Abstract
Background
Exacerbations of asthma remain common even in children and adolescents despite optimal medical management. Identification of host risk factors for exacerbations is incomplete, particularly for seasonal episodes.
Objective
Define host risk factors for asthma exacerbations unique to their season of occurrence.
Methods
This is a retrospective analysis of patients aged 6-20 years who comprised the control groups of the Asthma Control Evaluation trial and the Inner City Anti-IgE Therapy for Asthma trial. Univariate and multivariate models were constructed to determine if patient demographic and historical factors, allergic sensitization, fractional exhaled nitric oxide, spirometric measurements, asthma control, and treatment requirements were associated with seasonal exacerbations.
Results
The analysis included 400 patients (54.5% male; 59.0% African American; median age 13 years). Exacerbations occurred in 37.5% of participants over the periods of observation and were most common in the fall (28.8% of participants). In univariate analysis, impaired pulmonary function was significantly associated with greater odds of exacerbations for all seasons, as was an exacerbation in the previous season for all seasons except spring. In multivariate analysis, exacerbation in the previous season was the strongest predictor in fall and winter while a higher requirement for inhaled corticosteroids was the strongest predictor in spring and summer. The multivariate models had the best predictive power for fall exacerbations (30.5% variance attributed).
Conclusions
Among a large cohort of inner city children with asthma, patient risk factors for exacerbations vary by season. Thus, individual patient information may be beneficial in strategies to prevent these seasonal events.
Clinical Implications
Inner city children remain at risk for asthma exacerbations despite appropriate therapy. Because their risk factors vary by season, strategies to prevent them may need to differ as well.
Capsule summary
Risk factors for asthma exacerbations among inner city children varied by season. Fall exacerbations were the most common and the most predictable based on a compilation of historical and clinical variables.
Keywords: asthma, seasons, biomarkers, asthma exacerbations, IgE, exhaled nitric oxide, allergy, eosinophils, pulmonary function
INTRODUCTION
Asthma exacerbations remain a major factor contributing to the morbidity and even mortality of pediatric patients with asthma.1 Moreover, asthma exacerbations account for a large proportion of the direct and indirect medical costs associated with the disease.2,3 Although guideline-directed care reduces the frequency of asthma exacerbations among children, these events continue to occur in many patients.4 Given the importance of exacerbations to the overall burden of asthma, attention has focused on the causes of these events, including viral respiratory infections, airborne allergens, pollutants, and stress.5 While asthma exacerbations can occur at any time during the year, seasonal patterns exist, and in children, exacerbation rates are highest in the fall and lowest in the summer.6-8 The seasonal rise in fall exacerbations is highly consistent, and, based on studies from Canada, has been referred to as the “September Epidemic.”9 Fall exacerbations have been attributed to an increased frequency of rhinovirus respiratory infections among children when they return to school.10,11 Other factors, however, such as allergic sensitization and an increase in exposure to environmental allergens, have also been proposed to work in combination with viral respiratory infections to trigger fall exacerbations of asthma.12 Although the environmental contributors to asthma exacerbations are well recognized and appreciated, the patient characteristics associated with a susceptibility to these events on a seasonal basis have not been completely defined, nor has the importance of these factors in determining possible differences in the timing of exacerbations.
An achievement of optimal asthma control includes efforts to reduce the frequency of exacerbations.13 Understanding which patient characteristics are associated with an increased risk for asthma exacerbations is a critical step to prevent these events and may include seasonal modification of treatment. Towards that goal, a predominant finding in predicting who is at risk for asthma exacerbations has been the observation that prior exacerbations are the best predictor of future exacerbations.14-17 While this finding has important utility, the tautological nature of this finding suggests that incompletely defined additional factors may also predict exacerbations. Further, there has been little research that explores risk factors for exacerbations in individual seasons, other than the fall, or whether these risk factors can be predicted, and, as a consequence, if this information may improve approaches to prevent seasonal exacerbations. Consequently, we hypothesized that specific patient characteristics may be associated with seasonal exacerbations of asthma. Such characteristics may act to identify which patients are at high risk for seasonal asthma exacerbations and may, therefore, serve as a framework to identify more effective treatment strategies to prevent these events.
METHODS
Overview
This analysis examines risk factors for asthma exacerbations in 400 control group participants from two recent studies from the Inner City Asthma Consortium: Asthma Control Evaluation4 (ACE, n=253) and Inner City Anti-IgE Therapy for Asthma18 (ICATA, n=147). Seasonal and year-round predictors are considered using both univariate and multivariate analytic techniques. Of note, in both ACE and ICATA, all participants (including controls) were given guideline-based treatment by asthma specialists prior to randomization and throughout the year-long follow-up. In the ACE trial, the fraction of exhaled nitric oxide (FeNO) was added to the evaluation regimen to determine treatment for the intervention group. For ICATA, omalizumab was added to guideline treatment for the intervention group. In both studies, the guideline-treated control and intervention groups were closely monitored for symptoms and exacerbations during the follow-up year. Both studies were approved by each institution's Institutional Review Board. Study details, including study sites and inclusion and exclusion criteria are included in Table 1 in the online supplement. Both studies enrolled largely minority participants with persistent asthma from urban neighborhoods. Important differences between the trials include the ages of enrolled participants (12-20 years for ACE, 6-20 years for ICATA), the proportion of Hispanics enrolled (22.9% for ACE, 42.2% for ICATA), and the requirement that patients in ICATA have a combined body weight and total serum IgE level suitable for omalizumab dosing and a positive skin prick test to at least one perennial allergen (however, 85% of patients in ACE also met this requirement).
ACE Population and Study Design
A total of 546 participants with asthma, 12-20 years of age, were enrolled in the ACE study at ten large urban research centers in the United States.4 The ACE trial had a randomized, double-blind, parallel-group design. At screening visits, each participant was assessed for asthma symptoms, previous treatment, pulmonary function, allergen skin prick test sensitivity, total serum IgE, and allergen-specific IgE. Participants were evaluated at seven additional visits, at six to eight week intervals, with symptom assessments and pulmonary function testing at each visit. Treatment was adjusted according to guideline-based algorithms13 with or without taking into consideration the FeNO values. All study drugs were provided. Exacerbations were defined as a need for systemic corticosteroids and/or hospitalization.
ICATA Population and Study Design
ICATA was a randomized, double-blind, placebo-controlled, parallel-group, multicenter trial that compared omalizumab to placebo added to guidelines-based therapy in 419 inner-city children, adolescents, and young adults (ages 6-20 years) with persistent allergic asthma.18 Similar to ACE, at screening visits, each participant was assessed for asthma symptoms, previous treatment, pulmonary function, allergen skin prick test sensitivity, total serum IgE, and allergen-specific IgE. Asthma medications covered by the participants’ insurance plans were prescribed but were not supplied with the exception of omalizumab or placebo study injections and oral prednisone for exacerbations. Like ACE, exacerbations were defined by the need for systemic corticosteroids and/or hospitalization.
Statistical Analyses
Participants from ACE and ICATA were pooled for all analyses to increase sample size and to improve generalizability. Exacerbation predictors are described as either “baseline” or “previous season.” Baseline predictors were measured at or before study randomization, while previous season predictors were post-randomization measures collected prior to the start of the season of interest. For example, FEV1/FVC from the summer was used to predict exacerbations in the fall. The exacerbation outcome was binary (one or more exacerbation vs. no exacerbations in a given season). The initial 90 days of follow-up exacerbation data were not included as outcomes because there was no prior season prediction data; however data from this period was used as predictors for the following season. A minimum of 45 days of follow-up for exacerbations in a given season was required for inclusion in the analysis. All participants with at least 5 months of follow-up were included. A random effect for participant was added account for within-participant correlation in models looking across multiple seasons.
Univariate associations for each season were calculated using logistic regression models and reported as odds ratios (ORs) and 95% confidence intervals (CIs). In order to obtain overall associations accounting for the repeated measurements and to calculate appropriate interaction p-values, mixed models were constructed. Multivariate models were calculated using the R package “hier.part.”19 Hierarchical partitioning was performed on the variables that had any significant seasonal association, calculating the independent contribution of each factor in predicting exacerbations. This technique, rather than seeking a best fit, uses all possible models in a logistic regression to distinguish the predictor variables that have the highest independent contribution.
We scored participant risk using a simple algorithm. We started with 18 risk factors that were significant univariate predictors of exacerbations, and then removed 10 of the items that were collinear, leaving 8 measures. Skin test and total IgE were combined and presented as a single measure for “allergy”, leaving the following 7 items which were scored using tertiles: age, exacerbation history in the previous season, ICS treatment step, FEV1/FVC, FeNO, blood eosinophils, and allergy (Supplemental Table 2).
Log-transformations of skewed data (FeNO, total IgE, allergen-specific IgE, sum of the five allergen-specific IgEs) were used for univariate analyses. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute Inc; Cary, NC) and the R system for statistical computing version 3.0.2. The calculation of relative importance was conducted using the R add-on package hier.part.
RESULTS
Participants Characteristics (Table 1)
Table 1.
Characteristics of study participants
| ACE N=253 | ICATA N=147 | Combined N=400 | |
|---|---|---|---|
| Age of participants at screening (years)* | 14.0 [13.0;15.0] | 11.0 [8.00;14.0] | 13.0 [12.0 - 15.0] |
| Gender: Male | 134 (53.0%) | 84 (57.1%) | 218 (54.5%) |
| Race/Ethnicity: Black | 153 (60.5%) | 83 (56.5%) | 236 (59.0%) |
| Hispanic | 58 (22.9%) | 62 (42.2%) | 120 (30.0%) |
| Other/Missing | 42 (16.6%) | 2 (1.4%) | 44 (11.0%) |
| Caretaker completed at least high school | 167 (74.2%) | 103 (72.0%) | 270 (73.4%) |
| Household income < $15,000 annually | 133 (56.1%) | 80 (54.4%) | 213 (55.5%) |
| 1+ Household member employed | 200 (79.1%) | 115 (78.2%) | 315 (78.8%) |
| 1+ Smoker in Household | 93 (36.8%) | 65 (44.2%) | 158 (39.5%) |
| BMI percentile at screening* | 89.6 [63.3;97.5] | 86.6 [60.1;97.5] | 88.8 [62.9 - 97.5] |
| Asthma-related symptoms (number of days in preceding 2 weeks) at randomization* † | 2.0 [0.0;3.0] | 2.0 [0.0;4.0] | 2.0 [0.0 - 4.0] |
| ACT® (score in preceding month) at randomization ‡: Controlled | 193 (76.6%) | 95 (64.6%) | 288 (72.2%) |
| Uncontrolled | 59 (23.4%) | 52 (35.4%) | 111 (27.8%) |
| FEV1 (% predicted value) at randomization* | 96.4 [85.2;106.0] | 90.9 [80.9;103.0] | 94.2 [83.8 - 105.0] |
| FEV1/FVC (×100) at randomization* | 81.4 [75.5;86.2] | 79.4 [72.7;84.1] | 80.7 [74.5 - 85.8] |
| FeNO (ppb) at randomization* | 18.8 [10.6;37.3] | 21.4 [10.3;39.1] | 19.6 [10.5 - 37.9] |
| ICS Treatment Regimen at randomization§:Low dose | 77 (30.4%) | 42 (28.6%) | 119 (29.8%) |
| Medium dose | 73 (28.9%) | 83 (56.5%) | 156 (39.0%) |
| High dose | 103 (40.7%) | 22 (15.0%) | 125 (31.2%) |
| One or more hospitalization in 6 months before recruitment | 37 (14.6%) | 30 (20.4%) | 67 (16.8%) |
Values are medians [interquartile range]or frequencies [percent].
The number of days with symptoms was calculated as the largest of the following variables during the previous 2 weeks: number of days with wheezing, chest tightness, or cough; number of nights of sleep disturbance; and number of days when activities were affected. This symptom scale ranges from 0 to 14 days per 2-week period.
Scores on the Childhood Asthma Control Test (C-ACT) and the Asthma Control Test (ACT) were combined.
Six treatment steps were established, consistent with National Asthma Education and Prevention Program guidelines13 to standardize prescribing patterns according to levels of asthma severity. Steps 1 and 2 applied to mild asthma, step 3 to moderate asthma, and steps 4 through 6 to severe asthma. At step 0, the recommendation was for no asthma-control medication or albuterol as needed; at step 1, budesonide — 180 μg once a day; at step 2, budesonide — 180 μg twice a day; at step 3, budesonide — 360 μg twice a day; at step 4, fluticasone–salmeterol (Advair, GlaxoSmithKline) — 250 μg fluticasone and 50 μg salmeterol twice a day; at step 5, Advair — 250 μg and 50 μg twice a day plus montelukast once a day; and at step 6, Advair — 500 μg and 50 μg twice a day plus montelukast once a day. The doses for montelukast were 5 mg per day forchildren hildren er day forontelukast once a day. The doses for montelu
The 400 participants from the ACE and ICATA control treatment groups included in these analyses were typically low income (55.5% with income < $15,000), minority (59% black, 30% Hispanic) and overweight (median BMI percentile at screening 88.8%). Their median age was 13 years. In the 6 months before study entry, 16.8% were hospitalized for asthma. At study screening, the mean Asthma Control Test (ACT) score was low (18.6 ± 4.2), but rose after 3 to 4 weeks of guideline-based treatment prior to randomization (mean of 20.9 ± 3.5).
Frequency of Exacerbations
During the follow-up periods after randomization in the individual clinical trials, 149 of the 400 participants (37.5%) experienced an asthma exacerbation that required systemic corticosteroid use (Table 2). The highest frequency of exacerbations (28.8% of participants) occurred during the fall months (September-November). Spring was the second highest season for exacerbations, with 19.9% of the children having exacerbations followed by winter (15.9% of participants) and, finally, summer (14.5% of participants).
Table 2.
Univariate associations between seasonal use of prednisone during the double blind phase and explanatory variables*
| Overall | Fall | Winter | Spring | Summer | Interaction ‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) of patients with an exacerbation | 151/400 (37.8%) | 65/226 (28.8%) | 34/214 (15.9%) | 56/281 (19.9%) | 27/188 (14.5%) | ||||||
| Baseline Predictors † | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age (yrs.) | 0.96 (0.89,1.02) | 0.17 | 1.02 (0.91,1.15) | 0.73 | 0.91 (0.78,1.06) | 0.22 | 0.90 (0.82,0.98) | 0.02 | 1.10 (0.94,1.29) | 0.22 | |
| Gender (Male vs. Female) | 0.80 (0.54,1.17) | 0.25 | 0.77 (0.43,1.38) | 0.38 | 0.90 (0.43,1.89) | 0.79 | 0.97 (0.54,1.75) | 0.93 | 0.44 (0.18,1.03) | 0.06 | |
| Race (Black vs. Other) | 0.85 (0.57,1.25) | 0.41 | 0.81 (0.45,1.46) | 0.49 | 1.04 (0.49,2.22) | 0.911 | 0.99 (0.55,1.78) | 0.97 | 0.47 (0.19,1.17) | 0.105 | |
| BMI Percentile (85% or higher vs. not) | 1.16 (0.79,1.73) | 0.44 | 1.18 (0.66,2.13) | 0.57 | 1.28 (0.61,2.69) | 0.52 | 1.10 (0.61,1.98 | 0.74 | 0.98 (0.43,2.23) | 0.97 | |
| Allergen skin tests (no of positive tests of 14) | 1.02 (0.96,1.08) | 0.51 | 1.10 (1.01,1.19) | 0.03 | 0.95 (0.84,1.07) | 0.42 | 1.00 (0.90,1.10) | 0.93 | 0.97 (0.85,1.09) | 0.58 | *** |
| Mite skin tests (Yes vs. No) | 0.75 (0.50,1.10) | 0.14 | 1.37 (0.77,2.45) | 0.28 | 0.56 (0.26,1.19) | 0.13 | 0.59 (0.32,1.07) | 0.09 | 0.67 (0.29,1.56) | 0.35 | |
| Rodent skin tests (Yes vs. No) | 1.34 (0.90,2.01) | 0.14 | 2.05 (1.14,3.68) | 0.02 | 1.11 (0.52,2.37) | 0.78 | 1.03 (0.56,1.87) | 0.93 | 0.85 (0.36,2.01) | 0.71 | |
| Cockroach skin tests (Yes vs. No) | 1.15 (0.77,1.75) | 0.50 | 1.93 (1.03,3.61) | 0.04 | 0.99 (0.47,2.10) | 0.98 | 0.95 (0.51,1.75) | 0.87 | 0.74 (0.31,1.77) | 0.50 | *** |
| Mold skin tests (Yes vs. No) | 1.05 (0.71,1.55) | 0.82 | 1.50 (0.84,2.68) | 0.17 | 0.65 (0.30,1.41) | 0.27 | 0.95 (0.53,1.71) | 0.86 | 1.07 (0.46,2.49) | 0.88 | |
| Total IgE (Log-transformed kU/l) | 1.66 (1.14,2.48) | 0.01 | 1.90 (1.08,3.36) | 0.03 | 1.91 (0.94,3.86) | 0.07 | 1.19 (0.66,2.16) | 0.56 | 1.32 (0.58,2.99) | 0.51 | |
| SIgE Der f (Log-transformed kU/l) | 1.22 (0.98,1.54) | 0.08 | 1.46 (1.02,2.09) | 0.04 | 1.28 (0.83,1.96) | 0.26 | 0.99 (0.71,1.39) | 0.97 | 1.29 (0.83,2.02) | 0.26 | |
| SIgE Der p (Log-transformed kU/l) | 1.21 (0.96,1.52) | 0.10 | 1.43 (0.99,2.05) | 0.05 | 1.29 (0.84,2.00) | 0.25 | 0.98 (0.69,1.39) | 0.92 | 1.27 (0.80,1.99) | 0.31 | |
| SIgE cockroach (Log-transformed kU/l) | 1.17 (0.94,1.47) | 0.15 | 1.48 (1.06,2.06) | 0.02 | 1.08 (0.71,1.63) | 0.72 | 1.03 (0.74,1.44) | 0.85 | 0.98 (0.62,1.55) | 0.92 | |
| SIgE Alternaria (Log-transformed kU/l) | 1.13 (0.90,1.41) | 0.30 | 1.09 (0.77,1.53) | 0.63 | 0.89 (0.57,1.40) | 0.62 | 1.06 (0.76,1.48) | 0.74 | 1.64 (1.02,2.63) | 0.04 | |
| Blood Eosinophils (% of white blood cells) | 1.07 (1.00,1.13) | 0.03 | 1.08 (0.99,1.17) | 0.09 | 1.01 (0.91,1.13) | 0.80 | 1.04 (0.95,1.14) | 0.4 | 1.13 (1.01,1.27) | 0.04 | |
| Previous Season Predictors † | |||||||||||
| Asthma-related symptoms (days in preceding 2 wks.) | 0.98 (0.92,1.04) | 0.51 | 1.00 (0.89,1.12) | 0.95 | 1.03 (0.93,1.15) | 0.59 | 0.99 (0.90,1.09) | 0.84 | 1.00 (0.87,1.14) | 0.98 | |
| ACT® in preceding month (uncontrolled vs. controlled) | 0.85 (0.53, 1.33) | 0.48 | 0.84 (0.36, 1.99) | 0.70 | 1.33 (0.60, 2.94) | 0.48 | 0.80 (0.36,1.76) | 0.58 | 1.03 (0.41,2.62) | 0.95 | |
| FEV1 (% predicted value) | 0.98 (0.97,0.99) | <0.001 | 0.98 (0.96,1.00) | 0.01 | 0.97 (0.95,0.99) | 0.01 | 0.99 (0.97,1.00) | 0.11 | 0.98 (0.96,1.01) | 0.15 | |
| FEV1/FVC (×100) | 0.96 (0.93,0.97) | <0.001 | 0.97 (0.94,1.00) | 0.03 | 0.94 (0.91,0.99) | 0.01 | 0.96 (0.93,0.99) | 0.02 | 0.94 (0.89,0.99) | 0.02 | |
| FeNO (Log-transformed ppb) | 1.61 (0.98,2.68) | 0.06 | 3.63 (1.64,8.04) | 0.001 | 0.56 (0.21,1.49) | 0.25 | 1.06 (0.49,2.31) | 0.89 | 2.54 (0.86,7.53) | 0.09 | *** |
| Exacerbations previous year (Yes vs. No) | 2.61 (1.85,3.68) | <0.001 | 2.89 (1.60,5.23) | < 0.001 | 1.85 (0.89,3.87) | 0.10 | 4.34 (2.26,8.30) | < 0.001 | 1.71 (0.75,3.92) | 0.20 | *** |
| Exacerbations in previous season(Yes vs. No) | 2.50 (1.65,3.61) | <0.001 | 5.37 (2.53,11.39) | < 0.001 | 2.66 (1.21,5.83) | 0.02 | 1.53 (0.73,3.20) | 0.26 | 2.53 (1.05,6.08) | 0.04 | *** |
| Inhaled Corticosteroid Treatment (treatment steps) § | 1.15 (1.04,1.27) | 0.004 | 1.06 (0.90,1.23) | 0.49 | 1.10 (0.91,1.34) | 0.33 | 1.18 (1.01,1.38) | 0.03 | 1.34 (1.06,1.70) | 0.01 | |
| Composite Asthma Severity Index in preceding 2 months | 1.08 (1.00,1.16) | 0.05 | 1.08 (0.94,1.25) | 0.27 | 1.07 (0.93,1.23) | 0.37 | 1.11 (1.00,1.24) | 0.05 | 1.16 (0.99,1.36) | 0.07 | |
Values are odds ratios for exacerbations during each overall/season (columns) for the listed predictors (rows). Predictors are continuous variables unless noted.
For the overall column, all predictors are measured at either study enrollment or randomization. For the seasonal columns (Fall, Winter, Spring and Summer) baseline predictors are measured at study enrollment; the baseline measure is used regardless of the seasonal. Previous season predictors are measured during the season before the exacerbation outcome season; for example, maximum symptom days from the summer are used to predict fall exacerbations and so on.
Test of interaction among the seasonal predictors of exacerbations.
Significant interactions (p<0.10) are noted with the interpretation being that the effect of the predictor is different by season.
Six treatment steps were established, consistent with the guidelines of the National Asthma Education and Prevention Program.13
Univariate Associations with Exacerbations
Full year
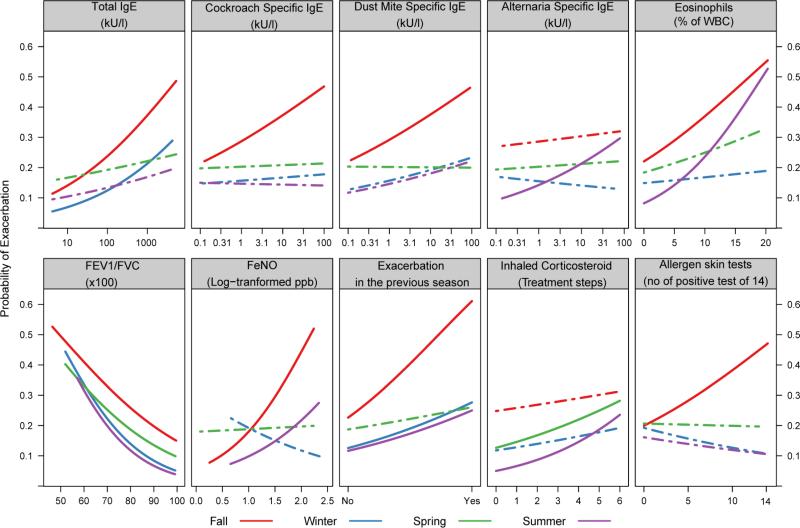
When examining exacerbations over the course of the year, without regard to season, the univariate factors most associated with exacerbations included an exacerbation during the prior year (OR 2.61, 1.85-3.68), decreased pulmonary function [FEV1, OR 0.98, 0.97-0.99 and FEV1/FVC (x100), OR (for a 1 point change) 0.96, 0.93-0.97], elevated total IgE (OR 1.66, 1.14-2.48), higher requirement for ICS (OR 1.15, 1.04-1.27), elevated Composite Asthma Severity Index (CASI)20 (OR 1.08, 1.00-1.16) and elevated blood eosinophils (OR 1.07, 1.00-1.13). In contrast, different patient characteristics emerged when the timing of the exacerbation was evaluated in relationship to the season of its occurrence. Table 2 and Figure 1 show the results of the univariate models to predict exacerbations for each individual season.
Figure 1.
Univariate associations of baseline predictors (total IgE, specific IgE's, number of positive skin tests, and eosinophils) and previous season predictors (FEV1/FVC, FeNO, exacerbation in the previous season and ICS step requirement) with the probability of exacerbation by season. Solid lines are for marginally significant associations (p<0.10), and dashed lines are for non-significant associations.
Fall Season (September-November)
During the fall, when the highest proportion of participants experienced an exacerbation (28.8%), the strongest risk factors for an exacerbation included an exacerbation during the prior season, which was nearly twice as strong as such events over the previous year (prior season, OR 5.37, 2.53-11.39, vs. previous year OR 2.89, 1.60-5.23); increasing number of positive allergen skin tests (OR 1.10, 1.01-1.19); positive allergen skin tests for rodent (OR 2.05, 1.14-3.68) or cockroach (OR 1.93, 1.03-3.61); elevated total IgE (OR 1.90, 1.08-3.36) and allergen-specific IgE to house dust mite (Der f) and cockroach (OR 1.46, 1.02-2.09, and OR 1.48, 1.06-2.06, respectively); elevated FeNO (OR 3.63, 1.64-8.04); and decreased pulmonary function (FEV1 OR 0.98-0.96,1.00 and FEV1/FVC (x100) CI 0.97 (0.94-1.00).
Winter Season (December-February)
Although 15.9% of the participants experienced an asthma exacerbation during the winter, there were few univariate associations to predict these events. Again, an exacerbation during the previous season (fall) was related to winter exacerbations (OR 2.66, 1.21-5.83), but not exacerbations in the previous year. The only other relationships observed with winter exacerbations were with decreased pulmonary function measures (FEV1 percent predicted OR 0.97, 0.95-0.99 and FEV1/FVC (x100), OR 0.94, 0.91-0.99).
Spring Season (March-May)
Unlike the fall and winter, spring exacerbations were most closely associated with an exacerbation during the previous year (OR 4.34, 2.26-8.30), but not an exacerbation in the prior season. Other associations with spring exacerbations were a decrease in exacerbation risk with increasing age (OR 0.90, 0.82-0.98), and increases in risk with a lower FEV1/FVC ratio (OR 0.96, 0.93-0.99), higher requirement for ICS (OR 1.18, 1.01-1.38) and elevated CASI (OR 1.11, 1.00-1.24).
Summer season (June-August)
Although fewer participants had an exacerbation during the summer (14.5%), a number of factors related to exacerbations at this time of year: impaired pulmonary function, as measured by FEV1/FVC (x100) ratio (OR 0.94, 0.89-0.99); allergic sensitivity to Alternaria (OR 1.64, 1.02, 2.63); elevated blood eosinophils (OR 1.13, 1.01-1.27); requirement for higher levels of ICS to maintain control (OR 1.34, 1.06-1.70); and an exacerbation in the previous season (OR2.53, 1.05-6.08).
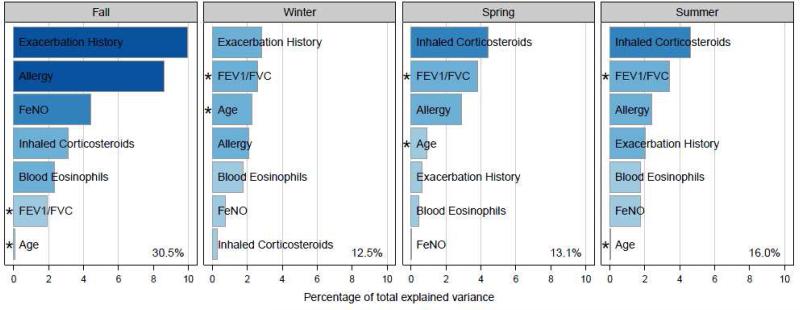
Multivariate prediction models
Seven risk factors, which had been significant in one or more univariate seasonal models, were selected to serve as independent predictors in multivariate analysis [age, exacerbation history in the previous season, ICS treatment step, FEV1/FVC, FeNO, blood eosinophils, and allergy (measured by combining the explained variance of positive skin tests with total IgE)]. First, the relative strength of these predictors was assessed, and different patterns by season emerged (Figure 2). The multivariate models explained a significant portion of the variance in all four seasons, with the best predictive power occurring in the fall (30.5% variance explained). A history of an exacerbation in the last season was the most important predictor in the fall and winter, while higher requirement for ICS was the most important factor in the spring and summer. Allergic sensitization and FEV1/FVC ratio played a major role in all seasons.
Figure 2.
Multivariate models for associations of baseline and previous season predictors of exacerbations. Baseline predictors were allergy (skin tests and total IgE) and age. Previous season predictors were FEV1/FVC ratio, FeNO, exacerbation history and ICS step requirement. Bars length and color indicates the strength of the association between the predictor and seasonal exacerbation as measured by variance explained. The total predictive power of a multivariate model containing all 7 predictors is annotated in the bottom right hand corner. Negative associations are indicated with an asterisk.
Prediction of Exacerbations
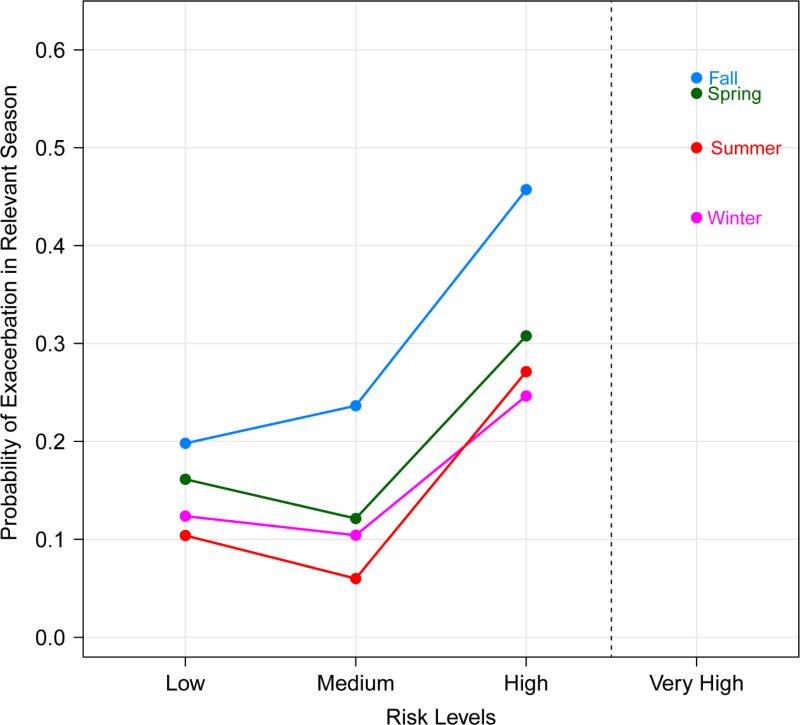
Participant risk was assessed with the same 7 risk factors used in the multivariate models. Each participant was given a risk score by rating the participant as high, medium or low risk for each of the risk factors and then adding up risk scores (2 points for high, 1 point for medium and 0 points for low) in all 7 components (Supplemental Table 2). These risk scores show a difference in exacerbation rates for high and low risk participants (Figure 3 and Supplemental Table 3). In the fall, for example, participants in the highest risk tertile had a 46% exacerbation rate while the participants in the lowest risk tertile had a 19% rate (OR 3.41, 1.73-6.72). Similar patterns are seen in the other seasons with 3.21, 2.32 and 2.31 fold increases in exacerbation rate for high-risk participants in the summer, winter and spring respectively (highest tertile vs. lowest tertile, Supplemental Table 3).
Figure 3.
Seasonal exacerbation prevalence by risk levels. Each participant had an exacerbation risk level calculated for each season using a simple index of 7 risk factors (allergy, age, FEV1/FVC ratio, FeNO, exacerbation in the previous season, and ICS step requirement). Seasonal risk levels were then divided in to tertiles (labeled “Low”, “Medium” and “High” risk) which are plotted against observed exacerbation rates in ACE and ICATA. An additional “Very High” risk group was added for participants with a risk index in the top 10 percent.
DISCUSSION
Our analysis of exacerbations on a seasonal basis derived from the control treatment arms of the ACE4 and ICATA18 studies from the Inner City Asthma Consortium reveals a number of important factors relevant to the identification of inner city children at high risk for asthma exacerbations. First, our ability to predict exacerbations by commonly used patient characteristics varies greatly by season. In the fall these factors predict 30.5% of the variance, a highly clinically significant percentage, while in the spring and winter, they have less predictive ability. Surprisingly, in summer, a time of lower asthma disease activity, our predictive ability approximated that in spring or winter (Figure 3). Second, predictors vary across the seasons. A recent exacerbation and impaired pulmonary function are important predictors in nearly every season, but allergic sensitization, peripheral blood eosinophils, FeNO, and required ICS treatment step (a surrogate for asthma severity) vary more widely across the seasons. Finally, the level of a patient's risk factors in the immediately preceding season is important for predicting an asthma exacerbation in the subsequent season. For example, a history of a summer exacerbation, higher FeNO values, and impaired pulmonary function are all relatively strong predictors of fall exacerbations. This information provides further evidence that, among high risk individuals, consideration should be given to escalating treatment prior to the fall season in order to prevent a subsequent exacerbation, rather than reducing asthma long-term control therapy in the summer. Our findings also suggest that follow-up visits for youth with asthma should be strategically timed in order to address specific risk factors, which are relevant to the individual patient, for the upcoming season.
This close examination of patient risk factors throughout the year suggests a necessity for more careful seasonal analysis of features associated with asthma exacerbations. In children and adolescents, viral respiratory infections are, perhaps, the most important factor associated with exacerbations. Johnston et al21 reported that in children aged 9-11 years, 80-85% of asthma exacerbations are associated with upper respiratory viral infections. Of the infections identified, rhinovirus (RV) has been the most commonly detected infectious agent in exacerbations,22,23 but other viruses, including human metapneumovirus, influenza, and parainfluenza, may play a smaller role.10,24 Our findings point to the likelihood that other, but highly relevant, factors also work in combination with the anticipated viral infections to trigger exacerbations. Our analyses demonstrates that some of these factors are important all year around such as pulmonary functions and previous exacerbations while others vary by season such as allergy and FeNO.
During the fall, risk factors included evidence for a role for multiple allergic sensitizations (dust, rodent, and cockroach). These seasonal associations may be due to higher exposures in the fall as youth return to school and spend more time indoors, and they may explain the high prevalence of fall exacerbations as both rhinovirus infections and seasonal allergic symptoms are present at this time of year. The importance of IgE sensitization to fall exacerbations is also supported by our report that omalizumab nearly eliminated the fall seasonal flare of these events in inner-city children and adolescents.18 Allergic sensitization was otherwise not significantly associated with seasonal exacerbations with the exception of Alternaria in the summer when humidity encourages its growth indoors.
We did not find a relationship between body mass index (BMI) or environmental tobacco smoke and risk for exacerbation either overall or for any individual season. In contrast, in a far larger cohort of 10,700 patients aged 5-17 years, Schatz et al25 found a linear increase in the proportion of patients experiencing exacerbations during fall and winter by BMI category (normal, overweight, and obese). These different findings may reflect the different racial/ethnic composition of the populations and the greater proportion of overweight/obese subjects in our cohort.
Previous reports of correlates of exacerbations have tended to evaluate the association of exacerbations over longer periods, typically over one year, and not by season. In addition, correlates were typically measured either at the start of the study period or at the time of the exacerbation. These studies,14, 26 similar to ours, found factors seemingly important throughout the year. For example, Covar et al14 sought to determine the factors associated with exacerbations in children with persistent asthma by analyzing data collected during the 48-week Childhood Asthma Research and Education network's Pediatric Asthma Controller Trial (PACT) study.27 Among the 48% of children aged 6-14 years who experienced exacerbations, multivariate analysis found only that a history of an asthma exacerbation requiring systemic corticosteroids in the past year to be associated with a subsequent exacerbation. Our analysis, in contrast, focused on exacerbations by season and found specific patient characteristics associated with increased seasonal risk. For example, higher FeNO as measured in the summer months and baseline allergic sensitization to cockroach, dust mite, and rodent were specifically associated with a high risk for fall exacerbations. The observation that FeNO may have a seasonal role in predicting exacerbations is novel. In a recent study28 of a similar inner-city and largely minority pediatric cohort, McCormack et al found no relationship between elevated FeNO and exacerbations. They did not, however, stratify by season. Other studies have found the role of FeNO in predicting exacerbations among adults to be more variable.29, 30
Another unique feature of our approach was an effort to identify the impact of combinations of risk factors, which, we propose, enhance the ability to predict asthma exacerbations. We derived a predictive index which indicates that the odds of an exacerbation increases 2-3 times when comparing individuals between the highest and lowest tertiles of the index scores. The additive, and even synergistic, nature of exacerbation risk factors has been suggested by other studies examining a more limited number of risk factors. For example, the risk of hospitalization for an asthma exacerbation increased approximately 3-4 times in individuals sensitized and exposed to cockroach allergen as compared to individuals not sensitized and/or not exposed.31 Similarly, a viral infection can further increase exacerbation risk in the presence of both allergen sensitization and exposure.32 Exposure to high levels of sulfur dioxide or nitrogen oxides at the time of an upper respiratory infection, has also been reported to increase the risk of an asthma exacerbation.33 A recent secondary analysis of 7,446 largely adult patients with asthma found an exponential increase in risk of an exacerbation that reaches 45% within the following six months based on index composed of 5 predictors: BMI, Asthma Control Questionnaire (ACQ),34 postbronchodilator FEV1, reliever use, and GINA treatment step.26
A major strength of this study is that the population of 400 inner city children and adolescents with moderate to severe persistent asthma were extensively characterized and closely managed with guideline-based care by asthma specialists throughout the observation period. Despite this careful supervision with standardized management approaches, over one-third (37.5%) experienced at least one exacerbation during the study period. As anticipated, the highest rate of exacerbations (28.8% of participants) occurred during the fall months and the lowest rate in the summer. However exacerbations were not infrequent in the summer. In a previous study of a similar population, a decrease in reported wheezing in summer was found but even in the summer levels still remained significant.8 The occurrence of an exacerbation during the prior summer was the best predictor of fall exacerbations. Although predictability of an asthma exacerbation in the other seasons was not as strong as during the fall, our study was able to identify risk factors of importance for each of these seasons. Those factors were different than the features associated with high risk for fall exacerbations.
Our study is a retrospective, post-hoc analysis and has limitations, and some caution must be used when generalizing these findings to other populations of children and adolescents with asthma as well as to adults. First, our study population included urban and largely minority children and adolescents with moderate to severe asthma. The associated characteristics for high risk of a seasonal exacerbation may differ in other populations. Second, the median age of our combined cohort was 13 years, older than the peak age for exacerbations in pediatric asthma. Of note, however, younger children are well represented in the final combined cohort: 87 of 400 (21.8%) were 6-11 years old. Third, our population was aggressively treated under a standardized protocol based on a guidelines-based approach with a high degree of adherence. As a consequence of this high adherence, we did not find that inadequate treatment or poor adherence to prescribed treatment was a major risk factor for exacerbations in contrast to other studies.15 In fact, the treatment factor in our analyses reflects the underlying severity of the disease that requires control of the impairment domain. Therefore, this prompts the question whether other features of the disease predispose these patients to a higher risk for an asthma exacerbation, and that these domains of asthma control are distinct. Fourth, our population did not include patients with intermittent or mild persistent asthma. While it is known that these groups have lower rates of exacerbations than the population included in these analyses, our findings do not examine their risk factors for exacerbation. Fifth, treatment adjustments or systemic corticosteroid courses administered in the previous season may influence the variables measured in a subsequent season. Nevertheless, the point of our study was to assess seasonal risk factors for exacerbation within the context of ongoing guideline-driven care. As such, from a clinical perspective, we could not prevent the interplay of the pre-seasonal factors. They evolved as they did while the patients were managed by our guideline-based treatment algorithms. Sixth, patients in ACE and ICATA were followed for a maximum of one year under guideline-driven therapy, making it impossible to assess the effect of exacerbations in the corresponding season of the prior year. Patients would not have been under our guideline-driven treatment algorithm during that period, however. Seventh, we examined host risk factors exclusively and did not examine environmental factors. A final limitation is that we do not have specific IgE's to tree pollen or other seasonal allergens available as allergic risk factors.
This study identifies host factors placing individual patients at high risk for an asthma exacerbation at specific seasons within the year. Our observations may inform development of preventative strategies that can then more effectively reduce the risk for these seasonal exacerbations. Our findings also highlight the need to continue the search for new predictors of exacerbations especially in the winter and spring. Validation and extension of these findings to other patient populations should result in developing a more individualized approach to asthma management that takes into consideration identifiable factors that could, in turn, affect improved management of the risk domain of asthma control versus impairment. Finally, the identification of risk factors by seasons may provide a platform to gain greater insight and knowledge of exacerbations, which appear to differ by etiology depending upon the season of occurrence.
Supplementary Material
Acknowledgments
Sources of funding: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C and HHSN272201000052I. Additional support was provided by the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, UL1 RR024982, M01RR00071, 5M01RR020359-04, 1UL1RR025771, 1UL1RR025780, 1UL1RR024156, and UL1RR031988. Lincoln Diagnostics provided Multi-Test ® and GlaxoSmithKline (GSK) provided study drug for ACE participants. Novartis Pharmaceuticals provided study drug, under a clinical trial agreement with the University of Wisconsin–Madison and Dey Pharma provided EpiPens for ICATA participants.
Abbreviations
- ACE
Asthma Control Evaluation study
- FeNO
fraction of exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ICAC
Inner City Asthma Consortium
- ICATA
Inner City Anti-IgE Therapy for Asthma study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3:1–67. [PubMed] [Google Scholar]
- 2.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. The Journal of Allergy and Clinical Immunology. 2012;129:1229–35. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Wang LY, Zhong Y, Wheeler L. Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2:A11. [PMC free article] [PubMed] [Google Scholar]
- 4.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, Morgan WJ, Kattan M, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sears MR. Epidemiology of asthma exacerbations. The Journal of Allergy and Clinical Immunology. 2008;122:662–8. doi: 10.1016/j.jaci.2008.08.003. quiz 9-70. [DOI] [PubMed] [Google Scholar]
- 6.Bloomberg GR, Trinkaus KM, Fisher EB, Jr., Musick JR, Strunk RC. Hospital readmissions for childhood asthma: a 10-year metropolitan study. American Journal of Respiratory and Critical Care Medicine. 2003;167:1068–76. doi: 10.1164/rccm.2201015. [DOI] [PubMed] [Google Scholar]
- 7.Cohen HA, Blau H, Hoshen M, Batat E, Balicer RD. Seasonality of asthma: a retrospective population study. Pediatrics. 2014;133:e923–32. doi: 10.1542/peds.2013-2022. [DOI] [PubMed] [Google Scholar]
- 8.Gergen PJ, Mitchell H, Lynn H. Understanding the seasonal pattern of childhood asthma: results from the National Cooperative Inner-City Asthma Study (NCICAS). Journal of Pediatrics. 2002;141:631–6. doi: 10.1067/mpd.2002.127510. [DOI] [PubMed] [Google Scholar]
- 9.Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, et al. The September epidemic of asthma exacerbations in children: a search for etiology. The Journal of Allergy and Clinical Immunology. 2005;115:132–8. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse WW, Lemanske RF, Jr., Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sears MR, Johnston NW. Understanding the September asthma epidemic. The Journal of Allergy and Clinical Immunology. 2007;120:526–9. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61:722–8. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Heart, Lung and Blood Institute . National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health; Bethesda, MD: 2007. NIH Publication 07-4051. [Google Scholar]
- 14.Covar RA, Szefler SJ, Zeiger RS, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. The Journal of Allergy and Clinical Immunology. 2008;122:741–7. e4. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forno E, Celedon JC. Predicting asthma exacerbations in children. Current Opinion in Pulmonary Medicine. 2012;18:63–9. doi: 10.1097/MCP.0b013e32834db288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forno E, Fuhlbrigge A, Soto-Quiros ME, Avila L, Raby BA, Brehm J, et al. Risk factors and predictive clinical scores for asthma exacerbations in childhood. Chest. 2010;138:1156–65. doi: 10.1378/chest.09-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haselkorn T, Zeiger RS, Chipps BE, Mink DR, Szefler SJ, Simons FE, et al. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. The Journal of Allergy and Clinical Immunology. 2009;124:921–7. doi: 10.1016/j.jaci.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. New England Journal of Medicine. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevan A, Sutherland M. Hierarchical partitioning. The American Statistician. 1991;45:90–6. [Google Scholar]
- 20.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. The Journal of Allergy and Clinical Immunology. 2012;129:694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. British Medical Journal. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. The Journal of Allergy and Clinical Immunology. 2005;116:267–73. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. The Journal of Allergy and Clinical Immunology. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JV, Crowe JE, Jr., Enriquez R, Minton P, Peebles RS, Jr., Hamilton RG, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. The Journal of Infectious Diseases. 2005;192:1149–53. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatz M, Zeiger RS, Zhang S, Chen W, Yang S-J, Camargo CA. Overweight/Obesity and Risk of Seasonal Asthma Exacerbations. The Journal of Allergy Clinical Immunology: In Practice. 2013;1:618–22. doi: 10.1016/j.jaip.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Bateman ED, Buhl R, O'Byrne PM, Humbert M, Reddel HK, Sears MR, et al. Development and validation of a novel risk score for exacerbations: The risk score for exacerbations. The Journal of Allergy and Clinical Immunology. doi: 10.1016/j.jaci.2014.08.015. [In Press] [DOI] [PubMed] [Google Scholar]
- 27.Sorkness CA, Lemanske RF, Jr., Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. The Journal of Allergy and Clinical Immunology. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 28.McCormack MC, Aloe C, Curtin-Brosnan J, Diette GB, Breysse PN, Matsui EC. Guideline-recommnded fractional exhaled nitric oxide is a poor predictor of health-care use among inner-city children and adolescents receiving usual asthma care. Chest. 2013 Sep;144(3):923–9. doi: 10.1378/chest.12-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. American Journal of Respiratory and Critical Care Medicine. 2013;187:804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 30.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. New England Journal of Medicine. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 31.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. New England Journal of Medicine. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 32.Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarlo SM, Broder I, Corey P, Chan-Yeung M, Ferguson A, Becker A, et al. The role of symptomatic colds in asthma exacerbations: Influence of outdoor allergens and air pollutants. The Journal of Allergy and Clinical immunology. 2001;108:52–8. doi: 10.1067/mai.2001.116574. [DOI] [PubMed] [Google Scholar]
- 34.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respiratory Medicine. 2005;(99):553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.