Abstract
Alpha-Galactosyl Ceramide (α-GalCer) is a prototypical synthetic ligand of invariant natural killer T (iNKT) cells. Upon presentation by the MHC class I-like molecule CD1d, this glycolipid stimulates iNKT cells to secrete a vast amount of both pro-inflammatory Th1 and anti-inflammatory Th2 cytokines. Recently, we discovered that selected 6″-modified α-GalCer analogues may produce markedly Th1-biased responses due to the formation of either an additional anchor with CD1d or by establishing extra interactions with the T-cell receptor of iNKT cells. Here, we report a practical synthesis towards 6″-O-carbamate and galacturonamide analogues of α-GalCer and their evaluation as iNKT cell agonists in mice.
Keywords: Glycolipids, α-GalCer, iNKT-cells, CD1d
Graphical abstract
Introduction
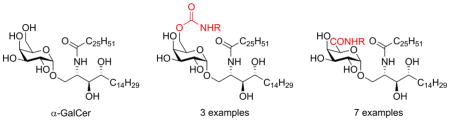
α-GalCer or KRN7000 (1, Figure 1) is a synthetic glycolipid derived from a group of galactosylceramides found in marine sponge extracts showing promising anti-tumor activity in a B16-melanoma murine model and currently under investigation in different early phase human trials for patients with metastatic cancer.1,2 α-GalCer is the prototypical antigen for iNKT-cells, a subset of T-lymphocytes, which show features of both innate and adaptive immunity. Whereas conventional T-cells are activated by recognition of peptide antigens presented by MHC class I or II molecules, iNKT-cells recognize lipid and glycolipid antigens in the context of CD1d expressed on antigen presenting cells.3,4 CD1d shows striking structural analogy with the MHC class I protein,5 however, it comprises a deeper and more lipophilic binding groove in line with the differences in antigen presentation. Indeed, crystal structures of both mouse and human CD1d bound to α-GalCer show that the lipid chains of α-GalCer are accommodated in two hydrophobic pockets. The F′ pocket binds the phytosphingosine chain while the fatty acyl chain fills the A′ pocket. As a result the galactose sugar is oriented towards the surface of CD1d, available for recognition by the TCR of iNKT cells.6,7
Figure 1.
Structures of KRN7000 (1), OCH (2) and α-C-GalCer (3)
Engagement of the CD1d-α-GalCer complex by the TCR of iNKT cells results in a rapid production of Th1 and Th2 cytokines both by iNKT cells itself and by activation of bystander immune cells.8 The robust cytokine production shows promise for a broad range of therapeutic applications, but despite its potent immune response α-GalCer showed limited therapeutic outcome in the clinic.9,10 The concomitant release of both Th1 and Th2 cytokines, which have opposing effects in vivo, is believed to account for its poor efficacy. Indeed, α-GalCer analogues capable of skewing the cytokine profile have improved therapeutic potential.11,12, 13 OCH (2), characterized by a truncated phytosphingosine chain, improves disease in animal models of experimental autoimmune encephalitis and arthritis as a result of a Th2 biased cytokine secretion.14,15 Stabilizing the anomeric bond by substituting a methylene unit for the glycosidic oxygen gave rise to α-C-GalCer (3), a compound with a marked Th1 response and superior malaria protection in mice.16
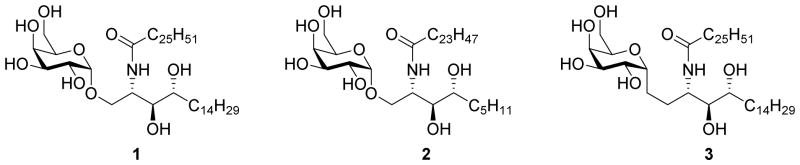
Modifications of the galactose moiety are mostly focusing on the 6″-position,17,18 as other alterations generally result in poor antigenicity. Previously, we discovered that a C-6″-naphthylurea derivative (NU-α-GalCer, 4, Figure 2) induces a potent Th1 response both in a murine and a human setting resulting in a superior tumor protection in the B16 mouse melanoma model.19 Crystallographic studies of the ternary CD1d-NU-α-GalCer-TCR complex revealed that the naphthyl moiety occupies a narrow binding pocket in CD1d above the A′ roof by induced fit, thereby enhancing the affinity for CD1d. The latter led us to investigate other modifications at the C-6″ position. Here we describe the synthesis of a series of C-6″-carbamate and C-5″-uronamide derivatives.
Figure 2.
Structures of NU-α-GalCer (4), C-6″-carbamate (5) and C-5″-uronamide derivatives (6) of α-GalCer
Results and Discussion
Chemistry
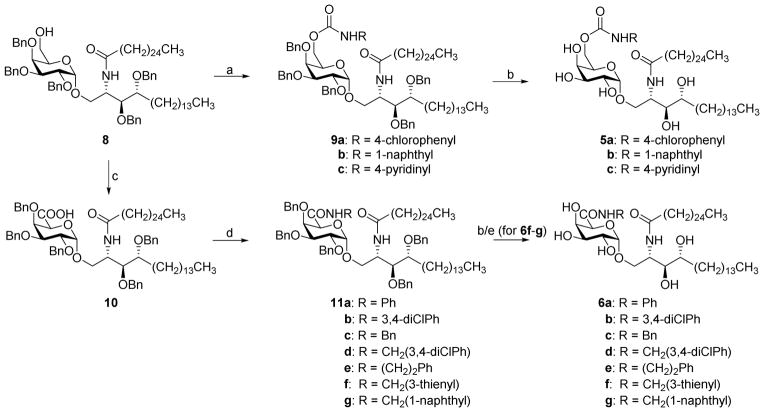
Both the C-6″-carbamates 5a-5c and the C-5″-amides 6a-6g can be accessed from the common intermediate 8, which is swiftly obtained upon treatment of the 4,6-benzylidene precursor with Cu(OTf)2 and BH3.THF. We previously started from the same intermediate for the synthesis of galacturonic acid and 6″-triazole analogues of α-GalCer.20,21 The primary OH group of 8 was converted into carbamates 9a and 9b upon reaction with the appropriate isocyanate, while compound 9c was obtained by treating 8 with 4-aminopyridine and 1,1′-carbonyldiimidazole (CDI).
The synthesis of the uronamides 11a-11g commenced with the TEMPO/BAIB oxidation of the primary hydroxyl group of 8 to afford the galacturonic acid intermediate 10.21 Successive coupling with the appropriate amine in the presence of 2-(6-chloro-1-H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate (HCTU) gave access to amides 11a-g. Palladium-catalyzed hydrogenation furnished the desired carbamates 5a-5c and amides 6a-6e. However, this deprotection method failed to remove the benzyl protecting groups of the sulfur-containing compound 11f, even under high pressure (50 bar), while overreduction of the naphthyl moiety was observed during hydrogenation of intermediates 9b and 11g. In case of naphthyl carbamate 9b, careful monitoring of the reaction yet allowed obtaining the final product. For the naphthyl amide compound, however, undesired reduction of the naphthyl ring was observed before all benzyl groups were removed, thereby impeding isolation of the envisaged analogue. This led us to explore alternative deprotection conditions for compounds 11f and 11g. Treatment with aluminum chloride and dimethylaniline allowed to produce the desired products 6f and 6g, albeit in low yields.
Biological Evaluation
The biological activity of all target glycolipids was assessed by measuring IFN-γ and IL-4 serum levels after intraperitoneal injection of 5 μg in mice (Figure 3). Except for 6a and 6f, the C-5″-amides are generally weaker antigens than α-GalCer with a Th1 cytokine bias, mainly originating from significantly lower IL-4 levels compared to α-GalCer.
Figure 3.
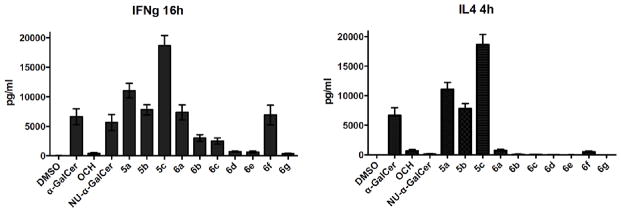
IFN-γ and IL-4 secretion, measured at respective 16 h and 4 h, after intraperitoneal injection of 5 μg of the glycolipids in mice (glycolipids were tested in at least 2 different experiments with 5–8 mice per group for each glycolipid).
The C-6″-carbamates clearly exhibit stronger antigenic effects than the uronamides, but their cytokine profiles resemble that of α-GalCer.
It was shown that the remarkably high INF-γ release of the C-6″-carbamates (5a-c) stems from a higher APC-derived IL-12 production, compared with that of α-GalCer.22 Notable, high IL-12 secretion was observed with 5c, largely exceeding that induced by NU-α-GalCer. The crystal structure of the ternary mCD1d-5b-TCR complex indicates that the binding mode of 5b in the parallels that of NU-α-GalCer. In particular, its naphthylcarbamate moiety also induces a hydrophobic pocket in CD1d by displacement of Met69 (Figure 4),23 indicating that the naphthyl moiety drives the observed induced fit rather than the nature of the linker, given that the length of the latter is appropriate. Probably, this extra interaction with CD1d compensates for the observed loss of the hydrogen bond between the 4″-OH group and Asn-30 of the TCR. Although the in vivo data indicate that 5b is a potent antigen, at present we have no explanation for its distinct cytokine profile compared to NU-α-GalCer.
Figure 4.
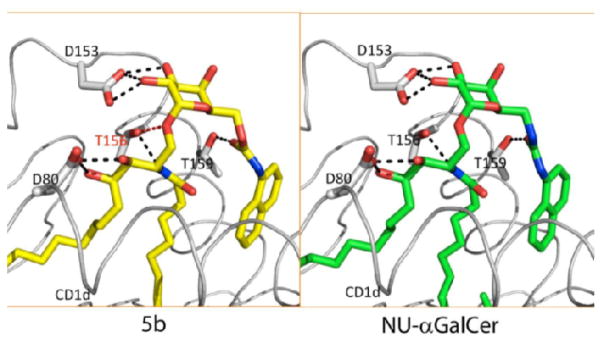
Crystal structure of 5b bound to CD1d, compared with that of NU-α-GalCer.
Previously, compound 5c (PyrC-α-GalCer) was shown to be tilted towards the TCR thereby forming intimate contacts with the TCR instead of forming a third anchor inside CD1d.22 The carbamate moiety of 5a, on the other hand, was situated laterally above the CD1d binding group, suggesting that the smaller pyridine as well as the phenyl ring are not sufficient to induce the structural change within the A′ roof of CD1d, in contrast to both 5b and NU-α-GalCer, which have the larger naphthyl ring.
Conclusions
In summary, we have synthesized a series of C-6″-carbamate and C-5″-galacturonamide derivatives of α-GalCer from a common intermediate 8, further illustrating the highly convergent potential of the latter. Generally, the antigenic potency of the carbamates is superior to that of the uronamides, although within each series significant variations in cytokine profiles are observed depending on the substitution patterns.
The binding behavior of the naphthylcarbamate 5b in the ternary complex is strikingly similar to that of NU-α-GalCer (4). The naphthyl moiety of both analogues occupies an extra binding pocket in CD1d. Determinants for the induction of this hydrophobic pocket possibly involve the size of the aromatic group and the correct positioning of a carbonyl that acts as H-bond acceptor. By varying the carbamate substituents it may be possible to increase the interaction with either the TCR or CD1d. A notable advantage of the carbamate derivatives over their urea analogues is that the synthesis of the former is significantly shorter.
Experimental Section
Chemical Synthesis
General
Precoated Macherey-Nagel SIL G/UV254 plates were used for TLC, and spots were examined under UV light at 254 nm and further visualized by sulfuric acid-anisaldehyde spray. Column chromatography was performed on Biosolve silica gel (32 – 63 μm, 60 Å). NMR spectra were obtained with a Varian Mercury 300 Spectrometer. Chemical shifts are given in ppm (δ) relative to the residual solvent signals, in the case of CDCl3: δ = 7.26 ppm for 1H and δ = 77.4 ppm for 13C and in the case of pyridine-d5: δ = 8.74, 7.58 and 7.22 ppm for 1H and δ = 149.9, 135.5 and 123.5 ppm for 13C. Exact mass measurements were performed on a Waters LCT Premier XE TOF equipped with an electrospray ionization interface and coupled to a Waters Alliance HPLC system. Samples were infused in a CH3CN/HCOOH (1000/1) mixture at 10 mL/min. All melting points were determined on a Büchi B-545 melting point apparatus and are uncorrected.
Procedure for the synthesis of carbamates 9a and 9b
To a solution of compound 8 (0.07 mmol) in DMF (1 mL) was added the appropriate isocyanate (0.18 mmol). After stirring overnight, the reaction mixture was evaporated to dryness under reduced pressure. Purification by column chromatography (hexanes/EtOAc: 8/2) afforded carbamates 9a (74 %) and 9b (67 %).
(2S,3S,4R)-3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-O-(4-chlorophenylcarbamoyl)-α-D-galactopyranosyl)-2-(hexacosanamido)octadecane-1,3,4-triol (9a)
1H NMR (300 MHz, CDCl3): δ 8.29 (s, 1H, NH), 7.31-7.10 (m, 29H, arom. H), 5.71 (d, J = 6.6 Hz, 1H, NH), 4.88 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.79 (d, J = 3.9 Hz, 1H, H-1″), 4.75 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.67 (d, J = 12.0 Hz, 1H, CH2-Ph), 4.62 (d, J = 11.9 Hz, 1H, CH2-Ph), 4.61 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.57 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.52 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.51 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.42 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.41 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.33-4.26 (m, 2H, H-2, H-6″), 4.01-3.90 (m, 3H, H-2″, H-1), 3.86-3.75 (m, 4H, H-3, H-3″, H-4″, H-5″), 3.69 (dd, J = 1.6 Hz and 11.4 Hz, 1H, H-6″), 3.51-3.46 (m, 1H, H-4), 1.96-1.84 (m, 2H, COCH2), 1.58-1.06 (m, 72H, CH2), 0.77 (t, J = 6.9 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.93, 153.51, 138.79, 138.73, 138.69, 138.47, 138.22, 137.63, 129.90, 128.96, 128.68, 128.64, 128.60, 128.57, 128.13, 128.04, 127.99, 127.90, 127.84, 127.73, 127.66, 119.69, 100.52, 80.54, 79.72, 79.48, 77.67, 77.45, 77.25, 76.83, 76.65, 75.20, 74.52, 73.88, 73.66, 73.62, 72.38, 70.26, 70.17, 65.87, 60.62, 52.34, 37.04, 32.17, 32.16, 30.88, 29.97, 29.96, 29.94, 29.92, 29.89, 29.79, 29.61, 29.60, 29.48, 26.17, 25.81, 22.93, 21.28, 14.43, 14.36.
Exact mass (ESI-MS) for C92H133ClN2O10 [M+H]+ found, 1461.9786; calcd, 1461.9727.
(2S,3S,4R)-3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-O-(1-naphthylcarbamoyl)-α-D-galactopyranosyl)-2-(hexacosanamido)octadecane-1,3,4-triol (9b)
1H NMR (300 MHz, pyridine-d5): δ 10.66 (s, 1H, NH), 8.99 (d, J = 8.4 Hz, 1H, NH), 8.84 (d, J = 8.4 Hz, 1H, arom. H), 8.37 (d, J = 7 Hz, 1H, arom. H), 7.96 (d, J = 7.9 Hz, 1H, arom. H), 7.78 (d, J = 8.1 Hz, 1H, arom. H), 7.66-7.58 (m, 2H, arom. H), 7.55-7.29 (m, 26H, arom. H), 5.43 (d, J = 3.4 Hz, 1H, H-1″), 5.17 (d, J = 11.1 Hz, 1H, CH2-Ph), 5.13 (d, J = 10.2 Hz, 1H, CH2-Ph), 5.00-4.82 (m, 4H, CH2-Ph, H-6″, H-2), 4.78-4.68 (m, 5H, H-6″, CH2-Ph), 4.64-4.58 (m, 2H, H-5″, CH2-Ph), 4.54 (dd, J = 2.0 and 8.4 Hz, 1H, H-3), 4.48-4.43 (m, 3H, H-1, H-2″, CH2-Ph), 4.34 (dd, J = 2.7 Hz and 10.2 Hz, 1H, H-3″), 4.25-4.20 (m, 2H, H-1, H-4″), 3.97-3.93 (m, 1H, H-4), 2.66-2.56 (m, 2H, COCH2), 2.15-1.18 (m, 72H, CH2), 0.90 (t, J = 6.7 Hz, 3H, CH3), 0.89 (t, J = 6.4 Hz, 3H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 172.21, 154.34, 138.55, 138.41, 138.21, 138.12, 127.65, 127.50, 127.29, 126.89, 126.80, 126.75, 126.58, 125.16, 125.11, 97.63, 80.00, 78.76, 77.89, 76.09, 74.88, 73.94, 73.24, 72.26, 71.67, 70.70, 68.77, 61.38, 50.13, 49.97, 48.02, 38.95, 35.66, 34.03, 30.97, 28.99, 28.87, 28.80, 28.74, 28.66, 28.43, 25.62, 25.27, 23.71, 21.78, 13.12.
Exact mass (ESI-MS) for C96H136N2O10 [M+H]+ found, 1478.0220; calcd, 1478.0273.
Procedure for the synthesis of carbamate 9c
To a solution of compound 8 (50 mg, 0.04 mmol) in DMF (0.5 mL) was added CDI (31 mg, 0.18 mmol). After stirring overnight, the reaction mixture was heated until 70 °C and 4-aminopyridine was added. The reaction mixture was stirred at 70 °C during 48 hours followed by evaporation to dryness under reduced pressure. Purification by column chromatography (hexanes/EtOAc: 7/3) afforded carbamate 9c (26 mg, 33 %).
(2S,3S,4R)-3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-O-(4-pyridinylcarbamoyl)-α-D-galactopyranosyl)-2-(hexacosanamido)octadecane-1,3,4-triol (9c)
1H NMR (300 MHz, CDCl3): δ 7.87 (s, 1H, NH), 7.35-7.15 (m, 28H, arom. H), 6.93 (s, 1H, arom. H), 5.69 (d, J = 8.2 Hz, 1H, NH), 4.90 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.81 (d, J = 3.7 Hz, 1H, H-1″), 4.76 (d, J = 11.1 Hz, 1H, CH2-Ph), 4.73 (d, J = 11.3 Hz, 1H, CH2-Ph), 4.69 (d, J = 11.3 Hz, 1H, CH2-Ph), 4.65 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.58 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.55 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.48 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.40 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.39 (d, J = 11.5 Hz, 1H, CH2-Ph), 4.29-4.20 (m, 2H, H-2, H-6″), 4.12-4.06 (m, 1H, H-6″), 3.99 (dd, J= 3.3 Hz and 9.8 Hz, 1H, H-2″), 3.94 (m, 1H, H-5″), 3.85 (dd, J = 2.5 Hz and 10.1 Hz, 1H, H-3″), 3.81 (dd, J = 4.91 Hz and 11.0 Hz, 1H, H-1), 3.75 (app. s, 1H, H-4″), 3.68-3.64 (m, 1H, H-3), 3.62-3.56 (m, 1H, H-1), 3.48-3.43 (m, 1H, H-4), 1.86-1.74 (m, 2H, COCH2), 1.54-1.09 (m, 72H, CH2), 0.83-0.76 (m, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.09, 148.33, 138.68, 138.62, 138.52, 138.01, 137.32, 130.68, 128.76, 128.73, 128.68, 128.66, 128.64, 128.60, 128.54, 128.16, 128.07, 128.05, 127.97, 127.82, 127.78, 127.68, 117.33, 99.09, 80.11, 79.67, 79.13, 74.56, 73.83, 73.48, 72.12, 68.33, 68.16, 66.68, 60.63, 56.66, 50.42, 36.97, 32.16, 31.82, 30.51, 30.04, 29.96, 29.93, 29.89, 29.84, 29.67, 29.64, 29.60, 29.59, 26.02, 25.94, 22.92, 22.88, 21.27, 14.22, 14.35.
Exact mass (ESI-MS) for C91H133N3O10 [M+H]+ found, 1429.0229; calcd, 1429.0064.
General procedure for the synthesis of amides (11a-11g)
To a solution of 10 (150 mg, 0.11 mmol) in DMF (0.3 mL) and CH2Cl2 (0.7 mL) was added DIPEA (22 mg, 0.17 mmol). After stirring for 10 minutes at room temperature, HCTU (72 mg, 0.17 mmol) was added and the mixture was stirred for 30 minutes. Then the appropriate amine (0.17 mmol) was added and the solution was continued stirring overnight. After completion of the reaction, the mixture was evaporated to dryness. The residue was partitioned between H2O and EtOAc and the aqueous layer was extracted with EtOAc. The organic layer was washed with brine and dried over Na2SO4. Purification by column chromatography (hexanes/EtOAc) and concentration under reduced pressure furnished the desired amides 11a (87 %), 11b (84 %), 11c (53 %), 11d (82 %), 11e (82 %), 11f (85 %) and 11g (82 %).
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-phenyl-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11a)
1H NMR (300 MHz, CDCl3): δ 8.20 (s, 1H, NH), 7.53 (d, J = 1.0 Hz, 2H, arom. H), 7.39-7.08 (m, 28H, arom. H), 5.73 (d, J = 8.2 Hz, 1H, NH), 4.98 (d, J = 3.5 Hz, 1H, H-1″), 4.87 (d, J = 10.8 Hz, 1H, CH2-Ph), 4.84 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.79 (d, J = 12.7 Hz, 1H, CH2-Ph), 4.71 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.66 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.62 (d, J = 12.0 Hz, 1H, CH2-Ph), 4.58 (d, J = 10.8 Hz, 1H, CH2-Ph), 4.55 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.51-4.45 (m, 3H, CH2-Ph and H-4″), 4.37 (app. d, J = 1.1 Hz, 1H, H-5″), 4.26 (m, 1H, H-2), 4.07 (dd, J = 3.5 Hz and 10.1 Hz, 1H, H-2″), 3.98 (dd, J = 2.7 Hz and 10.0 Hz, 1H, H-3″), 3.90-3.76 (m, 3H, H-1, H-3), 3.55-3.49 (m, 1H, H-4), 1.92-1.78 (m, 2H, COCH2), 1.68-1.22 (m, 72H, CH2), 0.88 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.07, 166.69, 138.73, 138.70, 138.55, 138.50, 138.40, 137.44, 129.16, 128.67, 128.65, 128.63, 128.61, 128.45, 128.35, 128.15, 128.07, 128.03, 128.01, 127.92, 127.90, 127.81, 127.70, 124.76, 120.19, 99.21, 79.97, 79.20, 78.55, 76.63, 76.10, 75.91, 74.10, 73.38, 72.72, 72.24, 72.14, 68.46, 50.19, 36.90, 32.16, 30.41, 30.05, 29.96, 29.94, 29.89, 29.82, 29.65, 29.61, 29.60, 26.11, 25.85, 22.93, 14.36.
Exact mass (ESI-MS) for C91H132N2O9 [M+Na]+ found, 1419.9889; calcd, 1419.9831.
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-(3,4-dichlorophenyl)-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11b)
1H NMR (300 MHz, CDCl3): δ 8.17 (s, 1H, NH), 7.74 (d, J = 2.2 Hz, 1H, arom. H), 7.40-7.14 (m, 27H, arom. H), 5.70 (d, J = 8.2 Hz, 1H, NH), 4.95 (d, J = 3.4 Hz, 1H, H-1″), 4.89-4.46 (m, 11H, CH2-Ph and H-4″), 4.35 (d, J = 1.2 Hz, 1H, H-5″), 4.31-4.25 (m, 1H, H-2), 4.04 (dd, J = 3.4 Hz and 10.0 Hz, 1H, H-2″), 3.96 (dd, J = 2.6 Hz and 10.2 Hz, 1H, H-3″), 3.91 (dd, J = 4.9 Hz and 10.6 Hz, 1H, H-1), 3.80-3.74 (m, 2H, H-1 and H-3), 3.56-3.52 (m, 1H, H-4), 1.94-1.77 (m, 2H, COCH2), 1.60-1.08 (m, 72H, CH2), 0.88 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.09, 167.17, 138.68, 138.66, 138.50, 138.43, 138.28, 136.81, 132.93, 130.62, 128.70, 128.68, 128.65, 128.64, 128.42, 128.40, 128.11, 128.09, 128.06, 128.01, 127.97, 127.94, 127.87, 127.70, 121.80, 119.37, 99.25, 79.92, 79.69, 78.49, 75.99, 74.11, 73.32, 72.94, 72.29, 68.67, 53.64, 50.20, 36.89, 32.16, 30.61, 30.05, 29.96, 29.94, 29.91, 29.89, 29.82, 29.66, 29.61, 29.59, 26.12, 25.85, 22.92, 14.35.
Exact mass (ESI-MS) for C91H130Cl2N2O9 [M+Na]+ found, 1487.9138; calcd, 1487.9046.
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-benzyl-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11c)
1H NMR (300 MHz, CDCl3): δ 7.37-7.17 (m, 30H, arom. H), 6.79 (app. t, J = 5.9 Hz, 1H, NH), 5.74 (d, J = 8.1 Hz, 1H, NH), 4.88 (d, J = 3.7 Hz, 1H, H-1″), 4.85-4.42 (m, 12H, CH2-Ph, H-4″, NH-CH2), 4.35-4.28 (m, 2H, H-5″ and NH-CH2), 4.26-4.18 (m, 1H, H-2), 4.04 (dd, J = 3.4 Hz and 9.9 Hz, 1H, H-2″), 3.95 (dd, J = 2.6 Hz and 10.0 Hz, 1H, H-3″), 3.82-3.71 (m, 3H, H-1 and H-3), 3.49 (m, 1H, H-4), 1.93-1.78 (m, 2H, COCH2), 1.65-1.14 (m, 72H, CH2), 0.87 (t, J = 6.6 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.05, 168.75, 138.80, 138.76, 138.74, 138.51, 137.76, 128.87, 128.63, 128.59, 128.40, 128.25, 128.15, 128.06, 128.01, 127.96, 127.87, 127.84, 127.75, 127.67, 99.05, 80.00, 78.98, 78.61, 76.26, 76.03, 75.60, 74.04, 73.47, 72.59, 72.01, 71.92, 68.18, 60.61, 50.12, 43.37, 36.88, 32.16, 31.82, 31.14, 30.27, 30.06, 29.96, 29.94, 29.89, 29.84, 29.68, 29.61, 29.59, 26.16, 25.87, 22.92, 22.88, 14.43, 14.35.
Exact mass (ESI-MS) for C92H134N2O9 [M+Na]+ found, 1433.9846; calcd, 1433.9982.
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-(3,4-dichlorobenzyl)-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11d)
1H NMR (300 MHz, CDCl3): δ 7.38-7.22 (m, 26H, arom. H), 7.14 (d, J = 8.2 Hz, 1H, arom. H), 6.98 (dd, J = 1.9 Hz and 8.2 Hz, 1H, arom. H), 6.82 (t, J = 6.1 Hz, 1H, NH), 5.75 (d, J = 8.0 Hz, 1H, NH), 4.92 (d, J = 3.3 Hz, 1H, H-1″), 4.89 (d, J = 11.0 Hz, 1H, CH2-Ph), 4.82 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.79 (d, J = 12.1 Hz, 1H, CH2-Ph), 4.74 (d, J = 12.1 Hz, 1H, CH2-Ph), 4.72 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.65 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.56 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.55 (d, J = 11.0 Hz, 1H, CH2-Ph), 4.50-4.43 (m, 4H, CH2-Ph, NH-CH2 and H-4″), 4.30-4.26 (m, 2H, H-5″ and H-2), 4.20 (dd, J = 5.4 Hz and 15.1 Hz, 1H, NH-CH2), 4.05 (d, J = 3.4 Hz and 10.1 Hz, 1H, H-2″), 3.96 (d, J = 2.5 Hz and 10.0 Hz, 1H, H-3″), 3.88-3.73 (m, 3H, H-1 and H-3), 3.55–3.58 (m, 1H, H-4), 1.99-1.80 (m, 2H, COCH2), 1.72-1.16 (m, 72H, CH2), 0.89 (t, J = 6.6 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.06, 168.95, 138.73, 138.69, 138.50, 138.47, 138.24, 132.72, 131.67, 130.87, 129.97, 128.66, 128.64, 128.62, 128.43, 128.13, 128.10, 128.03, 127.96, 127.91, 127.84, 127.68, 127.33, 99.14, 79.96, 79.33, 78.60, 77.69, 77.27, 76.84, 76.24, 75.98, 75.60, 74.06, 73.44, 72.73, 72.12, 71.97, 68.28, 50.18, 42.19, 36.91, 32.17, 30.42, 30.06, 29.97, 29.95, 29.90, 29.85, 29.69, 29.62, 29.60, 26.15, 25.88, 22.94, 14.37.
Exact mass (ESI-MS) for C92H132Cl2N2O9 [M+H]+ found, 1479.9365; calcd, 1479.9388.
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-phenethyl-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11e)
1H NMR (300 MHz, CDCl3): δ 7.38-7.12 (m, 30H, arom. H), 6.61 (t, J = 6.0 Hz, 1H, NH), 5.78 (d, J = 8.3 Hz, 1H, NH), 4.89 (d, J = 3.3 Hz, 1H, H-1″), 4.87 (d, J = 10.8 Hz, 1H, CH2-Ph), 4.82 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.77 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.73 (d, J = 10.5 Hz, 1H, CH2-Ph), 4.72 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.64 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.59 (d, J = 10.8 Hz, 1H, CH2-Ph), 4.56 (d, J = 11.5 Hz, 1H, CH2-Ph), 4.51-4.49 (m, 1H, H-4″), 4.48 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.46 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.27–4.29 (m, 2H, H-2 and H-5″), 4.03 (dd, J = 3.5 Hz and 10.0 Hz, 1H, H-2″), 3.94 (dd, J = 2.7 Hz and 10.1 Hz, 1H, H-3″), 3.82 (dd, J = 2.9 Hz and 6.5 Hz, 1H, H-3), 3.79-3.71 (m, 2H, H-1), 3.53-3.45 (m, 3H, NH-CH2 and H-4), 2.73 (ddd, J = 7.2 Hz, 13.5 Hz and 28.0 Hz, 2H, CH2), 1.98-1.80 (m, 2H, COCH2), 1.65-1.19 (m, 72H, CH2), 0.88 (t, J = 6.8 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 172.99, 168.65, 138.88, 138.80, 138.77, 138.75, 138.53, 138.51, 128.92, 128.78, 128.64, 128.62, 128.61, 128.39, 128.37,128.14, 128.06, 127.99, 127.95, 127.88, 127.85, 127.70, 126.70, 98.97, 80.11, 78.92, 78.61, 77.68, 77.45, 77.25, 76.83, 76.31, 76.03, 75.73, 74.06, 73.47, 72.53, 72.04, 71.81, 68.03, 50.11, 40.61, 36.91, 36.03, 32.16, 30.29, 30.07, 29.97, 29.94, 29.92, 29.89, 29.85, 29.69, 29.64, 29.61, 29.60, 26.21, 25.88, 22.93, 14.36.
Exact mass (ESI-MS) for C93H136N2O9 [M+H]+ found, 1426.0337; calcd, 1426.0324
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-(thiophen-3-ylmethyl)-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11f)
1H NMR (300 MHz, CDCl3): δ 7.30-7.13 (m, 25H, arom. H), 7.07 (dd, J = 1.4 Hz and 5.0 Hz, 1H, arom. H), 6.81 (dd, J = 1.3 Hz and 3.4 Hz, 1H, arom. H), 6.79- 6.75 (m, 2H, arom. H and NH), 5.66 (d, J = 8.3 Hz, 1H, NH), 4.81 (d, J = 3.3 Hz, 1H, H-1″), 4.77 (d, J = 11.0 Hz, 1H, CH2-Ph), 4.73 (d, J = 12.5 Hz, 1H, CH2-Ph), 4.68 (d, J = 12.0 Hz, 1H, CH2-Ph), 4.64 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.58-4.42 (m, 7H, CH2-Ph, NH-CH2, H-4″), 4.40 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.37 (d, J = 11.6 Hz, 1H, CH2- Ph), 4.20 (d, J = 0.8 Hz, 1H, H-5″), 4.18-4.12 (m, 1H, H-2), 3.95 (dd, J = 3.4 Hz and 10.0 Hz, 1H, H-2″), 3.87 (dd, J = 2.6 Hz and 10.0 Hz, 1H, H-3″), 3.75-3.63 (m, 3H, H-1 and H-3), 3.48-3.40 (m, 1H, H-4), 1.88-1.69 (m, 2H, COCH2), 1.58-1.07 (m, 72H, CH2), 0.81 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.02, 168.56, 140.27, 138.77, 138.76, 138.54, 138.52, 128.65, 128.63, 128.62, 128.39, 128.33, 128.19, 128.07, 128.01, 127.90, 127.88, 127.77, 127.69, 127.12, 126.52, 125.44, 99.06, 79.96, 79.01, 78.57, 77.70, 77.28, 76.86, 76.23, 76.03, 75.61, 74.05, 73.45, 72.58, 72.02, 71.89, 68.21, 50.10, 38.03, 36.90, 32.18, 32.17, 30.29, 30.08, 29.98, 29.96, 29.93, 29.91, 29.86, 29.70, 29.63, 29.61, 26.15, 25.88, 22.95, 14.38.
Exact mass (ESI-MS) for C90H132N2O9S [M+H]+ found, 1417.9757; calcd, 1417.9732
(2S,3S,4R)-(2,3,4-tri-O-benzyl-N-(naphthalen-1-ylmethyl)-α-D-galactopyranuronamidyl)-3,4-di-O-benzyl-2-(hexacosanamido)octadecane-3,4-diol (11g)
1H NMR (300 MHz, CDCl3): δ 7.98 (d, J = 8.2 Hz, 1H, arom. H), 7.85 (d, J = 7.8 Hz, 1H, arom. H), 7.77 (d, J = 8.2 Hz, 1H, arom. H), 7.50-7.23 (m, 29H, arom. H), 6.85 (t, J = 5.6 Hz, 1H, NH), 5.78 (d, J = 8.4 Hz, 1H, NH), 4.92-4.36 (m, 15H, H-1″, CH2-Ph, NH-CH2, H-4″, H-5″), 4.28-4.20 (m, 1H, H-2), 4.04 (dd, J = 3.2 Hz and 10.0 Hz, 1H, H-2″), 3.98 (dd, J = 2.0 Hz and 10.0 Hz, 1H, H-3″), 3.82-3.69 (m, 3H, H-1 and H-3), 3.53-3.47 (m, 1H, H-4), 1.97-1.79 (m, 2H, COCH2), 1.62-1.18 (m, 72H, CH2), 0.90 (t, J = 6.5 Hz, 6H, CH3)
13C NMR (75 MHz, CDCl3): δ 173.05, 168.73, 138.84, 138.75, 138.73, 138.53, 138.50, 134.02, 133.14, 131.56, 128.98, 128.95, 128.69, 128.65, 128.60, 128.43, 128.28, 128.18, 128.08, 127.98, 127.88, 127.84, 127.77, 127.71, 126.84, 126.51, 126.15, 125.67, 123.50, 99.02, 79.94, 78.89, 78.59, 77.71, 77.29, 76.86, 76.30, 76.06, 75.61, 74.06, 73.47, 72.59, 72.00, 71.95, 68.20, 50.07, 41.30, 36.88, 32.18, 30.22, 30.08, 29.99, 29.96, 29.91, 29.87, 29.71, 29.64, 29.62, 26.15, 25.89, 22.95, 14.39.
Exact mass (ESI-MS) for C96H136N2O9 [M+H]+ found, 1462.0370; calcd, 1462.0324
General procedure for debenzylation: (5a–5c and 6a–6e)
A solution of the protected amide/carbamate (0.03 mmol) in CHCl3 (0.4 mL) and EtOH (1.2 mL) was hydrogenated under atmospheric pressure in the presence of palladium black (10 mg). Upon reaction completion, the mixture was diluted with pyridine and filtered through celite. The filter cake was rinsed with CHCl3 and EtOH and the filtrate was evaporated to dryness. After purification by column chromatography (DCM/MeOH), final compounds 5a (81%), 5b (68%), 5c (86%), 6a (86%), 6b (63%), 6c (49%), 6d (80%) and 6e (79%) were afforded as white powders.
(2S,3S,4R)-1-O-(6-O-(4-chlorophenylcarbamoyl)-α-D-galactopyranosyl)-2-(hexacosanamido)octadecane-1,3,4-triol (5a)
1H NMR (300 MHz, pyridine-d5): δ 10.73 (s, 1H, NH), 8.58 (d, J = 8.2 Hz, 1H, NH), 7.91 (d, J = 8.7 Hz, 2H, arom. H), 7.40 (d, J = 8.9 Hz, 2H, arom. H), 6.99 (br. s, 1H, OH), 6.75 (br. s, 1H, OH), 6.62 (d, J = 3.7 Hz, 1H, OH), 6.45 (d, J = 6.6 Hz, 1H, OH), 6.27 (d, J = 6.8 Hz, 1H, OH), 5.51 (d, J = 3.9 Hz, 1H, H-1″), 5.21-5.18 (m, 1H, H-2), 5.03 (dd, J = 8.1 and 11.0 Hz, 1H, H-6″), 4.79 (dd, J = 3.5 Hz and 11.0 Hz, 1H, H-6″), 4.66-4.57 (m, 3H, H-1, H-2″, H-5″), 4.39-4.24 (m, 5H, H-3″, H-4″, H-1, H-3, H-4), 2.46 (t, J = 7.5 Hz, 2H, COCH2), 1.98-1.18 (m, 72H, CH2), 0.88 (t, J = 6.3 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 172.33, 153.39, 138.14, 128.14, 128.05, 126.14, 119.21, 100.24, 74.89, 71.53, 70.08, 69.72, 69.27, 68.86, 67.57, 64.78, 50.50, 35.64, 32.88, 30.96, 30.94, 29.31, 29.18, 28.98, 28.87, 28.84, 28.76, 28.74, 28.70, 28.63, 28.57, 28.45, 28.43, 25.37, 25.18, 21.77, 13.11.
Exact mass (ESI-MS) for C57H103ClN2O10 [M+H]+ found, 1011.7393; calcd, 1011.7374; m.p. 162.0°C – 164.0°C.
(2S,3S,4R)-1-O-(6-O-(1-naphthylcarbamoyl)-α-D-galactopyranosyl)-2-(hexacosanamido)octadecane-1,3,4-triol (5b)
1H NMR (300 MHz, pyridine-d5): δ 10.68 (s, 1H, NH), 8.74-8.70 (m, 1H, arom H), 8.58 (d, J = 8.6 Hz, 1H, NH), 8.26 (d, J = 7.3 Hz, 1H, arom. H), 7.94 (d, J = 7.5 Hz, 1H, arom. H), 7.74 (d, J = 8.4 Hz, 1H, arom. H), 7.62-7.51 (m, 3H, arom. H), 5.58 (d, J = 3.9 Hz, 1H, H- 1″), 5.28-5.22 (m, 1H, H-2), 5.17-5.06 (m, 1H, H-6″), 4.94 (dd, J = 4.2 Hz and 11.2 Hz, 1H, H-6″), 4.84-4.65 (m, 3H, H-1, H-5″, H-2″), 4.48-4.37 (m, 4H, H-1, H-3, H-3″, H-4″), 4.33-4.28 (m, 1H, H-4), 2.51-2.46 (m, 2H, COCH2), 1.93-1.23 (m, 72H, CH2), 0.91-0.86 (m, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 172.27, 154.65, 149.43, 135.00, 133.63, 127.56, 125.15, 125.11, 125.04, 123.71, 100.24, 75.11, 71.50, 70.10, 69.75, 69.29, 68.90, 64.83, 50.45, 35.66, 30.95, 30.94, 29.19, 28.95, 28.86, 28.83, 28.76, 28.74, 28.68, 28.62, 28.58, 28.45, 28.43, 25.32, 25.22, 21.76, 13.10.
Exact mass (ESI-MS) for C61H106N2O10 [M+H]+ found, 1027.7919; calcd, 1027.7926; m.p. 159.0 °C–161.0 °C.
(2S,3S,4R)-1-O-(6-O-(4-pyridinylcarbamoyl)-α-D-galactopyranosyl)-2-(hexacosanamido)octadecane-1,3,4-triol (5c)
1H NMR (300 MHz, pyridine-d5): δ 11.18 (s, 1H, NH), 8.83 (d, J = 8.3 Hz, 1H, NH), 8.67 (dd, J = 1.4 Hz and 4.8 Hz, 2H, arom. H), 7.93 (dd, J = 1.4 Hz and 4.8 Hz, 2H, arom. H), 5.55 (d, J = 3.8 Hz, 1H, H-1″), 5.26-5.20 (m, 1H, H-2), 5.08 (dd, J = 8.2 Hz and 11.0 Hz, 1H, H- 6″), 4.77 (dd, J = 3.4 Hz and 11.0 Hz, 1H, H-6″), 4.72- 4.62 (m, 3H, H-1, H-2″, H-5″), 4.45-4.30 (m, 5H, H-3″, H-4″, H-1, H-3, H-4), 2.53 (t, J = 7.5 Hz, 2H, COCH2), 1.95-1.23 (m, 72H, CH2), 0.88 (t, J = 6.5 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 173.94, 154.68, 151.19, 147.76, 113.56, 101.56, 76.57, 72.96, 71.66, 70.43, 53.40, 46.06, 37.19, 32.45, 30.72, 30.50, 30.38, 30.34, 30.27, 30.24, 30.17, 30.09, 29.94, 26.89, 26.75, 23.26, 14.61.
Exact mass (ESI-MS) for C56H103N3O10 [M+H]+ found, 978.7662; calcd, 978.7722; m.p. decomposition.
(2S,3S,4R)-(N-phenyl-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6a)
1H NMR (300 MHz, pyridine-d5): δ 9.88 (s, 1H, NH), 8.49 (d, J = 8.8 Hz, 1H, NH), 8.12 (dd, J = 1.1 Hz and 8.6 Hz, 2H, arom. H), 7.36-7.30 (m, 3H, arom. H and OH), 7.10 (t, J = 7.3 Hz, 1H, arom. H), 6.79 (d, J = 5.2 Hz, 1H, OH), 6.43 (d, J = 5.9 Hz, 1H, OH), 6.12 (d, J = 5.4 Hz, 1H, OH), 5.47 (d, J = 3.8 Hz, 1H, H-1″), 5.27- 5.24 (m, 1H, H-2), 5.02-4.99 (m, 3H, OH, H-4″ and H-5″), 4.66-4.55 (m, 2H, H-1 and H-2″), 4.44 (app. d, J = 9.2 Hz, 1H, H-3″), 4.32-4.21 (m, 3H, H-1, H-3, H-4), 2.49-2.44 (m, 2H, COCH2), 1.40-1.12 (m, 72H, CH2), 0.88 (t, J = 6.6 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 173.77, 169.23, 139.70, 129.36, 120.95, 101.60, 76.53, 74.21, 72.76, 71.71, 71.36, 69.97, 69.18, 51.49, 37.07, 34.44, 32.44, 32.43, 30.67, 30.45, 30.35, 30.31, 30.24, 30.22, 30.19, 30.11, 30.07, 29.93, 29.91, 26.80, 26.71, 23.25, 14.60.
Exact mass (ESI-MS) for C56H102N2O9 [M+H]+ found, 947.7612; calcd, 947.7658; m.p. 136.0 °C–138.0 °C.
(2S,3S,4R)-(N-(3,4-dichlorophenyl)-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6b)
1H NMR (300 MHz, pyridine-d5): δ 10.19 (s, 1H, NH), 8.52 (d, J = 8.7 Hz, 1H, NH), 8.46 (d, J = 2.4 Hz, 1H, arom. H), 7.92 (dd, J = 2.4 Hz and 9.0 Hz, 1H, arom. H), 7.39 (d, J = 9.0 Hz, 1H, arom. H), 7.28 (bs, 1H, OH), 6.85 (bs, 1H, OH), 6.45 (d, J = 4.2 Hz, 1H, OH), 6.13 (bs, 1H, OH), 5.40 (d, J = 3.6 Hz, 1H, H-1″), 5.27-5.23 (m, 1H, H-2), 5.03-4.96 (m, 3H, H-4″, H-5″ and OH), 4.62 (dd, J = 3.6 Hz and 10.5 Hz, 1H, H-2″), 4.57 (dd, J = 5.1 Hz and 10.8 Hz, 1H, H-3), 4.44 (dd, J = 1.8 Hz and 9.6 Hz, 1H, H-3″), 4.26-4.21 (m, 3H, H-1 and H-4), 2.49-2.44 (m, 2H, COCH2), 2.29-1.18 (m, 72H, CH2), 0.89 (t, J = 6.6 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 171.94, 168.21, 138.12, 131.03, 129.39, 125.19, 121.60, 121.10, 119.06, 100.22, 75.33, 72.80, 71.31, 70.27, 68.78, 68.42, 49.86, 35.56, 33.15, 30.95, 30.94, 29.17, 28.95, 28.86, 28.83, 28.76, 28.74, 28.70, 28.64, 28.58, 28.45, 28.43, 25.29, 25.21, 21.76, 13.10.
Exact mass (ESI-MS) for C56H100Cl2N2O9 [M+Na]+ found, 1015.6865; calcd, 1015.6884; m.p. 155.0 °C – 157.0 °C.
(2S,3S,4R)-(N-benzyl-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6c)
1H NMR (300 MHz, pyridine-d5): δ 8.69 (dd, J = 5.9 Hz and 6.8 Hz, 1H, NH), 8.47 (d, J = 8.7 Hz, 1H, NH), 7.59 (d, J = 5.9 Hz, 2H, arom. H), 7.30-7.17 (m, 4H, arom. H and OH), 6.40 (bs, 1H, OH), 5.58 (d, J = 3.9 Hz, 1H, H- 1″), 5.03-5.00 (m, 2H, H-2 and OH), 4.98-4.95 (m, 4H, H-4″, OH and NH-CH2), 4.69 (dd, J = 3.8 Hz and 9.9 Hz, 1H, H-2″), 4.63-4.56 (m, 2H, H-3 and H-5″), 4.44 (dd, J = 3.1 Hz and 10.0 Hz, 1H, H- 3″), 4.34-4.27 (m, 3H, H-1 and H-4), 2.48-2.43 (m, 2H, COCH2), 1.93-1.09 (m, 72H, CH2), 0.89 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 172.12, 169.18, 138.92, 127.50, 126.55, 125.87, 123.04, 121.66, 100.15, 75.12, 72.52, 71.26, 70.08, 69.96, 68.61, 67.55, 49.94, 41.71, 35.57, 32.98, 30.96, 30.94, 29.20, 28.98, 28.87, 28.83, 28.76, 28.74, 28.70, 28.63, 28.58, 28.45, 28.43, 25.32, 25.22, 21.77, 13.11.
Exact mass (ESI-MS) for C57H104N2O9 [M+H]+ found, 961.7808; calcd, 961.7815; m.p. 128.0 °C – 130.0 °C.
(2S,3S,4R)-(N-(3,4-dichlorobenzyl)-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6d)
1H NMR (300 MHz, pyridine-d5): δ 8.96 (dd, J = 5.7 Hz and 7.1 Hz, 1H, NH), 8.48 (d, J = 8.6 Hz, 1H, NH), 7.77 (d, J = 1.9 Hz, 1H, arom. H), 7.41 (dd, J = 2.0 Hz and 8.3 Hz, 1H, arom. H), 7.32 (d, J = 8.2 Hz, 1H, arom. H), 6.75 (app. s, 1H, OH), 6.43 (d, J = 5.7 Hz, 1H, OH), 6.13 (d, J = 4.8 Hz, 1H, OH), 5.60 (d, J = 3.8 Hz, 1H, H-1″), 5.26 (app. d, J = 4.6 Hz, 1H, H-2), 5.01-4.92 (m, 3H, NH-CH2, H-4″ and H-5″), 4.69-4.56 (m, H2, H-2″ and H-1), 4.49-4.42 (m, 2H, H-3″ and NH-CH2), 4.33-4.29 (m, 3H, H-1, H-3 and H- 4), 2.46 (app. t, J = 7.5 Hz, 2H, COCH2), 1.97-1.16 (m, 72H, CH2), 0.89 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 173.43, 170.90, 141.50, 132.57, 130.84, 130.65, 130.10, 127.77, 101.72, 76.86, 74.10, 72.75, 71.68, 71.44, 70.08, 51.32, 42.16, 37.07, 34.66, 32.45, 32.46, 30.71, 30.48, 30.38, 30.34, 30.27, 30.25, 30.20, 30.14, 30.10, 29.96, 29.94, 26.80, 26.72, 23.28, 14.61.
Exact mass (ESI-MS) for C57H102Cl2N2O9 [M+H]+ found, 1029.7021; calcd, 1029.7041; m.p. 144.0 °C – 146.0 °C.
(2S,3S,4R)-(N-phenethyl-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6e)
1H NMR (300 MHz, pyridine-d5): δ 8.50 (d, J = 8.7 Hz, 1H, NH), 8.13 (t, J = 6.0 Hz, 1H, NH), 7.32-7.18 (m, 5H, arom. H), 7.03 (br. s, 1H, OH), 6.41 (br. s, 1H, OH), 6.13 (br. s, 1H, OH), 5.54 (d, J = 3.9 Hz, 1H, H-1″), 5.27 (app. d, J = 4.4 Hz, 1H, H-2), 4.99-4.91 (m, 2H, H-4″ and H-5″), 4.65 (dd, J = 3.8 Hz and 10.0 Hz, 1H, H-2″), 4.60 (dd, J = 5.4 Hz and 10.7 Hz, 1H, H-1), 4.41 (dd, J = 3.1 Hz and 10.0 Hz, 1H, H-3″), 4.39-4.31 (m, 1H, H-4), 4.30 (dd, J = 4.2 Hz and 10.7 Hz, 1H, H-1), 3.72 (dd, J = 6.5 Hz and 14.4 Hz, 2H, NH-CH2), 2.94 (app. t, J = 7.5 Hz, 2H, CH2), 2.48 (t, J = 7.1 Hz, 2H, COCH2), 1.97-1.18 (m, 72H, CH2), 0.89 (t, J = 6.6 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 173.42, 170.26, 140.50, 136.52, 129.67, 129.16, 126.87, 123.50, 101.65, 76.91, 73.90, 72.78, 71.53, 71.49, 70.11, 69.17, 51.29, 41.44, 37.08, 36.72, 34.66, 32.46, 32.45, 30.71, 30.48, 30.37, 30.34, 30.27, 30.25, 30.21, 30.15, 30.11, 29.96, 29.94, 26.81, 26.73, 23.27, 14.61.
Exact mass (ESI-MS) for C58H106N2O9 [M+H]+ found, 975.7974; calcd, 975.7977; m.p. 144.0 °C – 146.0 °C.
Procedure for debenzylation of 6f and 6g
To a solution of the protected amide (0.07 mmol) in CH2Cl2 (1 mL) was added aluminum chloride (109 mg, 0.82 mmol) and dimethylaniline (0.13 mL, 1.00 mmol). After stirring for 8 hours, the reaction mixture was quenched with a 1N solution of HCl (1.5 mL) followed by extraction with EtOAc. Next, the combined organic layers were washed with a saturated NaHCO3 solution and brine, dried over Na2SO4 and evaporated to dryness. The resulting residue was submitted to column chromatography (CH2Cl2/MeOH: 28/2), yielding amides 6f (10 mg, 15 %) and 6g (8.5 mg, 11 %).
(2S,3S,4R)-(N-(thiophen-3-ylmethyl)-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6f)
1H NMR (300 MHz, pyridine-d5): δ 8.86 (t, J = 6.2 Hz, 1H, NH), 8.45 (d, J = 8.5 Hz, 1H, NH), 7.25 (app. dd, J = 1.2 Hz and 5.2 Hz, 2H, arom. H), 7.11 (br. s, 1H, OH), 6.91 (dd, J = 3.6 Hz and 5.1 Hz, 1H, arom. H), 6.68 (br. s, 1H, OH), 6.39 (br. s, 1H OH), 6.12 (br. s, 1H, OH), 5.55 (d, J = 3.8 Hz, 1H, H-1″), 5.28-5.21 (m, 1H, H-2), 5.0-4.82 (m, 4H, NH-CH2, H-4″ and H-5″), 4.66 (dd, J = 3.8 Hz and 9.9 Hz, 1H, H-2″), 4.56 (dd, J = 5.4 Hz and 10.5 Hz, 1H, H-1), 4.41 (d, J = 3.2 Hz and 9.9 Hz, 1H, H-3″), 4.35-4.19 (m, 2H, H-3 and H-4), 4.25 (dd, J = 4.2 Hz and 10.6 Hz, 1H, H-1), 2.44 (app. t, J = 7.4 Hz, 2H, COCH2), 1.92-1.19 (m, 72H, CH2), 0.89 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 171.87, 168.91, 141.99, 125.87, 124.66, 123.74, 100.23, 75.37, 72.51, 71.25, 70.01, 69.97, 68.60, 67.72, 53.86, 49.76, 36.91, 35.55, 33.13, 30.95, 30.94, 29.20, 28.97, 28.87, 28.83, 28.75, 28.74, 28.69, 28.63, 28.58, 28.45, 28.43, 25.29, 25.20, 21.76, 13.10.
Exact mass (ESI-MS) for C55H102N2O9S [M+H]+ found, 967.7431; calcd, 967.7379; m.p. 126.0°C – 128.0°C.
(2S,3S,4R)-(N-(naphtalen-1-ylmethyl)-α-D-galactopyranuronamidyl)-2-(hexacosanamido)octadecane-3,4-diol (6g)
1H NMR (300 MHz, pyridine-d5): δ 8.49 (d, J = 8.7 Hz, 1H, NH), 8.29 (dd, J = 2.7 Hz and 6.7 Hz, 1H, NH), 7.94 (d, J = 7.2 Hz, 1H, arom. H), 7.87 (dd, J = 3.1 Hz and 6.3 Hz, 1H, arom. H), 7.77 (d, J = 8.3 Hz, 1H, arom. H), 7.59-7.38 (m, 4H, arom. H), 5.54 (d, J = 3.7 Hz, 1H, H- 1″), 5.44 (dd, J = 6.7 Hz and 15.6 Hz, 1H, NH-CH2), 5.29-5.21 (m, 1H, H-2), 5.13-4.95 (m, 3H, NH-CH2, H- 4″ and H-5″), 4.68 (dd, J = 3.7 Hz and 9.8 Hz, 1H, H- 2″), 4.60 (dd, J = 5.4 Hz and 10.6 Hz, 1H, H-1), 4.45 (dd, J = 3.1 Hz and 10.0 Hz, H-3″), 4.43-4.29 (m, 2H, H-3 and H-4), 4.28 (dd, J = 4.4 Hz and 10.7 Hz, 1H, H-1), 2.46 (app. t, J = 7.1 Hz, 2H, COCH2), 1.97-1.19 (m, 72H, CH2), 0.89 (t, J = 6.6 Hz, 6H, CH3).
13C NMR (75 MHz, pyridine-d5): δ 173.42, 170.60, 134.50, 132.12, 129.31, 128.21, 126.85, 126.35, 126.27, 125.92, 101.77, 76.84, 74.18, 72.79, 71.63, 71.50, 70.12, 69.29, 55.37, 51.34, 41.33, 37.07, 34.62, 32.47, 30.82, 30.71, 30.48, 30.38, 30.34, 30.27, 30.25, 30.21, 30.15, 30.11, 29.96, 29.94, 26.81, 26.73, 23.28, 14.62.
Exact mass (ESI-MS) for C61H106N2O9 [M+H]+ found, 1011.7974; calcd, 1011.7971; m.p. 121.0 °C – 123.0 °C.
Biological evaluation
Mice
C57BL/6 and mice were in house bred (in accordance with the general recommendations for animal breeding and housing) or purchased from the Harlan Laboratory,. Experiments were conducted according to the guidelines of the Ethical Committee of Laboratory Animals Welfare of Ghent University. Mice used for experiments were between 5 and 12 weeks old.
In vivo activation of iNKT cells and serum analysis
For in vivo activation of iNKT cells C57BL/6 mice were intraperitoneally injected with 5 μg glycolipid (dissolved in PBS with DMSO). Subsequently mice were bled at 4hours and 16 hours after injection. Serum was stored at −20 and analyzed for IL-4 and IFN-γ levels by ELISA.
Scheme 1. Reagents and conditions.
(a) i. RNCO, DMF, 67%–74% or ii. RNH2, CDI, DMF, 70 °C, 33%; (b) Pd black, H2, EtOH/CHCl3, 49%–86%; (c) TEMPO, BAIB, CH2Cl2/H2O, 87%; (d) RNH2, HCTU, DIPEA, DMF, 53%–87 %; (e) AlCl3, dimethylaniline, CH2Cl2, 11%–15%.
Acknowledgments
Joren Guillaume is a fellow of the Agency for Innovation by Science and Technology (IWT) of Flanders. S.V.C. and D.E. received support of the Belgian Stichting tegen Kanker and the FWO Flanders. S. A. was a fellow of FWO Flanders. D.M.Z. was funded by NIH grant RO1 AI074952.
Abbreviations
- iNKT cell
invariant natural killer T-cell
- MHC
major histocompatibility complex
- Th
T helper
- CDI
1,1′-carbonyldiimidazole
- TEMPO
(2,2,6,6-Tetramethylpiperidin-1-yl)oxy
- BAIB
bis(acetoxy)iodobenzene
- HCTU
2-(6-chloro-1-H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate
- INF
interferon
- IL
interleukin
- APC
antigen presenting cell
- BMDC
bone marrow dendritic cell
- TCR
T cell receptor
- NU-α-GalCer
1-O-(6-naphtureido-6-deoxy-α-D-galactopyranosyl)-2-hexacosylamino-D-ribo-1,3,4-octadecanetriol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Natori T, Morita M, Akimoto K, Koezuka Y. Tetrahedron. 1994;50:2771. [Google Scholar]
- 2.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J Med Chem. 1995;38:2176. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. Nat Rev Immunol. 2004;4:231. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Mo-toki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 5.Zeng ZH, Castaño AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Science. 1997;277:339. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 6.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. Nat Immunol. 2005;6:819. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 7.Zajonc DM, Cantu C, III, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Nat Immunol. 2005;6:810. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Proc Natl Acad Sci USA. 2008;105:11287. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. Clin Cancer Res. 2005;11:1910. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 10.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. Clin Cancer Res. 2002;8:3702. [PubMed] [Google Scholar]
- 11.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, Ho DD, Tsuji M. Proc Natl Acad Sci USA. 2010;107:13010. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmieg J, Yang G, Franck RW, Tsuji M. J Biomed Biotechnol. 2010;2010:283612. doi: 10.1155/2010/283612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchet-Cadeddu A, Hénon E, Dauchez M, Renault JH, Monneaux F, Haudrechy A. Org Biomol Chem. 2011;9:3080. doi: 10.1039/c0ob00975j. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 15.Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Arthritis Rheum. 2004;50:305. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, Taniguchi M, Bendelac A, Van Kaer L, Koezuka Y, Tsuji M. Proc Natl Acad Sci USA. 2000;97:8461. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tashiro T, Nakagawa R, Inoue S, Shiozaki M, Watarai H, Taniguchi M, Mori K. Tetrahedron Lett. 2008;49:6827. [Google Scholar]
- 18.Trappeniers M, Van Beneden K, Decruy T, Hillaert U, Linclau B, Elewaut D, Van Calenbergh S. J Am Chem Soc. 2008;130:16468. doi: 10.1021/ja8064182. [DOI] [PubMed] [Google Scholar]
- 19.Aspeslagh S, Li YL, Yu ED, Pauwels N, Trappeniers M, Girardi E, Decruy T, Van Beneden K, Venken K, Drennan M, Leybaert L, Wang J, Franck RW, Van Calenbergh S, Zajonc DM, Elewaut D. EMBO J. 2011;30:2294. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauwels N, Aspeslagh S, Vanhoenacker G, Sandra K, Yu ED, Zajonc DM, Elewaut D, Linclau B, Van Calenbergh S. Org Biomol Chem. 2011;9:8413. doi: 10.1039/c1ob06235b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauwels N, Aspeslagh S, Elewaut D, Van Calenbergh S. Bioorg Med Chem. 2012;20:7149. doi: 10.1016/j.bmc.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Aspeslagh S, Nemčovič M, Pauwels N, Venken K, Wang J, Van Calenbergh S, Zajonc DM, Elewaut D. J Immunol. 2013;191:2916. doi: 10.4049/jimmunol.1203134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birkholz A, Nemčovič M, Yu ED, Girardi E, Wang J, Khurana A, Pauwels N, Chitale S, Farber E, Franck RW, Tsuji M, Howell A, Van Calenbergh S, Kronenberg M, Zajonc DM. doi: 10.1074/jbc.M115.654814. submitted elsewhere. [DOI] [PMC free article] [PubMed] [Google Scholar]