Abstract
Nitric oxide (NO) has been shown to inhibit Giardia lamblia in vitro and in vivo. This study sought to determine if Giardia infection induces arginase 1 (ARG1) expression in host macrophages to reduce NO production. Stimulations of RAW 264.7 macrophage-like cells with Giardia extract induced arginase activity. Real-time PCR and immunohistochemistry showed increased ARG1 and nitric oxide synthase 2 (NOS2) expression in mouse intestine following infection. Flow cytometry demonstrated increased numbers of macrophages positive for both ARG1 and NOS2 in lamina propria following infection, but there was no evidence of increased expression of ARG1 in these cells.
Keywords: Giardia lamblia, arginase 1, macrophages
1. Introduction
The protozoan parasite Giardia lamblia (syn. G. intestinalis, G. duodenalis) is a common cause of diarrheal disease worldwide. G. lamblia can infect humans and many other mammals, with prevalence rates in humans that range from 2–7% in developed countries to 20–30% in developing countries [1]. G. lamblia is transmitted through faecal contamination of food or water. As such infection rates are highest in countries where water purification is limited, leading to G. lamblia being included in the World Health Organization’s Neglected Disease Initiative since 2004 [2]. Intestinal pathology during infection is driven in part by the host immune system [3]. Yet the immunological processes that control infection and pathology are not entirely identified.
Arginine has diverse functions in mammalian physiology and plays an important role in host immunity. The production of Nitric Oxide (NO) by Nitric Oxide Synthase 2 (NOS2) from arginine is a key element of the innate immune response as NO is toxic to many pathogens. In fact arginine is the sole amino acid substrate for NO production [4]. The depletion of arginine as a means of limiting NO production is a survival strategy employed by many pathogenic organisms including viruses, bacteria, fungi, and protists [5]. Arginine limitation can be achieved through a number of pathways that include pathogen production of arginase (ARG), pathogen mediated induction of host ARG in macrophages, or consumption of host arginine by the pathogen [6, 7]. Nitric oxide is known to be cytostatic and potentially cytotoxic to G. lamblia in culture [8–11]. Yet the activation of host ARG as an evasive mechanism of G. lamblia has not been explored in vivo.
Host macrophages are often divided into two classes based on the expression of ARG1 and NOS2: classically activated M1 macrophages which express NOS2 and alternatively activated M2 macrophages which express ARG1 [5, 12]. These cells are thought to have antagonistic roles in the immune response with M1 macrophages being involved in pathogen control and M2 macrophages serving to limit excessive NO production and support healing. The ability of some pathogens to influence the expression of ARG1 and NOS2 can play an important role in immune subversion and parasite survival.
Previous work has shown that host NO can inhibit G. lamblia survival in vitro, and that arginine limitation negates this effect [9]. Recent studies on the influence of G. lamblia on epithelial cell ARG activity report a down regulation of host ARG in response to G. lamblia [13]. However these studies exclusively used in vitro culture systems of non-immune cells. In this study we aimed to determine if G. lamblia can directly induce macrophage ARG1 in vitro and if infection can lead to an increase in ARG1 expression in host macrophages in vivo.
2. Materials and methods
2.1 Mice and infections
C57BL/6J mice were obtained from Jackson Laboratories and kept under specific-pathogen-free conditions at Georgetown University. All experiments were performed with the approval of the Georgetown University Animal Care and Use Committee. G. lamblia (strain GS/M/H7) was cultured and used for infections as previously described [14]. Mice were infected via gavage with 1 × 106 parasites in phosphate buffered saline.
2.2 RAW 264.7 cell culture
The murine macrophage cell line RAW 264.7 was maintained in complete Dulbecco’s modified Eagle’s medium (Invitrogen). Only cells between passage 5 and 25 were used for arginase activity assays. For all experiments cells were grown to confluence in 12 well culture plates. Fresh media containing 10 ng/ml IL-13 (R&D Systems) or 100 μg/ml G. lamblia lysate was added to culture wells. Lysate was generated by washing trophozoites extensively in sterile PBS to remove culture media followed by repeated freeze thaw cycles to lyse trophozoites. Freeze thaw cycles were performed by freezing the trophozoite pellet in liquid nitrogen. The pellet was then thawed in a 37° C water bath and vortexed for 15 seconds. This process was repeated 6 times, and total cell extract was used for all assays. The protein concentration of the lysate was measure via Bradford assay (Biorad) and the absence of LPS contamination was confirmed using a Pierce™ LAL Chromogenic Endotoxin Quantitation Kit per the manufacturer’s protocol (Thermo Fischer). Following an 8 hour incubation, macrophages were lysed in 10 mM Tris-HCL pH 7.4, 0.4% w/v Triton X-100 containing protease inhibitor cocktail (Calbiochem), and arginase activity was measured using the Quantichrom Arginase Assay Kit (Bioassay Systems) per the manufacturer’s protocol. NO production was quantified by measuring nitrite, a stable breakdown product of NO, in culture supernatants using the Griess Reagent System per the manufacturer’s protocol (Promega).
2.3 Real-time PCR
Small intestinal tissue was collected from mice at days 0, 3, and 7 following infection, and total RNA was extracted and reverse transcribed to cDNA using a reverse transcription kit (Applied Biosystems). For ARG1 the primers were F-CAGAAGAATGGAAGAGTCAG R-CAGATATGCAGGGAGTCACC and yielded a 250 bp product. For NOS2 the primers were F-TGCATGGACCAGTATAAGGCAAGC R-GCTTCTGGTCGATGTCATGAGCAA and yielded a 223 bp product. For GADPH the primers were F-ACCCAGAAGACTGTGGATGG R-TCAGCTCTGGGATGACCTTG and yielded a 124 bp product. Real-time PCR was performed on cDNA with the primer pairs listed above on a CFX 96 cycler (Biorad). For each PCR reaction 2 ul cDNA, 900 nM primers, and 10 μl Syber-Green master mix (Sensifast) and an 65 °C anneal/extend were used. All samples were assayed in triplicate and analyzed using the ΔΔCT method to assess relative fold change in ARG1 and NOS2.
2.4 Immunohistochemistry
Small intestine tissue was fixed in 10% formalin, embedded in paraffin, and sectioned. Tissue sections were dewaxed in xylene and rehydrated. Antigen retrieval was performed by boiling slides in antigen unmasking solution (Vector Labs). Tissue sections were stained with antibodies against ARG1 and NOS2 followed by appropriate biotin conjugated secondary antibodies (all Santa Cruz Biotech). The tissue was labeled with Strep-Avidin HRP and visualized using a DAB peroxidase kit (Vector Labs). All tissue was counterstained with hematoxylin (Vector Labs).
2.5 Isolation of intestinal lamina propria cells
Ten cm duodenal segments were obtained and pooled from 4 mice per experimental group. The pooled duodena were fractionated to collect lamina propria cells as described [15]. Briefly, the Peyer’s patches were removed and the remaining intestinal fragments were washed with 1 mM DTE in order to remove the epithelial layer. The tissue was washed with 5 mM EDTA to strip off the epithelium, leaving an intact lamina propria fraction to be digested by liberase TL (Roche). After enzymatic digestion, the lamina propria fraction was separated on a Percoll (Sigma-Aldrich) gradient in order to enrich for leukocytes and remove dead cells.
2.6 Flow cytometry
Lamina propria cell preparations were washed in PBS, and 10 × 106 cells were stained with LIVE/DEAD Fixable Yellow Stain (Invitrogen) for 30 minutes at room temperature in the dark. Surface stains with fluorophore conjugated antibodies against F4/80 and CD11b (Biolegend) were performed for 30 minutes at 4° C. Cells were fixed in 4% paraformaldehyde and permeabilized using Perm Buffer (Biolegend). Intracellular staining with fluorophore conjugated antibodies ARG1 (R&D systems) and NOS2 (Santa Cruz Biotech) were performed for 1 hour at room temperature. Staining with antibodies was done in the presence of a CD16 blocking antibody (TruStain fcX, Biolegend). Stained cells were analyzed using a FACStar Plus dual laser system (Becton Dickinson) and FCS express version 4.0 software (DeNovo Software).
2.7 Statistical analysis
Data were analyzed by Student t test. Analyses were performed using GraphPad Prism. P values of <0.05 were considered significant
3. Results
3.1 G. lamblia induces arginase activity in macrophage like cells
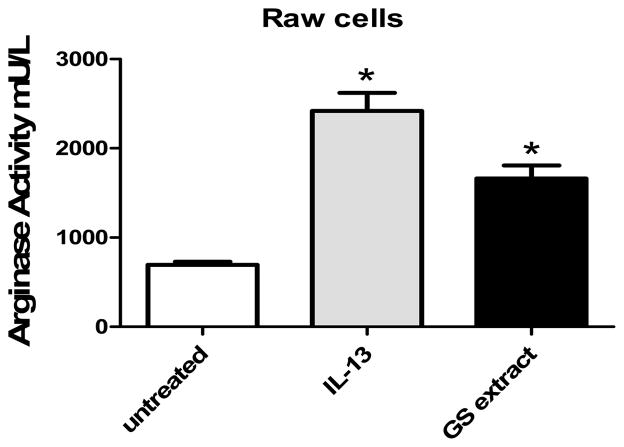
To determine if G. lamblia could directly induce ARG1 expression in macrophages, RAW 264.7 cells were stimulated with either parasite lysate (100 μg/ml) or IL-13 (10 ng/ml). IL-13 is known to induce arginase activity in RAW 264.7 cells [16] and served as a control for this experiment. RAW 264.7 cells were grown to confluence and then stimulated for 8 hours with parasite lysate or IL-13. Our data show that G. lambia extract is sufficient to induce arginase activity in RAW 264.7 cells (Fig. 1). While IL-13 led to a 3 fold increase in arginase activity, extract led to a 2 fold increase in activity. Cells treated with either parasite extract or IL-13 had increased arginase activity compared to untreated control cells. To determine if RAW 264.7 cells produced NO in response to G. lamblia extract, NO levels from culture supernatants were quantified. However we found no detectable NO production by RAW 264.7 cells in response to either IL-13 or parasite extract (data not shown). These data indicate that parasite extract has the ability to induce ARG1 activity in a direct manner.
Figure 1.
G. lamblia extract increases arginase activity in RAW 264.7 cells. RAW 264.7 cells were treated with IL-13 (10 ng/ml) or GS extract (100 μg/ml whole parasite lysate) for 8 hours. Following stimulations, cell lysates were used to assess arginase activiy. IL-13 and GS extract induce a significant increase in arginase activity compared to unstimulated control cells. *, P < 0.05 compared to unstimulated controls. n= 4 wells/treatment. Data are representative of 3 independent experiments.
3.2 G. lamblia infection induces arginase expression in the mouse small intestine
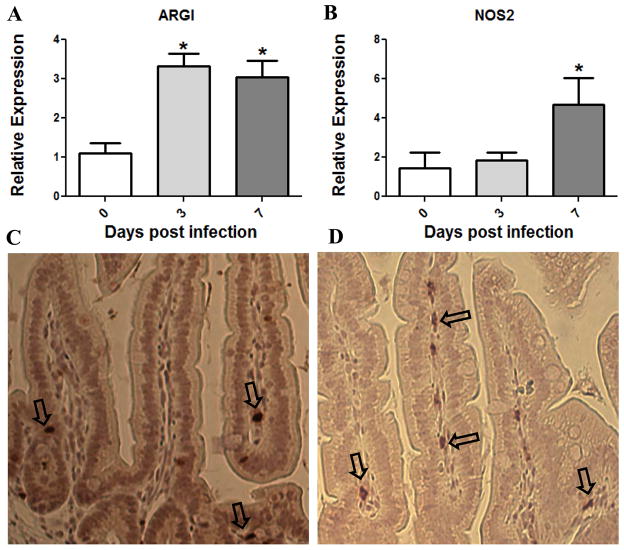
Since G. lamblia extract could stimulate arginase activity in vitro, we next wanted to determine if ARG1 expression was enhanced in infected animals. Small intestinal tissue was collected from mice at days 3 and 7 following infection and from uninfected controls and total RNA was extracted. These time points were selected to reflect early and peak parasite burden in our model. Real time PCR was used to quantify the relative changes in ARG1 and NOS2 mRNA throughout infection. ARG1 mRNA expression increases 3 fold as early as 3 days post-infection and this increase persists for at least 7 days (Fig. 2A). NOS2 mRNA also increased following infection, but not until day 7 of infection (Fig. 2B). These data indicate that G.lamblia infection leads to increased intestinal ARG1 and NOS2 but with varying kinetics.
Figure 2.
Expression of ARG1 and NOS2 in mouse intestine following Giardia lamblia infection. (A and B) Jejuna of mice infected with G. lamblia trophozoites for 0, 3 or 7 days were homogenized. Total RNA was extracted and reverse transcribed to cDNA. ARG1 and NOS2 were quantified via real time-PCR. Relative expression values are normalized to GAPDH and expressed as fold change in expression from control mice. (A) ARG1 expression increased significantly over control mice at both days 3 and 7 of infection. (B) Expression of NOS2 was increased significantly only on day 7 of infection. *, P < 0.05 compared to uninfected mice. N=4 mice/group. Data are representative of 3 independent experiments. ARG1 and NOS2 expression is localized to intestinal lamina propria. (C–D) Mouse small intestinal tissue from day 7 post infection was fixed in formalin and embedded in paraffin prior to sectioning and staining. (C) Immuno-staining for ARG1. (D) Staining for NOS2. Arrows indicate positive cells.
3.3 ARG1 and NOS2 expression localize to the lamina propria
To further investigate ARG1 and NOS2 expression in the small intestine, the tissue specific location of cells expressing these enzymes needed to be determined. Immunohistochemical analysis of formalin-fixed paraffin embedded mouse duodena revealed that both ARG1 and NOS2 are expressed in cells of the lamina propria and that the majority of ARG1 and NOS2 positive cells are located in this intestinal compartment (Fig. 2C and 2D). Confirming that ARG1 and NOS2 localize to lamina propria allowed for the design of experiments to collect ARG1 and NOS2 expressing cells from this tissue for further analyses.
3.4 ARG1 and NOS2 expressing macrophages accumulate in the lamina propria of the small intestines after infection with G. lamblia
To determine if ARG1 and NOS2 were expressed by macrophages in mouse small intestine lamina propria, duodena of 4 mice per treatment were collected and pooled, and lamina propria was fractionated and stained with antibodies against F4/80 and CD11b. These two cell surface markers are co-expressed on tissue macrophages and were selected as indicators of macrophage populations for this study. Following infection with G. lamblia, F4/80+ CD11b+ macrophages accumulate in the lamina propria of the mouse small intestine, increasing from 6% in uninfected mice to almost 14% of total lamina propria cells at 7 days post infection (Fig. 3A). Furthermore ARG1 and NOS2 are expressed by these macrophages (Fig. 3A). Thus G. lamblia Infection leads to a large increase of ARG1+ NOS2+ macrophages within the duodenal lamina propria.
Figure 3.
Infection with G. lamblia leads to an increase in ARG1 and NOS2 expressing intestinal macrophages. The duodena of 4 mice were pooled and fractionated to remove epithelium. The lamina propria was collected and stained with CD11b, F4/80, ARG1 and NOS2 antibodies for flow cytometry. Each plot represents the lamina propria from the duodena of 4 mice. (A) At day 7 of infection intestinal macrophages have increased compared to uninfected control animals. ARG1+ and NOS2+ macrophages increase in the lamina propria of the mouse small intestine. (B–C) Plots represent lamina propria gated on F4/80+ and CD11b+ macrophages. (B) ARG1 and NOS2 protein levels in macrophages as assessed by flow cytometry are shown relative to unstained controls (grey hitstogram). (C) ARG1 and NOS2 are co-expressed in duodenal macrophages. Data are representative of 3 independent experiments.
3.5 ARG1 expression is not induced in macrophages following G. lamblia infection
To determine if changes in ARG1 mRNA translates to changes in ARG1 protein in macrophages, we analyzed ARG1 levels in macrophages of mouse small intestine. Infection did not induce an increase in ARG1 protein levels in macrophages, but a majority of duodenal macrophages express ARG1 in uninfected and day 7 infected animals, 87.94% and 91.48% respectively (Fig 3B). However NOS2 protein did increase in macrophages following infection from 67.18% in uninfected to 84.21% at day 7 (Fig. 3B). These data demonstrate that while ARG1 expressing macrophages accumulate in the small intestine following infection, there is not increased ARG1 expression within these cells.
3.6 ARG1 and NOS2 are co-expressed in macrophages of the lamina propria
Because ARG1 and NOS2 expression are linked to macrophage function, determining the proportion of ARG1+ and NOS2+ macrophages during G. lamblia infection would aide in the functional characterization of these cells. Intriguingly a high proportion of duodenal macrophages co-express ARG1 and NOS2, 58.56% of uninfected and 73.94% of Day 7 macrophages (Fig. 3C). These data indicate that ARG1 and NOS2 are co-expressed by the macrophage population of the small intestine and this population is influenced by G. lamblia infection.
4. Discussion
Intestinal macrophages have specialized roles in defense and regulation of inflammation and are divided phenotypically and functionally based on those roles. The expression of ARG1, associated with regulation of inflammation, and NOS2, associated with host defense, within these cells can be used to classify their function [17]. These enzymes compete for the same substrate, and induction of host ARG by pathogens is thought to influence macrophage function in a manner that benefits the pathogen [6]. This study sought to determine if G. lamblia induced the expression of host ARG in macrophages. We found that while ARG expression is induced in RAW 264.7 cells in vitro, intestinal macrophages do not show induced ARG1 in response to G. lamblia infection. However ARG1+ NOS2+ macrophages accumulate in the small intestine during G. lamblia infection.
Data from our lab examining the role of NOS2 in clearance of infection has shown that while NOS2 mRNA expression increases following G. lamblia infection its role in parasite elimination is redundant with α-defensins [10, 11]. Furthermore in vitro studies on the relationship between G. lamblia and nitric oxide (NO), which is produced when NOS consumes arginine, have demonstrated that NO can have cytotoxic or cytostatic effects on the parasite [8, 9]. Thus we were interested in exploring the idea that G. lamblia could escape the effect of NO through altering host ARG expression.
Previous studies examining the relationship between host ARG and G. lamblia have used culture systems employing epithelial cell lines [13], a cell type not associated with in vivo ARG expression in response to pathogens. In this study the macrophage cell line RAW 264.7 demonstrated increased ARG activity in response to G. lamblia. This is consistent with studies in Toxoplasma gondii, Trypanosoma cruzi, and T. brucei gambiense where the parasite could directly stimulate changes in ARG activity of host macrophages[18–20]. However G. lamblia has an infection cycle quite different from the aforementioned pathogens. Thus it is important to demonstrate the relevance of these findings in an in vivo infection model where host cells and G. lamblia can interact naturally.
This study found that ARG1 mRNA expression in mouse small intestine tissue was induced by G. lamblia infection. ARG1 mRNA increased by day 3 of infection and this increase was maintained through day 7. However ARG1 protein expression as measured by flow cytometry was not induced in duodenal macrophages of infected mice. Given that the relative changes in ARG1 mRNA induced by infection were not large in magnitude perhaps any change in protein that might result from increased mRNA expression is not detectable via this methodology. Furthermore it is possible that increased ARG1 mRNA could be attributable to the increased proportion of ARG1 expressing macrophages in the small intestine following infection.
Unexpectedly ARG1+ NOS2+ macrophages accumulate in the lamina propria of the small intestine following G. lamblia infection. These data are intriguing as co-expression of these two enzymes could indicate an intermediate or immunosuppressive macrophage phenotype. ARG1+ NOS2+ macrophages have been reported in tuberculous granulomas during Mycobacterium tuberculosis infection and are thought to serve an anti-inflammatory function and limit lung pathology [21]. ARG1 and NOS2 co-expression has also been described in monocytes which infiltrate the heart during Trypanosoma cruzi infection and in monocytes associated with tumor development [22, 23]. In both of these cases, the co-expression of ARG1 and NOS2 is associated with immunosuppressive function. The ARG1+ NOS2+ phenotype of macrophages of the mouse small intestine could indicate that these cells serve a regulatory or suppressive function. Furthermore, the expansion of this cell population during G. lamblia infection could help to explain why there is little if any mucosal inflammation seen in acute parasite infection [24].
While the accumulation of macrophages during G. lamblia infection has not been previously reported, other studies have shown that epithelial cells express several chemokines in response to G. lamblia that differ from the chemokine profile of other inflammatory enteric pathogens, including CCL2, CCL20, and CXCL1-3 [25]. Furthermore CCL2 which is a strong recruiter of monocytes to tissue was induced in epithelial cells following exposure to G. lamblia which could contribute to the accumulation of ARG1+ NOS2+ macrophages in the intestinal lamina propria [25,26]. Finally, as G. lamblia has been shown to deplete arginine when cultured in the presence of intestinal epithelial cells [27]. This depletion could work in combination with ARG1+ NOS2+ macrophages to reduce inflammatory immune responses in the intestinal mucosa during G. lamblia infection.
Intestinal macrophages shape the host immune response to infection, yet we know little about how these cells respond to G. lamblia infection. In this study we were interested in understanding the how the ARG1/NOS2 axis was influenced by G. lamblia. Our data demonstrate that while in vitro induction of host ARG1 by G. lamblia can occur; this may not be relevant to ARG1 function in vivo, demonstrating the importance of in vivo validation of in vitro culture systems. Future work which aims to better characterize host macrophage responses during G. lamblia infection will help us to more fully grasp the importance of these immune sentinels in immune regulation and infection control.
Acknowledgments
We would like to acknowledge Karen Creswell for excellent assistance with flow cytometry. This work was funded by NIH grant AI 094492. The experiments were carried out with the help of the following Shared Resource. Histopathology and tissue, microscopy, and imaging, genomics and epigenomics and flow cytometry supported by NIH-P30 CA51008 and by NCATS 8 UL1 TR000101.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest affecting the conduct of this research or the publication of these results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solaymani-Mohammadi S, Singer SM. Giardia duodenalis: the double-edged sword of immune responses in giardiasis. Exp Par. 2010;126:292–7. doi: 10.1016/j.exppara.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasit. 2006;22:203–8. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Solaymani-Mohammadi S, Singer SM. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J Immunol. 2011;187:3769–75. doi: 10.4049/jimmunol.1100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutrition. 2007;137:1681S–6S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 5.Das P, Lahiri A, Lahiri A, Chakravortty D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Path. 2010;6:e1000899. doi: 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincendeau P, Gobert AP, Daulouede S, Moynet D, Mossalayi MD. Arginases in parasitic diseases. Trends Parasit. 2003;19:9–12. doi: 10.1016/s1471-4922(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 7.Ropolo AS, Touz MC. A lesson in survival, by Giardia lamblia. Sci World J. 2010;10:2019–31. doi: 10.1100/tsw.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes PD, Assreuy J. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz J Med Biol Res. 1997;30:93–9. doi: 10.1590/s0100-879x1997000100015. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, et al. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol. 2000;164:1478–87. doi: 10.4049/jimmunol.164.3.1478. [DOI] [PubMed] [Google Scholar]
- 10.Li E, Zhou P, Singer SM. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J Immunol. 2006;176:516–21. doi: 10.4049/jimmunol.176.1.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tako EA, Hassimi MF, Li E, Singer SM. Transcriptomic analysis of the host response to Giardia duodenalis infection reveals redundant mechanisms for parasite control. mBio. 2013;4:e00660–13. doi: 10.1128/mBio.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–54. [PubMed] [Google Scholar]
- 13.Stadelmann B, Hanevik K, Andersson MK, Bruserud O, Svard SG. The role of arginine and arginine-metabolizing enzymes during Giardia - host cell interactions in vitro. BMC Micro. 2013;13:256. doi: 10.1186/1471-2180-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P, Li E, Zhu N, Robertson J, Nash T, Singer SM. Role of interleukin-6 in the control of acute and chronic Giardia lamblia infections in mice. Infect Immun. 2003;71:1566–8. doi: 10.1128/IAI.71.3.1566-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan BS, Lefrancois L. Isolation of mouse lymphocytes from small intestine tissues. In: Coligan JE, et al., editors. Curr Protoc Immunol. Unit3. Chapter 3. John Wiley and Sons; 2012. p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barksdale AR, Bernard AC, Maley ME, Gellin GL, Kearney PA, Boulanger BR, et al. Regulation of arginase expression by T-helper II cytokines and isoproterenol. Surgery. 2004;135:527–35. doi: 10.1016/j.surg.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido VV, Dulgerian LR, Stempin CC, Cerban FM. The increase in mannose receptor recycling favors arginase induction and Trypanosoma cruzi survival in macrophages. Int J Biol Sci. 2011;7:1257–72. doi: 10.7150/ijbs.7.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzmuller P, Biron DG, Courtois P, Koffi M, Bras-Goncalves R, Daulouede S, et al. Virulence and pathogenicity patterns of Trypanosoma brucei gambiense field isolates in experimentally infected mouse: differences in host immune response modulation by secretome and proteomics. Mic Inf. 2008;10:79–86. doi: 10.1016/j.micinf.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191:773–84. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuervo H, Guerrero NA, Carbajosa S, Beschin A, De Baetselier P, Girones N, et al. Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J Immunol. 2011;187:2656–65. doi: 10.4049/jimmunol.1002928. [DOI] [PubMed] [Google Scholar]
- 23.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberhuber G, Kastner N, Stolte M. Giardiasis: a histologic analysis of 567 cases. Scand J Gastro. 1997;32:48–51. doi: 10.3109/00365529709025062. [DOI] [PubMed] [Google Scholar]
- 25.Roxstrom-Lindquist K, Ringqvist E, Palm D, Svard S. Giardia lamblia-induced changes in gene expression in differentiated Caco-2 human intestinal epithelial cells. Infect Immun. 2005;73:8204–8. doi: 10.1128/IAI.73.12.8204-8208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadelmann B, Merino MC, Persson L, Svard SG. Arginine consumption by the intestinal parasite Giardia intestinalis reduces proliferation of intestinal epithelial cells. PLoS One. 2012;7:e45325. doi: 10.1371/journal.pone.0045325. [DOI] [PMC free article] [PubMed] [Google Scholar]