Abstract
Activation of macrophages is a prerequisite for their antitumor effects. Several reagents, including agonistic anti-CD40 monoclonal antibody (anti-CD40), CpG oligodeoxynucleotides (CpG) and monophosphoryl lipid A (MPL), can stimulate activation of macrophages. Our previous studies showed synergy between anti-CD40 and CpG and between anti-CD40 and MPL in macrophage activation and antitumor efficacy in mice. In the present study, we asked whether there was synergy among these three reagents. The activation of adherent peritoneal exudate cells (PEC) obtained from mice injected with anti-CD40 and then treated with CpG and/or MPL in vitro was determined by their ability to suppress proliferation of tumor cells and to produce various cytokines and chemokines in vitro. Cell sorting and histology followed by functional testing showed that macrophages were the main cell population in PEC activated by CD40 ligation in vivo. A combination of anti-CD40, CpG or MPL activated PEC to suppress proliferation of B16 cells and produce nitric oxide far greater than the single reagents or any of the double combinations of these reagents. In addition, the combination of all three reagents activated PEC to secrete IL-12, IFN-γ and MCP-1 to a greater degree than any single reagent or any two combined reagents. These results demonstrate that macrophages can be synergistically activated by anti-CD40, CpG and MPL, suggesting that this novel combined approach might be further investigated as potential cancer therapy.
Keywords: Macrophages, anti-CD40, immunotherapy, monophosphoryl lipid A, CpG
1. Introduction
Macrophages can be activated to mediate antitumor effects. In response to various signals, macrophages can belong to two distinct phenotypes: classically activated M1 or alternatively activated M2 macrophages. The M1 phenotype is characterized by the expression of high levels of proinflammatory cytokines, high production of reactive nitrogen and oxygen intermediates, and strong tumoricidal activity. In contrast, M2 macrophages are considered to be involved in wound healing as well as tumor progression (Sica and Mantovani, 2012). The phenotype of macrophages infiltrating tumors is pliable and, if instructed properly, macrophages can mediate robust antitumor activity through their ability to eliminate malignant cells, inhibit angiogenesis, and prevent fibrosis (Long and Beatty, 2013).
Macrophages can be activated to mediate antitumor effects by some therapeutic reagents including anti-CD40, CpG and MPL (Rakhmilevich et al., 2012). The cell surface molecule CD40 is a member of the tumor necrosis factor receptor superfamily and is broadly expressed by immune cells, in particular B cells, dendritic cells, and monocytes/macrophages. Ligation of CD40 on monocytes and macrophages leads to their activation, resulting in secretion of proinflammatory cytokines including TNF, IL-6, IL-12 and IL-1, and chemokines such as MCP-1, as well as production of NO (Alderson et al., 1993; Suttles and Stout, 2009). We have shown that macrophages activated by agonistic anti-CD40 mediate cytostatic effects against tumor cells in vitro and induce suppression of tumor growth in tumor-bearing mice, including antitumor activity against murine neuroblastomas, melanomas, and several other tumor types (Buhtoiarov et al., 2005; Lum et al., 2006a Lum et al., 2006b;). Importantly, macrophages activated by anti-CD40 can mediate antitumor effects in patients with pancreatic cancer (Beatty et al., 2011; Beatty et al., 2013;Vonderheide et al., 2013).
All the members of TLR are expressed on macrophages, being responsible for mediating the activation of antitumor macrophages by molecules derived from certain microbes (Long and Beatty, 2013). TLR9 can recognize unmethylated CpG dinucleotides, a common pattern in bacterial and viral DNA, while TLR4 can recognize LPS which is the biologically active portion of the gram-negative bacterial cell wall. MPL is a detoxified derivative of LPS that lacks many of the endotoxic properties of LPS yet retains both its adjuvant and immunostimulatory activities. Both CPG and MPL can induce M1-like activation of macrophages with production of nitric oxide (NO), TNF-alpha, IL-6 or IL-12 (Aybay and Imir, 1998; Holtick et al., 2011; Ryu et al., 2011). Moreover, CpG delivered by nanoparticles can stimulate macrophages to inhibit tumor growth in a mouse tumor model (Lin et al., 2013). Macrophages activated by anti-CD40, CpG or MPL may offer a promising strategy for cancer immunotherapy.
Our previous studies showed synergy between anti-CD40 and CpG (Buhtoiarov et al., 2006) or MPL (Van De Voort et al., 2013) in activating macrophages to suppress proliferation of tumor cells. The synergy between anti-CD40 and CpG is evidenced by increased production of IFN-γ, IL-12, TNF and NO by macrophages, as well as by augmented apoptogenic effects of macrophages against tumor cells in vitro. Anti-CD40 and CpG also synergize in vivo in retardation of tumor growth in mice bearing the syngeneic B16 melanoma.(Buhtoiarov et al., 2006) Anti-CD40 and MPL synergize to activate macrophages inhibiting melanoma cell proliferation and secreting a significant amount of NO in vitro. Combining Anti-CD40 and MPL in mice bearing B16 melanoma slowed tumor growth and prolonged survival more effectively than treating with either agent individually (Van De Voort et al., 2013). Thus, activation of macrophages by anti-CD40 is enhanced by subsequent treatment with CpG or MPL.
Since macrophages can be activated by anti-CD40, CpG or MPL individually and synergistically by combination of anti-CD40 with either CpG or MPL, we wondered whether there is a synergy among all three molecules, anti-CD40, CpG and MPL, in activation of macrophages. In the present study, we showed that, indeed, peritoneal macrophages activated by the 3-agent combination of anti-CD40, CpG and MPL had enhanced in vitro tumoristatic function as well as enhanced production of IL-12, IFN-γ, MCP-1 and NO compared to the combinations of any two these reagents.
2. Materials and methods
2.1. Mice and tumor cell lines
Female C57BL/6 mice (6–8 wk old) were obtained from Taconic Farms (Germantown, NY). Mice were housed in the University of Wisconsin (UW) - Madison animal facilities at the Wisconsin Institutes for Medical Research. All experimentation was performed in accordance to protocols approved by the National Institutes of Health and by the Animal Care and Use Committees of UW-Madison. The B16 melanoma tumor cell line was grown in RPMI-1640 complete medium supplemented with 10% fetal calf serum (Sigma-Aldrich, St Louis, MO), 2mM L-glutamine, and 100 U/mL of penicillin/streptomycin (all from Life Technologies Inc., Grand Island, NY) at 37°C in a humidified 5% CO2 atmosphere.
2.2. Antibodies and Reagents
FGK 45.5 hybridoma cells producing the agonistic anti-CD40 antibody were a gift from Dr F. Melchers (Basel Institute for Immunology, Basel, Switzerland). The monoclonal antibody was obtained from ascites of nude mice injected with the hybridoma cells, and the ascites were then enriched for IgG by ammonium sulfate precipitation (Lum et al., 2006a). MPL from Salmonella enterica serotype minnesota (#L6895) was purchased from Sigma-Aldrich and reconstituted as previously described (Holtick et al., 2011). Rat IgG was purchased from Sigma-Aldrich. Unmethylated CpG-1826 oligodeoxynucleotide (TCCATGACGTTCCTGACGTT) was purchased from TriLink Biotechnologies (San Diego, CA).
2.3. Activation of macrophages with anti-CD40 and CpG or MPL
Mice were injected IP with 0.5 mg (or indicated doses) of anti-CD40 in 0.5mL PBS. After 3 days, PEC were obtained by peritoneal cavity lavage with 5mL of cold RPMI-1640 complete medium. Collected PEC were placed into 96-well flat-bottom cell culture plates (Corning Inc., Corning, NY) at a concentration of 2×105 cells/well in 0.1mL medium. The peritoneal macrophage population was enriched by adhesion to the plastic wells for 1.5-2 hours, followed by washing and removal of nonadherent cells. In our previous studies, flow cytometry indicated that approximately 40% of the PECs adhere and 95% of these adherent cells were macrophages, based on their expression of the F4/80 marker (Lum et al., 2006a).
The adherent macrophages were then cocultured with B16 melanoma cells (104 cells/well) for 48 hours in complete RPMI-1640 medium, alone or in the presence of indicated doses of CpG or MPL. Assay plates were incubated at 37°C in a humidified 5% CO2 atmosphere.
2.4. Macrophage-induced tumor cytostasis assay
Tumoristatic activity of macrophages was determined by the inhibition of DNA synthesis in tumor cells. Briefly, the adherent PEC were stimulated as described above and simultaneously co-cultured with B16 cells for 48 hours. Then, the wells were pulsed with [3H]-TdR (PerkinElmer, Boston, MA; 1μCi/well) for the last 6 hours of incubation. [3H]-TdR incorporation was determined by liquid β-scintillation counting of total cells after harvesting onto glass fiber filters (Packard, Meriden, CT), using the Packard Matrix 9600 Direct β-counter (Packard). PEC incorporate negligible amounts of [3H]-TdR compared with rapidly dividing B16 cells.(Rakhmilevich et al., 2008; Lum et al., 2006a).
2.5. NO production assay
Peritoneal macrophages were prepared and cocultured with B16 cells as described in the macrophage cytostatic assay. Supernatants were collected after 48 hours, and nitrite accumulation was measured using Griess Reagent (Sigma-Aldrich). Equal volumes of supernatants and Griess Reagent (50 mL each) were mixed for 10 minutes, and the absorbance at 570nm was measured by a microplate reader and compared with a standard nitrite curve ranging in concentration from 0 to 125 μM.
2.6. Cytokine determination by CBA
IL-12, TNF, IFN-γ, MCP-1, IL-10, and IL-6 in supernatants from the coculture of PEC and B16 cells were measured using the mouse inflammation kit CBA (BD Bioscience, San Diego, CA) according to the manufacturer's instructions. The data were acquired by flow cytometry and analyzed using CBA software supplied by BD Bioscience.
2.7. FACS
PECs from anti-CD40–treated C57BL/6 mice were collected and stained for 40 minutes at 4°C with anti-CD11b-APC (clone M1/70; BioLegend, San Diego, CA), anti-Gr-1-PE (clone RB6-8C5) and anti-CD19-PECy7 (clone 1D3) fluorochromes (eBioscience). FACS sorting was performed with a FACSAria cell sorter (BD). Cells were then sorted into purified populations according to their immunophenotype and forward-scatter and side-scatter characteristics. The purity of the sorted populations was confirmed by standard flow cytometry. For functional assays involving sorted cells, 5×104 sorted PECs and 1×104 B16 tumor cells were plated in triplicate in 96-well flat-bottomed plates. The [3H]-TdR incorporation and NO assays were performed as described in the sections previously.
2.8. Histology
Wright-Giemsa Stain (Sigma-Aldrich) was performed on the cytospin preparations of sorted cells. Briefly, PECs were centrifuged at 800 rpm for 3 minutes, fixed in 100% methanol for 2 minutes, and stained with Wright-Giemsa Stain (Sigma-Aldrich) for 45 seconds. Cells were further stained with an equal volume mixture of Wright-Giemsa Stain and water for 10 minutes, washed, and destained for 5 minutes. Pictures of cells were taken at ×40 magnification using the Magnafire 2.1 computer software (Optronics, Goleta, CA).
2.9. Statistical Analysis
Multiple comparisons were performed by one-way ANOVA, two-way ANOVA or three-way ANOVA followed by Post-tests correcting for multiple comparisons and determining significant differences between groups within an experiment. GraphPad Prism 5.04 software and SAS software version 9.4 were used. Data are presented as mean ± SE, and the significance threshold is defined based on the statistical method.
3. Results
3.1. Macrophages are the main population in PEC activated by anti-CD40 in vivo
To identify the specific subset of effector cells that can be activated on day 3 after anti-CD40 treatment, PECs were collected from mice that had been injected with anti-CD40 3 days earlier, stained and sorted into 4 subpopulations based on their expression of immunophenotypic surface markers, including (1) CD11bhigh Gr-1-/int; (2) CD11bint Gr-1+; (3) CD11b- Gr-1- CD19+ and (4) CD11bint Gr-1-CD19+ (Fig. 1A). The sorted PECs were further characterized by histologic staining. Population 1 contained CD11bhigh Gr-1-/int activated macrophages with large size; Population 2 was CD11bint Gr-1+ monocytes/macrophages with smaller size; Population 3 was CD11b- Gr-1- CD19+ B cells; Population 4 was CD11bint Gr-1-CD19+ B cells (Fig. 1B). Each sorted cell population was tested in medium, CpG (5 μg/mL) or MPL (5 μg/mL) for the ability to inhibit B16 proliferation and produce NO. Compared to other cell subpopulations, CD11bhigh Gr-1-/int macrophages (population 1) induced stronger antitumor effects (Fig. 1C) and produced larger quantities of NO (Fig. 1C,D). Monocytes/macrophages (subset 2) were also activated by anti-CD40 but to a lesser extent than subset 1. Macrophages from anti-CD40-treated mice were highly activated without help of CpG or MPL; however, in similar experiments (not shown) in naïve and tumor-bearing mice MPL and CpG increased macrophage stimulation. These results indicate that macrophages are the major subset of PEC effector cells activated by anti-CD40 and CpG or MPL.
Figure 1. CD11b+ macrophages are activated after anti-CD40 and CpG or MPL treatment.
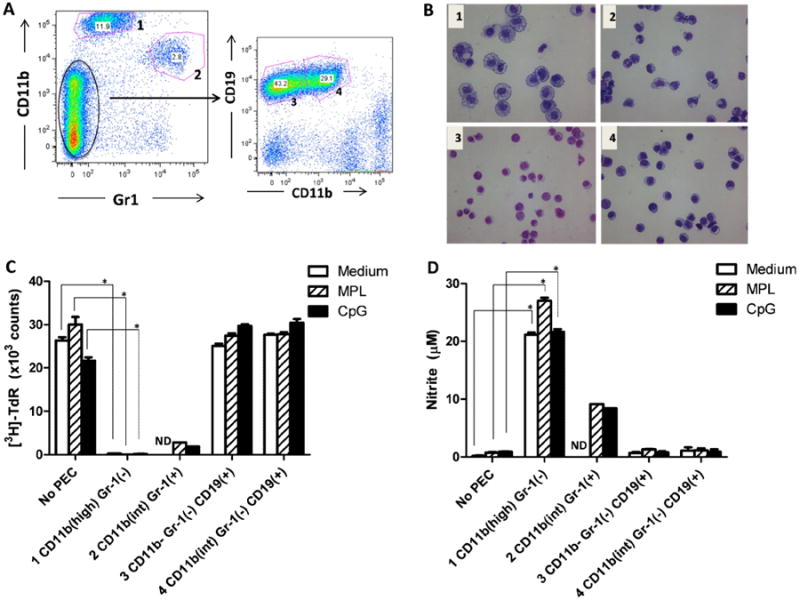
A. PEC from C57BL/6 mice injected IP with 0.5 mg anti-CD40 three days earlier were collected, stained and sorted into 4 subpopulations by a FACSAria cell sorter. The cells were divided as follows: (1) CD11bhigh Gr-1-/int; (2) CD11bint Gr-1+. A separate group of cells showed a CD11bint/-Gr-1- phenotype. These were further divided by CD19 and CD11b into populations (3) CD11b- Gr-1- CD19+ and (4) CD11bint Gr-1- CD19+. B. Each of the 4 sorted cell populations from Fig. 1A was stained with Wright-Giemsa stain to identify cell types based on morphology (×40 magnification). C. Each sorted cell population was tested in medium, CpG (5 μg/mL) or MPL (5 μg/mL) for the ability to inhibit B16 proliferation ([3H]-thymidine incorporation). D. Each sorted population was tested in medium, CpG (5 μg/mL) or MPL (5 μg/mL) for the ability to produce NO. A one-way ANOVA followed by Tukey test was used to compare [3H]-TdR counts or nitrite concentration between different populations cultured in the same stimulus (medium, CpG or MPL) in comparison to the control cultures of B16 cells in medium, CpG or MPL, but in the absence of any PEC. *P<0.001. ND, not done. Similar results were obtained in two independent experiments performed in triplicate.
3.2. Synergistic activation of PEC by combination of anti-CD40 plus CpG, anti-CD40 plus MPL, or CpG plus MPL
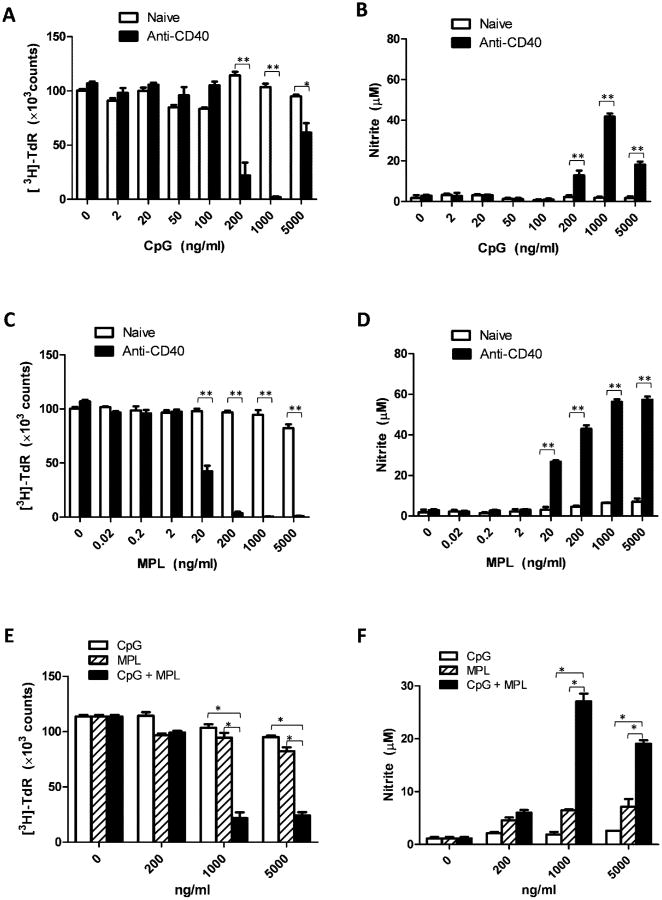
To test if all three reagents, anti-CD40, CpG and MPL, synergistically activate macrophages, we first thought to demonstrate the synergy of each pair of these immunostimulators. PEC collected from naïve mice or mice injected with anti-CD40 three days earlier were co-cultured with B16 cells and CpG at concentrations of 0, 2, 20, 50, 100, 200, 1000 or 5000 ng/ml. After 48 hours of coculture, the tumoristatic effect of the PEC was measured by the [3H]-TdR incorporation test. The NO level in the supernatant of the coculture was determined by Griess test. PEC stimulated with anti-CD40 and CpG (200-5000 ng/ml) had much stronger suppression of B16 cell proliferation than PEC stimulated with anti-CD40 or CpG alone (Figure 2A). NO production by PEC activated with anti-CD40 and CpG (200-5000 ng/ml) was significantly higher than that by PEC stimulated with anti-CD40 or CpG alone (Figure 2B). These results demonstrate a synergistic activation of PEC with anti-CD40 and CpG. The CpG 1826 used in this study has the phosphorothioate backbone, being resistant to nuclease. This phosphorothioate modification can inhibit CpG at high concentrations (Sester et al., 2000) which can explain that the activation of PEC from mice pretreated with anti-CD40 was decreased at higher concentration of CpG (5000ng/ml, Figures 2A,B).
Figure 2. Synergistic activation of PEC with anti-CD40 and CpG, anti-CD40 and MPL, and CpG and MPL.
A, B: Synergy between anti-CD40 and CpG. PEC were collected from naïve C57BL/6 mice (“naïve”) or mice injected with 0.5 mg anti-CD40 (“anti-CD40”) three days earlier. The adherent PEC were then cocultured with B16 cells in the absence or presence of CpG at the indicated concentrations for 48 hours. The wells were pulsed with [3H]-TdR (1μCi/well) for the last 6 hours of incubation to measure proliferation of B16 cells (A). Results are presented as the mean total number of β-counts over 5 minutes ± SE. Supernatants from the coculture of PEC and B16 cells were tested for nitrite concentration using Griess test (B). Data shown are represented as mean nitrite concentration ± SE. C, D: Synergy between anti-CD40 and MPL. PEC were collected from naïve C57BL/6 mice (“naïve”) or mice injected with 0.5 mg anti-CD40 (“anti-CD40”) three days earlier. The adherent PECs were cocultured with B16 cells in absence or presence of MPL at the indicated concentrations. The tumoristatic (C) and NO-inducing (D) effects of PEC are shown (mean ± SE). E, F: Synergy between CpG and MPL. PEC were collected from naïve C57BL/6 mice. Adherent PEC were cocultured with B16 cells in the absence or presence of CpG, MPL or their combination at the indicated concentrations for 48 hours. The tumoristatic (E) and NO-inducing (F) effects of PEC are shown (mean ± SE). The data were analyzed by two-way ANOVA followed by Bonferroni test. In A, B, C and D: *P <0.00125, **P<0.00025 for the difference between anti-CD40+CpG vs anti-CD40 or CpG alone at the indicated dose of CpG, and between anti-CD40+MPL vs anti-CD40 or MPL alone at the indicated dose of MPL. In E and F: *P<0.0005 for the indicated combination of CpG + MPL vs CpG or MPL alone. The experiment was carried out in triplicate and repeated three times.
Similarly, PEC stimulated with anti-CD40 and MPL (20-5000 ng/ml) induced stronger suppression of B16 cell proliferation (Figure 2C) and produced more NO (Figure 2D) than PEC stimulated with anti-CD40 or MPL alone. These results show a synergistic activation of PEC with anti-CD40 and MPL.
PEC collected from naïve mice were co-cultured with B16 cells in the presence of CpG, MPL or CpG + MPL at concentrations of 0, 200, 1000, or 5000 ng/ml. PEC stimulated with CpG and MPL (at the concentration of 1000 ng/ml for each reagent) induced stronger suppression of B16 cell proliferation (Figure 2E) and produced more NO (Figure 2F) than PEC stimulated with CpG or MPL at either 1000 or 5000 ng/ml alone. These results demonstrate a synergistic activation of PEC with CpG and MPL.
3.3. Synergistic activation of PEC by combination of anti-CD40, CpG and MPL
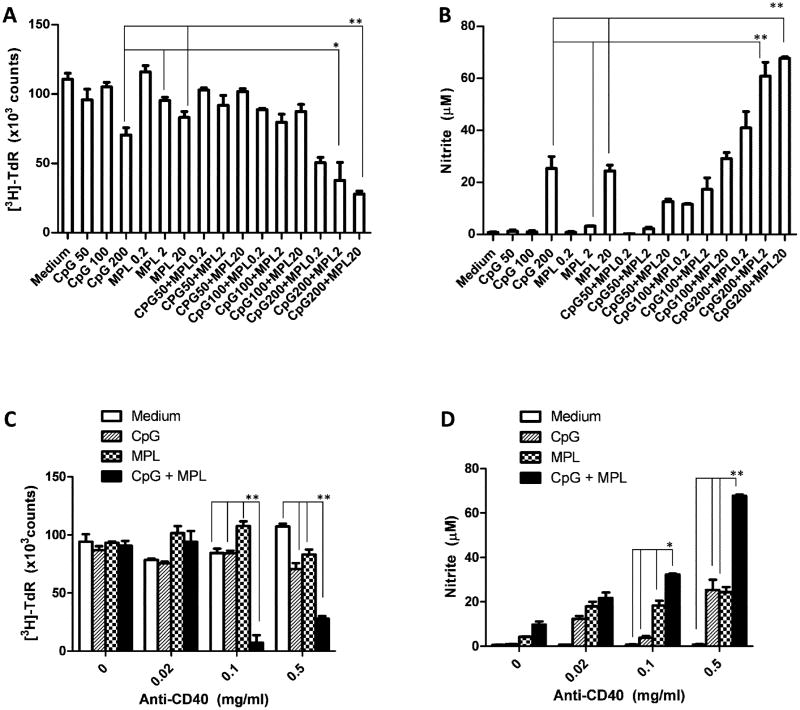
After showing synergy between anti-CD40 and CpG, anti-CD40 and MPL, and CpG and MPL, we asked if all three reagents will induce synergistic activation of macrophages. PEC collected from naïve mice or mice injected with anti-CD40 (0.5 mg/mouse) three days earlier were co-cultured with B16 cells in absence or presence of CpG (50, 100 or 200 ng/ml), MPL (0.2, 2 or 20 ng/ml) or their combination. Figure 3 demonstrates synergistic activation of macrophages to inhibit B16 cell proliferation (Figure 3A) and secrete NO (Figure 3B) with the 3-agent combination of anti-CD40 (0.5 mg/mouse), CpG (200 ng/ml) and MPL (20 ng/ml) compared to double combination of anti-CD40 (0.5 mg/mouse) and CpG (200 ng/ml) or MPL (20 ng/ml). Similar synergy was shown by the 3-agent combination of anti-CD40 (0.5 mg/mouse), CpG (100 ng/ml) and MPL (2 ng/ml) compared to 2-agent combination of anti-CD40 (0.5 mg/mouse) and CpG (100 ng/ml) or MPL (2 ng/ml).
Figure 3. Synergy among anti-CD40, CpG and MPL in tumoristasis and NO production of PEC.
A, B: Synergy among anti-CD40, CpG and MPL. PEC were collected from C57BL/6 mice injected with anti-CD40 (0.5mg IP) three days earlier. Adherent PEC were cocultured with B16 cells in the absence or presence of CpG, MPL or their combination at the indicated concentrations (ng/ml) for 48 hours. The wells were pulsed with [3H]-TdR (1μCi/well) for the last 6 hours of incubation to measure proliferation of B16 cells (A). Results are presented as the mean total number of β-counts over 5 min ± SE. Supernatants from the coculture of PEC and B16 cells were tested for nitrite concentration using Griess test (B). Data shown are represented as mean nitrite concentration ± SE. A two-way ANOVA followed by Tukey's post-testing method was performed. At the indicated doses of CpG and MPL, the 3-agent combination of anti-CD40, CpG and MPL is compared to the double combination of anti-CD40 and CpG or anti-CD40 and MPL. *P<0.05; **P<0.001. C, D: Synergy among anti-CD40, CpG and MPL at low doses of anti-CD40. PEC were collected from C57BL/6 mice injected with anti-CD40 at the indicated doses three days earlier Adherent PEC were then cocultured in triplicate wells with B16 cells in the absence or presence of CpG (200ng/ml), MPL (20ng/ml) or their combination for 48 hours. The tumoristatic (C) and NO-inducing (D) effects of activated macrophages are shown. The data were analyzed by two-way ANOVA followed by Bonferroni test. At the indicated dose of anti-CD40, the 3-agent combination of anti-CD40, CpG and MPL is compared to anti-CD40 alone, anti-CD40 and CpG, or anti-CD40 and MPL. *P<0.005, **P<0.0005. The experiment was carried out in triplicate and repeated three times.
As anti-CD40 was found to induce dose-dependent toxicity (largely a cytokine release clinical picture) in initial clinical trials (Vonderheide et al., 2007), we asked if the observed synergy among anti-CD40, CpG and MPL might be used to reduce the in vivo dose of anti-CD40 while preserving the antitumor efficacy. PEC collected from naive mice or mice injected with anti-CD40 at the doses of 0.02, 0.1 or 0.5 mg per mouse three days earlier were co-cultured with B16 cells in the absence or presence of CpG (200ng/ml), MPL (20ng/ml) or their combination for 48 hours. The results show that anti-CD40 at the doses 0.1 and 0.5 mg/mouse synergistically induced both suppression of tumor cell proliferation (Figure 3C) and increased NO production (Figure 3D) when combined with CpG and MPL, compared to anti-CD40 alone, anti-CD40 and CpG or anti-CD40 and MPL. These findings indicate that the dose of anti-CD40 can be reduced at least 5 fold while preserving antitumor efficacy (as measured in vitro) when combined with CpG and MPL, suggesting that this synergistic combination might be further evaluated in vivo for antitumor properties with reduced toxicity.
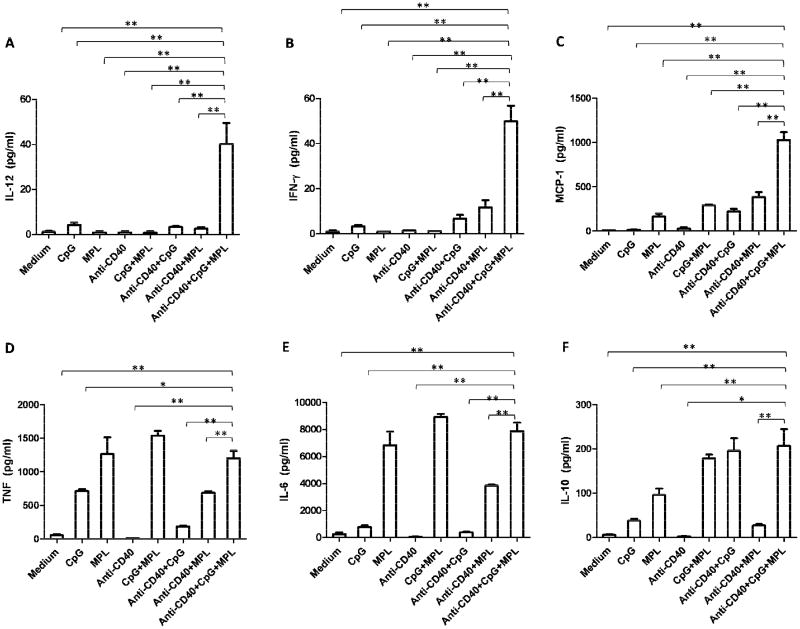
The results presented so far show the synergy of anti-CD40, CpG and MPL in activating macrophages to suppress tumor cell proliferation and produce NO in vitro (Figure 3). As activated macrophages produce various M1 and M2 cytokines and chemokines, we hypothesized that a synergistic activation of antitumor macrophages with anti-CD40, CpG and MPL will be accompanied by the synergistic production of M1 but not M2 cytokines. CBA methodology was used to determine the level of inflammatory cytokines including IL-12, TNF, IFN-γ, MCP-1, IL-10, and IL-6 in the supernatants from the coculture of PEC and B16 cells. There were significantly higher levels of IL-12, IFN-γ and MCP-1 (Figure 4A,B,C) in the coculture containing PEC activated with anti-CD40, CpG and MPL compared to those activated with any one or two reagents. PEC activated by anti-CD40, CpG and MPL produced as much IL-6 and TNF as MPL, alone or in combination with CpG (Figure 4D,E). On the other hand, CpG in combination with anti-CD40 or MPL stimulated PEC to produce as much IL-10 as a combination of anti-CD40, CpG and MPL (Figure 4F). These results show that anti-CD40, CpG and MPL synergistically activated PEC to produce M1 inflammatory cytokines, IFN-γ, and IL-12, and the chemokine MCP-1.
Figure 4. Synergy among anti-CD40, CpG and MPL in Cytokine and chemokine production of PEC.
PEC were collected from naïve C57BL/6 mice or mice injected with anti-CD40 (0.5mg) three days earlier. Adherent cells were then cocultured in triplicate wells with B16 cells in the absence or presence of CpG (200ng/ml), MPL (20ng/ml) or their combination for 48 hours. Supernatants were tested for cytokines and chemokines including IL-12 (A), IFN-γ (B), MCP-1 (C), TNF (D), IL-6 (E) and IL-10 (F) using the CBA method. Data are represented as mean ± SE. A three-way ANOVA followed by Dunnett's post-testing was performed. *P<0.01; **P<0.001. The experiment was carried out in triplicate and repeated two times.
4. Discussion
Macrophages can be activated by various agents including anti-CD40, CpG and MPL to mediate antitumor activity. In the present study we found that all these three reagents synergistically activated mouse peritoneal macrophages, as evidenced by enhanced tumoristasis, production of NO and secretion of pro-inflammatory cytokines and chemokines in vitro. These findings suggest that a 3-reagent combination of anti-CD40, CpG and MPL might be a new strategy to investigate as a means to activate macrophages for immunotherapy of cancer.
Activation of macrophages is induced by anti-CD40, CpG or MPL via different signaling pathways. Ligation of CD40 expressed in macrophages causes its oligomerization, which leads to recruitment of specific TRAF proteins including TRAF6 and TRAF2/3/5. These TRAF proteins cause activation of NF-κB, MAPKs (ERK1/2) and phosphoinositide 3-kinase/Akt pathway, resulting in production of inflammatory mediators, including IL-1, TNF, IL-6, IL-12, NO and MCP-1 (Suttles and Stout, 2009). The TLR9 signaling pathway is responsible for stimulation of CpG. TLR9 located in the endosomes of macrophages recognizes CpG ODN and transmits its signals through a specific interaction with adaptor molecules MyD88, which in turn interacts with IRAKs. The IRAKs subsequently recruits TRAF6, which activates TAK1, resulting in activation of downstream signalings of NF-κB and MAPKs (Erk1/2, JNK, and p38). These downstream signalings are responsible for activation of the macrophages (Rossol et al., 2011; Lu et al., 2008). Upon engagement of MPL with TLR4 displayed on surface of macrophages, MyD88-dependent and MyD88-independent pathways are initiated. For the MyD88-dependent pathway, TLR4 recruits adaptor proteins TIRAP and MyD88, which recruits IRAKs and TRAF6. The activated TRAF6 results in activation of MAP3Ks such as TAK1, ASK1 and Tpl2, which lead to parallel downstream signaling pathways of NF-κB and MAPKs (p38, JNK, and ERK). These downstream signaling induce expression of inflammatory mediators including IL-1, IL-6, TNF-α, MCP-1, IL-12 and NO. As for the MyD88-independent pathway, TLR4 recruits the adaptor protein TRIF and TRAM, which then recruit TRAF6, resulting in activation of TRAF6 and TAK1, with subsequent bifurcation to the NF-κB and MAPKs (p38 and JNK) pathways. Alternatively, TRIF can bind TRAF3, leading to the sequential activation of TBK1 and IRF3 and thus expression of IRF3-dependent genes such as type I IFN (Vilaysane and Muruve, 2009; Kumagai et al., 2008).
There may be several molecular mechanisms underlying the synergy of anti-CD40, CpG and MPL in the activation of mouse macrophages. First, anti-CD40 primes macrophages to respond to CpG or MPL by up-regulating the expression of TLR9 (Buhtoiarov et al., 2006) or TLR4 (Van De Voort et al., 2013). Our previous studies show that synergy between anti-CD40 and CpG in macrophage activation requires that anti-CD40 treatment precedes stimulation with CpG: TLR9 expression is increased in macrophages in a time-dependent manner, peaking 3 days after anti-CD40 treatment and correlating with anti-CD40/CpG–induced antitumor effects (Buhtoiarov et al., 2006). There is a similar pattern of anti-CD40–mediated upregulation of TLR4, a receptor for MPL (Van De Voort et al., 2013). Second, MPL might prime macrophages to respond to CpG by up-regulating expression of TLR9. Thus, LPS and CpG synergistically induced TNF-α production by murine DC (An et al., 2002) and synergistically stimulated osteoclast cells to produce IL-12, TNF and IL-6 (Krisher and Bar-Shavit, 2014). Observed up-regulation of TLR9 gene expression on the osteoclast cell or DC by LPS explains the synergy in the induction of cytokine production. Since MPL is a derivative of LPS and engages the same receptor, TLR4, MPL may induce elevated expression of TLR9 to prime macrophages to respond to CpG. Third, MPL might prime macrophages to respond to CpG by the crosstalk between TLR4 and TLR9 signaling pathways. CpG can induce activation of Erk1/2, JNK, and p38 MAPK signaling in macrophages, which are responsible for the production of pro-inflammatory cytokines such as TNF and IL-6. LPS pretreatment of macrophages amplifies CpG-induced JNK activation in the macrophages. Therefore, MPL might synergistically activate macrophages by priming the macrophages to respond to CpG via signaling molecules downstream of TLR9 (De Nardo et al., 2009). The signal molecules in the cytoplasm of the macrophage may be fully utilized when the three agents initiate their different signaling pathways. In addition, these signaling pathways may compensate or enhance one another via crosstalk resulting in macrophage activation.
Our results show that the synergy of anti-CD40, CpG and MPL in activation of macrophages was evident at low doses of all the reagents. Previous studies reported a synergy in activation of PEC between anti-CD40 and CpG or MPL at high doses (5 μg/ml) in vitro (Buhtoiarov et al., 2006; Van De Voort et al., 2013). In this study, the synergy between three reagents was significant at the concentration of CpG 100-200 ng/ml and MPL 2-20 ng/ml. In addition, the synergy of all the three reagents in activation of macrophages was still significant when the concentration of anti-CD40 in vivo was decreased 5 fold. Since the side effects of treatment with agonistic CD40 antibodies in the clinic are dose-related (Vonderheide et al., 2007), reduction of its dose in a combination of all the three reagents may combine macrophage-activating effect with low toxicity in clinic immunotherapy.
Synergistic activation of macrophages by anti-CD40, CpG and MPL may endow the macrophages with enhanced antitumor potential. We have previously shown that the antitumor effects of anti-CD40-activated macrophages involves apoptosis, NO and TNFα (Buhtoiarov et al., 2006; Lum et al., 2006a). In a recent study using a myeloma model we have shown that separation of macrophages (activated with anti-CD40 and either CpG or MPL) from tumor cells with a transwell plate abrogated macrophage-mediated cytotoxicity (Jensen et al., manuscript submitted for publication), suggesting that antitumor effect of anti-CD40-activated macrophages is contact-dependent. Our results show that macrophages activated by a combination of anti-CD40, CpG and MPL have augmented tumoristatic activity compared to the single reagent or two reagents. Consistently, the activated macrophages produced high level of NO and TNF-α, which are cytotoxic to tumor cells (MacMicking et al., 1997; Urban et al., 1986). In addition, macrophages activated synergistically by anti-CD40, CpG and MPL also produced high level of IL-12, IFN-γ and MCP-1, which are signatures of M1 polarization. M1 macrophages are believed to have strong antitumor effect (Long and Beatty, 2013; Sica and Mantovani, 2012). Therefore, our findings suggest that this novel triple combination for macrophage activation might be considered in tumor immunotherapy.
Highlights.
Mouse macrophages can be synergistically activated by anti-CD40, CpG and MPL to produce nitric oxide and cytokines, and to suppress growth of tumor cells in vitro, suggesting that this novel combined approach might be further investigated as potential cancer therapy.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grants CA032685, CA87025, CA166105, CA14520, a Stand Up To Cancer – St. Baldrick's Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113), and grants from the Midwest Athletes for Childhood Cancer Fund, The Crawdaddy Foundation, and Hyundai Hope on Wheels, and China Scholarship Council. We thank Dr. Kimberly McDowell and Ms. Lakeesha Carmichael for helping with statistical analysis.
Abbreviations
- Anti-CD40
CD40 monoclonal antibody
- CBA
cytometric bead array
- CpG
CpG oligodeoxynucleotides
- ERK
extracellular signal-regulated kinase
- IkB
inhibitor of kappa B
- IKK
IkB-kinase
- IRAK
interleukin-1 receptor-associated kinase
- JNK
c-Jun N-terminal kinase
- FACS
fluorescence-activated cell sorting
- [3H]-TdR
tritiated thymidine
- IP
intraperitonially
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinases
- MPL
monophosphoryl lipid A
- MyD88
myeloid differentiation primary response gene 88
- NF-kB
nuclear factor kappa-light-chain-enhancer B cells
- NO
nitric oxide
- PEC
peritoneal exudate cells
- TAK
TGF beta activated kinase
- TBK
TANK-binding kinase
- TIRAP
MyD88 adapter-like toll/IL-1 receptor adaptor protein
- TLR
toll-like receptor
- TRAF
TNF receptor-associated factor
- TRAM
toll-IL-1R domain-containing adaptor inducing IFN-related adaptor molecule
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Disclosure: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H, Yu Y, Zhang M, Xu H, Qi R, Yan X, Liu S, Wang W, Guo Z, Guo J, Qin Z, Cao X. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybay C, Imir T. Comparison of the effects of Salmonella minnesota Re595 lipopolysaccharide, lipid A and monophosphoryl lipid A on nitric oxide, TNF-alpha, and IL-6 induction from RAW 264.7 macrophages. FEMS Immunol Med Microbiol. 1998;22:263–73. doi: 10.1111/j.1574-695X.1998.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O'Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–95. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–18. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174:6013–22. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- De Nardo D, De Nardo CM, Nguyen T, Hamilton JA, Scholz GM. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183:8110–8. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR. Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs. 2011;20:361–72. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- Krisher T, Bar-Shavit Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J Cell Biochem. 2014;115:2146–54. doi: 10.1002/jcb.24891. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Takeuchi O, Aaira S. TLR9 as a key receptor for the recognition of DNA. Advanced Drug Delivery Reviews. 2008;60(7):795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Lin AY, Almeida JP, Bear A, Liu N, Luo L, Foster AE, Drezek RA. Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS One. 2013;8:e63550. doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KB, Beatty GL. Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology. 2013;2:e26860. doi: 10.4161/onci.26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yeh W, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. Tumoristatic effects of anti-CD40 mAb-activated macrophages involve nitric oxide and tumour necrosis factor-alpha. Immunology. 2006a;118:261–70. doi: 10.1111/j.1365-2567.2006.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006b;79:1181–92. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Rakhmilevich AL, Alderson KL, Sondel PM. T-cell-independent antitumor effects of CD40 ligation. Int Rev Immunol. 2012;31:267–78. doi: 10.3109/08830185.2012.698337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhmilevich AL, Buhtoiarov IN, Malkovsky M, Sondel PM. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer Immunol Immunother. 2008;57:1151–60. doi: 10.1007/s00262-007-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- Ryu M, Kulkarni OP, Radomska E, Miosge N, Gross O, Anders HJ. Bacterial CpG-DNA accelerates Alport glomerulosclerosis by inducing an M1 macrophage phenotype and tumor necrosis factor-alpha-mediated podocyte loss. Kidney Int. 2011;79:189–98. doi: 10.1038/ki.2010.373. [DOI] [PubMed] [Google Scholar]
- Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphorothioate backbone modification modulates macrophage activation by. CpG DNA J Immunol. 2000;165(8):4165–4173. doi: 10.4049/jimmunol.165.8.4165. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol. 2009;21:257–64. doi: 10.1016/j.smim.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Urban JL, Shepard HM, Rothstein JL, Sugarman BJ, Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986;83:5233–7. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Voort TJ, Felder MA, Yang RK, Sondel PM, Rakhmilevich AL. Intratumoral delivery of low doses of anti-CD40 mAb combined with monophosphoryl lipid a induces local and systemic antitumor effects in immunocompetent and T cell-deficient mice. J Immunother. 2013;36:29–40. doi: 10.1097/CJI.0b013e3182780f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaysane A, Muruve DA. The innate immune response to DNA. Seminars in Immunology. 2009;21:208–214. doi: 10.1016/j.smim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62:949–54. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O'Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]