-131,780,480 | 200 | 200 | 24 |
| R: TATCATAACAACAACTCCATCCCTA | ||||||
| 2 | F: AGGAGGGAGTTTTTTTTGGT | chr11(+): 131,781,124-131,781,399 | 276 | 200 | 27 | |
| R: AAACAAATACCCACAAACCCC | ||||||
| 3 | F: TTTTTGTTTTGGAAGTGTTT | chr11(+): 131,781,398-131,781,668 | 272 | 200 | 14 | |
| R: TACAACCCAAAAAACCAAC | ||||||
| S100P | 1 | F: ATGAGGATGTTATTGTGGTTTAGTG | chr4(+): 6,695,348-6,695,642 | 295 | 150 | 5 |
| R: CACCTTCCTCCTAAAAACTAACAAA | ||||||
| 2 | F: AGGAAGGTGGGTTTGAATTTAGTAT | chr4(+): 6,695,634-6,695,787 | 154 | 150 | 6 | |
| R: CCTAATAACTCCTTCTCCATCAACA | ||||||
Bisulfite converted DNA was amplified in a 25 μL PCR reaction using 2 μL converted DNA, 1× ZymoTaq (Zymo), and primers at 150-500 nM each (Table 1). Targets were amplified with hot start PCR with touchdown to 55°C for annealing and a final extension at 72°C for 10 minutes. A single PCR product from each sample was confirmed on an ethidum bromide stained 1.5% agarose gel.
Differential methylation was first examined using COBRA, in which the specific conversion or conservation of cytosines results in the creation of restriction endonuclease sites whose products are then visualized on an agarose gel (Xiong and Laird, 1997). Eight microliters of PCR product were digested in 25 μL reactions according to the manufacturer's instructions except that all digestion times were increased to four hours using restriction enzymes (Hpy99I, HpyCH4IV, BstUI, and TaqαI) from New England Biolabs (Ipswich, MA). The entire reaction was resolved on a SYBR Safe stained (Invitrogen) 1-5% agarose gel depending on the expected fragment sizes. Universally methylated or unmethylated DNA (Zymo) was included as a positive or negative control, respectively.
Amplicons that appeared differentially methylated by COBRA were further analyzed by bisulfite sequencing (Frommer et al., 1992). PCR amplicons were passed through DNA Clean and Concentrator-5 (Zymo) spin columns, eluted in nuclease free water, and quantified on a Nanodrop 2000 spectrophotometer. TopoTA cloning was performed at a 1:3 M vector:insert ratio. Plasmids were transformed into TOP10 One Shot Chemically Competent Cells (Invitrogen) and grown up on kanamycin and ×-gal containing plates overnight. The following day ≥ eight colonies were amplified overnight with the TempliPhi Kit (GE Healthcare, Pittsburgh, PA) according to the manufacturer's recommendations. Amplified product was diluted 1:2 with nuclease free water and 7 μL was sequenced with 1 μL M13 reverse primer using Big Dye V1.1 Terminator Kit (Life Technologies). Sequence reactions were purified using a performa DTR Ultra 96-well spin plate (EdgeBio) and electrophoresed on a Perkin-Elmer ABI 3130 sequencer. Sanger sequencing reactions were carried out by the NIEHS Sequencing Lab Group.
Sequencing results were viewed and edited in CLC Main Workbench Vs 6.9 (CLC Bio, Cambridge, MA). Sequences were then uploaded into the Bisulfite Sequencing DNA Methylation Analysis (BISMA) program (Rohde et al., 2010), where sequence identity, conversion rate, and gaps were evaluated and data images were generated. CpG locations are reported relative to the transcription start site of each gene. The overall percentage of methylation across each clone was calculated and the non-parametric Kruskal-Wallis test was used to look for differences across the four cell types. Dunn's post-hoc test was then used to compare the percentage of methylation in the transformants relative to the control cells.
Gene expression following treatment with a hypomethylating agent
To determine the extent to which DNA methylation contributed to differential gene expression cells were treated with 5-aza-dC as described previously (Benbrahim-Tallaa et al., 2007). After incorporation into DNA, 5-aza-dC inhibits DNA methyltransferase (DNMT) activity leading to DNA hypomethylation. Briefly, cells were plated in triplicate at 50% confluence and treated every 48 hours with 0 (DMSO) or 0.5 μM 5-aza-dC. After six days cells were collected and processed for analysis of gene expression as described above except that data was analyzed by two way ANOVA and all multiple comparisons post-hoc test to compare 5-aza-dC treated cells to untreated cells of the same type.
Analysis of microRNA (miRNA) expression
To determine if ALDH1A1 expression is regulated by miRNA expression, total RNA including nucleotides >18 bases in length was isolated from untreated and 5-aza-dC treated cells using the miRNeasy mini kit (Qiagen) following the manufacturer's recommendations. RNA was quantified and quality was evaluated as described above.
MicroRNAs that may regulate ALDH1A1 were identified by searching the literature (miR-29a) (Muniyappa et al., 2009) or searching within the microRNA (www.microRNA.org) and MicroCosm Targets (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/) databases as outlined by Kuhn et al. (Kuhn et al., 2008). Only microRNAs identified by both databases were evaluated. TaqMan microRNA real time RT-PCR assays (Applied Biosystems) were performed on 5-aza-dC treated or untreated samples for the seven miRNAs obtained by this method (Supplementary Table 2). Briefly, 10 ng RNA was used for each 15 μL reverse transcription reaction that contained target specific primers. Subsequently, 1.33 μL of reverse transcribed target were used in each 20 μL real time RT-PCR reaction that contained target specific primers and probe. The miRNA RNU6B was found to be constitutively expressed in the cell types investigated and was used to normalize the expression of the other miRNAs as described above.
Results
Differential gene expression in inorganic transformants
Our preliminary work using next generation sequencing of the transcriptome in CAsE-PE cells identified multiple dysregulated genes (Merrick et al., 2013). We selected five highly expression-altered genes that were associated with stem cell function (ALHD1A1 and NES), extracellular matrix (HYAL1), cell adhesion (NTM), and aggressive cancers (S100P) for in depth analysis in the current work. Relative expression of these five genes was assessed by real time RT-PCR. Three genes were found to be upregulated and two genes downregulated in the Cd and iAs transformants (Table 2). The magnitude of upregulation of HYAL1 and S100P was similar in both the Cd and iAs transformants. Expression of HYAL1 was increased 49- or 25-fold and expression of S100P increased 50- or 41-fold in Cd or iAs transformed cells, respectively. Expression of both NTM and NES was decreased in the metal transformants. Expression of NTM was decreased >1000-fold in Cd and iAs transformants. For example, NTM was detected at a cycle threshold (Ct) value of 27 in controls, but was completely undetected in Cd or iAs transformants. Expression of NES was decreased 5.2 or 7.4-fold in Cd or iAs transformants, respectively. Expression of ALDH1A1 was upregulated 10-fold in iAs transformants and downregulated 6.1-fold in Cd transformants. With the exception of ALDH1A1, expression of these genes in the cells transformed with the directly mutagenic organic carcinogen MNU (B26 cells) was more similar to what was observed in the controls (Table 2). That is, the magnitude of effect in the B26 cells was smaller than in the inorganic transformants.
Table 2.
Relative gene expression in transformed cells.
| Gene Name | CTPE | CAsE-PE | B26 |
|---|---|---|---|
| ALDH1A1 | −6.1* (−15, −2.4) | 10* (5.4, 22) | −20* (−117, −2.8) |
| HYAL1 | 49* (47, 51) | 25* (24, 27) | 1.1 (−1.1, 1.2) |
| NES | −5.2* (−8.3, −3.2) | −7.4* (−14, −3.9) | 1.7* (1.0, 3.0) |
| NTM | >−1000*a | >−1000*a | −23* (−36, −15) |
| S100P | 49.5* (43, 57) | 41.0* (36, 47) | −3.6* (−5.4, −2.4) |
Fold change relative to control RWPE-1 cell equal to 1.0.
p <0.05 by one-way ANOVA of log transformed fold change values followed by comparison to RWPE-1 control cells by Dunnett's multiple comparisons post-hoc test. (95% CI)
NTM detected at a cycle threshold of 27 in control RWPE-1 cells and undetected in CTPE or CAsE-PE cells.
Differential methylation profiles detected by COBRA and bisulfite sequencing
DNA was isolated, bisulfite converted, and COBRA was used to identify differentially methylated CpG sites within 1000 bases of the transcription start site of selected genes. When possible, two or more amplicons were evaluated in order to increase coverage of the CpG sites near the transcription start site. In COBRA, restriction digestion sites are only maintained after bisulfite conversion when a CpG site is methylated, thus allowing for the detection of differential methylation patterns. Representative agarose gels indicating differential methylation by COBRA are shown in Figures 1B, 2B, 3B, and 4B. COBRA also detected sites that are not differentially methylated, as in Figures 3B (amplicon #1 digested with Hpy99I, amplicon #2 digested with TaqαI, and amplicon #3 digested with HpyCH4IV), and 4B (amplicon #1 digested with Hpy99I or BstUI and amplicon #2 digested with HpyCH4IV).
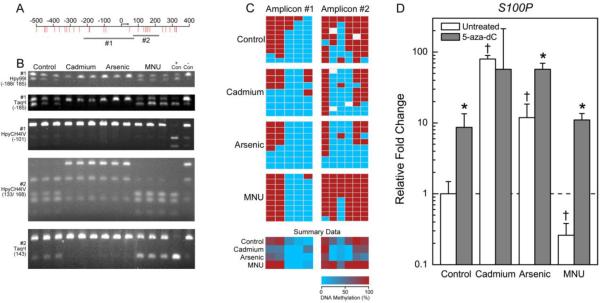
Figure 1.
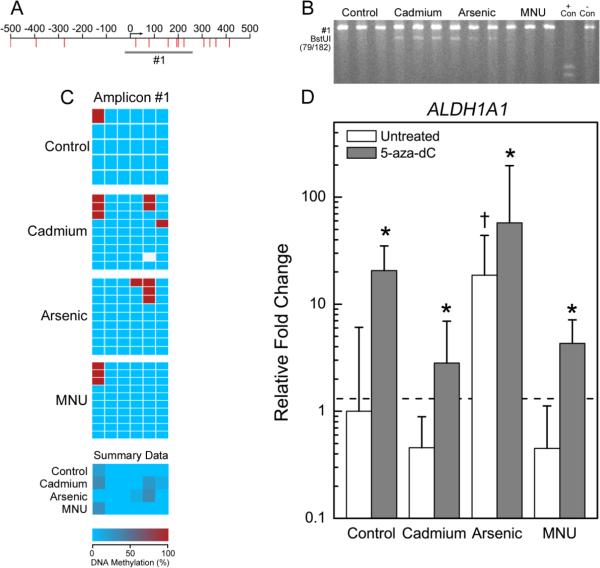
Differential methylation pattern of S100P in transformed cell lines. A) Genomic locus showing location of CpGs (red lines) relative to the transcription start site (arrow). Genomic DNA was bisulfite converted and PCR amplified (gray bars). B) Representative agarose gels from COBRA analysis. PCR amplified bisulfite converted DNA was enzymatically digested with BstUI, Hpy99I, HpyCH4IV, or TaqαI as indicated. Triplicate samples of each cell type and universally methylated (+ Con) or unmethylated (− Con) DNA were separated on each gel. C) Bisulfite sequencing results. Each box represents a CpG site where red is a methylated CpG site and blue is an umethylated CpG site. White boxes represent locations where a base call could not be accurately made. Each row is a different clone that was sequenced. Summary data for each cell type is also displayed. D) Relative gene expression in untreated cells (open bars) or cells treated with 0.5 μM 5-aza-dC (gray bars). Data is geometric mean of the fold changes + 95% confidence interval. Data were analyzed by two way ANOVA and all multiple comparisons post-hoc analysis. *p<0.05 for comparisons of treated and untreated cells of the same type and †p<0.05 for comparison of untreated transformants to untreated controls.
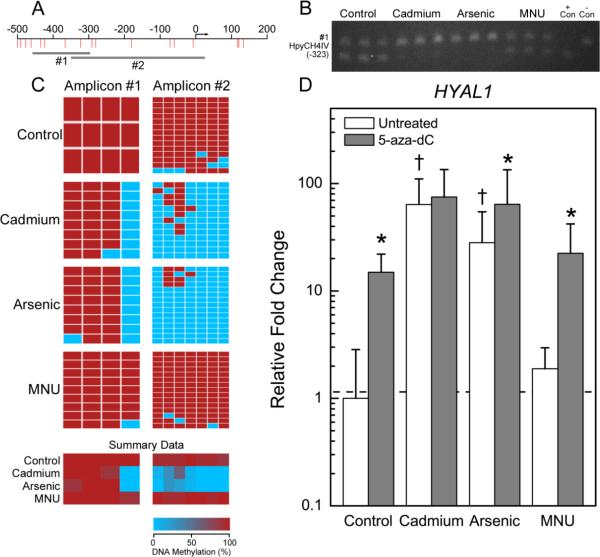
Figure 2.
Differential methylation pattern of HYAL1 in transformed cell lines. See Figure 1 legend for description of panels A-D.
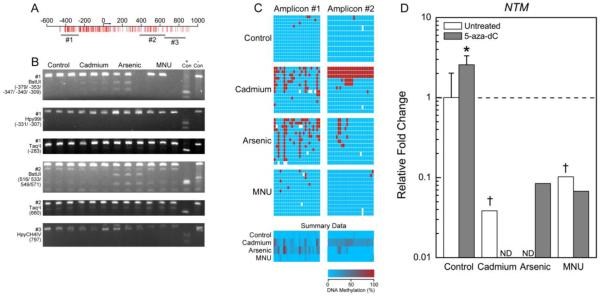
Figure 3.
Differential methylation pattern of NTM in transformed cell lines. See Figure 1 legend for description of panels A-D. ND indicates not detected.
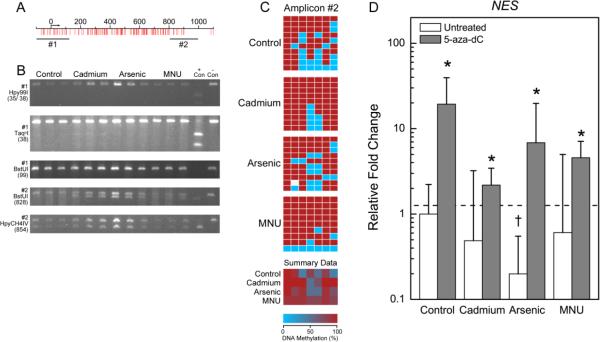
Figure 4.
Differential methylation pattern of NES in transformed cell lines. See Figure 1 legend for description of panels A-D.
Bisulfite sequencing was performed on amplicons that were identified as differentially methylated by COBRA. Bisulfite sequencing confirmed the methylation status of all CpGs interrogated by both methods, provided information on sites that could not be interrogated by COBRA, and indicated that S100P, HYAL1, NTM, and NES were differentially methylated in Cd and iAs transformants when compared to controls (Table 3). S100P and HYAL1 were hypomethylated whereas NTM and NES were hypermethylated in the inorganic transformants. In general, the methylation status in B26 cells was quite similar to controls for the genes examined.
Table 3.
Percentage (%) of methylated CpGs across the entire amplicon.
| Gene name | Amplicon | RWPE-1 | CTPE | CAsE-PE | B26 |
|---|---|---|---|---|---|
| S100P | Amplicon #1 | 42.0 | 25.7 | 22.5 | 42.2 |
| S100P | Amplicon #2 | 71.7 | 43.9 | 29.2* | 90.0 |
| HYAL1 | Amplicon #1 | 100 | 71.9* | 71.9* | 97.2 |
| HYAL1 | Amplicon #2 | 92.9 | 15.4* | 7.6* | 97.1 |
| NTM | Amplicon #1 | 1.4 | 19.0* | 25.5* | 2.4 |
| NTM | Amplicon #2 | 0.4 | 29.4* | 5.7 | 0.6 |
| NES | Amplicon #2 | 68.6 | 88.6 | 75.4 | 84.1* |
| ALDH1A1 | Amplicon #1 | 3.3 | 11.3 | 7.4 | 5.0 |
The percentage of methylated CpGs in each clone were compared by Kruskal-Wallis test
p <0.05 relative to RWPE-1 by Dunn's post-hoc analysis.
Methylation analyses revealed that S100P was hypomethylated in Cd and iAs transformants at CpG sites +132 and +149 (relative to the transcription start site) (Figure 1C). Likewise, HYAL1 was hypomethylated in inorganic transformants at CpG sites −324, −291, −281, −181, −72, −60, and −7 (Figure 2C).
NTM was generally unmethylated in controls and B26 cells (Table 3), but there was increased methylation present in both of the inorganic transformants, specifically at CpG sites −383, −355, −305, and −273 (Figure 3C). Whereas some CpG sites were only hypermethylated in the Cd transformants (−380, −341, +541, +545, +548, +550, and +559), other sites were only hypermethylated in the iAs transformants (−378, −353, −317, −260, +515, and +517). Bisulfite sequencing also revealed an interesting pattern of methylation within CTPE cells in which there appears to be at least three unique populations of cells: those with methylation at all CpG sites from +433 to +659, those with methylation only at CpG sites from +541 to +559, and those that are unmethylated at CpG sites from +433 to +659.
NES was hypermethylated at +827 and +878, in the Cd transformants (CTPE) (Figure 4C). Because there was no indication of differential methylation by COBRA, NES amplicon #1 was not sequenced.
ALDH1A1 was largely unmethylated across all cell types (Figure 5C, Table 3). COBRA suggested increased methylation in inorganic transformants at CpGs at +78 and/or +201. Bisulfite sequencing suggested partial methylation at the CpG at +201 in the Cd and iAs transformants.
Figure 5.
Methylation pattern of ALDH1A1 in transformed cell lines. See Figure 1 legend for description of panels A-D.
Effect of treatment with hypomethylating agent
As a means of confirming that altered gene expression was a result of differential DNA methylation, each of the various cell lines were treated with 5-aza-dC to inhibit DNMT activity thereby resulting in DNA hypomethylation. Cells were treated with 5-aza-dC for six days at which time gene expression was again evaluated.
For the two up-regulated genes, S100P and HYAL1, treatment with 5-aza-dC had an expected minimal effect on the expression in the inorganic transformants. Treatment with 5-aza-dC did, however, result in a marked increase in S100P and HYAL1 gene expression in the control and B26 cells (Figures 1D and 2D). For example, expression of S100P was increased 8.6- and 11-fold and expression of HYAL1 was increased 14.9- and 22-fold in the 5-aza-dC treated control and B26 cells, respectively, relative to the untreated RWPE-1 control cells.
The opposite effect was observed for the hypermethylated gene NES and the unmethylated gene ALDH1A1. The expression of NES and ALDH1A was increased in all cell types following 5-aza-dC treatment (Figures 4D and 5D). Expression of NTM was relatively unchanged in control and B26 cells following 5-aza-dC treatment, and remained undetectable in the CTPE and CAsE-PE cells (Figure 3D).
Expression analysis of miRNAs targeting ALDH1A1
The expression of miRNAs that potentially target ALDH1A1 was evaluated in cells that were untreated or treated with 5-aza-dC. There were no cell type specific changes in expression of miR-21, −23a, −23b, −29a, −132, −221 or −222. Treatment of cells with 5-aza-dC had no effect on the expression of the miRNAs assessed (Supplemental Figure 1).
Discussion
The present work indicates the altered gene expression observed with acquired malignant phenotype in Cd and iAs transformation of human prostate epithelial cells can arise from epigenetic factors, and specifically in this case, altered DNA methylation status. Here, five genes that were highly dysregulated in preliminary next generation sequencing of the transcriptome of CAsE-PE cells (Merrick et al., 2013) underwent in-depth DNA methylation profile analysis. The genes of interest were linked to stem cell function/dysfunction, extracellular matrix, cell adhesion, or aggressive cancers and can be relevant to cellular transformation (Ekici et al., 2004; Liu et al., 2004; Kasper, 2008; Ma and Allan, 2011; Jiang et al., 2012). Altered DNA methylation near the transcriptional start site appeared critical in regulating four of the five genes in the inorganic transformants. When the parental line is transformed with the direct acting alkylating carcinogen MNU there is little evidence of altered DNA methylation. Thus, although all three transformed cell lines produce xenograft carcinomas in mice, the mechanism by which they acquire a malignant phenotype appears quite distinct. This is consistent with prior work showing little evidence of ODD during Cd- or iAs-induced transformation of these prostate epithelial cells or other cell types (Qu et al., 2005; Kojima et al., 2009; Person et al., 2013) Given the large differences in exposure time needed to achieve transformation by MNU, Cd, or iAS (four hours, eight weeks, or 29 weeks, respectively) it is not surprising that the mechanism driving transformation with each agent is unique (Achanzar et al., 2001; Webber et al., 2001; Achanzar et al., 2002)). In the case of all five genes interrogated, exposure to Cd or iAs altered the gene expression in such a way that would favor carcinogenesis. Yet it is unclear if the changes in promoter region DNA methylation and expression of the genes observed in the current work are a cause or consequence of transformation. In this regard, a time course study would be of great value, comparing acquisition of cellular and molecular cancer cell characteristics with iAs and Cd and epigenetic changes.
The genes studied in the present work all have potential relevance to carcinogenesis. For instance, S100P is over-expressed in many malignancies including prostate carcinomas and is linked with particularly aggressive tumors (Jiang et al., 2012) increased cell growth, and anchorage independent growth of prostate cancer cells in soft agar (Basu et al., 2008). Proteins in the S100 family are often regulated by DNA methylation (Lesniak, 2011). Indeed, S100P is hypomethylated in human prostatic cancers and gene expression is increased in a non-cancerous prostate cell line by 5-aza-dC treatment (Wang et al., 2007). In our study S100P expression was highly increased in both Cd and iAs transformants and the gene was hypomethylated upstream of the transcription start site. Furthermore, 5-aza-dC treatment increased S100P expression in all cells except the Cd transformant, which already had a very high level of expression. This suggests that S100P is regulated by DNA methylation in the inorganic transformed cells, which would increase the anchorage independent cell growth and tumor forming potential in these cells.
The HYAL1 gene encodes an enzyme that degrades hyaluronan, a component of the extracellular matrix. HYAL1 expression is considered an angiogenic prognostic marker for prostate cancer (Ekici et al., 2004). HYAL1 expression was greatly increased in the inorganic transformants and was hypomethylated upstream of the transcriptional start site. HYAL1 expression was increased in all cell types after 5-aza-dC treatment, but most notably in the control and B26 cell lines that were highly methylated in the promoter region. The observed hypomethylation occurs in the region that is important in the transcriptional regulation of HYAL1 in prostate cancer cells. These CpGs occur in a region of overlapping SP1/Egr-1 transcription factor binding sites and near NFκB and AP-2-binding sites (Lokeshwar et al., 2008). The methylation status of these key CpGs regulates which transcription factors bind in prostate cells that differentially express HYAL1, suggesting a mechanism by which differential methylation of HYAL1 results in altered gene expression. Increased HYAL1 in the inorganic transformants likely promotes growth and invasion by remodeling the extracellular environment.
Our preliminary genome wide expression and methylation analyses in control and iAs transformed cells suggested that two poorly expressed genes, NTM and NES, were hypermethylated in CAsE-PE cells (Merrick et al., 2013). NTM is a cell surface adhesion molecule and a member of the IgLON immunoglobin superfamily (Liu et al., 2004). Little is known about NTM expression in normal or cancerous prostate tissue. In our work, NTM expression was detected at modest levels in control cells but was undetectable in inorganic transformants. Since NTM acts in cell adhesion this could suggest that the iAs and Cd transformed cells may have lost contact inhibition, which would be consistent with their greater capacity for invasion (Achanzar et al., 2001; Achanzar et al., 2002; Liuet al., 2004). NTM was largely unmethylated in the control and B26 cells and hypermethylated in the inorganic transformants, however, treatment with 5-aza-dC did not consistently activate NTM expression in the inorganic transformants. An interesting pattern of methylation was, however, detected in the Cd transformed cells raising the possibility that there may be multiple subsets of differentially methylated cells. Specific epigenomic and growth characteristics of such putative subpopulations within Cd transformants should be explored.
NES is a marker of a wide range of multi-lineage progenitor cells (Wiese et al., 2004), is detected in various tumor types including prostate cancers, and is often associated with a poor prognosis (Kasper, 2008). Transcriptional regulation of NES in adults is not well understood (Wiese et al., 2004), but seems to be linked to disease progression in prostate cancer cells (Kleeberger et al., 2007). In this work, decreased NES expression in the inorganic transformants was linked to gene hypermethylation. Indeed, preliminary genome-wide analysis in our laboratory shows a 27-fold increase in methylation near the promoter region of NES in the iAs transformed cells (Merrick et al., 2013). NES expression was increased by 5-aza-dC treatment in all cell types, suggesting DNA methylation regulates NES expression. However other controlling factors may also be at play, since in a mouse neuronal cell line histone acetylation, rather than DNA methylation, is required to regulate NES expression (Han et al., 2009). Whereas initial reports indicated that NES is involved in prostate cancer cell migration, invasion, and metastasis (Kleeberger et al., 2007), more recent work demonstrates an inhibitory role for NES in prostate cancer cell migration and invasion (Hyder et al., 2014). In the work by Hyder et al., (2014), decreased NES expression regulates phosphorylated focal adhesion kinase activity, integrin localization on the cell membrane, and extracellular matrix proteolysis resulting in larger cells that spread out upon the tissue culture growth surface and have increased invasive properties. Interestingly, the iAs transformed cells in our study often show a similar phenotype, with increased size, more extensive spreading, and increased invasion. The downregulation of NES in Cd and iAs transformants may, therefore, reflect a mechanism more similar to that identified by Hyder et al.
ALDH1A1 is a marker of normal and cancer stem cells (Ma and Allan, 2011). Despite being differentially expressed in the transformed cell lines, there were no differences in the methylation pattern of ALDH1A1 between the inorganic transformants and control cells. However, 5-aza-dC treatment increased ALDH1A1 expression in all four cell types, implicating methylation changes as relevant to expression and suggesting several possibilities. One is that the bisulfite sequencing failed to cover the key regulatory element. However, the closest CpG island is over 1.5 million bases upstream of the transcriptional start site, thus making direct regulation by promoter region DNA methylation less likely. More likely is the possibility that ALDH1A1 expression is regulated by an upstream signaling factor that is methylation sensitive. To explore this, expression of seven microRNAs predicted to target ALDH1A1 was assessed after 5-aza-dC treatment but none appeared to regulate ALDH1A1 expression.
An additional possibility is that the increased ALDH1A1 expression might be related to an increased number of cancer stem cells in the iAs transformants but not in the Cd and MNU transformants as previously reported (Tokar et al., 2010; Tokar et al., 2011). This may also explain why ALDH1A1 was differentially expressed, being upregulated in the CAsE-PE but downregulated in the CTPE and B26.. This, however, does not explain the further increase in ALDH1A1 expression following 5-aza-dC treatment and thus, the mechanism by which ALDH1A1 expression is increased in response to iAs or 5-aza-dC remains to be elucidated. The differential expression of ALDH1A1 amongst the transformants is likely further evidence that even between Cd and iAs different mechanisms are used to achieve malignant transformation, which is reflected in the length of time each chemical requires to induce transformation.
Cd and iAs are well known human carcinogens yet the carcinogenic mechanism is poorly understood. The RWPE-1-based isogenic cell model is useful for studying the epigenetic mechanisms associated with inorganic transformation of prostate cells since RWPE-1 cells have a relatively normal global DNA methylation profile (Severson et al., 2012). In this model, Cd transformants have increased global DNA methylation content, increased DNMT activity, and DNMT3b transcript expression (Benbrahim-Tallaa et al., 2007). In contrast, iAs transformants have decreased global DNA methylation and DNMT activity (Benbrahim-Tallaa et al., 2005). This global hypomethylation is often attributed to depletion of S-adenosyl-methionine (SAM), the methyl-donor during both conversion of cytosine to 5-methylcytosine and biomethylation of iAs. Chronic exposure to iAs can deplete SAM reducing the availability for DNA methylation (Reichard and Puga, 2010). Although RWPE-1 cells do not biomethylate iAs they do, however, have depleted levels of SAM due to adaptive mechanisms that shuttle more homocysteine to the transsulfuration pathway in the presence of chronic iAs for iAs efflux (Coppin et al., 2008; Kojima et al., 2009). Furthermore, although global hypomethylation occurs with chronic iAs exposure, there are also significant amounts of gene specific hypermethylation (Severson et al., 2012).
In summary, we studied altered DNA methylation near the transcriptional start site as a basis for the differential expression of five of the most highly dysregulated genes in inorganic transformed prostate cell lines. In this analysis the inorganic transformants appear more similar to each other while the B26 cells appear more similar to the control in both expression and DNA methylation status. This suggests that the mechanism of transformation by these inorganics is distinct from the organic alkylating carcinogen MNU. Finally, while the genes examined in this work are associated with the malignant process, it is unclear if the changes in methylation and expression are causes or consequences of oncogenic transformation.
Supplementary Material
Cd and iAs are known human carcinogens, yet neither appears directly mutagenic
Prior data suggest epigenetic modification plays a role in Cd or iAs induced cancer
Altered methylation of four misregulated genes was found in Cd or iAs transformants
The resulting altered gene expression may be relevant to cellular transformation
Acknowledgments
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government. The authors thank Drs. Olive Ngalame, Wei Qu, Michael Devito and John Bucher for review of this work. The authors thank Mr. John Otstot and the NIEHS Sequencing Lab Group for their help with DNA sequencing and Mr. Mathew Bell for his assistance in preparation of graphics.
Funding Information: This work was supported by the National Toxicology Program of the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: Cadmium (Cd), inorganic arsenic (iAs), kerinocyte-serum free medium (K-SFM), combined bisulfite restriction analysis (COBRA), Bisulfite Sequencing DNA Methylation Analysis (BISMA), Quantitation Tool for Methylation Analysis (QUMA)
References
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001;61:455–458. [PubMed] [Google Scholar]
- Basu GD, Azorsa DO, Kiefer JA, Rojas AM, Tuzmen S, Barrett MT, Trent JM, Kallioniemi O, Mousses S. Functional evidence implicating S100P in prostate cancer progression. Int J Cancer. 2008;123:330–339. doi: 10.1002/ijc.23447. (10.1002/ijc.23447) [DOI] [PubMed] [Google Scholar]
- Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Dill AL, Webber MM, Waalkes MP. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environ Health Perspect. 2007;115:1454–1459. doi: 10.1289/ehp.10207. (10.1289/ehp.10207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, Waalkes MP. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicol Appl Pharmacol. 2005;206:288–298. doi: 10.1016/j.taap.2004.11.017. (10.1016/j.taap.2004.11.017) [DOI] [PubMed] [Google Scholar]
- Coppin JF, Qu W, Waalkes MP. Interplay between cellular methyl metabolism and adaptive efflux during oncogenic transformation from chronic arsenic exposure in human cells. J Biol Chem. 2008;283:19342–19350. doi: 10.1074/jbc.M802942200. (10.1074/jbc.M802942200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, Soloway MS, Lokeshwar VB. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer. Int J Cancer. 2004;112:121–129. doi: 10.1002/ijc.20368. (10.1002/ijc.20368) [DOI] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Do JT, Arauzo-Bravo MJ, Lee SH, Meissner A, Lee HT, Jaenisch R, Scholer HR. Epigenetic hierarchy governing Nestin expression. Stem Cells. 2009;27:1088–1097. doi: 10.1002/stem.43. (10.1002/stem.43) [DOI] [PubMed] [Google Scholar]
- Hyder CL, Lazaro G, Pylvanainen JW, Roberts MW, Qvarnstrom SM, Eriksson JE. Nestin regulates prostate cancer cell invasion by influencing the localisation and functions of FAK and integrins. Journal of cell science. 2014;127:2161–2173. doi: 10.1242/jcs.125062. (10.1242/jcs.125062) [DOI] [PubMed] [Google Scholar]
- IARC Arsenic and arsenic compounds. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. 2012a;101C:41–94. [PubMed] [Google Scholar]
- IARC Cadmium and cadmium compounds. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization. International Agency for Research on Cancer. 2012b;101 C:121–146. [Google Scholar]
- Jiang H, Hu H, Tong X, Jiang Q, Zhu H, Zhang S. Calcium-binding protein S100P and cancer: mechanisms and clinical relevance. Journal of cancer research and clinical oncology. 2012;138:1–9. doi: 10.1007/s00432-011-1062-5. (10.1007/s00432-011-1062-5) [DOI] [PubMed] [Google Scholar]
- Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem cell reviews. 2008;4:193–201. doi: 10.1007/s12015-008-9033-1. (10.1007/s12015-008-9033-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199–9206. doi: 10.1158/0008-5472.CAN-07-0806. (10.1158/0008-5472.CAN-07-0806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima C, Ramirez DC, Tokar EJ, Himeno S, Drobna Z, Styblo M, Mason RP, Waalkes MP. Requirement of arsenic biomethylation for oxidative DNA damage. J Natl Cancer Inst. 2009;101:1670–1681. doi: 10.1093/jnci/djp414. (10.1093/jnci/djp414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DE, Martin MM, Feldman DS, Terry AV, Jr., Nuovo GJ, Elton TS. Experimental validation of miRNA targets. Methods. 2008;44:47–54. doi: 10.1016/j.ymeth.2007.09.005. (10.1016/j.ymeth.2007.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y, Oda M, Okano M. QUMA: quantification tool for methylation analysis. Nucleic Acids Res. 2008;36:W170–175. doi: 10.1093/nar/gkn294. (10.1093/nar/gkn294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak W. Epigenetic regulation of S100 protein expression. Clinical epigenetics. 2011;2:77–83. doi: 10.1007/s13148-011-0023-9. (10.1007/s13148-011-0023-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- Liu J, Li G, Peng X, Liu B, Yin B, Tan X, Fan M, Fan W, Qiang B, Yuan J. The cloning and preliminarily functional analysis of the human neurotrimin gene. Science in China. Series C, Life sciences / Chinese Academy of Sciences. 2004;47:158–164. doi: 10.1360/03yc0072. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. (10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Gomez P, Kramer M, Knapp J, McCornack MA, Lopez LE, Fregien N, Dhir N, Scherer S, Klumpp DJ, Manoharan M, Soloway MS, Lokeshwar BL. Epigenetic regulation of HYAL-1 hyaluronidase expression. identification of HYAL-1 promoter. J Biol Chem. 2008;283:29215–29227. doi: 10.1074/jbc.M801101200. (10.1074/jbc.M801101200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem cell reviews. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. (10.1007/s12015-010-9208-4) [DOI] [PubMed] [Google Scholar]
- Merrick BA, Tokar EJ, Phadke DP, Shah RR, Wang M, Gordon O, Wright GM, Burke M, Baxter SA, Pelch KE, Tice RR, Waalkes MP. Epigenetics of Arsenic Carcinogenesis and Location of Methylated DNA Sites Underlying Gene Expression Changes. The Toxicologist. 2013;132:113. [Google Scholar]
- Misra RR, Smith GT, Waalkes MP. Evaluation of the direct genotoxic potential of cadmium in four different rodent cell lines. Toxicology. 1998;126:103–114. doi: 10.1016/s0300-483x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Muniyappa MK, Dowling P, Henry M, Meleady P, Doolan P, Gammell P, Clynes M, Barron N. MiRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur J Cancer. 2009;45:3104–3118. doi: 10.1016/j.ejca.2009.09.014. (10.1016/j.ejca.2009.09.014) [DOI] [PubMed] [Google Scholar]
- Pelch KE, Schroder AL, Kimball PA, Sharpe-Timms KL, Davis JW, Nagel SC. Aberrant gene expression profile in a mouse model of endometriosis mirrors that observed in women. Fertil Steril. 2010;93:1615–1627. e1618. doi: 10.1016/j.fertnstert.2009.03.086. (10.1016/j.fertnstert.2009.03.086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RJ, Tokar EJ, Xu Y, Orihuela R, Ngalame NN, Waalkes MP. Chronic cadmium exposure in vitro induces cancer cell characteristics in human lung cells. Toxicol Appl Pharmacol. 2013;273:281–288. doi: 10.1016/j.taap.2013.06.013. (10.1016/j.taap.2013.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Diwan BA, Reece JM, Bortner CD, Pi J, Liu J, Waalkes MP. Cadmium-induced malignant transformation in rat liver cells: role of aberrant oncogene expression and minimal role of oxidative stress. Int J Cancer. 2005;114:346–355. doi: 10.1002/ijc.20736. (10.1002/ijc.20736) [DOI] [PubMed] [Google Scholar]
- Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. (10.2217/epi.09.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. (10.1289/ehp.1002114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde C, Zhang Y, Reinhardt R, Jeltsch A. BISMA--fast and accurate bisulfite sequencing data analysis of individual clones from unique and repetitive sequences. BMC bioinformatics. 2010;11:230. doi: 10.1186/1471-2105-11-230. (10.1186/1471-2105-11-230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, Klein CB. Genetic and epigenetic effects of environmental arsenicals. Metallomics. 2011;3:1135–1141. doi: 10.1039/c1mt00074h. (10.1039/c1mt00074h) [DOI] [PubMed] [Google Scholar]
- Severson PL, Tokar EJ, Vrba L, Waalkes MP, Futscher BW. Agglomerates of aberrant DNA methylation are associated with toxicant-induced malignant transformation. Epigenetics. 2012;7:1238–1248. doi: 10.4161/epi.22163. (10.4161/epi.22163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumylaite L, Kregzdyte R, Bogusevicius A, Poskiene L, Baranauskiene D, Pranys D. Association between cadmium and breast cancer risk according to estrogen receptor and human epidermal growth factor receptor 2: epidemiological evidence. Breast cancer research and treatment. 2014;145:225–232. doi: 10.1007/s10549-014-2918-6. (10.1007/s10549-014-2918-6) [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP. Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice. Toxicol Sci. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. (10.1093/toxsci/kfq315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Qu W, Liu J, Liu W, Webber MM, Phang JM, Waalkes MP. Arsenic-specific stem cell selection during malignant transformation. J Natl Cancer Inst. 2010;102:638–649. doi: 10.1093/jnci/djq093. (10.1093/jnci/djq093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP. Cadmium carcinogenesis. Mutat Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Wang Q, Williamson M, Bott S, Brookman-Amissah N, Freeman A, Nariculam J, Hubank MJ, Ahmed A, Masters JR. Hypomethylation of WNT5A, CRIP1 and S100P in prostate cancer. Oncogene. 2007;26:6560–6565. doi: 10.1038/sj.onc.1210472. (10.1038/sj.onc.1210472) [DOI] [PubMed] [Google Scholar]
- Webber MM, Quader ST, Kleinman HK, Bello-DeOcampo D, Storto PD, Bice G, DeMendonca-Calaca W, Williams DE. Human cell lines as an in vitro/in vivo model for prostate carcinogenesis and progression. The Prostate. 2001;47:1–13. doi: 10.1002/pros.1041. (10.1002/pros.1041) [DOI] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cellular and molecular life sciences : CMLS. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. (10.1007/s00018-004-4144-6) [DOI] [PubMed] [Google Scholar]
- Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.