Summary
Cardiac abnormalities are the most common birth defects. Derangement of circulatory flow affects many vital organs; without proper supply of oxygenated blood, the brain is particularly vulnerable. Although surgical interventions have greatly reduced mortality rates, patients often suffer an array of neurological deficits throughout life. Neuroimaging provides a macroscopic assessment of brain injury, and has shown that white matter is at risk. Oligodendrocytes and myelinated axons have been identified as major targets of white matter injury, but still little is known about how congenital heart anomalies affect the brain at the cellular level. Further integration of animal model studies and clinical research will define novel therapeutic targets and new standard of care to prevent developmental delay associated with cardiac abnormalities.
Keywords: congenital, heart, white matter, myelin, oligodendrocyte
Why congenital cardiac anomalies and white matter injury?
Congenital heart disease (CHD) is the most common major birth defect; nearly 8 in every 1000 infants born each year suffer a cardiac abnormality [1]. Although significant advances have greatly reduced hospital mortality risk [2], patients with CHD frequently suffer from a broad spectrum of subsequent neurological deficits; including motor, cognitive, behavioral, social, and attention abnormalities [3–6]. Due to improvements in the mortality rate, it is estimated that 1 in every 150 young adults will be affected by some form of CHD within the next decade [7]. The personal, familial, and societal costs/hardships of neurological morbidity within this expanding population are inestimable. Therefore, as stated by the Pediatric Heart Network of the National Heart, Lung, and Blood Institute [8], “one of the most important challenges in the 21st century for CHD patients is to improve neurological deficits.”
Neurological outcome in CHD patients is governed by multiple factors, including unusual fetal cerebral blood flow and oxygen saturation [9]. In normal fetuses, highly oxygenated blood is preferentially streamed to the developing brain; however, severe/complex CHD often alters blood flow (Figure 1), resulting in delivery of de-saturated blood to the brain [9,10]. These alterations have been shown to cause immature and delayed brain development in newborns [11–15]. Recent MRI studies demonstrate a high frequency of white matter (WM) injury (25–55%) in CHD patients [14,16–18]. In addition to the prenatal insults to WM development, clinical trials and animal models have identified peri-operative factors - such as cardiopulmonary bypass surgery - contributing to brain injury [17,19–22]. Cellular events associated with CHD-induced WM injury are largely unknown and unexplored, partially due to the technical and ethical difficulties of studying human tissue. Although several large and small animal models have been designed to mimic CHD, they are rarely utilized to study the impact of CHD on WM development, particularly at the cellular level. The purpose of this review is to summarize current knowledge in this field and highlight an urgent need to create a truly translational area of research in CHD-induced WM injury through further exploration and integration of animal models with findings in human subjects and in postmortem human tissue.
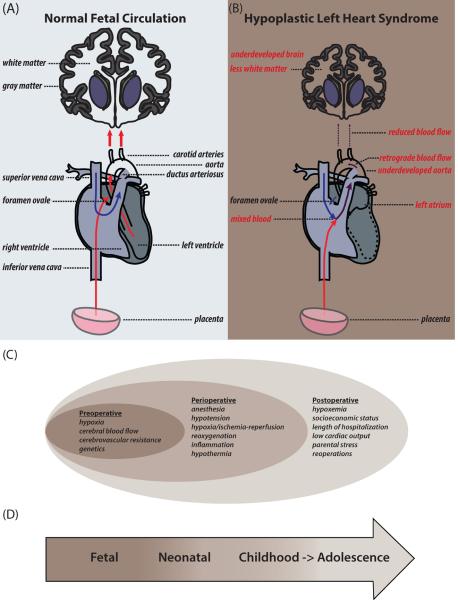
Figure 1.
Fetal cerebral circulation, white matter (WM) damage, and risk factors associated with neurological outcomes in congenital heart disease (CHD). (A,B) Cartoon illustrating fetal cerebral blood flow. In normal fetuses (A), oxygenated blood (red arrows) from the placenta is preferentially pumped to the left heart, exits through the carotid arteries, and flows to the brain. In fetuses with hyoplastic left heart syndrome (HLHS) (B), oxygenated and deoxygenated (blue arrows) blood mix (purple arrows) and is misdirected to the aorta, bypassing the carotid arteries. Retrograde flow of mixed blood exits through the carotid arteries at a low flow rate to the brain. In HLHS, developing fetal brains receive less and hypoxic blood, resulting in delayed WM maturation. (C,D) Risk factors associated with neurological deficits in CHD patients throughout life.
Congenital heart disease (CHD)
Worldwide, approximately 1.3 million infants are born with CHD each year and this population is steadily rising [23]. More than 75% of CHD children who survive the first year of life, including those with complex cardiac malformations, will live into adulthood [24]. Prolonged neurological deficits are commonly observed in patients with CHD and pose substantial socioeconomic and management challenges for patients, families, and society. Elucidating the cellular events underlying CHD-induced neurological deficits is not only a fundamental research endeavor: it is vital for the healthcare of this growing community of patients.
Several complex factors, often combinatorial and cumulative, contribute to neurological outcomes in patients with CHD (Figure 1), including: i) preoperative factors, such as unusual fetal cerebral blood flow; ii) perioperative factors involved with heart surgery; and iii) postoperative factors, such as length of hospital stay and parental stress [4,19,25]. Sophisticated imaging techniques are bringing prenatal events associated with neurological injury into focus [4,10]. Fetal cerebral blood flow involves preferential streaming of the most highly oxygenated blood to the developing brain [9]. However, heart abnormalities can alter these beneficial patterns, resulting in less or oxygen-deficient cerebral blood flow [9,10] (Figure 1). The first organ to form during embryonic development is the heart; through shared morphogenic programs, there is great time overlap between heart and brain development in human fetuses (for review, see [14,26]). Since the heart is nearly fully developed by the 7th week of gestation, cardiac abnormalities can disrupt fetal cerebral oxygen and nutrient delivery for more than 7 months during a period critical for brain development.
In order to survive, a majority of newborns with severe/complex CHD require cardiac surgery within the first few weeks of infancy. Since surgery cannot be performed on a beating heart it is necessary to stop the heart during cardiopulmonary bypass surgery (CPB) - a technique involving mechanical circulation and oxygenation of blood throughout the body while bypassing the heart and lungs. Although CPB facilitates heart surgery, this process mounts an inflammatory response associated with WM injury to the developing brain [27,28]. In addition to CPB, surgical correction of complex congenital cardiac anomalies may also require deep hypothermic circulatory arrest (DHCA). During this procedure the body is cooled to 15–18°C and blood circulation is completely stopped, which carries a risk of hypoxic/ischemic and reoxygenation injuries to the brain. Cardiac surgery itself introduces additional and compounding insults to the developing brain including gaseous and particulate microemboli.
To understand mechanisms underlying neurodevelopmental impairments in patients with CHD, it is imperative to fully investigate cellular events within the brain associated with CHD and corrective surgery. Understanding the combinatorial nature of these etiologies is also necessary for the development and improvement of treatment strategies for CHD.
Outcomes of CHD-induced brain injury: from conception to adolescence
Nearly all serious congenital cardiac abnormalities can be detected prenatally in the United States, and preoperative neurodevelopmental impairments are identifiable within days of birth [14,29]. In neonatal and early infant periods, motor asymmetries, absent suck, feeding difficulties, hyper- and hypotonia, poor visual orientation, and unusual cranial sizes are amongst the visible abnormalities that will likely result in neurological impairments later in life [9,30,31]. Following surgery for CHD, prospective clinical trials and retrospective clinical studies have documented worse outcomes for motor skills than cognitive abilities in children 1 and 4 years of age [5,6,32]. These findings are consistent with several recent studies using magnetic resonance imaging (MRI) in newborns and infants after cardiac surgery, which - as discussed below - also documented significant evidence of WM injury.
As infants mature into childhood, a battery of advanced neurodevelopmental and neuropsychological tests can readily identify neurological abnormalities in the CHD population that cannot be assessed in neonates. Children born with complex CHD requiring surgery may exhibit motor, cognitive, and behavioral difficulties encompassing problems in a broad range of skills: visual-spatial, fine and gross motor, memory, attention, language, executive, and psychosocial [9,33,34]. The broad range of neurological dysfunction documented in CHD patients is remarkably similar to the deficits observed in preterm survivors, in whom WM injury is a major source of morbidity [35–37].
Few studies have followed the neurodevelopmental outcome of children born with a CHD into adolescence. Increasing evidence that CHD newborns are at high risk of manifesting neurodevelopmental disorders [3] has spurred a consensus to the need of continuously monitoring these patients during childhood and throughout adolescence. The neurodevelopmental outcome of adolescents born with d-transposition of the great arteries (d-TGA) who underwent surgical correction was recently reported [3]. Despite a history of special academic services and psychotherapeutic counseling, these adolescents performed significantly lower than the population mean on a battery of tests scoring academic achievement, general memory, visual-spatial skills, social cognition, and attention [3,38]. A significant correlation between neurodevelopmental deficits in CHD patients and WM injury determined by conventional imaging techniques has not been identified [32,39]. However, a recent study demonstrated altered WM microstructure in CHD patients with diffusion tensor imaging (DTI) in regions of the brain displaying no obvious WM abnormalities by routine MRI [40]. Adolescents with CHD show a strong correlation between cognitive outcome and reduced WM microstructures assessed with DTI [41]. Additionally, a recent study reported a high incidence of depression and anxiety in adults with CHD [42].
Since WM is crucial for proper neural connectivity and communication [43], WM immaturity and injury likely account for the type and severity of neurological deficits exhibited in CHD patients. In light of findings from neurological assessments in patients with CHD, there is a clear need to define the cellular response and vulnerability to CHD-induced WM injury during critical developmental periods in order to develop therapeutic interventions to treat and promote recovery of CHD-induced WM damage.
Neuroimaging of white matter in CHD patients
Advances in neuroimaging technology have provided a wealth of information regarding brain development and injury in patients born with CHD (Box 1). Collectively, the most recent WM imaging studies in newborns have primarily focused on preoperative WM abnormalities and newly acquired WM injury patterns associated with, and following, cardiac surgery (summarized in Table 1).
Table 1.
Recent neuroimaging studies assessing white matter injury
| Cohorta | Imagingb | WM Findingsc | Refs | |
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| A. HLHS | ||||
| fetuses | MRI | • Progressive decline in WM volume from 25 to 37 weeks of gestation | N/A | [15] |
| fetuses | MRI | WMI (13–50%) • Prenatal diagnosis of WMI may be protective, depending on clinical practices |
N/A | [82] |
| neonates | WMI (46–50%) | |||
| neonates | MRI | WMI (19%) Males with aortic atresia were most vulnerable |
N/A | [83] |
| B. Mixed | ||||
| preterm neonates | MRI | WMI (42%) • Punctate WM lesions and structural abnormalities in central WM |
N/A | [84] |
| DTI | • Microstructural abnormalities in the splenium of all preterm patients with CHD | |||
| preterm neonates | DTI | • Reduced FA in cerebral WM including optic radiations and splenium | N/A | [85] |
| neonates | MRI | WMI (13%) | WMI (7%) | [86] |
| neonates | MRI | WMI (16%) | WMI (11–22%) | [16] |
| newborns | MRI | WMI (17%) | N/A | [87] |
| newborns | MRI | WMI | N/A | [52] |
| DTI | ↓ FA in WM tracts (optic radiations, internal capsul, corpus callosum, corticospinal) | |||
| newborns | DTI | ↓ WM FA | ↓ WM FA associated with new WMI | [88] |
| infants | MRI | WMI (24%) | WMI (30–31%) | [89] |
| infants | MRI | WMI (20%) | WMI (42–44%) | [17] |
| infants | MRI | WMI (19%) | N/A | [80] |
| HUS | • Not sensitive enough to detect same brain injuries | |||
| infants | HUS | WMI (6%) | N/A | [90] |
| infants | MRI | WMI (42%) | N/A | [91] |
| DTI | • Delayed WM microstructural maturation | |||
| adolescents | MRI | N/A | WMI (11%) | [92] |
| C. TGA, SVP | ||||
| neonates | MRI | WMI (23%) | WMI (31%) | [93] |
| infants | MRI | WMI (28%) | N/A | [51] |
| DTI | ↓ WM FA (optic radiations, perirolandic, posterior, frontal) | |||
| infants | MRI | N/A | WMI (28%) | [94] |
| adolescents | MRI | WMI (20%) | N/A | [40] |
| DTI | ↓FA in 18 WM regions | |||
Neuroimaging studies within the past 5 years.
HLHS: hypoplastic left heart syndrome; TGA: transposition of the great arteries; SVP: single ventricle physiology.
MRI: magnetic resonance imaging; DTI: diffusion tensor imaging; HUS: head ultrasound.
WM: white matter; WMI: white matter injury; CHD: congenital heart disease; FA: fractional anisotropy.
Diffuse and focal (e.g. – periventricular leukomalacia) WM injury represent the predominant signatures detected by MRI in CHD patients. Over the past five years, MRI studies have demonstrated that CHD patients often exhibit WM abnormalities and immaturity at birth (Table 1). Additionally, it has become widely recognized that newly acquired WM injury from perioperative factors is one of the most prevalent lesions seen in CHD patients (Table 1). A recent clinical study reported that WM injury was the most common brain injury in infants born with various forms of CHD before (20%) and after (44%) heart surgery [17]. Although WM injury has been reported in several clinical studies, it is important to consider the multivariate nature and complexity of these studies encapsulating (i) patient demographics: most studies include a mixed population of patients with several forms of CHD (Table 1); (ii) type of surgical intervention; and (iii) perioperative procedures. Prospective MRI studies performed on patients diagnosed with the same CHD and undergoing the same surgical procedure will be of great value in determining the vulnerability of WM in the developing brain.
Until recently, the maturation process of WM in the developing fetus was unknown, due to the technical difficulty of motion artifacts during fetal MRI scans [15,44]. Using advanced fetal MRI imaging on living fetuses with hypoplastic left heart syndrome (HLHS), a progressive decline in WM brain volume from 25 to 37 weeks of gestation was seen, when compared to control fetuses [15]. Interestingly, these differences were only significant after 30 weeks gestation. This study provides strong evidence that immature WM is particularly vulnerable in CHD patients prior to birth, and underscores the importance of imaging the brain longitudinally in utero.
In parallel with antenatal WM findings, CHD fetuses also display drastic delays in cortical folding, preceding a progressive decline in normal cortical and subcortical gray matter development [15]. The use of magnetic resonance spectroscopy (MRS) also demonstrates lower NAA:Cho – a measure of neuronal [metabolic] integrity – in CHD fetuses during the third trimester; a critical period with elevated oxygen demands during robust synapse formation/activity [13]. Though indirect, these findings strongly suggest the presence of CHD-induced neuronal abnormalities during fetal development.
Doppler ultrasound is an emerging technique that can predict brain volumetric abnormalities and neurodevelopmental outcomes of fetuses with certain CHDs [15,45–50]. This noninvasive technique offers important information regarding fetal hemodynamics by measuring the velocity of blood flow through cerebral arteries. CHD fetuses with lower cerebroplacental resistance (CPR < 1.0) were found to have less white, cortical gray, and subcortical gray matter volumes associated with an absence of antegrade blood flow to the developing brain [15]. Additionally, a reduction in fetal cerebral oxygen consumption correlates with smaller brain volumes in CHD fetuses [46]. Abnormal cerebrovascular resistance in the middle cerebral arteries of CHD fetuses was recently reported to precede lower cognitive development scores, assessed at 18 months of age [45]. MRI, MRS, and Doppler ultrasound offer useful measures of macroscopic brain structures/volumes, metabolic activity, and blood flow during fetal development. Routine and follow-up scans, commencing in the second trimester, will be invaluable in understanding the etiologies of CHD-induced brain injury and defining optimal windows for interventions and treatments of neurological sequelae.
The emergence of DTI has provided great insights into WM immaturity and injury in CHD patients. A DTI study recently demonstrated that delayed WM development was directly correlated with abnormal cardiac anatomy associated with reduced brain perfusion in term infants with CHD [51]. Another study reported lower FA values in several WM tracts within the brains of CHD infants, including optic radiations, corticospinal tracts, corpus callosum, and the internal capsule [52]. WM microstructure in the brains of adolescents following the arterial switch procedure to correct d-transposition of the great arteries has also been explored [40]. With DTI, this group demonstrated significantly lower FA values within 18 regions of WM underlying the cerebrum and cerebellum; importantly, only scant WM injury was seen with conventional MRI in this cohort [40]. These findings highlight the need for more sensitive imaging acquisitions in CHD patients in order to better understand the manifestation of specific, associated neural deficits.
Although neuroimaging studies have provided a wealth of knowledge regarding WM injury, there is an urgent need for further, more detailed investigations. It is becoming increasingly evident that MRI scans of CHD patients have a similar signature/pattern to those of the preterm brain [53]. Future studies with DTI and refined imaging techniques will greatly aid in acquiring a full picture of WM immaturity and newly acquired injuries on the microstructural level within specific WM tracts. Additionally, follow-up studies on postmortem CHD tissue will fuel investigations of how CHD affects WM at the cellular level; for example, DTI imaging paired with histological analysis.
The recent surge in animal imaging technologies offers several avenues for elucidating the multifactorial etiologies underlying brain impairments within the CHD population. Animal models allow for controlled conditions, such as genetic background, as well as a wide array of experimental approaches unfeasible or unethical in human patients. Unlike postmortem human tissue, euthanasia by perfusion in animals allows for rapid fixation of brain tissue to visualize microscopic changes to pathological insults at precise, static time points. Thus, there is a solid need for animal studies incorporating clinically relevant imaging approaches paired with high quality histological analysis of the cellular/molecular responses to CHD-induced brain injury. Studies of this nature will expedite, inform, and guide future clinical research in order to improve the neurological outcome of CHD patients.
The white matter: before and after
WM accounts for 50% of human brain volume. WM growth is a complex, multi-staged process during human brain maturation [43]. A primary constituent of WM is myelin, a lipid-rich membrane synthesized by specialized cells called oligodendrocytes. Myelin sheaths encase and insulate axons, which not only provides protection from their extracellular environment, but also enables efficient axonal communication. Additionally, myelin integrity is crucial for proper cognitive stability [54,55]. In humans, the onset of myelination is near midgestation; a surge of WM maturation persists into the second year of life. WM development and maintenance continue throughout adulthood [56].
Specialized imaging techniques - such as myelin water fraction imaging - have recently revealed the spatio-temporal patterns of normal WM development in humans throughout infancy and early childhood [57,58]. WM development occurs at different rates within each hemisphere and structure of the maturing brain. For example, myelination is robust in structures such as the cerebellum 3–4 months following birth, and more prominent in the frontal and temporal lobes around 6–8 months of age [57]. Hence, certain WM tracts in CHD patients are likely more vulnerable to operative factors depending on the age and time of surgical intervention.
The cellular events underlying myelination are well established and involve incremental stages of oligodendrocyte (OL) genesis and maturation (for review, see [59,60]). Oligodendrocyte precursors (OPCs) have the capacity to self-renew, and – after birth - primarily originate from the largest germinal zone of the postnatal mammalian brain, the subventricular zone (SVZ). Following cellular expansion, OPCs migrate into the WM where they undergo differentiation and myelination. Following WM injury, OPCs display a remarkable endogenous recovery potential, which recapitulates many of the processes during normal WM development ([61,62]; see below).
WM maturation delays and injury are frequently seen in CHD patients (Table 1). In order to understand the association between WM damage caused by CHD in humans, it will be necessary to understand WM injury at the cellular/molecular level. OLs simultaneously myelinate several axons, therefore loss of a single OL can have widespread effects on neural connectivity and communication. This can also impact axonal function, ultimately resulting in severe neurological impairment.
Animal models of CHD
Multiple animal models have been developed in order to mimic congenital heart abnormalities (Table 2), and determine the underlying causes and subsequent effects of these complex birth defects. These models encompass three broad categories: (i) genetic, (ii) environmental, and (iii) surgical. Since environmental factors can be readily explored in both models, we will focus on the genetic and surgical aspects to highlight the complementary nature of small and large animal studies.
Table 2.
Recent animal models of congenital heart disease and corrective surgery
| Species | WMa | Gene Proteinb | Key Findingsc | Refs |
|---|---|---|---|---|
| A. Genetic, Pharmacalogical | ||||
| Mouse | N/A | Gata4 G295S | atrial and ventral septation defects | [95] |
| Mouse | N/A | Hoxa1 | tetralogy of Fallot, interrupted aortic arch, abnormal subclavian artery | [96] |
| Mouse | N/A | Smad7 | septal chamber defects | [97] |
| Mouse | N/A | RAR | second heart field and septal defects | [98] |
| Mouse | N/A | Pax3 | double outlet right ventrical alignment defect | [99] |
| Mouse | N/A | Galnt1 | aortic and pulmonary valve stenosis | [65] |
| Mouse | N/A | FOXP1 | AVSD, HLHS | [100] |
| Mouse | N/A | RA, FA | TGA | [101] |
| B. Surgical | ||||
| Dog | N/A | N/A | surgery induced severe neurological injury and drastic increases in gene regulation | [102] |
| Pig | N/A | N/A | under hypothermic conditions, high pressure perfusion was not beneficial during long-term cerebral perfusion | [103] |
| Piglet | √ | N/A | area- and maturation-dependent, cerebral WM injury following surgery | [21] |
| Piglet | N/A | N/A | higher rates of antegrade cerebral perfusion increased cerebral blood flow | [104] |
| Piglet | N/A | N/A | blood brain barrier permeability was increased following surgery | [105] |
| Piglet | N/A | N/A | ischemic preconditioning was neuroprotective following surgery | [106] |
| Piglet | N/A | N/A | delayed hypothermia reduced cerebral perfusion | [107] |
| Piglet | N/A | N/A | regional blood flow was highest in the brain following surgery with selective cerebral perfusion at 32 degrees Celsius | [108] |
| Piglet | N/A | N/A | intravenous injection of granulocyte-colony stimulating factor, prior to surgery, reduced pro-apoptotic signalling in the brain - especially the striatum | [109] |
| C. Brain slice culture | ||||
| Mouse | √ | N/A | hypoxic exposure prior to surgery exacerbates WM injury; hypothermic conditions during surgery were protective of WM progenitor cells | [22] |
| Mouse | √ | N/A | WM and WM producing cells were most vulnerable in younger mice | [71] |
WM: white matter; N/A: not assessed or not applicable.
N/A: not applicable; RAR: retinoic acid receptor; RA: retinoic acid; FA: folic acid.
AVSD: Atrioventricular septal defect; HLHS: hypoplastic left heart syndrome; TGA: transposition of the great arteries.
Mouse models provide significant advantages: low cost, large litter sizes, genetic and molecular manipulation, and an eclectic toolkit of cutting edge techniques. More than 750 genetic syndromes have been associated with CHD patients [34], and a vast majority of mouse CHD models rely on manipulation or deletion of a single gene (Table 2). The primary aim of these studies has been to model the structural anomalies of the heart exhibited in CHD patients. Targeting genes within cardiac neural crest precursor cell pools has been a common approach and results in several types of developmental heart defects. Interestingly, both the heart and brain largely originate from a shared ancestral pool of cells [14] and craniofacial abnormalities are commonly associated with structural abnormalities of the heart in rodent models (Table 2).
Unlike humans, WM development within the mouse brain begins postnatally [63,64]. However, nearly all genetic mouse models of CHD result in embryonic lethality; therefore, the pathological effects of CHD on WM maturation cannot be assessed in these models. Additionally, these genetic alterations typically perturb/impair the development of several vital organs in addition to the structural alterations of the heart, such as the lungs. Therefore, in order to better understand the neurological deficits seen in CHD patients, it is necessary to develop a conditional rodent model in which only cardiac defects are apparent in utero and allow proper birth and postnatal development.
Recently, it was demonstrated that the Galnt1 gene is necessary for normal heart valve development and proper cardiac function in mice [65]. Although this study uncovered several of the cellular and molecular mechanisms underlying the formation and remodeling of heart valves, it holds great promise for putative brain abnormalities. Nearly 75% of Galnt1 null mice survive into adulthood with little evidence of abnormalities in other organs (Table 2). Future studies focused on white matter development in the brains of these mice will likely provide vast insights into the cellular and molecluar mechanisms underlying brain immaturity and injury in CHD patients.
Large animals, such as swine and lambs possess a developmental WM profile similar to humans and are large enough to undergo prenatal surgery [21,66–68]. Bilateral carotid artery occlusion models in sheep fetuses to induce cerebral ischemia have provided great insights into WMI, both on a macrostructural and cellular level [69,70]. It was recently demonstrated that prenatal cerebral ischemia impairs cortical growth associated with disturbances in dendritic arborization and synapse formation in sheep [70]. Another study reported a reduction in pre-myelinating OLs and arrested preOL differentiation localized to gliotic lesions in a sheep model of cerebral ischemia [69]; importantly, the histological findings strongly correlated with WMI detected by MRI.
Although large animal models are informative, powerful approaches to understanding the brain pathology of CHD, studies in these species have been largely limited to surgical approaches and systemic outcomes. Several groups have investigated how intra- and post-operative factors affect subsequent development, utilizing open-heart surgery approaches commonly performed to correct CHD (Table 2). Although there has been great success in emulating the surgical repair of CHDs in large animal studies, the post-operative neurological outcome was rarely assessed.
Considering the prevalence of WM developmental delays and injury in CHD patients, the near absence of knowledge and lack of emphasis on cerebral WM in animal models is alarming. While rodent models provide explanations of abnormal heart development, these studies are greatly limited by the longevity of subjects. On the other hand, large animal models have the potential to explore the peri- and post-operative vulnerability of cerebral WM during and following corrective surgery, respectively. For these reasons, there is still a scarcity of knowledge regarding CHD-induced WM impairment. Integrative approaches, capitalizing on the strengths of these complementary models will greatly aid in unveiling the underlying mechanisms resulting in the neurological impairments seen in infants born with a CHD and surviving heart surgery into adulthood.
Cellular and developmental analysis of WM injury in CHD
Our labs recently developed a cardiopulmonary bypass (CPB) surgery model in healthy neonatal piglets to assess the intraoperative contributions to WM injury [21]. WM maturation in piglets is area-dependent and greatly mirrors the progression seen in humans [21]. CPB-induced ischemia-reperfusion and reoxygenation injury demonstrated maturation-dependent vulnerability of WM; particularly, immature WM was most vulnerable. Histological analysis aimed at identifying different developmental stages of the oligodendrocyte lineage revealed a selective vulnerability of O4+ preoligodendrocytes (pre-OL) (Figure 2) [21]. Oligodendrocyte precursor cells (OPCs), however, displayed great resilience to the surgical insult and represent the endogenous recovery potential of WM tissue based on their potential to generate new oligodendrocytes. An arrest in OL maturation - paired with delayed myelination - was evident one month following surgery [21]. Additionally, this study identified modifiable, perioperative measures - reduced inflammation and maintenance of high oxygen-protective of WM injury during immature stages of brain development [21].
Figure 2.
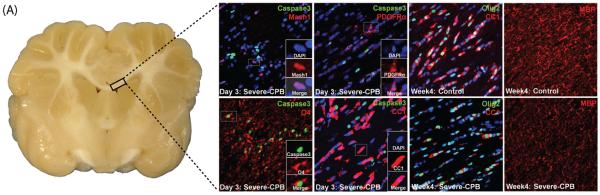
Immature oligodendrocytes (OLs) are highly vulnerable to cardiac surgery-induced brain injury which delays/impairs white matter (WM) development [21]. (A) Gross anatomy of perinatal pig brain. (B, C, F, G) Immunostains labeling apoptotic (Caspase3+) cells in the OL lineage within the corpus callosum (CC) 3 days following severe cardiopulmonary bypass (CPB) surgery. OL precursor (PDGFRα+) and progenitor cells (Mash1+, PDGFRα+), as well as mature OLs (CC1+), survive the surgical insult; however, immature (O4+) oligodendrocytes undergo cell death (O4+/Caspase3+) following CPB surgery (F). Fewer, mature OLs are present in the CC 4 weeks following surgery (H) compared to control piglets (D). Additionally, less myelin (MBP) is seen the corpus callosum following injury (I) when compared to control animals (E).
In order to further investigate the pre- and intraoperative effects of in utero hypoxia and CPB on WM development and injury, respectively, we also developed a rodent brain slice model [22,71]. Mice were reared in prolonged hypoxic conditions during a neonatal developmental time window overlapping with the period spanning third trimester to term birth in humans [22]. Viable brain slices were cultured in a perfusion system with artificial CSF where circulatory arrest could be simulated simply by oxygen-glucose deprivation (OGD). These combined approaches demonstrated pre-OL vulnerability paired with OPC resistance to insult in normal brains [22,71]. However, mature OLs and OPCs were significantly more vulnerable to OGD in brains that had developed under hypoxic conditions [22]. This shift in susceptibility to WM injury was temperature-specific and deep hypothermia during OGD was protective to OPCs [22].
Brain injury in CHD patients is remarkably similar to that seen in premature neonates. Both populations exhibit delays in WM maturation, and cardiac surgery often results in secondary WM injury. Cerebral hypoxia is an underlying commonality between these patients: in CHD patients, de-saturated blood is streamed to the brain in utero and following birth. After birth, premature infants often experience hypoxia due to underdeveloped lungs that cannot handle normal oxygen loads. Interestingly, studies modeling premature birth with hypoxia report similar findings to those seen in CHD models, even at the cellular level [61,62].
Proliferation and expansion of endogenous OPC pools is critical for replacing OLs and repairing damaged/absent myelin following hypoxia [61,62]. Although several key regulators of the OPC regenerative response have been identified, molecular manipulation in rodent models often relies on genetic or invasive techniques that may not be practical or ethical approaches in treating humans. Two recent studies explored the feasibility and efficacy of intranasal drug delivery to target OPCs to protect/repair WM following hypoxic or hypoxic-ischemic insults [62,72]. Intranasal infusion of human apotransferrin (aTf) prior to hypoxia-ischemia reduced WM injury and enhanced OPC survival and proliferation within the SVZ and corpus callosum [72]. Intranasal administration of heparin binding epidermal growth factor (EGF) immediately following hypoxic exposure reduced OL death, promoted OL generation, and improved functional/behavioral recovery [62].
A recent study demonstrated that OPCs are critical for the coordination of vascular growth in developing WM in mice [73]. OLs and OPCs often make direct contact with blood vessels which provide oxygen and nutrition, two key ingredients necessary for the high metabolic demand of membrane expansion during myelination. Interestingly, OPC-encoded hypoxia inducible factor (HIF) signaling was found to regulate angiogenesis while arresting OPC differentiation prior to myelination [73]. The newly formed blood vessels provide the appropriate oxygen levels to inhibit HIF activity which allows for OL differentiation and myelination in the presence of an adequate metabolic supply. These data offer a potential mechanism for delayed/arrested WM maturation in CHD fetuses; the absence of highly oxygenated cerebral blood flow stabilizes HIF activity, preventing OPC differentiation and subsequent myelination.
HIF-1α is a transcription factor that regulates several target gene products including erythropoietin (EPO) and vascular endothelial growth factor (VEGF) [74]. A recent clinical study found increased levels of HIF-1α and EPO gene expression in term newborns with CHD associated with hypoxia [74]. Erythropoietin treatment, shortly following preterm birth, has resulted in positive clinical outcomes in recent years and has promising neuroprotective potential [75–79]. Early administration of recombinant EPO (rEPO) reduced white and gray matter injury in preterm infants [75]. Additionally, follow-up studies have shown that rEPO treatment in infancy significantly improves neurodevelopmental and cognitive outcomes in toddlers and through early adolescence [76–78].
In view of the similarities in WM and cell-specific vulnerabilities seen in CHD and preterm animal models, it is likely that there is a common mechanism of injury. Future studies modeling CHD could greatly benefit through extrapolations of findings from the enormous population of preterm birth models. Additionally, engineering and testing noninvasive or less invasive drug administration will allow for quicker translation into the clinic.
Conclusions & Future Perspectives
Over the past few decades, surgical and clinical care has significantly improved the survival rates of children born with even the most complex forms of CHD. Clinical research has recently shifted from a focus on survival to routine evaluation of morbidity throughout development [34]. As stated by the American Heart Association, the growing population of infant cardiac surgery survivors demands new unified stratification methods and algorithms to classify, diagnose, and manage developmental disorders in CHD patients [34].
Clinical trials and animal studies have provided a wealth of bidirectional information regarding the neurological impairments seen in the CHD population; however, several large gaps in knowledge remain (Box 2). While clinical studies have identified many psychological and anatomical associations between neurological defects and heart abnormalities, they are limited in technical and experimental approaches. However, such findings have guided the design of several animal models that have begun elucidating the cellular and molecular mechanisms underlying these pathologies. There is a growing need for integrative approaches, such as animal models that recapitulate clinical outcome measures while providing information on the cellular/molecular level in order to develop novel therapies.
Considering the complexity of severe CHD and the array of putative comorbidities, identifying independent causes of WM injury is difficult and will require careful study design. Infants born with d-Transposition of the great arteries (d-TGA) represent a unique avenue for integrative approaches because they typically receive surgery within 2 weeks of birth; additionally, d-TGA is rarely associated with confounding genetic syndromes seen with other anomalies [9]. With few exceptions, our neuroanatomical information regarding WM development and injury comes from clinical studies evaluating pools of patients with different types of CHD. Retrospective studies, in which patients are subdivided by specific CHD, will also be of great value.
Although large animal models are limited in genetic approaches commonly utilized in rodents, they serve as a great platform for understanding the cellular dynamics underlying CHD-induced brain injury before and after surgical intervention. Additionally, higher mammals share several anatomical, physiological, and metabolic similarities to humans and are ideal candidates for testing human drugs while closely monitoring systemic and neurological responses seen in the clinic. Banking and indexing brain tissue from CHD patients will facilitate postmortem analysis of WM injury and improve the quality of new study designs. Integrating the genetic, cellular, and molecular approaches in animal models with novel imaging tools used on humans will mutually inform future research and provide large steps towards developing new therapies and treatment windows to improve the neurological outcomes seen in CHD patients.
Box1: Neuroimaging Techniques.
Since the first computed tomography (CT) scan in the 1970s, there have been tremendous advances in neuroimaging technologies. State-of-the-art imaging techniques not only provide anatomical details but also greatly aid in surgical, stereotactic precision. These technologies vary -in cost, utility, length of scan, resolution- and have given us noninvasive longitudinal windows into abnormalities during fetal and postnatal brain development within the CHD population. Additionally, neuroimaging studies offer the promise of predictive measures in CHD developmental disorders.
Acquisition of a head ultrasound (HUS) is most common across centers, least expensive, quickest, and can identify major structural abnormalities within the brain that may worsen following cardiac surgery [34]; however, the limitations of HUS in detecting the wide spectrum of structural brain abnormalities are increasingly recognized [80]. On the other hand, Doppler imaging by HUS is still a useful tool to measure fetal cerebral blood flow [10]. This technique is also utilized as an intraoperative monitoring system to standardize cerebral oxygen delivery for the individual patient [32,81].
Over the last decade there has been an increased utilization of magnetic resonance imaging (MRI) in clinical studies and trials. Although, until recently, MRI was not a routine procedure in neonates with CHD [34] - perhaps due to clinical feasibility, cost, and safety - several prospective studies have underscored the need for brain MRIs in these children. Magnetic resonance spectroscopy (MRS) is a specialized imaging technique which provides the concentration of metabolites, such as N-Acetylaspartic acid (NAA) and choline (cho), in target brain regions. Together, these complementary techniques allow for visualization as well as metabolic activity of specific brain structures.
Recent clinical and research laboratory studies have employed more sophisticated technologies, such as diffusion tensor imaging (DTI) which enables visualization and a quantitative measure of WM microstructures. DTI is a sensitive imaging technique that measures the diffusion of water molecules and provides high-resolution images of microstructures within the brain. With diffusion tractography, WM fiber bundles can be reconstructed and quantitatively analyzed in an un-biased manner. Fractional anisotropy (FA) is a common metric of DTI and describes the directional dependence of water displacement; lower FA values are indicative of WM immaturity or injury.
Box 2: Outstanding Questions.
Can we create an animal model that recapitulates the morphogenic, developmental aspects of CHD without directly affecting other organs or developmental processes?
What are the prenatal and neonatal cellular responses to CHD in the developing brain?
What are the molecular mechanisms underlying WM immaturity and vulnerability to CHD and how can we intervene?
How can we accurately assess the dynamic neurological outcomes of CHD and/or corrective surgery in animal models?
Prenatal or postnatal insults to the developing brain: which is most devastating in regards to developmental and behavioral disabilities?
How can we best extrapolate from, and integrate, neuroimaging findings/correlations in human patients with cellular/molecular approaches in animal models?
Highlights.
Complex CHD is often associated with developmental delay and behavioral problems
Currently, the neurological deficits displayed by CHD patients are irreversible
Animal studies offer powerful avenues to understand CHD brain impairments
Acknowledgements
This work was supported by R01NS045702 (V.G.), R01NS056427 (V.G.), R01HL104173 (R.A.J.), and the Baier Cardiac Research Fund (R.A.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marelli AJ, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 2.Khairy P, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabbutt S, et al. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 5.Newburger JW, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg CS, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. 2014;165:490–496. e498. doi: 10.1016/j.jpeds.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnes CA, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease) Circulation. 2008;118:2395–2451. doi: 10.1161/CIRCULATIONAHA.108.190811. [DOI] [PubMed] [Google Scholar]
- 8.Kaltman JR, et al. Report of the pediatric heart network and national heart, lung, and blood institute working group on the perioperative management of congenital heart disease. Circulation. 2010;121:2766–2772. doi: 10.1161/CIRCULATIONAHA.109.913129. [DOI] [PubMed] [Google Scholar]
- 9.McQuillen PS, et al. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol. 2010;29:79–85. doi: 10.1016/j.ppedcard.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donofrio MT, et al. Impact of congenital heart disease on fetal brain development and injury. Curr Opin Pediatr. 2011;23:502–511. doi: 10.1097/MOP.0b013e32834aa583. [DOI] [PubMed] [Google Scholar]
- 11.Miller SP, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 12.Licht DJ, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limperopoulos C, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuillen PS, Miller SP. Congenital heart disease and brain development. Ann N Y Acad Sci. 2010;1184:68–86. doi: 10.1111/j.1749-6632.2009.05116.x. [DOI] [PubMed] [Google Scholar]
- 15.Clouchoux C, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2013;23:2932–2943. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- 16.Andropoulos DB, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beca J, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 18.Gaynor JW. Periventricular leukomalacia following neonatal and infant cardiac surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:133–140. doi: 10.1053/j.pcsu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Kussman BD, et al. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation. 2010;122:245–254. doi: 10.1161/CIRCULATIONAHA.109.902338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman GM, et al. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmentaloutcome. J Thorac Cardiovasc Surg. 2013;146:1153–1164. doi: 10.1016/j.jtcvs.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi N, et al. White matter protection in congenital heart surgery. Circulation. 2012;125:859–871. doi: 10.1161/CIRCULATIONAHA.111.048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agematsu K, et al. Effects of preoperative hypoxia on white matter injury associated with cardiopulmonary bypass in a rodent hypoxic and brain slice model. Pediatr Res. 2014;75:618–625. doi: 10.1038/pr.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr. 2013;24:141–145. doi: 10.5830/CVJA-2013-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilboa SM, et al. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1):92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 26.Paige SL, et al. Molecular Regulation of Cardiomyocyte Differentiation. Circ Res. 2015;116:341–353. doi: 10.1161/CIRCRESAHA.116.302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahle WT, et al. Inflammatory response after neonatal cardiac surgery and its relationship to clinical outcomes. Ann Thorac Surg. 2014;97:950–956. doi: 10.1016/j.athoracsur.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 28.Chew LJ, et al. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci. 2013;35:102–129. doi: 10.1159/000346157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner HM. Fetal echocardiography: 20 years of progress. Heart. 2001;86(Suppl 2):II12–22. doi: 10.1136/heart.86.suppl_2.ii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen M, et al. Abnormal brain structure and function in newborns with complex congenital heart defects before open heart surgery: a review of the evidence. J Child Neurol. 2011;26:743–755. doi: 10.1177/0883073811402073. [DOI] [PubMed] [Google Scholar]
- 31.Owen M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr. 2014;164:1121–1127. e1121. doi: 10.1016/j.jpeds.2013.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andropoulos DB, et al. Neurodevelopmental outcomes after regional cerebral perfusion with neuromonitoring for neonatal aortic arch reconstruction. Ann Thorac Surg. 2013;95:648–654. doi: 10.1016/j.athoracsur.2012.04.070. discussion 654–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellinger DC, et al. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino BS, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 35.Litt J, et al. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil. 2005;38:130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- 36.Back SA, et al. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 37.Salmaso N, et al. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeMaso DR, et al. Psychiatric disorders and function in adolescents with d-transposition of the great arteries. J Pediatr. 2014;165:760–766. doi: 10.1016/j.jpeds.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soul JS, et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374–381. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivkin MJ, et al. Adolescents with D-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. J Thorac Cardiovasc Surg. 2013;146:543–549. e541. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollins CK, et al. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165:936–944. e931–932. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Donovan CE, et al. The impact of illness perceptions and disease severity on quality of life in congenital heart disease. Cardiol Young. 2015:1–10. doi: 10.1017/S1047951114002728. [DOI] [PubMed] [Google Scholar]
- 43.Filley CM. White matter dementia. Ther Adv Neurol Disord. 2012;5:267–277. doi: 10.1177/1756285612454323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mlczoch E, et al. Structural congenital brain disease in congenital heart disease: results from a fetal MRI program. Eur J Paediatr Neurol. 2013;17:153–160. doi: 10.1016/j.ejpn.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Williams IA, et al. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. 2012;40:304–309. doi: 10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun L, et al. Reduced Fetal Cerebral Oxygen Consumption is Associated With Smaller Brain Size in Fetuses With Congenital Heart Disease. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donofrio MT, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 48.Zeng S, et al. Assessment of cerebral blood flow perfusion in fetuses with congenital heart diseases by 3D power Doppler ultrasound. Ultrasound Obstet Gynecol. 2015 doi: 10.1002/uog.14798. [DOI] [PubMed] [Google Scholar]
- 49.Masoller N, et al. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2014;44:182–187. doi: 10.1002/uog.13373. [DOI] [PubMed] [Google Scholar]
- 50.Arduini M, et al. Cerebral blood flow autoregulation and congenital heart disease: possible causes of abnormal prenatal neurologic development. J Matern Fetal Neonatal Med. 2011;24:1208–1211. doi: 10.3109/14767058.2010.547961. [DOI] [PubMed] [Google Scholar]
- 51.Sethi V, et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res. 2013;73:661–667. doi: 10.1038/pr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulkey SB, et al. White matter injury in newborns with congenital heart disease: a diffusion tensor imaging study. Pediatr Neurol. 2014;51:377–383. doi: 10.1016/j.pediatrneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Counsell SJ, et al. New imaging approaches to evaluate newborn brain injury and their role in predicting developmental disorders. Curr Opin Neurol. 2014;27:168–175. doi: 10.1097/WCO.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 54.Brauer J, et al. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- 55.Fjell AM, et al. Reduced white matter integrity is related to cognitive instability. J Neurosci. 2011;31:18060–18072. doi: 10.1523/JNEUROSCI.4735-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geng X, et al. Quantitative tract-based white matter development from birth to age 2years. Neuroimage. 2012;61:542–557. doi: 10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deoni SC, et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deoni SC, et al. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63:1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 61.Jablonska B, et al. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scafidi J, et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol Appl Neurobiol. 1980;6:415–420. doi: 10.1111/j.1365-2990.1980.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 64.Craig A, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 65.Tian E, et al. Galnt1 is required for normal heart valve development and cardiac function. PLoS One. 2015;10:e0115861. doi: 10.1371/journal.pone.0115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Back SA, Rosenberg PA. Pathophysiology of glia in perinatal white matter injury. Glia. 2014;62:1790–1815. doi: 10.1002/glia.22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunn AJ, Bennet L. Fetal hypoxia insults and patterns of brain injury: insights from animal models. Clin Perinatol. 2009;36:579–593. doi: 10.1016/j.clp.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Back SA, et al. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics. 2012;9:359–370. doi: 10.1007/s13311-012-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riddle A, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011;70:493–507. doi: 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dean JM, et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5:168ra167. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murata A, et al. Rodent brain slice model for the study of white matter injury. J Thorac Cardiovasc Surg. 2013;146:1526–1533. e1521. doi: 10.1016/j.jtcvs.2013.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guardia Clausi M, et al. Intranasal administration of aTf protects and repairs the neonatal white matter after a cerebral hypoxic-ischemic event. Glia. 2012;60:1540–1554. doi: 10.1002/glia.22374. [DOI] [PubMed] [Google Scholar]
- 73.Yuen TJ, et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemus-Varela ML, et al. Expression of HIF-1 alpha, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin Biochem. 2010;43:234–239. doi: 10.1016/j.clinbiochem.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 75.Leuchter RH, et al. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. JAMA. 2014;312:817–824. doi: 10.1001/jama.2014.9645. [DOI] [PubMed] [Google Scholar]
- 76.Neubauer AP, et al. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67:657–666. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 77.Ohls RK, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133:1023–1030. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown MS, et al. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124:e681–687. doi: 10.1542/peds.2008-2701. [DOI] [PubMed] [Google Scholar]
- 79.Limperopoulos C. Pediatrics: neuroprotective effects of erythropoietin in preterm infants. Nat Rev Neurol. 2010;6:301–302. doi: 10.1038/nrneurol.2010.66. [DOI] [PubMed] [Google Scholar]
- 80.Rios DR, et al. Usefulness of routine head ultrasound scans before surgery for congenital heart disease. Pediatrics. 2013;131:e1765–1770. doi: 10.1542/peds.2012-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andropoulos DB, et al. Novel cerebral physiologic monitoring to guide low-flow cerebral perfusion during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;125:491–499. doi: 10.1067/mtc.2003.159. [DOI] [PubMed] [Google Scholar]
- 82.Algra SO, et al. Minimizing the risk of preoperative brain injury in neonates with aortic arch obstruction. J Pediatr. 2014;165:1116–1122. e1113. doi: 10.1016/j.jpeds.2014.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goff DA, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg. 2014;147:1312–1318. doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paquette LB, et al. Abnormal cerebral microstructure in premature neonates with congenital heart disease. AJNR Am J Neuroradiol. 2013;34:2026–2033. doi: 10.3174/ajnr.A3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paquette LB, et al. Abnormal Development of Thalamic Microstructure in Premature Neonates with Congenital Heart Disease. Pediatr Cardiol. 2015 doi: 10.1007/s00246-015-1106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bertholdt S, et al. Cerebral lesions on magnetic resonance imaging correlate with preoperative neurological status in neonates undergoing cardiopulmonary bypass surgery. Eur J Cardiothorac Surg. 2014;45:625–632. doi: 10.1093/ejcts/ezt422. [DOI] [PubMed] [Google Scholar]
- 87.Mulkey SB, et al. Multi-tiered analysis of brain injury in neonates with congenital heart disease. Pediatr Cardiol. 2013;34:1772–1784. doi: 10.1007/s00246-013-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dimitropoulos A, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glass HC, et al. Infection and white matter injury in infants with congenital cardiac disease. Cardiol Young. 2011;21:562–571. doi: 10.1017/S1047951111000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Latal B, et al. Can preoperative cranial ultrasound predict early neurodevelopmental outcome in infants with congenital heart disease? Dev Med Child Neurol. 2015 doi: 10.1111/dmcn.12701. [DOI] [PubMed] [Google Scholar]
- 91.Ortinau C, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2012;143:1264–1270. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.von Rhein M, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 93.Block AJ, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Drury PP, et al. Deep hypothermic circulatory arrest during the arterial switch operation is associated with reduction in cerebral oxygen extraction but no increase in white matter injury. J Thorac Cardiovasc Surg. 2013;146:1327–1333. doi: 10.1016/j.jtcvs.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 95.Misra C, et al. Congenital heart disease-causing Gata4 mutation displays functional deficits in vivo. PLoS Genet. 2012;8:e1002690. doi: 10.1371/journal.pgen.1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makki N, Capecchi MR. Cardiovascular defects in a mouse model of HOXA1 syndrome. Hum Mol Genet. 2012;21:26–31. doi: 10.1093/hmg/ddr434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang S, et al. Trigenic neural crest-restricted Smad7 over-expression results in congenital craniofacial and cardiovascular defects. Dev Biol. 2010;344:233–247. doi: 10.1016/j.ydbio.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li P, et al. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev Cell. 2010;18:480–485. doi: 10.1016/j.devcel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olaopa M, et al. Pax3 is essential for normal cardiac neural crest morphogenesis but is not required during migration nor outflow tract septation. Dev Biol. 2011;356:308–322. doi: 10.1016/j.ydbio.2011.05.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang SW, et al. Genetic abnormalities in FOXP1 are associated with congenital heart defects. Hum Mutat. 2013;34:1226–1230. doi: 10.1002/humu.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amati F, et al. Hif1alpha down-regulation is associated with transposition of great arteries in mice treated with a retinoic acid antagonist. BMC Genomics. 2010;11:497. doi: 10.1186/1471-2164-11-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allen JG, et al. Hawley H. Seiler Resident Award. Transcriptional profile of brain injury in hypothermic circulatory arrest and cardiopulmonary bypass. Ann Thorac Surg. 2010;89:1965–1971. doi: 10.1016/j.athoracsur.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haldenwang PL, et al. Effect of pressure management during hypothermic selective cerebral perfusion on cerebral hemodynamics and metabolism in pigs. J Thorac Cardiovasc Surg. 2010;139:1623–1631. doi: 10.1016/j.jtcvs.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 104.Sasaki T, et al. Optimal flow rate for antegrade cerebral perfusion. J Thorac Cardiovasc Surg. 2010;139:530–535. doi: 10.1016/j.jtcvs.2009.12.005. discussion 535. [DOI] [PubMed] [Google Scholar]
- 105.Okamura T, et al. Hypothermic circulatory arrest increases permeability of the blood brain barrier in watershed areas. Ann Thorac Surg. 2010;90:2001–2008. doi: 10.1016/j.athoracsur.2010.06.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jensen HA, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 107.Lee JK, et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med. 2011;39:2337–2345. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, et al. The impact of temperature and pump flow rate during selective cerebral perfusion on regional blood flow in piglets. J Thorac Cardiovasc Surg. 2013;145:188–194. doi: 10.1016/j.jtcvs.2012.09.055. discussion 194–185. [DOI] [PubMed] [Google Scholar]
- 109.Pastuszko P, et al. Effect of granulocyte-colony stimulating factor on expression of selected proteins involved in regulation of apoptosis in the brain of newborn piglets after cardiopulmonary bypass and deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2012;143:1436–1442. doi: 10.1016/j.jtcvs.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]