Abstract
Background
Individuals with communication disorders, such as aphasia, exhibit weak auditory cortex responses to speech sounds and language impairments. Previous studies have demonstrated that pairing vagus nerve stimulation (VNS) with tones or tone trains can enhance both the spectral and temporal processing of sounds in auditory cortex, and can be used to reverse pathological primary auditory cortex (A1) plasticity in a rodent model of chronic tinnitus.
Objective/Hypothesis
We predicted that pairing VNS with speech sounds would strengthen the A1 response to the paired speech sounds.
Methods
The speech sounds ‘rad’ and ‘lad’ were paired with VNS three hundred times per day for twenty days. A1 responses to both paired and novel speech sounds were recorded twenty four hours after the last VNS pairing session in anesthetized rats. Response strength, latency and neurometric decoding were compared between VNS speech paired and control rats.
Results
Our results show that VNS paired with speech sounds strengthened the auditory cortex response to the paired sounds, but did not strengthen the amplitude of the response to novel speech sounds. Responses to the paired sounds were faster and less variable in VNS speech paired rats compared to control rats. Neural plasticity that was specific to the frequency, intensity, and temporal characteristics of the paired speech sounds resulted in enhanced neural detection.
Conclusion
VNS speech sound pairing provides a novel method to enhance speech sound processing in the central auditory system. Delivery of VNS during speech therapy could improve outcomes in individuals with receptive language deficits.
Keywords: Speech, Primary auditory cortex, Plasticity, Vagal nerve stimulation
Introduction
Neurons in auditory cortex are selective to the spectral and temporal features of environmental sounds [1]. The tuning properties of these neurons can be altered by a variety of conditions [2]. The repeated presentation of sounds paired with stimulation of neuromodulatory systems is one of the best studied methods of selectively altering the response properties of auditory cortex neurons [3–5].
Deep brain stimulation or cranial nerve stimulation paired with the presentation of a sound can enhance the primary auditory cortex (A1) response to the paired sound. For example, repeated pairing of a tone with stimulation of nucleus basalis or locus coeruleus results in A1 frequency map plasticity that is specific to the paired tone [4,6,7]. Pairing vagus nerve stimulation (VNS) with a tone also dramatically increases the percentage of A1 that responds to the paired tone [5]. Pairing stimulation of the nucleus basalis or the vagus nerve with either slow or fast trains of tones either decreases or increases the temporal following rate of A1 neurons [6,8]. Pairing nucleus basalis stimulation with a spectrotemporally complex acoustic stimulus results in plasticity that is specific to the spectrotemporal transitions in the paired sound [9–11]. It is unknown whether VNS pairing can alter the A1 response to similarly spectrotemporally complex sounds, such as human speech. If VNS pairing of complex sounds also results in plasticity specific to the paired sounds, VNS pairing could be used to generate potentially therapeutic neural plasticity [12].
Auditory system plasticity accelerates auditory learning and could benefit patients with speech and hearing disorders [13–15]. Many studies have demonstrated that language impaired individuals have weak auditory cortex responses to sound that can be strengthened following extensive rehabilitation therapy [16–19]. Vagus nerve stimulation is a safe, well-tolerated procedure that is frequently used to treat patients with epilepsy or depression [20–22]. Pairing VNS with rehabilitation improves recovery from stroke in animal models [23,24]. Pairing VNS with tones has recently been shown to improve tinnitus symptoms in patients and animal models with chronic tinnitus [5,15]. It is possible that this VNS pairing therapy could also be used to treat other auditory processing disorders. We hypothesized that pairing VNS with speech sounds would enhance A1 responses to the paired speech sounds.
Materials and methods
Speech sounds
The paired speech sounds were the words ‘rad’ and ‘lad’ spoken by a female native English speaker, as used in our previous studies [25,26]. The sounds ‘rad’ and ‘lad’ were chosen because they are known to weakly activate A1 neurons [25], and are known to be perceptually difficult sounds to learn [27]. These characteristics make our results more relevant to conditions, such as dyslexia and autism, which exhibit weak responses to speech sounds that generate strong responses in typically developing individuals.
All sounds were presented so that the loudest 100 ms of the vowel was 60 dB SPL, and the onset of the initial consonant was approximately 40 dB SPL. The sounds were spectrally shifted up by one octave using the STRAIGHT vocoder to better match the rat hearing range [28].
Vagus nerve surgery
Sprague Dawley rats were implanted with a custom made platinum iridium bipolar cuff electrode around the left cervical vagus nerve, as in our previous studies [5,8,23]. Rats were anesthetized with pentobarbital (50 mg/kg), and received supplemental doses of dilute pentobarbital (8 mg/mL) as needed. Body temperature was maintained at 37°C using a heating pad, and rats received subcutaneous injections of dextrose and Ringer’s lactate for hydration, cefotaxime sodium to prevent infection, and atropine and dexamethasone to decrease bronchial secretions. Leads from the vagus nerve cuff electrode were tunneled subcutaneously to a headcap attached to the skull. Based on previous studies showing no difference between naïve rats and rats that either had implants which were not activated or rats that received VNS which was not paired with any particular event [5,8,24,29], the control rats in the current study did not undergo sham surgery. The University of Texas at Dallas Institutional Animal Care and Use Committee approved all protocols and recording procedures.
Vagus nerve stimulation
The words ‘rad’ and ‘lad’ were paired with vagus nerve stimulation 300 times per day for 20 days. The onset of vagus nerve stimulation was 50 ms before the onset of the speech sound. In previous studies, plasticity was indistinguishable when stimulation was 200 ms before sound onset through 50 ms after sound onset [4,5]. The stimulation burst was a brief 500 ms long pulse train (30 Hz) with a 100 μs biphasic pulse width at an intensity of 0.8 mA, as in our previous studies [5,8,23]. The amount of VNS used in this study is less than 1% of the FDA approved VNS protocol for epilepsy and depression. The speech sounds were delivered to the unrestrained rats free-field via a speaker (Optimus Bullet Horn Tweeter) located 20 cm above a 25 × 25 × 25 cm3 wire cage. Presentation of the speech sounds was randomly interleaved throughout each VNS speech pairing session, and there was no significant difference between the number of times per session that each rat heard ‘rad’ (147 ± 6) compared to ‘lad’ (144 ± 10, p = 0.75). The timing of each VNS-speech pairing trial was also randomized so that the rats could not predict when VNS-speech pairing would occur, with an average of 30 seconds between VNS-speech pairing trials. The control rats in this study did not undergo unpaired stimulation. Our previous studies and those of other labs have shown that sound presentation alone (without VNS stimulation) or VNS stimulation alone (without sound presentation) does not substantially alter A1 responses [5,7,8,30–32].
Physiology
Primary auditory cortex (A1) recordings were obtained from each rat 24 hours after the last VNS pairing session, as in our previous studies [5,8,26]. Auditory cortex responses were recorded from 263 A1 sites in 4 VNS speech paired rats and 536 A1 sites in 11 control rats. Similar to the vagus nerve surgery, rats were anesthetized with pentobarbital, and supplemental doses of dilute pentobarbital were provided throughout the experiment. Humidified air was delivered through a tracheotomy in order to facilitate breathing. To prevent brain swelling, a cisternal drain was performed. Right primary auditory cortex was exposed following a craniotomy and durotomy. Four Parylene-coated tungsten microelectrodes (1–2 MΩ, FHC) were used to record A1 responses, and were placed to evenly sample A1 while avoiding blood vessels. 1,296 tones were presented at each frequency and intensity combination between 1 – 32 kHz in 0.125 octave steps and 0 – 75 dB SPL in 5 dB steps. The paired speech sounds ‘rad’ and ‘lad’ and the novel speech sounds ‘dad’ and ‘sad’ were randomly interleaved twenty times each at every recording site. The novel sounds ‘dad’ and ‘sad’ were spoken by the same female native English speaker and were presented to determine whether plasticity was specific to the paired sounds. Sounds were presented using a speaker located 10 cm from the left ear of the rat.
Data analysis
For all analyses, A1 responses in VNS speech paired rats were compared with A1 responses in control rats. A1 recording sites were defined based on latency, tonotopy, and relative location [33,34]. The onset response strength to speech sounds was the number of evoked spikes fired during the first 40 ms of the response. Previously published research has demonstrated that both humans and animals can reliably discriminate between consonant sounds using only the first tens of milliseconds of the sound [25,35–37]. The response strength to the vowel was quantified as the number of spikes evoked during the 300 ms immediately following vowel onset [38]. The peak latency to speech sounds was the latency (in ms) with the maximum firing rate, while the onset latency variance was the square of the standard deviation of the onset latency response to the paired sounds across driven A1 recording sites (in ms2). Neural detection accuracy was calculated using a nearest-neighbor classifier, where 50% correct is chance performance and 100% correct is perfect neural detection [25,34]. Euclidean distance was used to compare the 40 ms onset response (40 1-ms bins) evoked by each of the speech sounds (‘dad’, ‘lad’, ‘rad’, and ‘sad’) with spontaneous firing recorded when no sound was presented. At each A1 recording site, an average sound template post stimulus time histogram (PSTH) was created from 19 of the 20 repeats recorded with and without sound presentation. The PSTH templates were compared to the remaining repeat using Euclidean distance, and each single trial response was assigned to the most similar responding PSTH template with the smallest Euclidean distance. Significance was determined using two-sample t-tests, using a Bonferroni correction for multiple comparisons.
Threshold was the lowest intensity (in dB SPL) that evoked a response at the characteristic frequency for each recording site. Bandwidth was measured 40 dB above each site’s threshold as the frequency range (in octaves) that evoked a response. Driven rate was the average response (in spikes/tone) to all of the tones in each site’s receptive field. The percent of A1 neurons responding and the number of spikes evoked per tone was calculated for each tone frequency at each tone intensity [5,26]. For Figures 6 and 7, a Benjamini-Hochberg correction was used to control the false discovery rate [39].
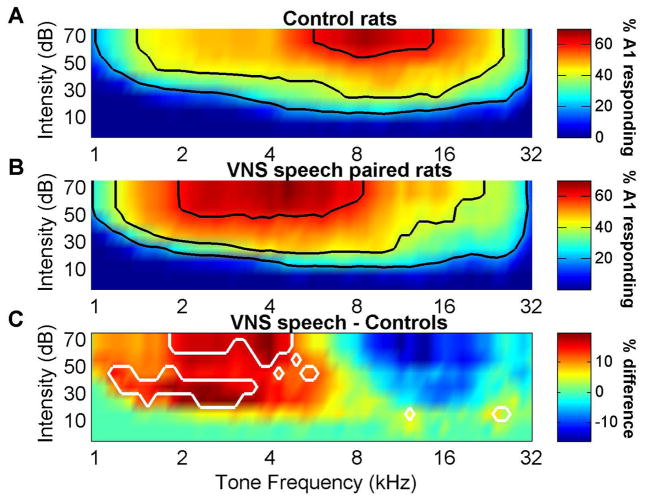
Figure 6.
The percentage of primary auditory cortex responding to low frequency tones increased in VNS speech paired rats. (a) The percent of primary auditory cortex neurons that respond to a tone of any frequency and intensity combination in control rats. Black contour lines indicate 20, 40, and 60% of primary auditory cortex responding. (b) The percent of primary auditory cortex neurons that respond to tones in VNS speech paired rats. (c) The difference in the percent of primary auditory cortex neurons that respond to tones between VNS speech paired rats and control rats. White contour lines surround the regions of tones that were significantly different compared to control rats (false discovery rate was used to correct for multiple comparisons).
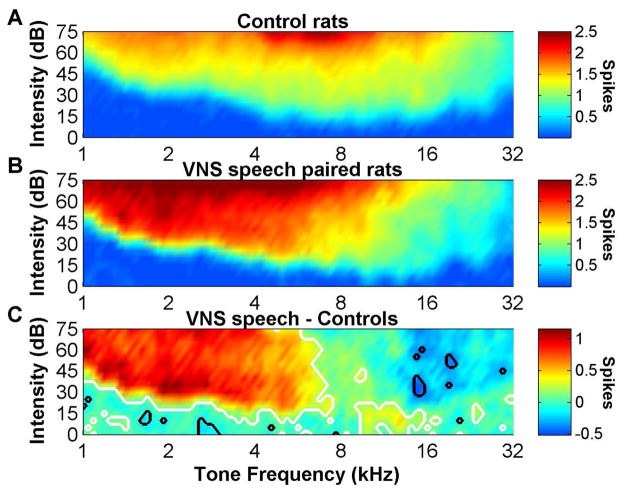
Figure 7.
The number of spikes evoked per tone in VNS speech paired rats increased for low frequency tones and decreased for high frequency tones. (a) The number of spikes evoked in response to any frequency and intensity combination of tones in control rats. (b) The number of spikes evoked in response to any frequency and intensity combination of tones in VNS speech paired rats. (c) The difference in the number of spikes evoked between VNS speech paired rats and control rats. White contour lines surround the tone regions that were significantly increased (false discovery rate was used to correct for multiple comparisons) compared to control rats, while black contour lines surround the tone regions that were significantly decreased compared to control rats.
Results
VNS pairing enhanced A1 responses to the paired speech sounds
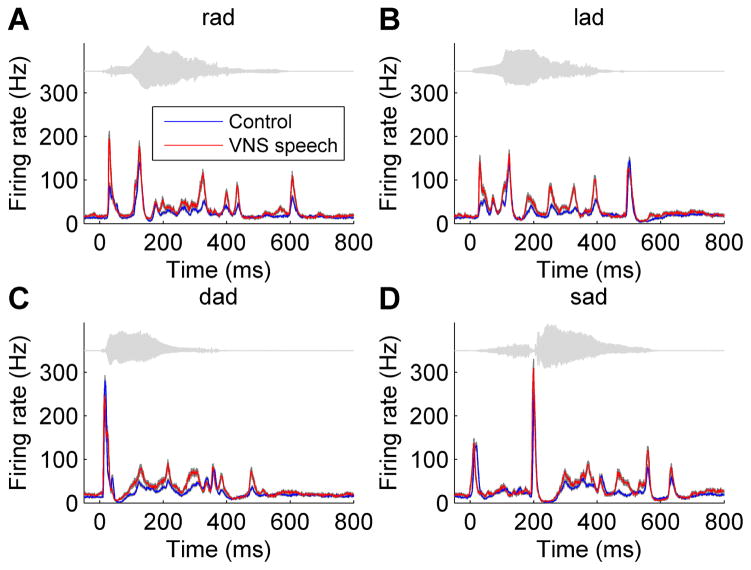
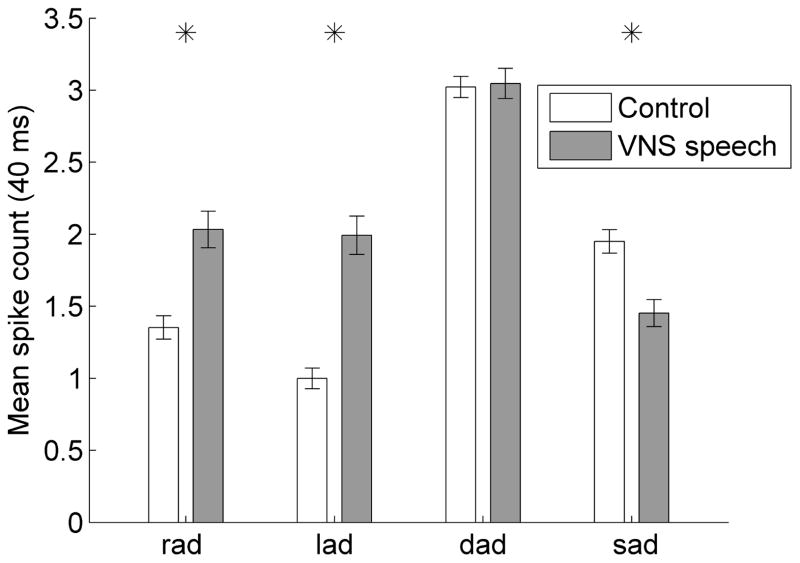
VNS paired with the speech sounds ‘rad’ and ‘lad’ significantly enhanced the A1 response strength to the paired sounds (Figure 1). Following 20 days of VNS-speech pairing, rats had a 50% stronger onset response to ‘rad’ and a 99% stronger onset response to ‘lad’ compared to control rats (p < 0.0001, average number of spikes fired in the first 40 ms of the neural response, Figure 2). Interestingly, this response strength enhancement did not generalize to novel speech sounds. For example, the onset response strength to the novel sound ‘dad’ did not significantly change in VNS speech rats, while the onset response strength to the novel sound ‘sad’ was actually 26% weaker in VNS speech rats compared to control rats (p = 0.0002, average number of spikes fired in the first 40 ms of the neural response, Figure 2). This pattern of response strength enhancement for the paired sounds but not the novel sounds was observed across a wide range of analysis durations for the consonant response (120 ms for ‘rad’, 110 ms for ‘lad’, 30 ms for ‘dad’, and 210 ms for ‘sad’; each in 10 ms increments). The vowel /ae/ was common across the four speech sounds, and the response strength to the vowel was stronger for both paired and novel speech sounds. The response strength to the vowel in ‘rad’ increased from 4.0 ± 0.2 (mean ± SEM) spikes in control rats to 6.9 ± 0.4 spikes in VNS speech paired rats (300 ms vowel response, p < 0.0001), the vowel response to ‘lad’ increased from 4.0 ± 0.2 spikes in controls to 6.3 ± 0.5 spikes in VNS speech paired rats (p < 0.0001), the vowel response to ‘dad’ increased from 4.6 ± 0.2 spikes to 7.2 ± 0.5 spikes in VNS speech paired rats (p < 0.0001), and the vowel response to ‘sad’ increased from 4.6 ± 0.2 spikes to 6.0 ± 0.4 spikes in VNS speech paired rats (p = 0.0002, Figure 1). This stronger response strength to the vowel in VNS speech paired rats was observed across a wide range of analysis durations for the vowel response (200 – 500 ms in 100 ms increments, p < 0.05).
Figure 1.
VNS speech pairing strengthened the response strength to the paired speech sounds. (a) The mean number of spikes evoked across recording sites in response to the paired speech sound ‘rad’ was significantly stronger in VNS speech paired rats compared to control rats. Gray shading behind each group indicates SEM across recording sites. The waveform for the speech sound ‘rad’ is plotted in gray above the response. (b) The number of spikes evoked in response to the paired speech sound ‘lad’ was significantly stronger in VNS speech paired rats compared to control rats. (c) While the number of spikes evoked in the 40 ms onset response to the novel speech sound ‘dad’ was not significantly different between VNS speech paired and control rats, the VNS speech paired rats exhibited a stronger response to the vowel portion of the sound. (d) While the number of spikes evoked in the 40 ms onset response to the novel speech sound ‘sad’ was significantly weaker in VNS speech paired rats compared to control rats, the VNS speech paired rats exhibited a stronger response to the vowel portion of the sound.
Figure 2.
The mean spike count in response to the VNS paired speech sounds ‘rad’ and ‘lad’ was significantly increased in VNS speech paired rats compared to control rats. The driven number of spikes was calculated for each speech sound using the 40 ms onset response to each sound. Error bars indicate SEM across recording sites; asterisks indicate speech sounds with response strengths that were significantly different between VNS speech paired and control rats (p < 0.05).
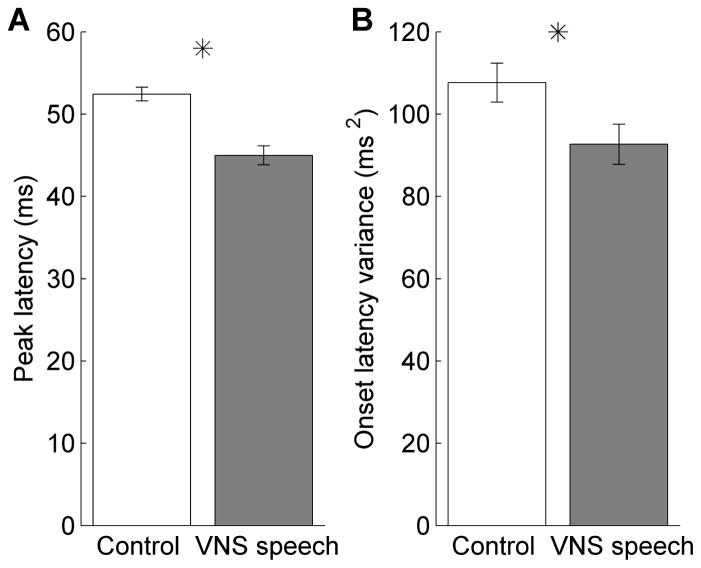
In addition to stronger A1 responses to the paired speech sounds, the A1 responses to the paired sounds were also faster. The peak firing latency to the paired sounds was significantly faster in VNS speech rats compared to control rats (44.9 ± 1.2 ms vs. 52.4 ± 0.8 ms, p < 0.0001, Figure 3a). A1 neurons also fired more reliably to the paired sounds in VNS speech rats compared to control rats (latency variance of 92.7 ± 4.9 ms2 vs. 107.7 ± 4.7 ms2, p = 0.03, Figure 3b). The peak firing latency to the novel sounds was unaltered in VNS speech rats compared to control rats (23.5 ± 1.1 ms vs. 23.7 ± 0.6 ms, p = 0.84). In contrast to the paired sounds, A1 neurons fired less reliably to novel sounds in VNS speech rats compared to control rats (latency variance of 36.4 ± 2.8 ms2 vs. 27.6 ± 1.7 ms2, p = 0.004).
Figure 3.
A1 responses to the paired speech sounds were significantly faster in VNS speech paired rats. (a) The peak latency was significantly shorter in VNS speech paired rats compared to control rats. Error bars indicate SEM across recording sites; asterisks indicate a statistically significant difference between VNS speech paired and control rats (p < 0.05). (b) The trial-by- trial variability in onset latency was significantly decreased in VNS speech paired rats compared to control rats.
VNS pairing alters A1 receptive fields
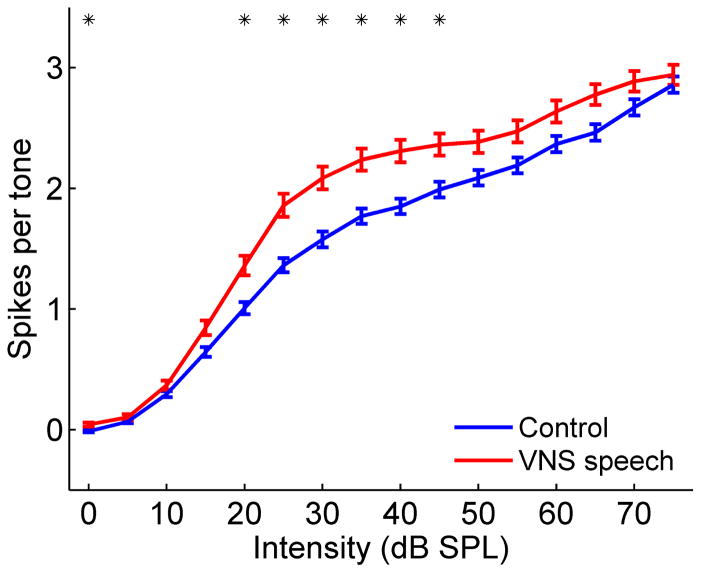
VNS speech pairing significantly altered primary auditory cortex responses to tones. A1 neurons were able to respond to tones that were 3.3 dB quieter in VNS speech paired rats compared to control rats (p < 0.0001, Table 1). These paired neurons were able to respond to frequencies spanning an additional 0.2 octaves compared to control neurons (p = 0.009, Table 1). VNS speech paired responses to tones were 1.1 ms faster (p = 0.01) and 0.4 spikes per tone stronger (p = 0.001) compared to responses in control rats (Table 1). A1 responses to tones were significantly stronger (p < 0.0031, Figure 4) in VNS speech paired rats compared to control rats at tone intensities ranging from 20 – 45 dB SPL, which matches the intensity of the initial consonants in the paired sounds ‘rad’ and ‘lad’ (Figure 5). A1 responses to tones were both stronger and faster following VNS speech pairing, and VNS speech paired neurons responded to quieter tones and a wider range of frequencies compared to control neurons.
Table 1.
VNS speech pairing induced receptive field plasticity.
| Control | VNS speech | p value | |
|---|---|---|---|
| Threshold (dB) | 18.61 | 15.27 | p < 0.0001 |
| Bandwidth 40 (octaves) | 2.54 | 2.72 | p = 0.009 |
| Peak latency (ms) | 19.95 | 18.88 | p = 0.01 |
| Driven rate (spikes/tone) | 3.07 | 3.47 | p = 0.001 |
Figure 4.
The response strength to tones was significantly stronger in VNS speech paired rats. VNS speech paired rats evoked more spikes per tone at intensities between 20 – 45 dB SPL compared to control rats. Responses are the average spikes evoked per tone for tones within 1 octave of each A1 recording site’s characteristic frequency. Asterisks indicate intensities that evoke a stronger response in VNS speech paired rats compared to control rats (p < 0.0031, Bonferroni correction). Error bars indicate SEM across recording sites.
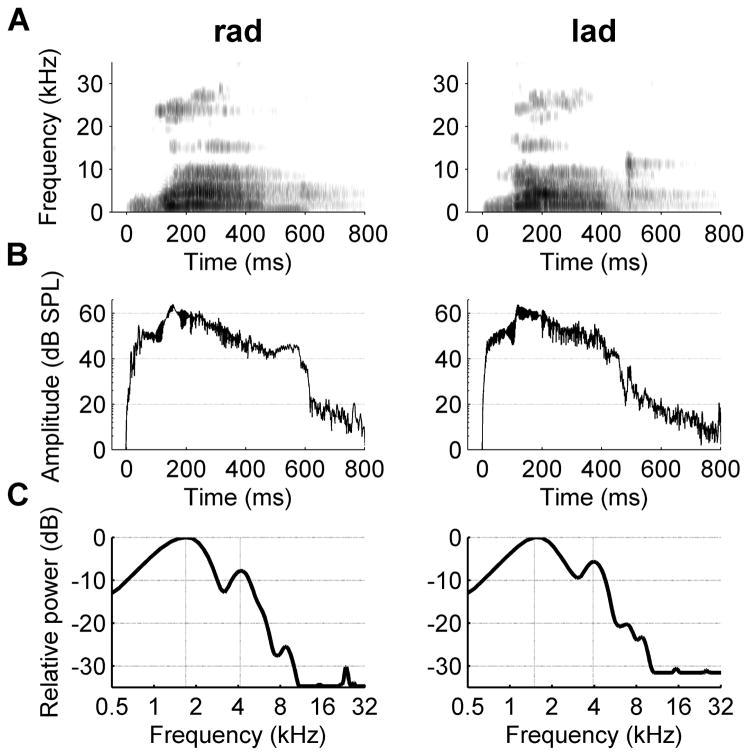
Figure 5.
Spectrograms, amplitude envelopes, and power spectrums for the paired speech sounds ‘rad’ and ‘lad’. (a) The spectrogram for the paired sounds ‘rad’ and ‘lad’. Time is represented on the x axis (−50 to 800 ms), and frequency is represented on the y axis (0 to 35 kHz). The intensity of the sound is plotted so that white is 70 dB SPL quieter than black. The amplitude envelope (b) and power spectrum (c) for ‘rad’ and ‘lad’.
Previous studies have demonstrated that vagus nerve or nucleus basalis stimulation paired with a tone increases the percent of A1 that responds to the paired tone [4,5]. Since the paired speech sounds ‘rad’ and ‘lad’ are low frequency biased sounds (Figure 5), it is possible that the stronger response strength to the paired speech sounds is simply due to an expansion of the percentage of A1 that responds to low frequency sounds. We quantified the percent of cortex responding to tones with all frequency and intensity combinations to determine if VNS speech pairing results in A1 frequency map plasticity. At 60 dB SPL, approximately 16% more A1 neurons responded to frequencies between 1.9 – 4.9 kHz in VNS speech paired rats compared to control rats (p < 0.05, Figure 6).
The number of spikes evoked per tone was then quantified to determine whether VNS speech paired rats have both more neurons that respond to low frequency sounds, as well as stronger responses to low frequency sounds. At 60 dB SPL, A1 neurons responded on average 50% stronger to low frequency tones below 6 kHz in VNS speech paired rats compared to control rats (p < 0.05, Figure 7).
The low frequency map expansion does not fully account for the enhanced speech responses
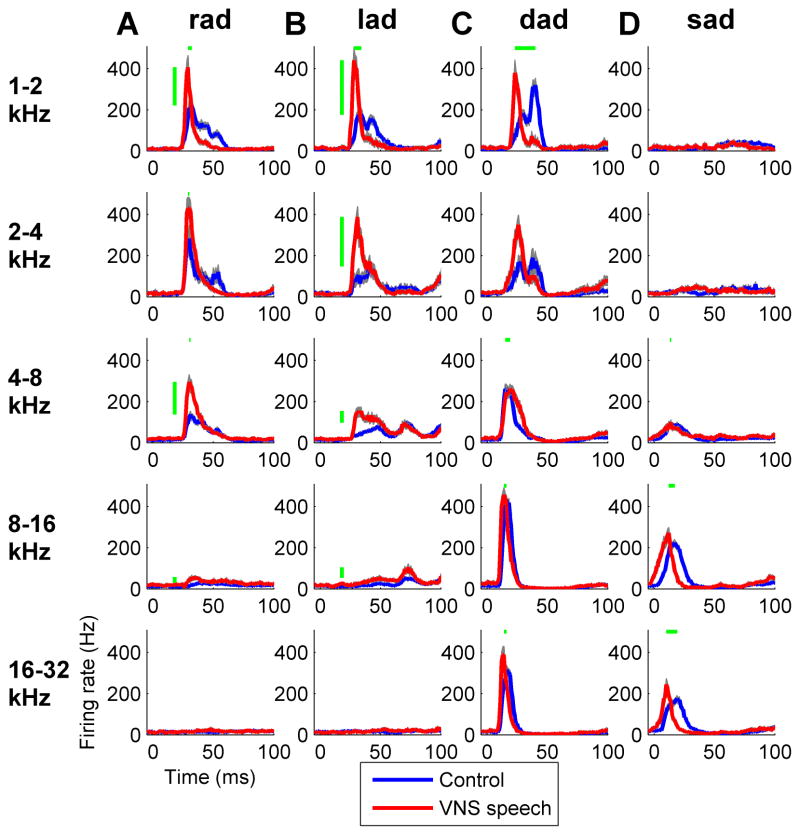
The enhanced A1 response strength to the paired speech sounds was not fully explained by the increased low frequency map representation. Low frequency neurons responded with a higher peak firing rate to the paired speech sounds ‘rad’ and ‘lad’ in VNS speech paired rats compared to control rats (p < 0.01, Figure 8a,b). Even A1 sites tuned to tone frequencies above 6 kHz exhibited a stronger peak firing rate to the paired speech sounds in VNS speech paired rats compared to control rats (p < 0.01, Figure 8a,b). In contrast, the peak firing amplitude to the novel speech sounds ‘dad’ and ‘sad’ was not significantly altered in VNS speech paired rats compared to control rats (p > 0.05, Figure 8c,d). The latency to peak response was decreased for both paired and novel speech sounds in VNS speech paired rats compared to control rats (p < 0.01, Figure 8).
Figure 8.
The onset response to each of the speech sounds across characteristic frequency. The paired sounds ‘rad’ (a) and ‘lad’ (b) evoked a strong response in low frequency tuned neurons. The novel sound ‘dad’ (c) evoked a response across all frequency ranges, while the novel sound ‘sad’ (d) evoked a response in high frequency tuned neurons. Vertical green lines indicate a significantly stronger peak firing rate across recording sites in VNS speech paired rats compared to control rats (Bonferroni correction for multiple comparisons, p < 0.01), while horizontal green lines indicate a significantly altered peak firing latency in VNS speech paired rats compared to control rats (Bonferroni correction for multiple comparisons, p < 0.01). Gray shading behind each line indicates SEM across recording sites.
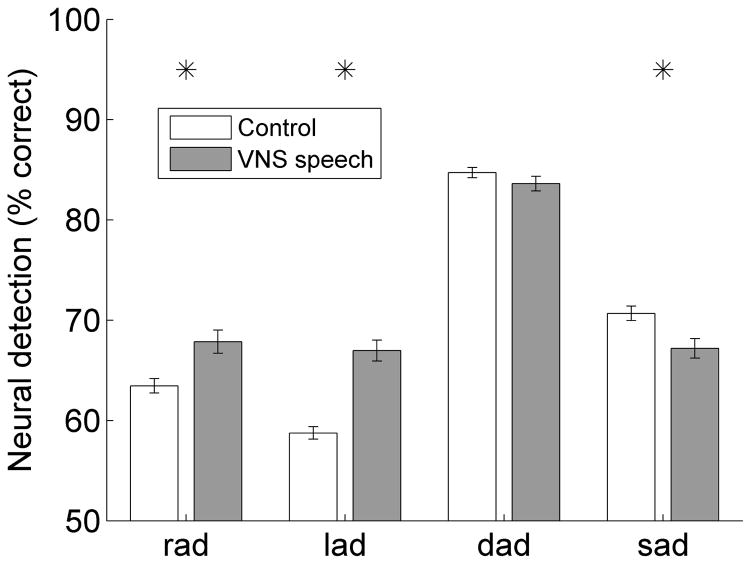
The stronger response strength, faster latency, and decreased latency variance of the evoked responses to the paired speech sounds improved the ability of a neural classifier to detect the onset of the paired sounds. Neural detection of the two paired sounds was significantly more accurate in VNS speech paired rats compared to control rats across all A1 recording sites (p < 0.001, Figure 9). Neural detection of the novel speech sound ‘dad’ was not altered (p = 0.22), while neural detection of the novel speech sound ‘sad’ was 4% less accurate in VNS speech paired rats compared to control rats (p = 0.005, Figure 9). This finding suggests that VNS speech pairing can increase the neural detection accuracy of the paired sounds by making A1 responses stronger, faster, and more reliable.
Figure 9.
Neural detection accuracy for the paired speech sounds ‘rad’ and ‘lad’ and the novel speech sounds ‘dad’ and ‘sad’. Neural detection performance significantly increases for the paired speech sounds ‘rad’ and ‘lad’, does not change for the novel speech sound ‘dad’, and significantly decreases for the novel speech sound ‘sad’. Error bars indicate SEM across recording sites; asterisks indicate a statistically significant difference between VNS speech paired and control rats (p < 0.05).
Discussion
Many studies document auditory cortex plasticity specific to the acoustic characteristics of the presented sounds. In this study, we extend these findings by showing that VNS paired with speech sounds enhanced the A1 response to the paired speech sounds. The A1 response evoked by the paired sounds ‘rad’ and ‘lad’ was stronger, faster and less variable following 20 days of VNS speech pairing. In contrast, the amplitude of the response evoked by novel speech sounds was not strengthened. A1 receptive fields were altered, and this plasticity was specific to the frequency and intensity characteristics of the paired sounds. A neural classifier was significantly more accurate at detecting the paired speech sounds, while neural detection accuracy was not enhanced for novel speech sounds.
Receptive field plasticity
The speech sounds used in this study were low frequency sounds with most of their energy below 4 kHz. Following VNS speech pairing, the A1 representation of low frequency sounds was expanded and these low frequency tuned neurons were also stronger. This finding is consistent with previous studies that paired tones with stimulation of the nucleus basalis or vagus nerve [3,5]. Extensive speech sound discrimination training also results in an expansion and strengthening of the low frequency A1 response [26].
In this study, the speech sounds were presented so that the loudest portion of the vowel was 60 dB SPL, while the initial consonants were between 20 and 45 dB SPL. Following VNS speech pairing, the A1 response to these middle intensities was stronger, which matches the intensity of the paired consonants. This finding matches previous findings showing intensity specific plasticity following tone intensity training [40].
Previous studies have documented that pairing a tone with nucleus basalis stimulation can increase receptive field size and decrease latency [4,41]. Each of the receptive field changes observed in this study are consistent with previously documented A1 changes following tone pairing or tone training.
Speech sound plasticity
This study as well as many previous studies have documented A1 plasticity that is specific to the acoustic characteristics of the paired sounds [9–11]. The stronger response strength evoked by the paired speech sounds in this study was not restricted to the low frequency map expansion. High frequency neurons responded stronger to both of the paired speech sounds after VNS speech pairing. These neurons did not respond more strongly to the novel speech sounds, but did decrease their latency to peak amplitude for the novel speech sounds. The high frequency tuned neurons had increased receptive field sizes (Table 1) so that they were able to respond to lower frequency sounds, such as the paired sounds ‘rad’ and ‘lad’, following VNS speech pairing. Neural detection ability of the paired speech sounds increased, while the neural detection ability of novel speech sounds was not enhanced.
Rehabilitation training / Intervention therapy
Many patient populations, such as individuals with aphasia (1 million people in the US [NINDS]), deaf individuals (500,000 people in the US [42]), and individuals with autism spectrum disorders (1 in 68 children in the US [43]), suffer from language deficits due to impaired cortical responses to sounds [44–46]. These individuals require extensive interventions in order to improve speech perception and cortical responses to sounds [16–19,47]. For example, auditory cortex responses are slow and weak in deaf individuals [48,49]. Cochlear implantation and speech therapy improve both cortical responses and speech perception outcomes; however, this process can take many months [50,51]. Pharmacologically enhanced therapy improves both speech outcomes and auditory cortex responses [52]. Individuals with autism spectrum disorders, particularly those with fragile X syndrome or Rett syndrome, have severe language deficits and impaired cortical responses to sound [53,54]. Many of these individuals also have epilepsy, and may already have a VNS implant to control their seizures [55,56]. Pairing speech therapy with VNS could potentially be used to enhance auditory cortex responses and speech perception outcomes in individuals with receptive language deficits.
Because of the precise timing and short stimulation duration, the VNS method reported here is only 1% of the FDA approved protocols for VNS stimulation therapy in epilepsy and depression. Although translation from animal models to clinical effectiveness is notoriously difficult, pilot studies suggest that VNS pairing therapy may translate from animals to humans. Pairing VNS with tones has recently been shown to improve tinnitus symptoms in both animals with tinnitus [5] and tinnitus patients [15], with additional clinical trials now underway (clinicaltrials.gov # NCT01962558). Pairing VNS with rehabilitation therapy has improved upper limb function in stroked animals [23,24,57], and studies are ongoing to evaluate the effectiveness of VNS paired with rehabilitation in stroke patients (clinicaltrials.gov identifier NCT01669161 & NCT02243020).
Conclusions
In this study, VNS speech sound pairing resulted in A1 responses to the paired speech sounds were stronger, faster, and less variable, while the amplitude of the response to novel speech sounds was not strengthened. Future clinical studies would be needed to determine if delivering VNS during speech therapy can enhance speech perception in patients with speech processing disorders.
VNS paired with speech sounds enhanced the A1 response to the paired speech sounds
A1 responses to the paired sounds were stronger, faster, and less variable
The response evoked by novel speech sounds was not strengthened
Neural plasticity was specific to the acoustic characteristics of the paired sounds
Acknowledgments
We would like to thank Michael Borland, Amanda Clark, Melyssa Fink, Kevin Huang, Juan Omana, Hinah Rasul, Sindhu Sudanagunta, Deepthi Vuppala, and Cary Walker for running the VNS pairing sessions, Sven Vanneste and Robert Rennaker for helpful discussions, and Pritesh Pandya for assistance with auditory cortex recordings. This research was supported by grants from the National Institutes of Health to MPK (Grant # R01DC010433, R43NS084566, R44DC010084). NDE is an employee of MicroTransponder Inc. and MPK is a paid consultant for MicroTransponder Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kilgard MP, Merzenich MM. Distributed representation of spectral and temporal information in rat primary auditory cortex. Hear Res. 1999;134:16–28. doi: 10.1016/s0378-5955(99)00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–24. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilgard MP, Merzenich MM. Cortical Map Reorganization Enabled by Nucleus Basalis Activity. Science (80-) 1998;279:1714–8. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 5.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–4. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1:727–31. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edeline J-M, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res. 2011;274:75–84. doi: 10.1016/j.heares.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol. 2012;233:342–9. doi: 10.1016/j.expneurol.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci U S A. 2002;99:3205–9. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moucha R, Pandya PK, Engineer ND, Rathbun DL, Kilgard MP. Background sounds contribute to spectrotemporal plasticity in primary auditory cortex. Exp Brain Res. 2005;162:417–27. doi: 10.1007/s00221-004-2098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandya PK. Speech sound representation and experience-dependent plasticity in the rat auditory system. The University of Texas; Dallas: 2005. [Google Scholar]
- 12.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–99. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed AC, Riley J, Carraway RS, Carrasco A, Perez CA, Jakkamsetti V, et al. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–31. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ridder D, Vanneste S, Engineer ND, Kilgard MP. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation. 2014;17:170–9. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- 16.Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150–9. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Q-J, Galvin JJ. Maximizing cochlear implant patients’ performance with advanced speech training procedures. Hear Res. 2008;242:198–208. doi: 10.1016/j.heares.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vollmer M, Beitel RE. Behavioral training restores temporal processing in auditory cortex of long-deaf cats. J Neurophysiol. 2011;106:2423–36. doi: 10.1152/jn.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115:1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 21.Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–28. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 22.Clark KB, Naritoku DK, Smith DC, Browning Ra, Jensen Ra. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 23.Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60:80–8. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, et al. Cortical activity patterns predict speech discrimination ability. Nat Neurosci. 2008;11:603–8. doi: 10.1038/nn.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engineer CT, Perez CA, Carraway RS, Chang KQ, Roland JL, Kilgard MP. Speech training alters tone frequency tuning in rat primary auditory cortex. Behav Brain Res. 2014;258:166–78. doi: 10.1016/j.bbr.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logan JS, Lively SE, Pisoni DB. Training Japanese listeners to identify English /r/ and /l/: a first report. J Acoust Soc Am. 1991;89:874. doi: 10.1121/1.1894649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawahara H. Speech representation and transformation using adaptive interpolation of weighted spectrum: Vocoder revisited. Proc ICASSP. 1997;2:1303–6. [Google Scholar]
- 29.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex. 2012;22:2365–74. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 30.Ranasinghe KG, Carraway RS, Borland MS, Moreno NA, Hanacik EA, Miller RS, et al. Speech discrimination after early exposure to pulsed-noise or speech. Hear Res. 2012;289:1–12. doi: 10.1016/j.heares.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J, Zhang Y. Sound-guided shaping of the receptive field in the mouse auditory cortex by basal forebrain activation. Eur J Neurosci. 2005;21:563–76. doi: 10.1111/j.1460-9568.2005.03878.x. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. J Neurophysiol. 2003;89:90–103. doi: 10.1152/jn.00968.2001. [DOI] [PubMed] [Google Scholar]
- 33.Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–38. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- 34.Centanni TM, Engineer CT, Kilgard MP. Cortical speech-evoked response patterns in multiple auditory fields are correlated with behavioral discrimination ability. J Neurophysiol. 2013;110:177–89. doi: 10.1152/jn.00092.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumstein SE, Stevens KN. Acoustic invariance in speech production: evidence from measurements of the spectral characteristics of stop consonants. J Acoust Soc Am. 1979;66:1001–17. doi: 10.1121/1.383319. [DOI] [PubMed] [Google Scholar]
- 36.Bertoncini J, Bijeljac-Babic R, Blumstein SE, Mehler J. Discrimination in neonates of very short CVs. J Acoust Soc Am. 1987;82:31–7. doi: 10.1121/1.395570. [DOI] [PubMed] [Google Scholar]
- 37.Porter BA, Rosenthal TR, Ranasinghe KG, Kilgard MP. Discrimination of brief speech sounds is impaired in rats with auditory cortex lesions. Behav Brain Res. 2011;219:68–74. doi: 10.1016/j.bbr.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez CA, Engineer CT, Jakkamsetti V, Carraway RS, Perry MS, Kilgard MP. Different timescales for the neural coding of consonant and vowel sounds. Cereb Cortex. 2013;23:670–83. doi: 10.1093/cercor/bhs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B …. 1995;57:289–300. [Google Scholar]
- 40.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–82. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001;86:326–38. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- 42.Blanchfield BB, Feldman JJ, Dunbar JL, Gardner EN. The severely to profoundly hearing-impaired population in the United States: prevalence estimates and demographics. J Am Acad Audiol. 2001;12:183–9. [PubMed] [Google Scholar]
- 43.Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 44.Nittrouer S, Sansom E, Low K, Rice C, Caldwell-Tarr A. Language Structures Used by Kindergartners With Cochlear Implants: Relationship to Phonological Awareness, Lexical Knowledge and Hearing Loss. Ear Hear. 2014:1–13. doi: 10.1097/AUD.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009;12:686–91. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- 46.Lai G, Schneider HD, Schwarzenberger JC, Hirsch J. Speech stimulation during functional MR imaging as a potential indicator of autism. Radiology. 2011;260:521–30. doi: 10.1148/radiol.11101576. [DOI] [PubMed] [Google Scholar]
- 47.Callan DE, Tajima K, Callan AM, Kubo R, Masaki S, Akahane-Yamada R. Learning-induced neural plasticity associated with improved identification performance after training of a difficult second-language phonetic contrast. Neuroimage. 2003;19:113–24. doi: 10.1016/s1053-8119(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 48.Naito Y, Hirano S, Honjo I, Okazawa H, Ishizu K, Takahashi H, et al. Sound-induced activation of auditory cortices in cochlear implant users with post- and prelingual deafness demonstrated by positron emission tomography. Acta Otolaryngol. 1997;117:490–6. doi: 10.3109/00016489709113426. [DOI] [PubMed] [Google Scholar]
- 49.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–9. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Oh S-H, Kim C-S, Kang EJ, Lee DS, Lee HJ, Chang SO, et al. Speech perception after cochlear implantation over a 4-year time period. Acta Otolaryngol. 2003;123:148–53. doi: 10.1080/0036554021000028111. [DOI] [PubMed] [Google Scholar]
- 51.Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46:494–9. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- 52.Tobey EA, Devous MD, Buckley K, Overson G, Harris T, Ringe W, et al. Pharmacological enhancement of aural habilitation in adult cochlear implant users. Ear Hear. 2005;26:45S–56S. doi: 10.1097/00003446-200508001-00007. [DOI] [PubMed] [Google Scholar]
- 53.Stach BA, Stoner WR, Smith SL, Jerger JF. Auditory evoked potentials in Rett syndrome. J Am Acad Audiol. 1994;5:226–30. [PubMed] [Google Scholar]
- 54.Van der Molen MJW, Van der Molen MW, Ridderinkhof KR, Hamel BCJ, Curfs LMG, Ramakers GJa. Auditory change detection in fragile X syndrome males: a brain potential study. Clin Neurophysiol. 2012;123:1309–18. doi: 10.1016/j.clinph.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 55.Levy ML, Levy KM, Hoff D, Amar AP, Park MS, Conklin JM, et al. Vagus nerve stimulation therapy in patients with autism spectrum disorder and intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. J Neurosurg Pediatr. 2010;5:595–602. doi: 10.3171/2010.3.PEDS09153. [DOI] [PubMed] [Google Scholar]
- 56.Wilfong Aa, Schultz RJ. Vagus nerve stimulation for treatment of epilepsy in Rett syndrome. Dev Med Child Neurol. 2006;48:683–6. doi: 10.1017/S0012162206001435. [DOI] [PubMed] [Google Scholar]
- 57.Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45:3097–100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]