Abstract
Background
Leukocyte telomere length (LTL) is a marker of cellular turnover and oxidative stress. Studies suggest major depressive disorder (MDD) is associated with oxidative stress, but examinations of MDD and LTL have yielded mixed results, likely because of differences in measurement methods and unmeasured confounding. This study examined LTL and telomerase activity in 166 individuals with MDD compared to 166 age- and gender-matched matched controls free of any psychiatric disorder, using well-validated assays and clinical assessment methods, and controlling for a range of potential confounders.
Methods
Subjects aged 18 to 70 were evaluated by trained raters and provided blood for LTL and telomerase activity measurement. LTL was assayed using Southern blot and replicated with qPCR, and telomerase activity was assayed with a repeat amplification protocol using a commercial kit.
Results
There was no significant difference in telomere length for individuals with MDD [mean (SD)=9.1 (3.0) kbp] compared to controls [mean(SD) =8.9(2.5) kbp] measured by Southern blot (p=0.65) or by confirmatory qPCR (p=0.91) assays. Controlling for potential confounders did not alter the results. Telomerase activity did not differ by MDD diagnosis overall (p=0.40), but the effect of MDD was significantly modified by gender (t(299)= 2.67, p=0.0079) even after controlling for potential confounders, with telomerase activity significantly greater only in males with MDD versus controls.
Conclusion
Our well-characterized, well-powered examination of concurrently assessed telomere length and telomerase activity in individuals with clinically significant, chronic MDD and matched controls failed to provide strong evidence of an association of MDD with shorter LTL, while telomerase activity was lower in men with MDD.
Keywords: Major depressive disorder, telomere length, telomerase
1. Introduction
Major depressive disorder (MDD) has been repeatedly associated with increased risk for medical morbidity (Evans et al., 2005) and mortality (Everson-Rose et al., 2004; Gump et al., 2005). This association has been found to remain true after accounting for potential health behavior confounds, such as smoking and lower levels of exercise (Penninx et al., 1998). Growing data suggest that this increased risk may be explained by an increased rate of age-related diseases such as cardiovascular disease (e.g. Wassertheil-Smoller et al., 2004) or cancer (e.g. Pinquart and Duberstein, 2010). Specifically, increasing evidence suggests that the elevated risk for age-related disease in MDD may be due in part to an abnormal stress and immune response (e.g. Chrousos, 1998; Pariante and Miller, 2001; Raison and Miller, 2003; Simon et al., 2006), which may accelerate biological aging through chronic wear on cells and tissue, increasing vulnerability to age-related disease (Kop et al., 2010; Maes et al., 2011). Leukocyte telomere length (LTL) has recently emerged as an index of cellular aging. Telomeres are nucleoprotein complexes consisting of long arrays of double stranded TTAGGG repeats associated with telomeric repeat-binding proteins and found at the ends of linear chromosomes. It is thought that telomeres function to ‘cap’ chromosomal termini and prevent end-to-end recombination, thereby counteracting or delaying shortening during cell division by maintaining chromosomal integrity (Blackburn, 2010).
Telomerase is an enzyme that adds DNA sequence repeats (“TTAGGG”) to the 3′ end of DNA strands in the telomere regions. Telomerase levels are usually insufficient to maintain telomere length in somatic cells, and progressive attrition occurs with each cell division, resulting in metered loss of telomeres that may serve as a cellular mitotic clock ultimately limiting the number of cell divisions and cellular life span. Further, while evidence suggests telomere length is in part genetically determined, increased cellular turnover in the presence of stress-related oxidative damage may exacerbate accelerated telomere shortening, suggesting that telomere-driven replicative senescence may be primarily a stress response (Lung et al., 2007; Vasa-Nicotera et al., 2005; von Zglinicki et al., 2005).
Leukocyte telomeres have been proposed to be an ideal marker of chronic stress-accelerated aging due to MDD because they serve as a marker of cellular turnover, oxidative stress, and telomerase regulation. To date, a number of studies have investigated the relationship between LTL and MDD using a variety of assays to measure telomere length [e.g. quantitative polymerase chain reaction (qPCR), Southern blot, and flourescent in situ hybridiziation (FISH)], assessing MDD with a range of measures (e.g. structured clinical interviews vs. self-report questionnaires), and differentially controlling for potential confounding variables (for review see Schutte and Malouff, 2015). Results have been mixed particularly with adjustment for the presence of concurrent medical morbidity. Multiple cross-sectional investigations have provided evidence that a history of MDD or current MDD is associated with shorter telomere length in comparison to controls (Garcia-Rizo et al., 2013; Hartmann et al., 2010; Karabatsiakis et al., 2014; Lung et al., 2007; Simon et al., 2006; Wikgren et al., 2012) including in an investigation that controlled for age, sex, education, body mass index (BMI), smoking, alcohol use, physical activity, and somatic diseases (Verhoeven et al., 2013). Further, in a recent meta-analysis using data from 25 studies (including the studies referenced here), Schutte & Malouff (2015) found a small association between depression and shorter LTL (r= -0.12, P < 0.001).
However, many studies report mixed results including Hoen et al. (2011) who found significantly shorter LTL in MDD compared to controls after controlling for age and sex, but the findings were only trending significance after controlling for BMI, smoking, diabetes, left ventricular ejection fraction, statin use, antidepressant use, physical inactivity, and anxiety [MDD: 0.85(0.02) vs. no MDD 0.89(0.01) T/S ratio, P=0.06]. Other investigations found no overall association between LTL and MDD but significant association with subpopulations with greater cumulative depression exposure (Wolkowitz et al., 2011) or on antidepressants (Needham et al., 2015). Additionally, Shalev et al. (2014) reported significant acceleration of telomere length erosion from age 26 to 38 in males with major depression, but not females. Other cross-sectional studies have failed to demonstrate any significant association between LTL and clinically diagnosed MDD (Malan et al., 2011; Schaakxs et al., 2015; Teyssier et al., 2012). Additionally, in a prospective longitudinal study, MDD diagnosis (determined by a self-administered version of the CIDI) at midpoint assessment did not predict shorter LTL at two year follow-up, while anxiety disorders did (Hoen et al., 2013). This mixed literature suggests that more research carefully examining the association of clinically diagnosed MDD and telomere length controlling for a range of potential confounds including BMI, medical morbidity, age, anti-inflammatory medication use, and environmental stressors such as loss and trauma, is needed to clarify this association.
In order to better isolate the effect of the chronic stress of MDD on telomere length, we designed this study selecting individuals with current MDD with an onset of the first MDD episode at least five years prior, extensively characterized the medical morbidity of our sample, and excluded individuals with characteristics that have previously been linked to telomere length, including severe obesity (Muezzinler et al., 2014), ongoing severe medical illnesses such as cancer, chronic inflammatory diseases, and diabetes (Kong et al., 2013). Use of medications known to impact inflammation and antidepressants was also exclusionary. We hypothesized that even after controlling for allowed medical morbidity, gender and age, individuals with MDD would have shorter telomeres than healthy controls free of psychiatric illness, distinctly implicating chronic MDD with telomere shortening as a marker of accelerated aging. Further, in contrast to the finding of greater telomerase activity with MDD by Wolkowitz et al (2012), we hypothesized that telomerase activity would be lower in MDD and would be related to shorter telomere length.
2. Materials and Methods
2.1 Participants
Participants age 18 to 70 years old with a primary diagnosis of MDD and gender-matched controls were recruited through advertisement and referral at the Center for Anxiety and Traumatic Stress Disorder and the Depression Clinical and Research Program at the Massachusetts General Hospital (MGH). Participants were first screened by telephone and those potentially eligible were assessed in person by trained experienced doctoral-level (MD or PhD) clinicians for psychiatric disorders using the Structured Clinical Interview for DSM-IV (First et al., 2002). Those meeting entry criteria completed interviewer-rated and self-report measures, and then underwent phlebotomy. Participants provided written informed consent, and were compensated $75 for study participation. All study procedures were approved by the Institutional Review Board of the MGH.
Study entry criteria included current chronic unipolar MDD, defined as lifetime illness duration of at least 5 years (calculated as time since initial onset of symptoms). Previous remission was allowed if the participant was in a current major depressive episode concurrent with screen and phlebotomy. Lifetime schizophrenia or any psychosis, mental retardation, organic medical disorders, and bipolar disorder were exclusionary, as well as current eating disorders, and alcohol or substance use disorders. Control participants were free of any DSM-IV Axis I psychiatric disorder. For both groups, lifetime alcohol and substance-use disorders were allowed if criteria were not met in the past 12 months. The maximum age was limited to 70 to prevent selective survival bias, and current cancer, chronic inflammatory disorders, epilepsy, diabetes, current unstable cardiovascular illness, or surgery within the prior 4 weeks, obesity (defined as BMI>35), and current pregnancy were exclusionary to reduce potential confounders. Use of any psychiatric medication in the 2 weeks (4 weeks for fluoxetine) prior to screen or of anti-inflammatory medications in the 3 days prior to blood draw was exclusionary. For antibiotic use or an acute infectious process such as an upper respiratory infection, participants returned for phlebotomy one week after symptom resolution or completion of any antibiotics. Lifetime psychiatric medication use was not exclusionary, but was recorded and adjusted for in the analyses within MDD participants, as less than 6 months vs. greater than 6 months of cumulative lifetime exposure.
2.2 Measures
The Structured Clinical Interview for DSM-IV (SCID; First et al., 2002) was used to assess psychiatric disorders, including the number and total cumulative length of major depressive episodes based on the SCID (module J). The 10-item clinician-administered Montgomery Asberg Depression Rating Scale (MADRS), a validated clinician-administered measure of depression (Montgomery and Asberg, 1979) with total scores ranging from 0 to 60, was used to assess depressive symptom severity.
The Cumulative Illness Rating Scale (CIRS; Fortin et al., 2005; Miller et al., 1992) has been well-established to accurately assess overall burden of illness with specific assessment of multiple medical comorbidities and severity to generate 13 specific medical category ratings, each rated with anchors from 0 (no problem) or 4 (extremely severe) and yielding a cumulative severity rating. The CIRS was administered by MDs formally trained in its use to assess medical comorbidity as a potential covariate. BMI, exercise frequency (days per week of strenuous exercise of at least 20-minutes duration), and smoking history (lifetime pack-years) were also assessed. Lifetime exposure to psychiatric medication was also formally assessed and categorized as less than 6 months of cumulative lifetime exposure or greater than 6 months of cumulative exposure.
The Traumatic Events Questionnaire (TEQ; Vrana and Lauterbach, 1994) was adapted to assess cumulative exposure to a range of 11 categories of childhood and adult traumatic life events in a self-rated format, with a cumulative score double weighted for multiple exposures. The short form of the Early Trauma Inventory Self-Report (ETISR-SF; Bremner et al., 2007) was used to assess exposure to trauma before age 18, including general trauma (11 items), physical abuse (5 items), emotional abuse (5 items), and sexual abuse (6 items). Those who reported a significant loss with the Loss History Form also completed the Inventory of Complicated Grief (ICG), a 19-item scale assessing CG symptom severity (Prigerson et al., 1995) with each item rated from 0=“not at all” to 4=“severe,” and a total score ranging from 0 to 76. The Perceived Stress Scale, a 10-item self-report measure, was used to assess perceptions of stress during the previous month (Cohen et al., 1983).
2.3 Collection of Blood Samples and DNA Extraction
Participants underwent venipuncture at rest between 9:00AM and 6:45PM. Total DNA for telomere assays was isolated from from anticoagulated whole blood (stored at -20) using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, cat # 69506).For telomerase assays, whole blood was centrifuged at 3500 rpm for 20 minutes using soft acceleration and deceleration. The buffy coat was then transferred to phosphate buffered saline (PBS) and centrifuged for 10 minutes at 2600 rpm using hard acceleration and deceleration. The resulting leukocyte pellet was then re-suspended in PBS and stored at −80 °C.
2.4 Telomere Length
We measured leukocyte telomere length using Southern blot in our final sample of 332 participants (166 MDD patients and 166 controls). Further, we validated our measure of telomere length using quantitative polymerase chain reaction (qPCR) in a subset of 250 individuals with sufficient DNA for a second analysis (125 MDD, 125 controls). Assays were performed blind to diagnosis.
2.4.1 Telomere Length by Southern blot
Telomere length was first assessed with standard quantitative telomere Southern blot analyses (Bodnar et al., 1998). To improve quantification of the gel results, in addition to exposure to autoradiographic film (Kodak Biomax-ER, Rochester, New York), the radioactive signal from the gels was quantified using a phosphorimager (Storm 860, GE Healthcare Life Sciences, Pittsburgh, PA). ImageQuant TL software (GE Healthcare Life Sciences) was then used to draw a grid (30 boxes) over each lane from 24 to 2.5 kb, allowing quantification of pixel density for each box (ODi). Molecular weight size standards were used to calibrate the molecular weight associated with each box (MWi) for each individual gel. Mean terminal restriction fragment (TRF) of each sample was calculated from background-corrected box optical density (cODi) using the formula TRF = Σ (cODi * MWi)/Σ (cODi) with the assistance of UTSWTELORUN software (available http://www4.utsouthwestern.edu/cellbio/shay-wright/research/sw_lab_methods.htm), a modification of previous TELORUN software (Ouellette et al., 2000).
2.4.2 Telomere Length by Quantitative Polymerase Chain Reaction (qPCR)
Telomere length was further measured in confirmatory analyses with qPCR, a method now commonly used in published studies (e.g. Cawthon, 2009). The ratio of telomere repeat copy number to a single gene copy number (T/S) was determined by a previously described modified, high-throughput version (Wang et al., 2008) of the qPCR telomere assay (Cawthon, 2002) run on the Applied Biosystems 7900HT Sequence Detection System (Foster City, CA, USA). Triplicate reactions of each assay were performed for each sample. LTL is reported as relative T/S (i.e. T/S of the sample divided by the T/S of a within-plate reference sample). The telomere and single-gene assay coefficients of variation (CVs) for triplicates were 0.86% and 0.49%, respectively. CVs for the exponentiated T/S ratio were 13.4%.
2.5 Telomerase Activity
Total-cell extracts from leukocytes lysed on ice were cleared of debris, assayed for total protein concentration (Pierce BCA Protein Assay Kit), and flash frozen at -80 until assay. The Telomeric Repeat Amplification Protocol (TRAP) (Quantitative Telomerase Detection Kit, Allied Biotech Inc.) was used to quantify telomerase activity in samples of equivalent total protein through qPCR-based amplification of telomeric repeats added onto an oligonucleotide substrate by the enzyme in a prior in vitro incubation step of fixed duration. All samples were run in technical triplicates with low standard deviation. Additionally, this methodology was verified by experimental replication of the first twenty matched pairs of samples. As telomerase is a heat-sensitive enzyme, an equal volume of each sample heated to 85° for 10 minutes additionally served as a within-plate negative control and sample-specific measure of background fluorescence.
2.6 Statistical Methods
Multivariate linear regression was used to examine whether telomere length and telomerase activity varied by diagnosis (MDD vs. controls). Telomere length (as measured both by Southern blot and by qPCR) and telomerase activity were natural log transformed to achieve normality. Prior to analysis, we tested whether significant differences existed by diagnosis in the baseline demographic and clinical covariates shown in Table 1, using t-tests for continuous covariates and Fisher's exact tests for categorical covariates. We also tested whether these covariates were significantly associated with telomere length and telomerase activity (after ensuring the relationship was linear). While traditionally a potential confounder would be defined as any covariate associated with both the outcome of interest (i.e., telomere length or telomerase activity) and with diagnosis, and which is not on the causal pathway (Clayton and Hills, 1998), we took a conservative approach and adjusted for any covariates significantly associated with either the outcome of interest or diagnosis (but were not on the causal pathway), and controlled for such covariates in the multivariate regression models. We also examined whether age and gender (matching variables) were effect moderators by including them in interaction terms with diagnosis. Finally, as a post-hoc analysis, the association between continuous depressive symptom severity and telomere length and telomerase activity was examined in the full sample using a multiple regression. The level of statistical significance was set to 0.05 (two-tailed) for all analyses. Analyses were conducted using SAS v9.2 (SAS Institute, Cary, NC).
Table 1. Characteristics of 166 Individuals with Major Depressive Disorder and 166 Matched Controls.
| Control (n=166) | MDD (n=166) | p-valuea | |||
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
|
| |||||
| Age (years), Mean (SD) | 41.3 | (13.7) | 41.3 | (13.8) | 0.99 |
| Sex, N (%) Female | 89 | (54) | 89 | (54) | 1.0 |
| Race, N (%) | 0.089 | ||||
| White | 113 | (68) | 130 | (78) | |
| Black or African American | 29 | (17) | 19 | (11) | |
| Asian | 11 | (7) | 7 | (4) | |
| Native American/Alaska Native | 0 | (0) | 2 | (1) | |
| Other | 13 | (8) | 7 | (4) | |
| Ethnicity, N (%) | 0.42 | ||||
| Not Hispanic/Latino | 150 | (90) | 152 | (92) | |
| Hispanic/Latino | 16 | (10) | 11 | (7) | |
| Education level, N (%) | 0.0010* | ||||
| Graduate school | 58 | (35) | 34 | (20) | |
| College graduate | 66 | (40) | 58 | (35) | |
| College, partial | 30 | (18) | 42 | (25) | |
| High school graduate or less | 11 | (7) | 28 | (17) | |
|
| |||||
| Clinical Characteristics | |||||
|
| |||||
| Years since MDD Onset, Mean (SD) | n/a | n/a | 21.8 | (14.3) | n/a |
| Years of Depressive Episodes, Mean (SD) | n/a | n/a | 12.6 | (12.8) | n/a |
| MADRS Total Score, Mean (SD) | 1.9 | (2.4) | 28.2 | (6.0) | n/a |
| Any anxiety disorder - current, N (%) | n/a | n/a | 83 | (50) | n/a |
| Any anxiety disorder - lifetime, N (%) | n/a | n/a | 92 | (55) | n/a |
| Anti-depressant use > 6 months, N (%) | n/a | n/a | 65 | (39) | n/a |
| Mood stabilizer use > 6 months, N (%) | n/a | n/a | 6 | (4) | n/a |
| Benzodiazepine use > 6 months, N (%) | n/a | n/a | 19 | (11) | n/a |
| Antipsychotic use > 6 months, N (%) | n/a | n/a | 10 | (6) | n/a |
| Lifetime alcohol or drug abuse/dependence, N (%) | 13 | (8) | 47 | (28) | <0.0001* |
| History of loss (based on ICG) | 121 | (73) | 126 | (76) | 0.30 |
| ICG Total score, Mean (SD) | 6.0 | (7.0) | 17.9 | (14.3) | <0.0001* |
| TEQ weighted score, Mean (SD) | 1.7 | (2.4) | 4.2 | (4.1) | <0.0001* |
| ETISR (# answered yes), Mean (SD) | 3.5 | (3.8) | 8.4 | (5.6) | <0.0001* |
| PSS Total score, Mean (SD) | 8.2 | (5.4) | 24.5 | (5.7) | <0.0001* |
|
| |||||
| Medical History | |||||
|
| |||||
| Height (inches), Mean (SD) | 67.3 | (3.7) | 67.0 | (3.7) | 0.50 |
| Weight (lbs), Mean (SD) | 163.8 | (33.7) | 166.4 | (37.1) | 0.51 |
| BMI, Mean (SD) | 25.4 | (4.2) | 25.9 | (4.6) | 0.26 |
| Menopausal status, N (%) | 0.72 | ||||
| Pre- / Peri-menopausal | 66 | (74) | 59 | (66) | |
| Post-menopausal / Hysterectomy | 21 | (24) | 22 | (25) | |
| Exercise level, N (%) | <0.0001* | ||||
| Never / Less than once a month | 17 | (10) | 45 | (27) | |
| Less than once a week | 23 | (14) | 34 | (20) | |
| Few times a week | 36 | (22) | 32 | (19) | |
| Several times a week | 70 | (42) | 35 | (21) | |
| Every day | 19 | (11) | 14 | (8) | |
| Smoking status, N (%) | 0.0017* | ||||
| Current smoker | 15 | (9) | 34 | (20) | |
| Past smoker | 30 | (18) | 38 | (23) | |
| Never smoked | 120 | (72) | 90 | (54) | |
| Pack years smoking (in smokers), Mean (SD) | 10.8 | (11.6) | 10.3 | (14.0) | 0.78 |
| Any lifetime medical illness, N (%) | 141 | (85) | 146 | (88) | 0.52 |
| Hypertension (BP > 150/90), N (%) | 31 | (19) | 18 | (11) | 0.044* |
| CIRS Total score, Mean (SD) | 2.6 | (2.1) | 3.6 | (2.9) | 0.0026* |
|
| |||||
| Biological Outcomes | |||||
|
| |||||
| log(Telomere length kbp) Southern, Mean (SD) | 8.9 | (3.0) | 9.1 | (2.5) | 0.65 |
| log(Telomere length T/S ratio) qPCR, Mean (SD) | 0.64 | (0.17) | 0.65 | (0.21) | 0.91 |
| log(Telomerase activity), Mean (SD) | 3.6 | (1.6) | 3.7 | (1.5) | 0.40 |
BMI: Body Mass Index; CIRS: Cumulative Illness Rating Scale; ETISR: Early Trauma Inventory self-report; ICG: Inventory of Complicated Grief; MADRS: Montgomery Asberg Depression Rating Scale; TEQ: Traumatic Events Questionnaire; MDD: Major Depressive Disorder; PSS: Perceived Stress Scale; BP: Blood Pressure; n/a = not analyzed; %'s may not add to 100% due to missing data
Based on Fisher's exact test for categorical data and Student's t-test for continuous data.
Significant (p<0.05)
The full sample for the primary analyses comprised 166 adults with current MDD and 166 age and gender-matched controls (total n=332). Participants were not, however, matched by race; in light of data published after study enrollment reporting that telomeres of African-Americans are significantly longer at birth but shorten faster than telomeres of Caucasians (Hunt et al., 2008; Rewak et al., 2014; Zhu et al., 2011), resulting in shorter telomeres on average by middle or late adulthood in African-Americans (Diez Roux et al., 2009; Geronimus et al., 2010), we conducted further analyses among Caucasian participants only (as we did not have enough statistical power to examine the associations for participants within other races). In this follow-up analysis within Caucasians, to conserve power we did not additionally restrict the sample to include only those MDD patients and Controls with an age/gender match (i.e., we included Caucasian MDD patients previously matched to non-Caucasians, as well as 1 Caucasian Control who was not previously matched to any MDD patient). Telomerase activity was assessed in 300 of the 332 participants in the full sample, excluding 16 of the MDD-Control pairs due to insufficient collected leukocyte samples.
3. Results
3.1 Participants and Symptom Characteristics
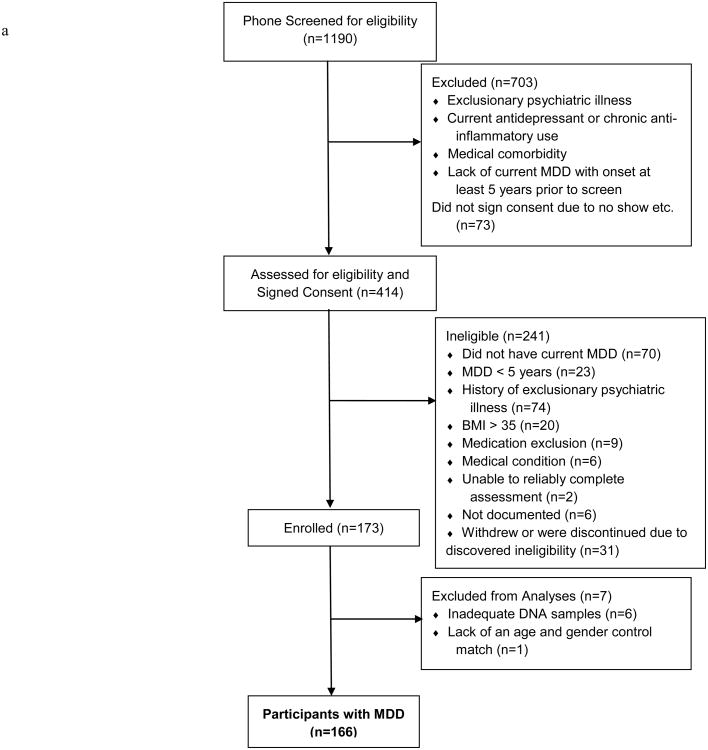
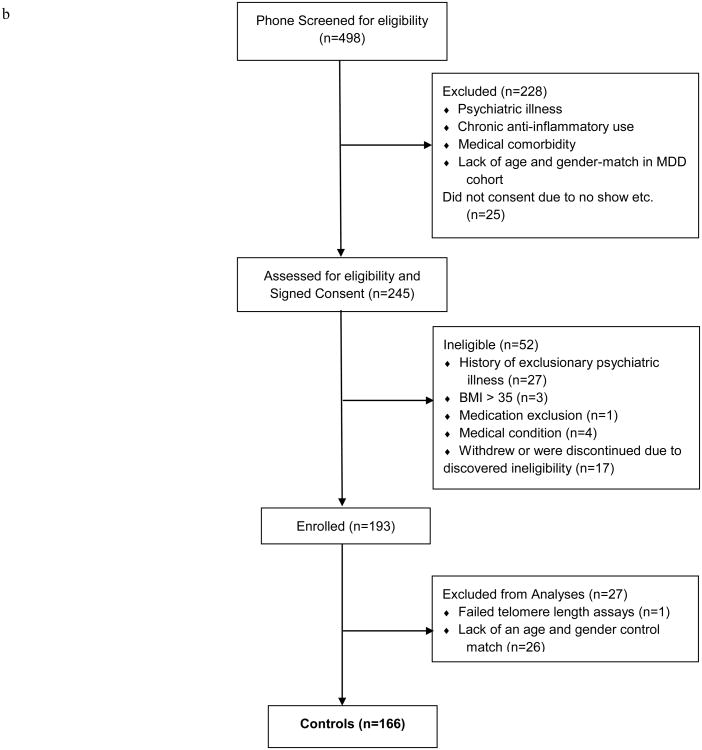
Figure 1 presents the study enrollment flowchart. Participants' characteristics by group are reported in Table 1. As expected, no significant differences were detected by diagnosis in age and gender, which were our matching factors. As seen in Table 1, at study entry, patients with MDD were significantly more likely than controls to have had a lower level of education, exercise less frequently, smoke, and have medical co-morbidity (CIRS total score), but less likely to have hypertension; they also were more likely to have lifetime alcohol and/or drug abuse or dependence, greater grief severity (ICG total score), trauma exposure (TEQ weighted score and ETISR number answered “yes”), and perceived stress (PSS total score).
Figure 1.
Summary of number of subjects assessed for eligibility, excluded, and enrolled, with reasons for exclusion from the study and exclusion from the analysis population, for subjects with MDD 1a) and healthy controls (1b). Note: MDD = major depressive disorder; BMI = body-mass index.
Mean time from MDD onset to study entry was 21.8 years (SD = 14.3, range 4 – 61). Cumulative duration of mood episodes ranged from 8 months to 53 years, with a mean of 12.6 (SD=12.8) years. Almost half (39%, n=65) of participants with MDD reported lifetime anti-depressant use of six months or more, 50% met criteria for a current anxiety disorder (including SAD, panic, agoraphobia, current GAD, PTSD, and OCD), and 55% for a lifetime anxiety disorder.
Table 2 presents a correlation table of continuous symptom measures with telomere length, telomerase, BMI and age for controls and MDD participants. Of note, measures of stress (PSS) and loss (ICG) were significantly associated with MADRS score in both groups. No symptom measures, however, including MADRS score were significantly correlated with telomere length, as measured by Southern or qPCR, or telomerase, with the exception of perceived stress, which was correlated at the level of a trend (r=0.14, p<0.10) in the MDD sample.
Table 2. Pearson Correlation Coefficients between Continuous Covariates in MDD and Control Subjects.
| MDD | log (Telomere Southern length) |
log (Telomere PCR length) |
log (Telomerase activity) |
MADRS Total |
sqrt (ICG Total) |
sqrt (TEQ weighted) |
sqrt (CIRS Total) |
sqrt (ETISR # yes) |
sqrt (Pack years smoking) |
PSS Total | BMI | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||||||
| Log (Telomere Southern length) | -- | 0.29** | -0.01 | -0.03 | 0.07 | <0.01 | -0.01 | 0.06 | 0.03 | -0.14* | <0.01 | <0.01 |
| Log (Telomere PCR length) | 0.08 | -- | 0.20** | -0.04 | -0.02 | -0.10 | < -0.01 | -0.06 | 0.06 | < -0.01 | -0.23** | -0.24** |
| Log (Telomerase activity) | 0.07 | -0.06 | -- | -0.01 | -0.13 | 0.01 | <0.01 | < -0.01 | -0.03 | 0.02 | -0.03 | <0.01 |
| MADRS Total | 0.01 | -0.03 | -0.06 | -- | 0.18** | 0.21** | -0.03 | 0.04 | -0.01 | 0.37** | 0.04 | 0.17** |
| sqrt (ICG Total) | 0.05 | < -0.01 | 0.02 | 0.22** | -- | 0.38** | 0.07 | 0.28** | -0.10 | 0.15* | 0.04 | 0.04 |
| sqrt (TEQ weighted) | -0.03 | -0.02 | 0.02 | < -0.01 | 0.27** | -- | 0.21** | 0.64** | 0.06 | 0.14 | 0.17* | 0.23** |
| sqrt (CIRS Total) | <0.01 | -0.06 | -0.08 | 0.21** | 0.10 | 0.37** | -- | 0.08 | 0.15 | -0.01 | 0.02 | 0.33** |
| sqrt (ETISR # yes) | 0.09 | -0.07 | 0.01 | 0.09 | 0.20** | 0.56** | 0.31** | -- | 0.08 | 0.15* | -0.02 | 0.04 |
| Sqrt (Pack years smoking) | -0.10 | -0.23** | -0.06 | -0.06 | -0.03 | 0.27** | 0.26** | 0.22** | -- | -0.03 | 0.02 | 0.18* |
| PSS Total | -0.01 | 0.05 | 0.05 | 0.33** | 0.38** | 0.05 | 0.12 | 0.21** | -0.03 | -- | 0.14* | -0.03 |
| BMI | 0.10 | -0.04 | -0.12 | 0.02 | 0.14 | 0.22** | 0.15** | 0.19** | 0.03 | -0.09 | -- | 0.25** |
| Age | -0.05 | -0.16* | -0.07 | 0.01 | 0.08 | 0.21** | 0.32** | 0.10 | 0.33** | -0.24** | 0.23** | -- |
p<0.05;
0.05≤p<0.10; bolded correlation coefficients had a p<0.10
3.2 Telomere Length by Diagnosis
There was no significant difference in telomere length for individuals with MDD [mean (SD)=9.1 (3.0)kbp] compared to controls [mean(SD) =8.9(2.5)kbp] measured by Southern blot 0=0.014, SE(β)=0.031, t(331)= 0.45, p=0.65], or with confirmatory measurement by qPCR [β= -0.0042, SE(β)=0.036, t(249) = -0.12, p=0.91]. The Pearson correlation between LTL by Southern and by qPCR length (both log transformed to achieve normality) was r=0.21 overall (p=0.0009).
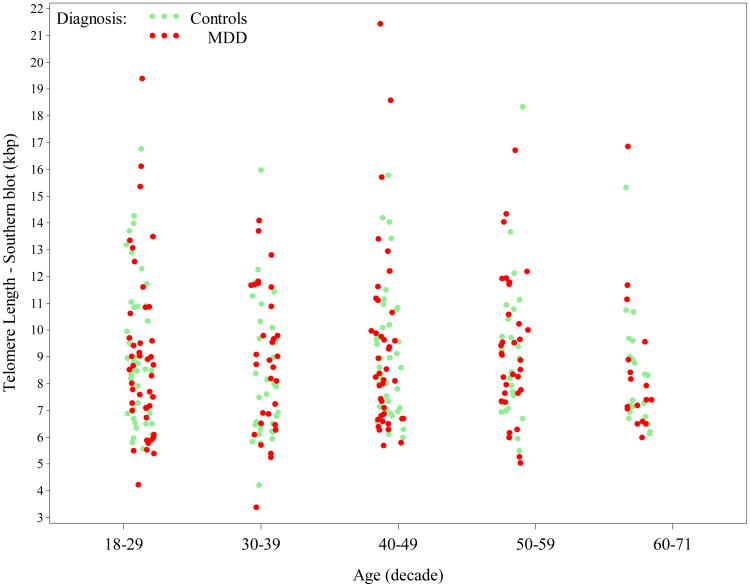
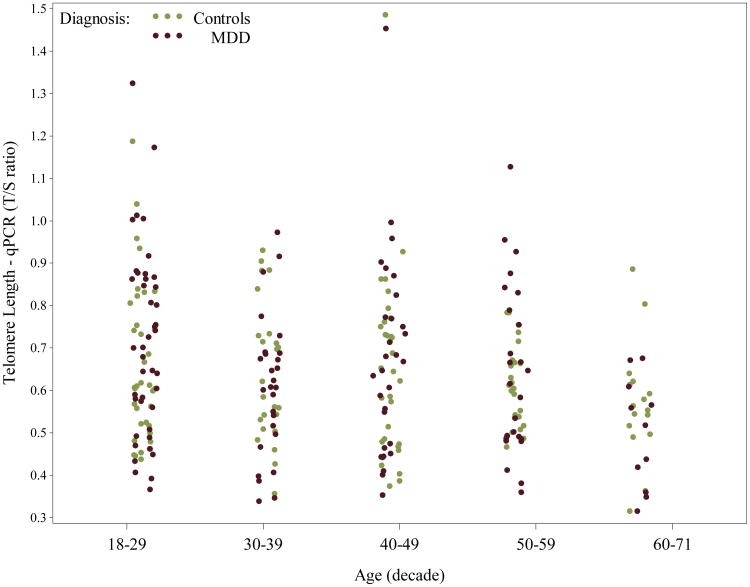
On average, females had slightly longer telomeres [mean (SD) = 9.2 (2.9)kbp] compared to males [mean (SD) = 8.7 (2.5)kbp], but differences by gender were non-significant [β=0.045, SE(β)=0.031, t(331)= 1.45, p=0.15]. When measured by qPCR, telomere length was very similar between females [mean (SD) = 0.66 (0.18) T/S ratio] and males [mean (SD) = 0.63 (0.20) T/S ratio]. While LTL by Southern blot did not vary significantly by continuous age [β= -0.00045, SE(β)=0.0011, t(331)= -0.40, p=0.69] - see Appendix Figure A2, which breaks out values by decade of age, LTL by qPCR did [β= -0.0043, SE(β)=0.0013, t(249)= -3.32, p=0.0010], being shortest in older participants, as seen in Appendix Figure A3. The relationship between LTL by Southern and MDD was not significantly modified by gender (interaction p=0.47) nor by age (interaction p=0.65). Results were similar for qPCR data.
In the post-hoc analysis examining whether the continuous MADRS total score (i.e. level of depressive symptoms) was significantly associated with LTL across the full sample, we again found a non-significant association with LTL in both Southern blot (R2=0.0003, p=0.76) and qPCR (R2=0.0004, p=0.76) analyses.
When we examined the potential for confounding, we found that education level, alcohol and/or drug abuse/dependence, grief symptoms (ICG total score), cumulative (TEQ weighted score) and early (ETISR number answered yes) trauma exposure, perceived stress (PSS total score, exercise level, smoking status, hypertension, and medical co-morbidity (CIRS total score) were significantly associated with MDD diagnosis, and that age (p=0.0010), weight (p=0.030), and BMI (p=0.020) were significantly associated with LTL by qPCR, and thus were potential confounders (note that none of the covariates shown in Table 1 were significantly associated with LTL by Southern blot). Due to possible collinearity with cumulative trauma exposure (TEQ total score), we chose not to adjust for early trauma exposure (ETISR number answered yes), and also did not control for perceived stress (PSS total score), because theoretically it is on the causal pathway - i.e. stress is a possible mechanism through which MDD acts on telomere length. Adjusting for the remaining potential confounders failed to alter our findings; LTL still did not significantly differ by diagnosis for either Southern blot [β=0.016, SE(β)=0.049, t(188)=0.33, p=0.74] or qPCR [β=-0.012, SE(β)=0.059, t(139)= -0.20, p=0.84].
Finally, when we repeated our analyses among Caucasian participants only, we again found no significant difference in telomere length between MDD and controls in either Southern blot (130 MDD, 114 Controls) or qPCR (108 MDD, 91 Controls) analyses, nor any effect modification by age or gender.
3.3 Telomerase Activity by Diagnosis
On average, MDD patients had greater telomerase activity compared to controls, as seen in Table 1, but differences were not statistically significant [β= -0.15, SE(β)=0.18, t(299)= -0.84, p=0.40]. Even after controlling for potential confounding by those covariates in Table 1 that differed significantly by diagnosis, with the exception of the ETISR number answered yes and PSS total score for reasons noted above (note that none of the covariates listed were significant univariate predictors of telomerase activity), telomerase activity did not significantly differ by diagnosis overall [β=0.22, SE(β)=0.28, t(172)=0.78, p =0.44]. A post-hoc analysis examining the association of continuous MADRS total score with telomerase activity in the full sample was also non-significant (R2 =0.0015, p=0.50), even after controlling for potential confounding.
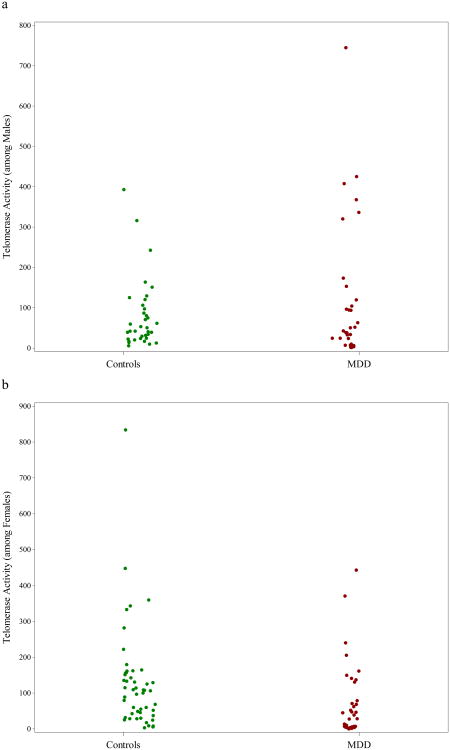
Telomerase activity did not vary significantly by gender [β= -0.062, SE(β)=0.18, t(299)=-0.34, p=0.73] nor by age [β= -0.0041, SE(β)=0.0066, t(299)= -0.62, p=0.53], but gender was a significant effect modifier (interaction p=0.0046), as seen in Figure 2. Male MDD patients had significantly greater telomerase activity than male controls (β=0.70, SE(β)=0.26, t(299)=2.67, p=0.0079), but female MDD patients had lower (but not significantly different) telomerase activity than female controls (β= -0.32, SE(β)=0.24, t(299)= -1.31, p=0.19). After adjustment for potential confounding, gender remained a significant moderator, with significantly increased telomerase activity among MDD males vs. controls [β=1.01, SE(β)=0.38, t(172)=2.65, p=0.0090], and non-significantly lower telomerase activity among MDD females vs. controls [β= -0.38, SE(β)=0.35, t(172)= -1.11, p=0.27]. When we repeated our analyses among Caucasian participants only, we again found significant effect modification by gender, with significantly increased telomerase activity among MDD males vs. controls, and non-significantly lower telomerase activity among MDD females vs. controls.
Figure 2.
Scatterplot illustrating the differential association between MDD and Telomerase activity based on gender (each dot/circle represents a subject). As seen in Figure 2a, mean Telomerase activity was significantly higher among males with major depressive disorder (MDD) compared to healthy male controls, whereas in Figure 2b, the opposite was true - mean Telomerase activity was lower among females with MDD than healthy female controls, though differences were non-significant. For color versions of the figures, please refer to the web version of the article.
3.4 Telomere Length and Telomerase Activity Within Participants with MDD
Among participants with MDD, neither total duration of mood episodes, years of MDD, nor anxiety comorbidity (current or lifetime) were significantly associated with either LTL (by Southern blot or qPCR) or telomerase level, even after controlling for potential confounding by gender, age, and anti-depressant use. After additionally examining associations with substance abuse/dependence co-morbidity, smoking status, exercise level, education level, presence of hypertension, ICG total score, TEQ weighted score, and CIRS total score within the MDD sample, we found that LTL by qPCR was significantly shorter among MDD patients with hypertension, on average [b= -0.18, SE(b)=0.090, t(121)= -2.01, p=0.047], and there was a slight trend for shorter LTL by qPCR associated with more years of MDD [β= -0.0036, SE(β)=0.0020, t(124)= -1.83, p=0.070]. Additional post-hoc associations examined by diagnosis can be found in Table 2. Within the MDD sample, telomerase activity (log transformed) was noted to be significantly positively correlated with LTL by qPCR (r=0.20, p<0.05), as seen in Table 2, and the association remained significant even after adjusting for age.
4. Discussion
Our well-powered concurrent examination of telomere length and telomerase activity in a well-characterized sample of individuals with chronic MDD and age- and gender-matched controls failed to find an association of MDD with telomere length. This finding is at odds with a recent meta analysis of 25 studies examining LTL and MDD (Schutte and Malouff, 2015) and a number of published studies reporting that individuals with a MDD diagnosis determined by structured or questionnaire supported diagnostic interviews possess shortened telomeres (Garcia-Rizo et al., 2013; Hartmann et al., 2010; Karabatsiakis et al., 2014; Lung et al., 2007; Simon et al., 2006; Verhoeven et al., 2013; Wikgren et al., 2012). On the other hand, our data are consistent with other published studies that have failed to demonstrate a significant association with depression diagnosis ascertained by validated, structured clinical interviews (Malan et al., 2011; Schaakxs et al., 2015; Teyssier et al., 2012). In addition, studies that employed a variety of self-report symptom screening methods or self administered diagnostic assessments, some of which include non-clinical, population based studies have also reported no significant association between LTL and depression (Hoen et al., 2013; Ladwig et al., 2013; Shaffer et al., 2012; Surtees et al., 2011).
Potential explanations for these mixed results include the differing methods of assessment of MDD (e.g. SCID vs. CES-D), as well as ascertainment of clinical samples versus non-clinical populations (for review see Kinser and Lyon, 2013; Schutte and Malouff, 2015). However, both positive and negative findings have been reported in studies using validated, structured clinical interviews to diagnose depression. Mixed results in depression and telomere studies may also be due in part to the presence of potentially uncontrolled confounding factors such as variably assessed medical illness and life stressors; however, not all studies with positive results failed to control for multiple confounding variables. For example, Verhoeven et al. (2013) found that both remitted and current MDD was associated with shorter telomeres than controls after adjusting analysis for age, sex, education, BMI, smoking, alcohol use, physical activity, and somatic diseases. Finally, telomere length assay methods also vary across studies. While Southern blot was initially the preferred method to measure telomere length and is a gold standard for reliability (Hartmann et al., 2010; Lung et al., 2007), quantitative polymerase chain reaction has become the most widely used method (Schutte and Malouff, 2015). In summary, using a replication strategy with two well-validated methods to measure telomere length, we found that individuals with MDD did not have shorter telomeres than their psychiatric disorder-free gender- and age-matched counterparts. Neither adjustment for a broad array of potential confounding factors (such as allowed medical comorbidity, age, gender, education, alcohol/drug-use disorders and life stressors including trauma and loss) nor restriction of the analyses to Caucasians revealed significant differences. While there was no significant difference in telomerase activity between individuals with MDD and controls in the bivariate analysis, the analysis controlling for potential confounds including grief symptoms, trauma exposure, alcohol and/or drug abuse/dependence, education level, and medical co-morbidity, revealed higher telomerase activity in males with MDD than in male controls, suggesting depression might be associated with increased telomerase activity. Although counter to our initial hypothesis and significant only for men with significant gender effects, these telomerase activity results are in line with those of Wolkowitz et al. (2012) who reported that telomerase activity was higher in 20 medication-free individuals with MDD compared to 18 controls after controlling for age and gender. It has been suggested that increased telomerase activity may reflect a compensatory response to cellular damage (Harley, 2005), and our results suggest that MDD may be associated with such increased activity. It is, however, unclear why certain factors associated with MDD (e.g. grief symptoms, trauma exposure, alcohol and/or drug abuse/dependence, and medical co-morbidity) might play a role in normalizing telomerase activity in individuals with MDD, or why we found significantly greater telomerase activity in men but not women.
Our study's strengths include recruitment of a sample with careful selection criteria, limiting potential confounding by factors such as obesity that may not be sufficiently adjusted for by statistical approaches alone, and extensive formal characterization of lifetime comorbidities, environmental stressors, and formalized cumulative medical illness ratings. Further, we used well-validated rating scales and rigorous enrollment criteria, requiring at least 5 years since the onset of the first major depressive episode and a current episode as determined by SCID administered by a trained clinical rater in order to assure that MDD was a primary diagnosis of sufficient severity and length to potentially impact telomere length. Our findings raise the possibility that residual confounding by factors known to be associated with both depression and telomere length might be contributing to findings in some prior studies of depression and telomere length with less rigorous assessment or entry criteria.
Study limitations include the cross-sectional nature of this study with depression history dependent on structured clinician interviews only, and the lack of assessment of other potential confounds such as paternal or maternal age or genetic contributors. Further, our rigorous entry criteria developed to limit confounding, including the exclusion of some medical conditions, may have selected an unrepresentative cohort of patients with MDD. Enrolled patients were required to be free of antidepressants at study entry in order not to interfere with experimental assays. This may have limited severity and chronicity of the sample; of note, less than half of the sample reported lifetime antidepressant use for over 6 months (40%). Of interest, a recent population based study of young adults with depression also found no difference in telomere length overall or in Caucasians specifically, except in the subsample currently taking antidepressants—a subset interpreted as having severest disease as indicated by prior medical presentation with treatment-warranting symptoms (Needham et al., 2015). Nonetheless, in our study, the cumulative duration of depressive episodes was long (mean of 12.6 (SD=12.8) years) and the mean time from MDD onset was 21.8 years (SD = 14.3, range 4 – 61), representative of chronic MDD. Further, depression severity as measured by the MADRS also failed to show a significant association with both telomere length and telomerase activity. Finally, the direct correlation between qPCR T/S ratio and Southern telomere kbp length results were modest, and non significant in controls; the confirmatory qPCR was also performed later at a different laboratory. It is worth noting that prior studies have also found wide variability in absolute results across laboratories, though standardized values examined as ranks were highly correlated, significant variance across labs remained and was largely explained by differences in qPCR and gel based methods (Martin-Ruiz et al., 2014)
In conclusion, despite some limitations, when extensively controlling for a range of measured covariates at entry and in analyses, including age, gender, trauma and loss, and medical comorbidity, our cross sectional analyses suggest that telomere length may not be independently associated with MDD. It is possible that some previous positive reports may include unmeasured confounding of the MDD-telomere length relationship, such as medical conditions or obesity, contributing to variability across studies. Finally, while not significant overall, telomerase activity was lower in men with depression even after adjustment for potential confounders. Further replication and prospective studies are needed to shed light on the relationship of MDD to cellular aging.
Highlights.
Telomere length was not associated with depression.
Telomerase activity was higher in men with depression even after adjustment for potential confounders
Residual confounding and differences in methodology might contribute to variability in reports of associations between telomere length and depression
Acknowledgments
The study was funded by a grant from the National Institute of Mental Health (5 RO1 MH077700-05).
Role of the funding source: The funding source had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Appendix
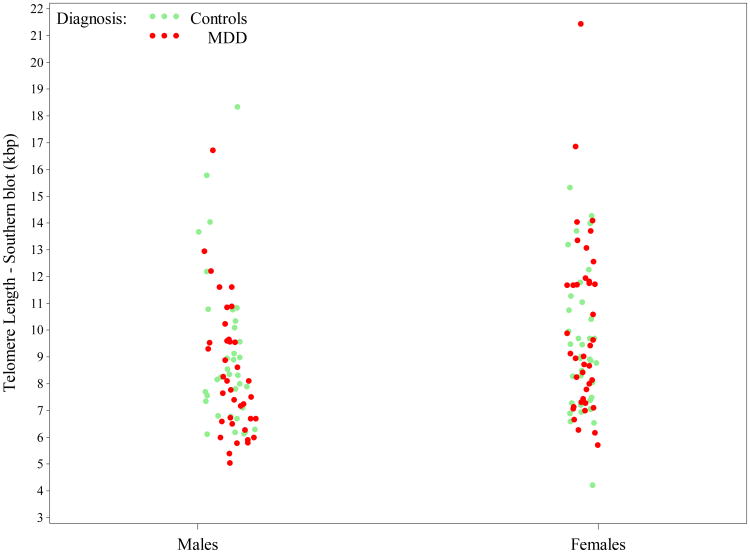
Appendix Figure A1.
Scatterplot of Telomere length, as measured by Southern blot (kilobase pair = kbp), by gender and diagnosis (each dot/circle represents a subject). Although not significantly different, females had slightly longer Telomeres than males overall, with differences more pronounced among MDD patients. For color versions of the figures, please refer to the web version of the article.
Appendix Figure A2.
Scatterplot of Telomere length, as measured by Southern blot (kilobase pair = kbp), by decade of age and diagnosis (each dot/circle represents a subject). Telomere Southern length did not vary significantly by age overall. For color versions of the figures, please refer to the web version of the article.
Appendix Figure A3.
Scatterplot of Telomere length, as measured by qPCR (ratio of telomere repeat copy number to a single gene copy number= T/S ratio), by decade of age and diagnosis (each dot/circle represents a subject). Telomere qPCR length was significantly shorter in older participants compared to younger participants overall. For color versions of the figures, please refer to the web version of the article.
Footnotes
Author Contributions: Naomi M. Simon: Dr. Simon was responsible for study design and oversaw all aspects of data collection, data management, data analysis, data interpretation, and manuscript writing.
Zandra E. Walton: Ms. Walton ran assays, participated in data interpretation, and assisted with manuscript writing.
Eric Bui: Dr. Bui contributed to data collection, data management, data analysis, data interpretation, and manuscript writing.
Jennifer Prescott: Dr. Prescott ran assays and assisted with manuscript writing.
Elizabeth Hoge: Dr. Hoge assisted with data collection, data interpretation, and manuscript writing.
Aparna Keshaviah: Ms. Keshaviah was responsible for data management and statistical analysis. She also assisted with manuscript writing.
Noah Schwarz: Mr. Schwarz assisted with data collection, data management, and manuscript writing.
Taylor Dryman: Ms. Dryman assisted with data collection, data management, and manuscript writing.
Rebecca A. Ojserkis: Ms. Ojserkis assisted with data collection, data management, and manuscript writing.
Benjamin Kovachy: Mr. Kovachy assisted with literature review, manuscript writing, and manuscript preparation.
David Mischoulon: Dr. Mischoulon assisted with data collection, data interpretation, and manuscript writing.
John Worthington: Dr. Worthington assisted with data collection and manuscript writing.
Immaculata DeVivo: Dr. DeVivo ran assays and assisted with manuscript writing.
Maurizio Fava: Dr. Fava contributed to study design, data interpretation, and manuscript writing.
Kwok-Kin Wong: Dr. Wong oversaw experiments, study design, data interpretation, and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture) Angew Chem Int Ed Engl. 2010;49:7405–7421. doi: 10.1002/anie.201002387. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Clayton D, Hills M. Statistical Models in Epidemiology. Oxford University Press; 1998. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8:251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, House JS, Mero RP. Depressive symptoms and mortality risk in a national sample: confounding effects of health status. Psychosom Med. 2004;66:823–830. doi: 10.1097/01.psy.0000145903.75432.1f. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute Biometrics Research Department; New York: 2002. [Google Scholar]
- Fortin M, Hudon C, Dubois MF, Almirall J, Lapointe L, Soubhi H. Comparative assessment of three different indices of multimorbidity for studies on health-related quality of life. Health and Qual of Life Outcomes. 2005;3:74. doi: 10.1186/1477-7525-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK, Heaphy CM, Bernardo M, Kirkpatrick B. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain Behav Immun. 2013;28:49–53. doi: 10.1016/j.bbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US Black Women Experience Stress-Related Accelerated Biological Aging?: A Novel Theory and First Population-Based Test of Black-White Differences in Telomere Length. Hum Nature. 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Eberly LE, Chang YF. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- Harley CB. Telomerase therapeutics for degenerative diseases. Current Mol Med. 2005;5:205–211. doi: 10.2174/1566524053586671. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27:1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, Blackburn E, Whooley MA. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med. 2011;73:541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, van der Harst P, de Jonge P. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med. 2013;43:689–697. doi: 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry. 2014;14:192. doi: 10.1186/1471-244X-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinser PA, Lyon DE. Major depressive disorder and measures of cellular aging: an integrative review. Nurs Res Pract. 2013;2013:469070. doi: 10.1155/2013/469070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. 2013;280:3180–3193. doi: 10.1111/febs.12326. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, Gottdiener JS. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med. 2010;72:626–635. doi: 10.1097/PSY.0b013e3181eadd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, Codd V, Hafner S, Albrecht E, Illig T, Samani NJ, Wichmann HE, Gieger C, Peters A. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Leunis JC, Geffard M. IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: new pathways that underpin the inflammatory and neuroprogressive pathophysiology. J Affect Disord. 2011;135:414–418. doi: 10.1016/j.jad.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Malan S, Hemmings S, Kidd M, Martin L, Seedat S. Investigation of telomere length and psychological stress in rape victims. Depress Anxiety. 2011;28:1081–1085. doi: 10.1002/da.20903. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Baird D, Roger L, Boukamp P, Krunic D, Cawthon R, Dokter MM, van der Harst P, Bekaert S, de Meyer T, Roos G, Svenson U, Codd V, Samani NJ, McGlynn L, Shiels PG, Pooley KA, Dunning AM, Cooper R, Wong A, Kingston A, von Zglinicki T. Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol. 2014 doi: 10.1093/ije/dyu191. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2014;15:192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- Needham BL, Mezuk B, Bareis N, Lin J, Blackburn EH, Epel ES. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry. 2015;20:520–528. doi: 10.1038/mp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette MM, Liao M, Herbert BS, Johnson M, Holt SE, Liss HS, Shay JW, Wright WE. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, Havlik RJ. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Reynolds CF, 3rd, Bierhals AJ, Newsom JT, Fasiczka A, Frank E, Doman J, Miller M. Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59:65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, Non AL, Kubzansky LD. Race-related health disparities and biological aging: Does rate of telomere shortening differ across blacks and whites? Biol Psychol. 2014;99:92–99. doi: 10.1016/j.biopsycho.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaakxs R, Verhoeven JE, Oude Voshaar RC, Comijs HC, Penninx BW. Leukocyte Telomere Length and Late-Life Depression. Am J Geriatr Psychiatry. 2015;23:423–32. doi: 10.1016/j.jagp.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. The Association Between Depression And Leukocyte Telomere Length: A Meta-analysis. Depress Anxiety. 2015;32:229–238. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW, Kirkland S, Honig LS, Shimbo D. Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS One. 2012;7:e48318. doi: 10.1371/journal.pone.0048318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014;19:1163–1170. doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci. 2011;66:1152–1162. doi: 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Chauvet-Gelinier JC, Ragot S, Bonin B. Up-regulation of leucocytes genes implicated in telomere dysfunction and cellular senescence correlates with depression and anxiety severity scores. PLoS One. 2012;7:e49677. doi: 10.1371/journal.pone.0049677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson JR, Mason A, Bodycote CL, Raleigh SM, Louis E, Samani NJ. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2013;19:895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Ladhoff J, d'Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Vrana S, Lauterbach D. Prevalence of traumatic events and post-traumatic psychological symptoms in a nonclinical sample of college students. J Trauma Stress. 1994;7:289–302. doi: 10.1007/BF02102949. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen H, Gao X, McGrath M, Deer D, De Vivo I, Schwarzschild MA, Ascherio A. Telomere length and risk of Parkinson's disease. Mov Disord. 2008;23:302–305. doi: 10.1002/mds.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, Robbins J, Aragaki A, Dunbar-Jacob J. Depression and cardiovascular sequelae in postmenopausal women. The Women's Health Initiative (WHI) Arch Intern Med. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71:294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson JC, Dhabhar FS, Blackburn EH. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17:164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang X, Gutin B, Davis CL, Keeton D, Thomas J, Stallmann-Jorgensen I, Mooken G, Bundy V, Snieder H, van der Harst P, Dong Y. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J Pediatr. 2011;158:215–220. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]