Abstract
Testosterone is a sex hormone involved in brain maturation via multiple molecular mechanisms. Previous human studies described age-related changes in the overall volume and morphological properties of white matter during male puberty. Based on this work, we have proposed that testosterone may induce an increase of radial growth and, possibly, modulate axonal transport. In order to determine whether this is the case we have used two different experimental approaches. With electron microscopy, we have evaluated sex differences in the structural properties of axons in the corpus callosum (splenium) of young rats, and tested consequences of castration carried out after weaning. Then we examined in vitro the effect of the non-aromatizable androgen Mibolerone on the structure and bidirectional transport of wheat-germ agglutinin vesicles in the axons of cultured sympathetic neurons. With electron microscopy, we found robust sex differences in axonal diameter (males>females) and g ratio (males>females). Removal of endogenous testosterone by castration was associated with lower axon diameter and lower g ratio in castrated (vs. intact) males. In vitro, Mibolerone influenced the axonal transport in a time- and dose-dependent manner, and increased the axon caliber as compared with vehicle-treated neurons. These findings are consistent with the role of testosterone in shaping the axon by regulating its radial growth, as predicted by the initial human studies.
Keywords: axon, myelin, testosterone, axon transport, white matter
Introduction
Maturation of white matter (WM) is a complex process involving changes in axonal diameter and myelin thickness, with progressive fine-tuning of the structure and function of the axon-myelin system (Ullén, 2009). Previous studies in our laboratory showed a positive correlation between testosterone levels and WM volume in adolescent males with the more efficient form of androgen receptor (AR). Furthermore, indirect evidence suggests that testosterone may influence WM volume in male adolescents by increasing axon diameter and not myelination (Perrin et al., 2009).
Axons are the main constituents of WM, occupying almost 90% of its total volume (Wang et al., 2008). Axons come in different sizes: some are thin, others thick, and different sizes are often mixed within the same fascicle (Peters et al., 1991). In myelinated axons, the ratio between axon diameter and fiber (axon + myelin thickness) diameter – the so-called g ratio – appears to increase as a function of axon diameter, indicating a relatively thinner myelin sheath in large axons (Berthold et al., 1983; Chatzopoulou et al., 2008; Gillespie and Stein, 1983). Distribution of axon diameter might relate to the heterogeneity of information transfer of a nerve bundle or fascicle (Perge et al., 2012). Besides the classical function as conductors of action potentials, axons also provide a physical conduit for the transport of a wide variety of cargos to the synapse. Thus, the axonal transport is a fundamental process to form the cellular networks of the nervous system and ensure a proper flow of information throughout the brain (reviewed in Paus et al. 2014). The key molecules for the microtubule-based axonal transport are motor proteins, members of the kinesins and cytoplasmic dynein superfamilies. Kinesins transport a cargo anterogradely toward the synapse, while dyneins move it retrogradely towards the cell body (Hirokawa et al., 2010). Maintenance of neuronal homeostasis depends on the fine regulation of motor proteins and cytoskeleton components engaged in the axon transport (Colin et al., 2008; Morfini et al., 2002; Perrot and Julien, 2009). Cytoskeletal elements have a dual role in the regulation of axon transport: they form the “tracks” along which the cargos are transported, and they are responsible for radial growth of the axon (Barry et al., 2010). In the context of the work described here, it is of interest that there is a reciprocal influence of the axonal radial growth on the axon transport and of the effect of the axon transport on axonal radial growth. On one hand thicker axons may accommodate a higher rate of axonal transport (Murayama et al., 2006). On the other hand, transport of cytoskeleton elements (containing tubulin and neurofilament proteins) plays an important role in regulating the axon diameter (Eyer and Peterson, 1994; Xia et al., 2003). As such, modulation of microtubule-based axonal transport under the influence of sex steroids during puberty and adolescence might affect the axon diameter and their distribution and, in turn, the performance of various WM pathways in a sex-dimorphic way.
Although the role of androgens in brain reorganization during the pubertal period has been recognized (Durdiakova et al., 2011; Herting et al., 2012; Peper et al., 2011), the exact mechanism of their action and how these differences are sustained at the microstructural level remain poorly understood. We have hypothesized that testosterone influences processes that determine axonal diameter and, in doing so, the macroscopic properties of WM assessed in vivo with MRI (Perrin et al. 2009, Paus and Toro 2009). Here we test this hypothesis using two experimental approaches. First we use electron microscopy to assess the effect of testosterone on axon morphology in the splenium of the corpus callosum of young male and female rats, and after real or sham castration (after weaning) of male (adult) rats. We have chosen the callosal splenium (posterior fifth) as a model system because this structure has been the subject of previous research on sexual dimorphism (Kim et al. 1996; Kim and Juraska 1997). Second, due to the close relationship between axon transport and axon radial growth, we evaluated the effects of androgens on axonal transport by quantifying the movement of wheat-germ agglutinin (WGA) vesicles in the axons of cultured sympathetic neurons treated with Mibolerone, a non-aromatizable synthetic androgen. Our results are consistent with the possibility that testosterone plays a key role in the pubertal maturation of WM pathways by modulating the cellular processes that affect the dynamic turnover of cytoskeletal matrix responsible for the axonal structure.
Materials and Methods
Animals
Experimental animals were young (70 post-natal days) male and female wild-type Wistar rats, ordered from Charles River (St. Constant, Quebec, Canada). Rats were assigned to the following experimental groups: Experiment 1 (6 intact males, M; and 6 intact females, F); and Experiment 2 (10 sham castrated M; 10 sham castrated F; and 10 castrated males, M gx). Real or sham castrations were done at pre-pubertal stage (21 post-natal days) at Charles River; rats were shipped on post-natal day 65.
Five days after delivery (70 days of age), rats were weighed, deeply anesthetized with isoflurane and perfused through the left cardiac ventricle with 0.9% NaCl at 37°C, followed without interruption by 1% freshly prepared para-formaldehyde and 1% glutaraldehyde in 0.1M phosphate buffer at 37°C. After perfusion, the brain was removed and a slice (200 μm thick) of the medial section of the right hemisphere was taken, exposing the corpus callosum (CC) in plain view. The splenium was removed and prepared for electron microscopy. Briefly, tissues were postfixed in osmium tetroxide, dehydrated through ethanol to propylene oxide and embebbed in Spurrs resin. Blocks containing the tissue samples were cut at 60 nm using an Ultracut E ultramicrotome (Leica, Nussloch, Germany) and collected on a 200-mesh grid (Agar Scientific, Stansted, UK). These sections were stained in 3% uranyl acetate and 1% lead citrate before proceeding with electron microscopy carried out on a Hitachi (Tokyo, Japan) H-600 transmission electron microscope operated at 75 kV.
Morphometric Analysis
To investigate the entire area of the splenium, one micrograph was taken at 10,000x magnification from eight different areas along the dorsal-to-ventral extent of the splenium in each rat; the sampled region did not include the hippocampal commissure (Figure 1A). For myelinated axons, diameter was calculated as follows. Using ImageJ software (http://rsb.info.nih.gov/ij/), the micrographs were transformed into binary images: the axon appeared white and myelin black. Next, we used the wand (tracing) tool to select thresholded pixels forming a contiguous area: we selected first a single pixel within an axon and then expanded this selection to all adjacent (white) pixels bound by the encircling myelin. The cross-sectional area of these pixels (i.e., the axon area) was measured automatically. In this manner, we calculated the cross-sectional area of about 1,000 myelinated axons in each rat. The g ratio, the ratio between the axon diameter and fiber diameter (axon diameter + myelin sheath), was estimated by measuring the minimum and maximum axon diameter and the minimum and maximum fiber diameter of each axon using the same ImageJ software; the g ratio was obtained by dividing the mean axon diameter by the mean fiber diameter. The g ratio was calculated in about 400 myelinated axons for each animal in Experiment 1 and in a subset of animals (n=5 per group) in Experiment 2. Axon diameter, estimated as the mean of the minimum and maximum axon diameter, was also measured about 50 unmyelinated axons in each rat in a subset of animals (n=5 per group) in Experiment 2.. Only axons with regular shape were measured. The distribution of axons was analyzed for each animal and the skewness was calculated as follows: Σ (x−μ)^3/(N−1)σ^3, where Σ means sum all the values, μ represents a sample mean and σ is the is the sample standard deviation. The rater was blind to the sex and castration status of the axons under examination.
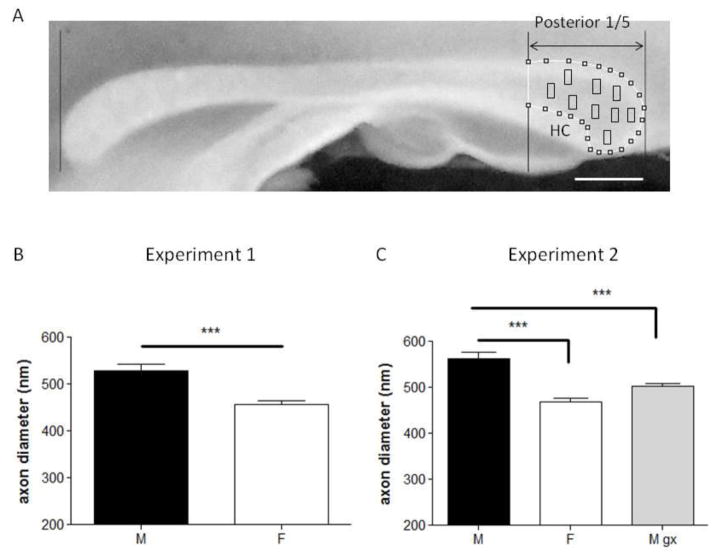
Figure 1.
A, Representative digital image of the rat corpus callosum in a midsagittal section. Rostral is to the left. The lines at the two edges were used to delineate the splenium. Axon diameter was assessed within the area delimited by the black rectangles. HC denotes dorsal hippocampal commissure. Scale bars, 1 mm.
B and C, Barplots showing the results of axon diameter in Experiment 1 and 2 respectively. C, Mean axon diameter of myelinated axons was greater in males compared with females. Gonadectomized males showed a significant decrease in the axon diameter as compared with intact males. *** p<0.001 vs. males. M = males; F = females; M gx = males gonadectomised.
Western blot analysis
We measured concentrations of AR protein in the fresh superior thoracic ganglion and cerebral cortex from adult rats, as well in the sympathetic cell culture from P5 rats using Western blotting as described previously (Vaillant et al., 1999). We used a rabbit polyclonal AR antibody, PG-21 (1:200; Upstate Biotechnology, NY, USA). Protein bands were visualized with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10000; Sigma, ON, Canada).
Immunocytochemistry
To determine the AR distribution in the cells, we performed immunohistochemistry on sympathetic cell cultures. Immunocytochemistry was performed as described previously (Vaillant et al., 2002). We used PG-21 (1:20) and a goat anti-mouse Alexa 488 (Life Technologies, Inc., ON, Canada) as the primary and secondary antibodies, respectively.
Axonal Transport: Primary neuron cultures
To determine whether testosterone affects axonal transport, we analyzed time-lapse imaging of fluorescent WGA vesicles in axons of cultured neurons from the superior cervical ganglia of neonatal rats (PN 5). This culture system was previously used to analyze both fast and slow axonal transport (Schwab et al., 1979; Wang and Brown, 2001, 2002). Sympathetic neuron cultures were prepared as described previously (Vaillant et al., 1999). Briefly, sympathetic neurons were dissected from superior cervical ganglia (SCG) of rat pups (postnatal days 3 to 5) supplied by Charles River Canada. Media used were supplemented with 2 mM L-glutamine (Cambrex, MD, USA) and 100 μg/ml penicillin/streptomycin (Cambrex). On Day 1, neurons were plated on collagen-coated 8-chamber cover slip (Nalge Nunc, NY, USA) at a density of 0.4 ganglia/well in Ultraculture medium (Cambrex) including 3% fetal bovine serum (FBS; Cambrex), 0.7 mM cytosine arabinoside (Sigma, ON, Canada) and 25 ng/ml nerve growth factor (NGF; CedarLane, ON, Canada). Neurons were plated in a corner of the well so that axons could be traced from the cell body to the growth cone, thus allowing us to determine the orientation of the axons in the time-lapse movies. On Day 3, the medium was changed to Ultraculture (w/o serum) including 20 ng/ml NGF, 0.4 μg/ml WGA Alexa-546-conjugated (Invitrogen-Molecular Probes, ON, Canada), and either Mibolerone, (Toronto Research Chemicals, ON, Canada) at concentration of 4nM (4M) or 10nM (10M) or ethanol vehicle control (Ctrl). On the same day, to observe the movement of vesicles following WGA internalization, cells were transferred to a 37°C air-heated stage on spinning disk confocal microscopy (Yokogawa, Japan) equipped with an x63 oil-immersion objective. WGA- Alexa-546-conjugated-vesicles were observed by using a fluoresceinisothio-cyanate (FITC) filter set. Consecutive confocal images were collected at a rate of 10 frames/sec. The data were collected for each condition at three different time points (06hrs, 24hr, 48hrs after application of WGA and Mibolerone/Vehicle).
Axonal Transport: Image Processing and Analysis
Data were collected from 20 tissues, each derived from a different litter. Each tissue was used for 360 “experiments” (wells) divided evenly across the three conditions (4M Mibolerone, 10M Mibolerone, Vehicle). An WGA-vesicle was considered mobile if it moved between two consecutive frames at a velocity > 0.1 μm/s. Motion analysis was performed by tracking the position of the vesicle in successive time-lapse image frames using Image-Pro 3D analyzer 7.0 software (Media Cybernetics, Inc). The rater was blind to the experimental conditions. Vesicle tracking data were grouped into periods of forward-moving and not-forward-moving movements (i.e., pauses). For each period of forward movement, distance (run length) and duration were calculated. To calculate an average velocity per vesicle (in μm/s), we divided the forward-run length by its duration. For each vesicle, we also calculated the percent time spent moving (In Motion%): duration of movement/duration of measurement X 100. In addition, we quantified peak velocity, maximum run-length and the ratio of anterograde to retrograde movement (Antero/Retro). Vesicle based statistics were tabulated automatically.
Axon diameter in primary neuron cultures
Axon diameter was evaluated in a subset of experiments derived from seven litters. For the analysis of axon diameter, control and Mibolerone-treated cells were fixed overnight in 2% formaldehyde, 0.1% glutaraldehyde. The next day, cells were prepared for the electron microscopy as described above. Axon diameter was calculated from micrographs taken at 20,000x magnification, using automatic analyses of the cross-sectional area as previously described. The axon diameter was calculated in about 2,000 axons in each condition. Since axons and dendrites are difficult to distinguish in cell cultures, only spatially isolated regions, far from the corner where neurons were plated were selected for observation. This minimized measurements of ambiguous neuronal processes. The rater was blind to the experimental conditions.
Statistical analyses
The data were analysed using JMP software (SAS Institute Inc). Standard methods were used to obtain summary statistics. Differences among groups were assessed by ANOVA or Student’s t test for continuous parameters, chi-square test for categorical parameters. The median values for axonal diameter and g ratio as well as the mean values of skeweness from M, F and M gx were compared using 1-way ANOVAs followed by Student’s t-tests (Bonferroni corrected for multiple comparisons). Logistic regression was used to assess the contribution of treatment and time for the prediction of directionality of vesicles movements. For all analyses, p< 0.05 (corrected) was considered significant.
Cohen’s d was also calculated as an indicator of effect size (the mean difference divided by the mean SD).
Results
I. Effect of testosterone on axon morphology in the splenium of young male and female rats
Animal body weight
As expected, body weight was higher in males than in females in both experiments (Experiment 1: M, 380.3 ± 5.2 g; F, 235 ± 6.4 g; n=6 per group; p=0.001); castrated males weighed less than intact males but more than females (Experiment 2: M, 396 ± 6.6 g; F, 240 ± 7.4 g; M gx 358 ± 5.7 g; n=10 per group; M vs. F, p= 0.001; M vs. M gx, p= 0.01; M gx vs. F, p= 0.001).
Axon diameter and g ratio
In Experiment 1, we observed sex differences in myelinated axon diameter, with males having larger axons than females (M, 529 ± 31.71; F, 456 ± 19.71; n=6 per group; p=0.001, percent difference = 14.82%, Cohen’s d effect size = 2.8; Figure 1C). This sex difference was confirmed in Experiment 2 (sham castrated male, M, 563 ± 41.84; sham castrated females, F, 469 ± 27.37; n=10 per group; p=0.001, percent difference = 18.22%, d= 2.7; Figure 2A [M, F]). Furthermore, in Experiment 2, we observed that castration (after weaning) was associated with smaller axon diameter (in young adulthood), as compared with intact males (M, 563 ± 41.84; M gx, 502 ± 21.24; n=10 per group; p=0.001, percent difference = 11.46%, d=1.84; Figure 2A) but did not differ from intact females (F, 469 ± 27.37; M gx, 502 ± 21.24; n=10 per group; p=0.069; Figure 2A). In contrast to myelinated axons, there were no sex differences in the diameter of unmyelinated axons (M, 229 ± 23.90; F 249 ±17.74; M gx, 502 ± 21.24; n=5 per group; p=0.5).
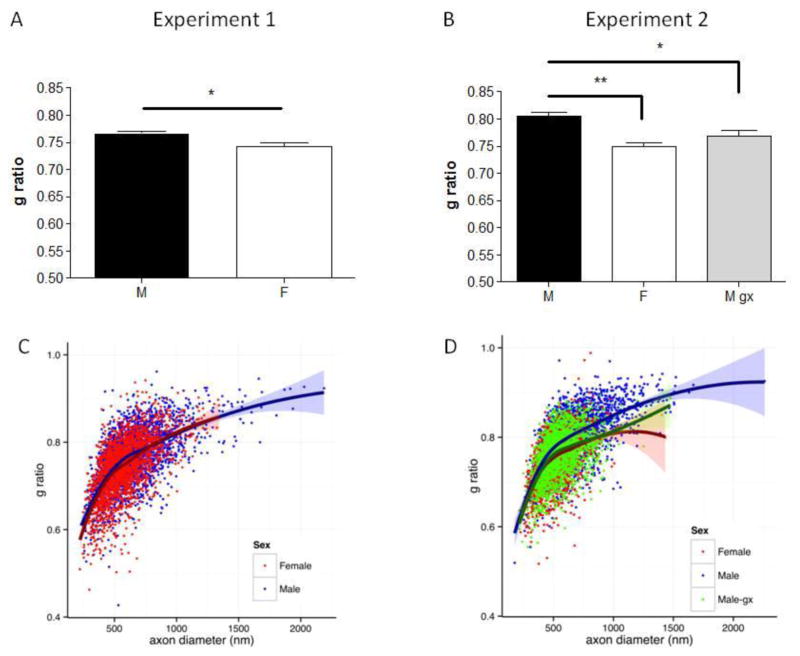
Figure 2.
A and B, Barplots showing the results of g ratio quantification in Experiment 1 and 2 respectively. The median values of g ratio from individual animals were used for analysis of variance. g ratio calculated in intact male and females was higher in males, as compared with females. Removal of endogenous T decreased g ratio in gonadectomized males to values similar to females. **p<0.01 vs. Males; *p<0.05 vs. Males. M = males; F = females; M gx = males gonadectomised.
C, Scatter plot of g ratio and axon diameter. The plot represents the grouped data from all axons of Experiment 1 in intact males (blue) and intact females (red). A Local Polynomial Regression curve (LOESS) is shown for each sex, with 95% confidence intervals as shaded bands.
D, Scatter plot of g ratio and axon diameter. The plot represents the grouped data from all axons of Experiment 2 in intact males (blue), gonadectomized males (green) and intact females (red). A Local Polynomial Regression curve (LOESS) is shown for each sex, with 95% confidence intervals as shaded bands. In both experiments, the average g ratio obtained in intact males appears to differ from both females and M gx for axons with diameters in the range of 400–800 nm. As can be seen from the plots, this range contains the majority of axons.
In addition to axon diameter, we evaluated g ratio in a subset of rats. In both Experiment 1 and 2, g ratio was higher in males compared with females. Castrated males had lower g ratio than intact males but did not differ from intact females (Experiment 1: M, 0.77 ± 0.01; F, 0.74 ± 0.02, n=6 per group, p=0.02, percent difference = 3.97%, d=1.89; Experiment 2: M, 0.81 ± 0.02; F, 0.75 ± 0.02; M gx 0.77 ± 0.02; n=5 per group; M vs. F, p= 0.004, percent difference = 7.69%, d=3.6; M vs. M gx p= 0.0195, percent difference = 5.06%, d=2.0; M gx vs. F, p= 0.209; Figures. 1D and 2B). Lastly, we plotted individual observations from both experiments to depict the relationship between axon diameter and g ratio (Figure 2C and D). The non-linear relationship between the two measures is demonstrated by the locally weighted scatterplot smoothing curves (LOESS). As expected, in both Experiment 1 and 2, g ratio increases as a function of axon diameter. Comparing males with both females and M gx shows divergence in the average g ratio for axons with diameters in the range of 400–800 nm. For statistical purposes, however, only Figures 2A and 2B should be considered as these represent group averages of g ratios (medians), with each median being based on about 400 myelinated axons per rat.
Axon diameter distribution
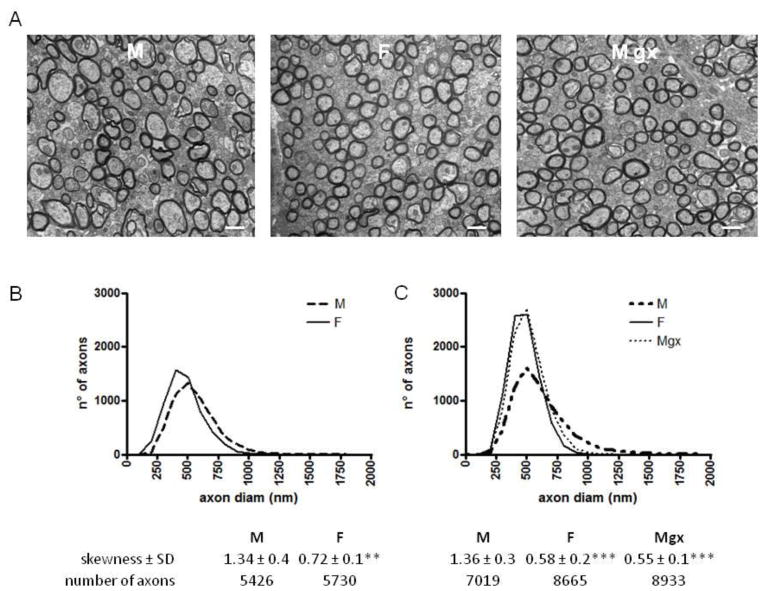
We then tested for the presence of sex differences in the distribution of axons with various diameters. For each group of rats, we characterized the distribution of axon diameter using the skewness ((diameter-m)/SD^3), a measure of the asymmetry of a distribution. Figure 3 provides an overview of the axon-diameter distributions in the two experiments. In both Experiment 1 and 2, the axon distribution of intact males spans a fairly broad range, with a positive skew toward larger diameter (Experiment 1: M skewness 1.34; Experiment 2: M skewness 1.36). In females, on the other hand, axon distribution was narrower and fairly symmetrical, with a smaller skew toward larger axons (Experiment 1: F skewness 0.72; Experiment 2: F skewness 0.58). Interestingly, in castrated males, axon distribution was nearly identical to that observed in intact females; it was narrow and fairly symmetrical, with a small skew (skewness of 0.55).
Figure 3.
A, Electron micrographs showing representative myelinated axons in cross sections of splenium of corpus callosum in an intact male, intact female and gonadectomized male rat.
B and C, Distribution of fiber diameters in Experiment 1 and Experiment 2. The distribution in intact males is broader, with a strong skew. Intact females and gonadectomized males show fairly symmetrical distribution, with a small skew. *** p<0.001 vs. males; **p<0.01 vs. males. M = males; F = females; M gx = males gonadectomized. Scale bars, 500 μm.
II. Effects of synthetic testosterone, Mibolerone, on axonal transport in primary neuron culture
Expression of AR in sympathetic neurons
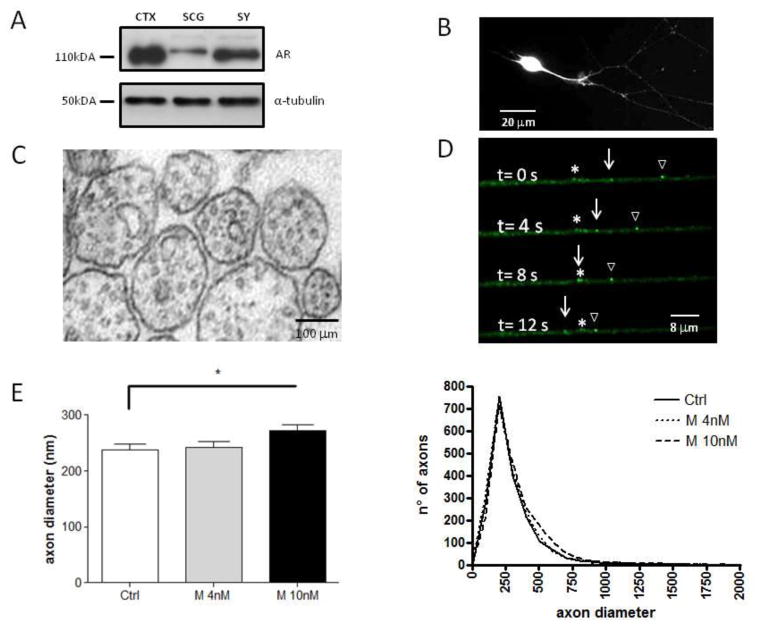
The presence of AR was evaluated on the total proteins extracted from superior cervical ganglion and sympathetic cell culture. Cerebral cortex of the rat was used as positive control (DonCarlos et al. 2006). Figure 4A shows results of the Western blots performed using the PG-21 antibody recognizing the first 21 amino acids of the human AR. Immunoreactive bands with a molecular weight close to 110 kDa, as expected for the AR protein (Sato et al., 1997), were present in all homogenates. A doublet was observed in lysates from SCG and sympathetic neurons (sy)reflecting the phosphorylated and unphosphorylated forms of the AR.
Figure 4.
A, Western blot showing expression of AR. Homogenates of superior cervical ganglion and sympathetic cell culture lysate show a band of the expected molecular weight for the AR protein (110 kDa), which coincides with the control band of brain cortex homogenate. α-tubulin was used as a loading control. CTX, cortex; SCG, superior cervical ganglion; Sy, sympathetic neurons.
B, Immunostaining for the AR epitope reveals AR expression throughout cell bodies and neurites. AR is homogeneously distributed in the cell body, and in a punctate pattern along the processes of sympathetic neurons. Scale bar = 20 μm.
C, Electron micrographs showing representative axonal cross-section from sympathetic neurons. Abundant round microtubule are visible as well as membranous organelles.
D, Example of WGA vesicles moving in anterograde direction, empty triangle sign and white arrow. Asterisk indicates a stationary WGA vesicle. The images shown here were selected from a time-lapse movie in which images were acquired at 10 images frames/second.
E, Barplots showing the results of axon diameter quantification in sympathetic neurons. Mean axon diameter of myelinated axons was greater in Mibolerone 10nM (n = 2065 axons) as compared with Control (n = 1915 axons) but not with Mibolerone 4nM (n = 1979 axons). *p < 0.05 vs. Mibolerone 10nM.
F, Distribution of fiber diameters in sympathetic cell culture. All three condition show similar distribution of diameters. Ctrl = Control; M 4nM = Mibolerone 4nM; M 10nM = Mibolerone 10nM.
To evaluate the distribution of ARs in the cells, PG-21 immunodetection was carried out in sympathetic cell culture (Figure 4B). As expected, a robust fluorescence was observed in the cell body. Moreover, a markedly punctuate distribution was also present along the axon indicating the presence of both nuclear AR and membrane AR in sympathetic neurons.
Time-lapse imaging of WGA-Alexa-546-conjugated-vesicles
To detect the movement of WGA-vesicles along the axon, we selected thin single axons; this allowed us to determine their polarity and to evaluate movement in both anterograde and retrograde direction. To quantify the WGA transport, 10 images frames/second were captured from a single axonal segment and converted into movies. We recorded time-lapse movies of 497 vesicles from 20 different experiments at 3 different time points (06hrs, 24hrs, 48hrs) each. The duration of the movies ranged from 30 to 60 sec. Table 1 summarized measurements of vesicle movements.
Table 1.
| Direction | Ctrl | M4 | M10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterograde | 06h | 24h | 48h |
00 00 |
06h | 24h | 48h |
00 00 |
06h | 24h | 48h |
| Number vesicles | 13 | 18 | 16 | 3 | 26 | 17 | 4 | 28 | 35 | ||
| In Motion (%) | 51 | 70 | 58 | 51 | 69 | 70 | 45 | 79 | 69 | ||
| Max run length (μm) | 14.5±9.7 | 16.8±9.0 | 14.1±4.8 | 18.9±6.5 | 16.36±6.9 | 18.9±8.2# | 19.53±26 | 23.7±11## | 18.6±8.3# | ||
| Average velocity (μ/sec) | 1±0.9 | 1.5±0.6 | 1.3±0.5 | 0.6±0.1 | 1.5±0.7 | 1.5±0.6 | 0.4±0.6 | 1.6±0.6*** | 1.4±0.5** | ||
| Peak velocity (μ/sec) | 1.4±1.2 | 1.8±0.7 | 1.7±0.7 | 0.7±0.2 | 1.9±0.6 | 1.9±0.6 | 1.0±0.3 | 1.9±0.6 | 1.7±0.7 | ||
| Retrograde | 06h | 24h | 48h | 06h | 24h | 48h | 06h | 24h | 48h | ||
| Number vesicles | 60 | 27 | 38 | 46 | 26 | 26 | 66 | 21 | 23 | ||
| In Motion (%) | 53 | 55 | 59 | 56 | 55 | 60 | 52 | 21 | 23 | ||
| Max run length (μm) | 12.7±6.9 | 13.4±7.3 | 14.5±8.1 | 12.9±8.1 | 12.2±6.1 | 16.1±9.7 | 14.4±10.5 | 15.0±10 | 15.5±9.7 | ||
| Average velocity (μ/sec) | 0.8±0.4 | 0.9±0.3 | 0.8±0.4 | 0.7±0.2 | 0.7±0.2 | 0.7±0.3 | 0.8±0.4 | 0.9±0.3 | 0.9±0.4 | ||
| Peak velocity (μ/sec) | 1.2±0.8 | 1.3±0.4 | 1.1±0.4 | 1.1±0.5 | 1.0±0.3 | 1.0±0.4 | 1.1±0.6 | 1.2±0.3 | 1.2±0.6 | ||
|
| |||||||||||
| Antero/Retro | 0.2 | 0.6 | 0.4 | 0.07 | 1 | 0.6 | 0.06 | 1.3 | 1.5 | ||
p<0.001 vs. 06h within the same condition;
p<0.01 vs. 06h within the same condition
p<0.01 vs. Ctrl within the same time point;
p<0.05 vs. Ctrl within the same time point
First, we inspected the movement of the WGA-vesicles in the axon. Consistent with in vivo studies, the movement of WGA-vesicles occurred in both directions (Gibson et al., 1984). Time-lapse imaging revealed a rapid movement of WGA-vesicles, which was often interrupted by prolonged pauses typical of membranous organelles (Lasek et al., 1984). For example, Figure 4D shows one WGA vesicle pausing and two moving in anterograde direction at different velocities. A few WGA-vesicles exhibited brief reversals of direction, but since these were rare and sustained for duration of less than 1 sec (and distributed equally across the three conditions), we excluded such reversal periods from the analysis.
Second, we analyzed the effect of Mibolerone on the directionality of vesicle movements. Logistic regression analysis revealed a significant conditions x time interaction (χ2 = 16.4, p= 0.0026) on the directionality. In particular, the effect of Mibolerone on the directionality of vesicle movements within each time points was as follows. At the first time point (06hrs), Mibolerone decreased (almost 10 fold) the ratio of anterograde to retrograde movement in both M4 and M10 conditions, relative to the control condition (Ctrl=0.2; M4=0.07; M10=0.06; Table 1); the contingency analysis of directionality showed a significant difference over the three conditions (χ2 = 6.9, p = 0.03). At the second time point (24 hrs), all groups increased the Antero/Retro ratio as compared with 06 hrs (Ctrl= 0.6; M4= 1; M10= 1.3), although no differences were observed across the three conditions with respect to the directionality (χ2 = 2.8, p = 0.25). Only the M10 treatment sustained a high Antero/Retro ratio of movement at 48 hrs (Ctrl= 0.4; M4= 0.6; M10= 1.5, χ2 = 11.2, p = 0.004).
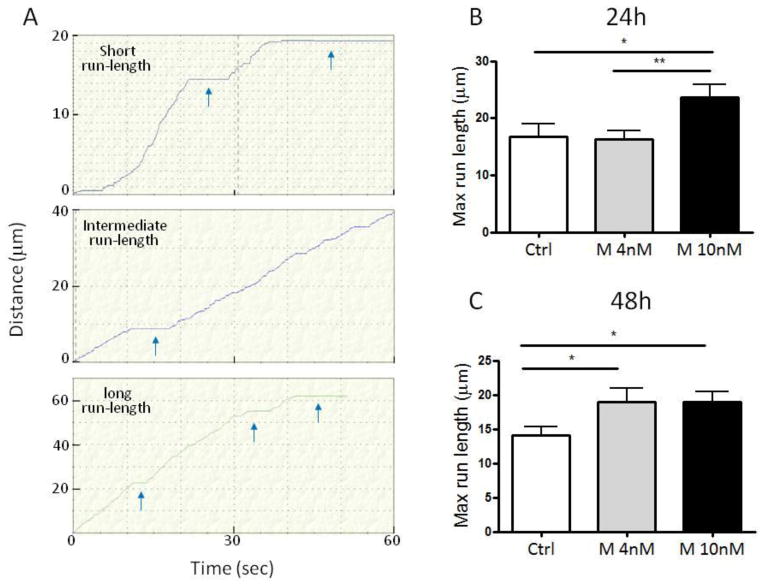
Next, we calculated the effect of treatment on the longest-distance traveled (max run-length, Figure 5) by vesicles. Figure 5A show the motile behavior of three representative anterograde-moving vesicles exhibiting different run lengths. We found an increase in max run-length by anterograde-moving vesicles at 48 hrs in both M4 and M10 conditions as compared with control (Figure. 5C; M4 vs. Ctrl p= 0.048; M10 vs. Ctrl p= 0.032). The maximum run-length was higher also at 24 hrs in M10 condition as compared with M4 and control (Figure. 5B; M10 vs. M4 p= 0.006; M10 vs. Ctrl p= 0.036). The run length is a measure of the processivity of motor proteins (Thorn et al., 2000). Thus, the increased run-length observed here indicates that Mibolerone treatment is able to enhance the processivity of kinesin proteins in a dose-dependent manner. The treatment with Mibolerone at the dose of 10 nM also appeared to increase the average velocity of the anterograde movement of WGA-vesicles at both 24 and 48 hrs, as compared with 06 hours (Table 1). Unlike the above observations about the treatment- and time-related changes in anterograde transport, the number of vesicles moving in a retrograde direction, their max run-length and average velocity, were remarkably homogeneous across the three treatment conditions at each time point, suggesting that androgens regulate the efficiency of axonal transport only in the anterograde direction (Table 1).
Figure 5.
A, Graphical representation of anterograde movement of three representative vesicles at 48 hrs. The Y-axis represent the distance of the vesicle from its initial location. The X-axis represents the time of the movie. In some cases the acquisition interval was less than 60 sec because the vesicle move outside of the imaged region.
B, Barplot of average max run-length (the longest distance traveled by vesicles between two pauses) in anterograde direction at 24 hrs. Control (n = 18); Mibolerone 4nM (n = 26); Mibolerone 10nM (n = 28). **p< 0.01 vs. Mibolerone 10nM; *p < 0.05 vs. Mibolerone 10nM.
C, Barplot of average max run-length in anterograde direction at 48 hrs. Ctrl (n = 16); Mibolerone 4nM (n = 17); Mibolerone 10nM (n = 35). *p < 0.05 vs. Mibolerone 10nM. Ctrl = Control; M 4nM = Mibolerone 4nM; M 10nM = Mibolerone 10nM.
Axon diameter
Since axonal transport plays an important role in regulating axon diameter (Eyer and Peterson, 1994; Xia et al., 2003), we measured axon diameter of the sympathetic neurons after 48h of treatment in presence of either ethanol (Ctrl) or Mibolerone at 4nM and 10nM. The quantitative morphometry of about 2000 cross-section area, revealed a significant increase of axon diameter in M10 condition as compared with Ctrl. (Ctrl, 237 ± 28.67; M10, 273 ± 26.88, n=7 per group, p=0.03, percent difference = 14.12%, d=1.28; Figure 4C). Treatment with Mibolerone at 4nM did not affect the axonal caliber. This is not surprising given that this dose increased the efficiency of WGA axonal transport (max run length) only after 48 hrs, with no effect on the mean velocity and Antero/Retro ratio. Only the high dose (10 nm) increased the run length at 24 hrs, and the mean velocity and the Antero/Retro ratio of movement at 48 hrs. The effect on axon radial growth observed with the 10-nm dose of Mibolerone suggests that, at least in sympathetic neurons, androgens influence the axonal radial growth without being aromatized to estrogen.
Axon diameter distribution
Lastly, we evaluated the distribution of axons in the three conditions. The distributions of axon diameters for the three conditions essentially superimpose (Fig. 4). They all peak near 250 nm and all have similar skew (Ctrl= 2.45; M4= 2.34 and M10= 3.32)
Discussion
Mechanisms underlying sexual dimorphism in the maturation of WM are of considerable interest because of sex-specific vulnerabilities to psychopathology during adolescence. As demonstrated in our previous studies in humans, WM sexual dimorphisms become more prominent during adolescence under the influence of testosterone (Perrin et al., 2008, 2009).
Given this dependence of WM maturation on levels of bioavailable testosterone, here we sought to understand how this difference is sustained at the microstructural level. We use the electron microscopy to measure the diameter of myelinated and unmyelinated axons, and g ratio of myelinated axons in the splenium of intact male and female rats, and after real or sham castration carried out after weaning. Our results revealed a sex difference in myelinated axonal diameter in early adulthood (M>F; Cohen’s d = 2.7). We also found a sex difference in g ratio (M>F; d = 3.7). Furthermore, removal of endogenous T by castration before puberty was associated with lower myelinated axon diameter and g ratio, as compared with intact males (d = 1.84 for axon diameter and d = 2.0 for g ratio).
To our knowledge, these observations are the first to show sex differences in the diameter and g ratio of myelinated axons. Previous studies have not reported such sex differences in the diameter of myelinated axons in young adult rats (Kim and Juraska, 1997; Kim et al., 1996). A possible reason for this discrepancy could be the strategy used to measure axon diameter. In the previous studies, the average axon diameter was estimated by measuring only the shortest diameter of each axon (in ~ 600 axons per animal). But measuring the actual diameter may not provide accurate estimates of axonal size because axons are not precisely circular. For this reason, we measured automatically the cross-sectional area of each axon and then calculated the diameter of the equivalent circle; we have done so in about 1,000 axons per animal. The relative power of our study, and the replication of these sex differences in Experiment 2, gives us some confidence in the validity of our findings. An alternative explanation may lie in the different age of rats examined. Kim and Juraska analyzed sex differences at 60 days of age, whereas we used 70 days old rats. Normally, testosterone levels rise gradually from postnatal day (PND) 20 to 40, and abruptly double by PND 50 (Matsumoto et al., 1986; Monosson et al., 1999), with a peak of testosterone concentration occurring around 50–60 days of age (Ojeada and Urbansky 1994). It is therefore possible that in these studies, animals have been analyzed before the maximal effect of testosterone on the organization of neural circuits took place. Androgens are produced in testis and adrenals; moreover, they could be also synthesized de novo in the brain (Baulieu, 1998). In the present study, we did not analyze changes on androgens levels after castration. In a previous study, others have demonstrated that gonadectomy is able to decrease considerably the levels of testosterone and its metabolite, dihydrotesterone, both in plasma and the central nervous system when compared with the intact animals (Caruso et al., 2010).
We speculate that the thicker axons found in males reflect a role of testosterone in modulating the cellular processes underlying the radial growth of axons rather than the myelin thickness.
On surface, this finding appears inconsistent with previous results reporting a strong effect of androgens in modulating myelination (Cerghet et al., 2006; Hussain et al., 2013). But these studies estimated the amount of myelin by the number of myelinated axons, oligodentrocytes or expression of myelin proteins rather than by measuring g ratio. In our study, we evaluated the effect of testosterone on myelin volume relatively to the axonal volume. As mentioned above, g ratio increases as a function of axon diameter, indicating that the myelin thickness does not scale linearly with the axon diameter (Berthold et al., 1983; Chatzopoulou et al., 2008; Gillespie and Stein, 1983). Here, we confirm this finding and extend it by showing that the average g ratios were higher in males as compared with both females and castrated males (Figure 2A and B), further demonstrating a role of exogenous testosterone in regulating this process.
Our data do not speak to the question of possible mechanisms by which androgens regulate axon caliber. A number of possibilities exist. Androgens might affect a turnover of cytoskeletal matrix responsible for the axonal structure. In this regard, it is known that phosphorylation of both the C-terminal regions and the head domain of neurofilaments contributes to the regulation of the interactions of neurofilaments with each other, neurofilaments with microtubules, and the interactions between microtubules and motor proteins, the latter responsible for axonal transport (Julien and Mushynski, 1998). These processes may modulate the dynamics of the formation of the neurofilament-based cytoskeletal lattice supporting mature axons (Shea and Chan, 2008; Yuan et al., 2012). Androgens can modulate phosphorylation-dependent changes in protein activity by AR-dependent activation of the mitogen-activated protein kinase (MAPK/ERK) signal transduction (Nguyen et al., 2005). Androgen receptors may also exert rapid nongenomic effects through the rapid rise of intracellular calcium concentration (Ca2+) (Foradori et al., 2008; Sarkey et al., 2008). Through these two distinct mechanisms, androgen can stimulate axon extension, axon branching and axonal radial growth (Estrada et al., 2006; Marron et al., 2005; Shughrue and Dorsa, 1994). It appears that androgens affect preferentially axon diameter and axon branching presumably by activation of distinct effector pathways (Lustig et al., 1994; Markus et al., 2002; Sun et al., 2003). We speculate that testosterone, by inducing neurofilament phosphorylation, can be in part responsible for the amount of neurofilaments undergoing axonal transport and in doing so, the turnover of cytoskeletal matrix responsible for the axonal radial growth. Furthermore, phosphorylation of neurofilaments is also upregulated by signals originating from myelin (Nixon et al., 1994; Sánchez et al., 2000; Starr et al., 1996); this may be one of the mechanisms responsible for the expansion of axonal diameter during development. But our in vitro results suggest that the presence of myelin is not necessary for androgen-related axonal growth. Thus, the higher diameter of axons found in cultured sympathetic neurons treated with the non-aromatizable Mibolerone (vs. vehicle-treated controls) suggests that, at least in vitro, the effect on axonal radial growth can be modulate by androgens through direct activation of AR without the interaction with glial cells. On the other hand, the absence of androgen-related effects on the diameter of unmyelinated axons observed in our in vivo experiments suggest that, in myelinated axons androgens may influence axon diameter both directly (via AR acting on cytoskeleton) and indirectly (via myelin acting on cytoskeleton.
A large body of literature also indicates a positive effect of ovarian hormones on myelin formation, synthesis of myelin protein and myelin thickness. These effects can, in turn, affect the g ratio (Baulieu and Schumacher, 2000; Crawford et al., 2010; Garcia-Segura and Melcangi, 2006; Marin-Husstege et al., 2004). Therefore we cannot exclude the possibility that estrogen-mediated increase of myelination might be in part responsible for sex difference in WM maturation during puberty.
Can the testosterone-dependent increase in axon diameter affect the axon-diameter distribution? When we compared the distribution across the three groups (M, F, M gx), the results were surprising: while males showed a skewed distribution typical for most myelinated fiber-tracts (Olivares et al. 2000; Wang et al. 2008; Perge et al. 2012), the distribution in females was fairly symmetrical. Such a skewed distribution of axon diameters has been observed previously in the corpus callosum of male monkeys (Macaca fascicularis and Macaca mulatta; Tomasi et al. 2012). In our study, removal of endogenous testosterone by castration resulted in a female-like distribution in these males. Thus, circulating testosterone appears to shape the distribution of axons with different diameter (i.e., a large-axon tail) rather than simply shifting the mean towards higher values. Since the axon diameter contributes more than the connection length to the broadening of the spectrum of conduction velocities (Caminiti et al., 2013), we speculate that the observed sex differences in the axon-diameter distributions might be reflected in the broader range of conduction velocities in males, as compared with females. Future work is necessary to elucidate further this hypothesis. Unlike what we observed in vivo, mibolerone did not influence the axon-diameter distributions in sympathetic neurons. Since axon-diameter distribution within nerve fascicles is influenced by the anatomical constraints, namely a fixed number of fibers in an available volume (Pajevic and Basser, 2013), it is possible that the lack of such a constraint influenced the distribution of axon diameter in neurons developing in the low-density culture.
Axon growth is a complex mechanism that requires a fine orchestration of stimuli to be adequately directed to modulate synthesis and transport of axonal structural components (Goldberg, 2003). Several lines of evidence have supported axonal transport mechanism as one of the key component of axonal radial growth (Eyer and Peterson, 1994; Hoffman, 1995, 1985; Hoffman et al., 1984; Medori et al., 1985; Xia et al., 2003). Therefore, we also sought to evaluate the possible influence of Mibolerone in regulating axonal transport. Mibolerone is an highly specific synthetic non-aromatizable androgen that, like testosterone, is a ligand of the AR (Markiewicz and Gurpide, 1997). We decided to use a synthetic ligand for AR to exclude any possible effect mediated through the aromatization of testosterone into estradiol. We showed that AR is present in both cell bodies and axons of rat sympathetic neurons indicating that androgens can exert their effects on these cells through genomic and nongenomic pathways. We used time-lapse microscopy to quantify the effect of Mibolerone on bidirectional transport of WGA vesicles in living neurons. Detailed quantification of vesicles’ behavior revealed two distinct phenomena that are presumably mediated by nongenomic and genomic pathways, respectively: 1) We found a depression of the motion in anterograde direction at the first time point (06 hrs); and 2) The anterograde movement of vesicles increased in dose-dependent manner becoming strongly favored over the retrograde transport in cells treated with 10 nM Mibolerone at 48hrs. A possible mechanism underlying depression of axonal transport at the short timescale may involve the activation of putative membrane-associated androgen receptors (mAR), which generally induces intracellular free calcium (Ca2+) flux (Estrada et al., 2006). These calcium oscillations are detected by specific sensor molecules, including protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CaMKII), able to induce signal transduction cascade and to modulate transcription activity implicated in many cellular processes (Mellström and Naranjo, 2001; Pelley et al., 2006). Recent work in mammalian neurons suggests that changes in local Ca2+ concentration as well as activation of CaMKII play an important part in regulating the dissociation of kinesins from microtubule or their cargoes facilitating the proper spatial and temporal dissociation of motors from their cargos (Guillaud et al., 2008; Wang and Schwarz, 2009). We speculate that the Mibolerone-mediated activation of Ca2+ levels may be responsible for the depression in anterograde motion induced by Mibolerone during the first six hours after its application. Surprisingly, long-term exposure to Mibolerone (48 hrs) resulted in an opposite effect: increasing the anterograde transport over the retrograde one. We found that, at high concentration (10 nM), the percentage of vesicles moving in anterograde direction was higher by 68% and 42% as compared, respectively, with the control condition and the low concentration of Mibolerone (4 nM). Moreover, we found an increase in the travelled distance by anterograde-moving vesicles in cells treated with Mibolerone 10 nM at 24 hrs and in both 10 nM and 4 nM treatments at 48 hrs as compared with control. In vitro studies suggest that cargoes that are attached to multiple motor-proteins are able to move further and faster than cargoes that engaged only one motor (Derr et al., 2012; Reis et al., 2012). It is possible that the long-term exposure to Mibolerone, through the activation of classical nuclear AR, is able to increase the expression of kinesin motor proteins. This may allow recruitment of multiple kinesin motors onto a single cargo leading to increases in both the rate of anterograde transport and the length of uninterrupted anterograde movements (run length). Since the force of kinesins is stronger than the opposing force of dynein motors, a small increase in the number of kinesins will be sufficient to change the directionality of transport in favour of anterograde transport (Holzbaur and Goldman, 2010). As mentioned above, axonal transport of cytoskeletal elements, mRNA, lipids and cofactors is essential for the axon growth (Martenson et al., 1993; McQuarrie and Grafstein, 1982; Vance et al., 2000; Zhang et al., 2001). In particular, expression and transport of neurofilaments, the major cytoskeletal protein within axons (Lee and Cleveland, 1996), appear to be intrinsic determinants of axon diameter; dysregulation of these processes results in an impaired radial growth of the axon (Cleveland et al., 1991; Eyer and Peterson, 1994; Hoffman, 1995; Hoffman et al., 1984; Xia et al., 2003). Available evidence suggests that neurofilaments are transported in both anterograde and retrograde directions through direct interactions with kinesin and dynein motor proteins (Jung et al., 2005; Wagner et al., 2004). (Jung et al., 2005; Wagner et al., 2004). The dynein-dynactin complex is responsible for the retrograde (minus end-directed) motion of neurofilaments on microtubules while a number of kinesin-related proteins transport neurofilaments in the anterograde (plus end) direction (Shah et al., 2000). Kinesin-I is one of the kinesin-family proteins involved in moving neurofilaments along the axon in anterograde direction, as suggested by studies of the kinesin-I heavy chain (KIF5A) knockout mice and analyses of the effect of kinesin-I mutation on neurofilament transport in cultured neurons (Wang and Brown, 2010; Xia et al., 2003). In addition to these classical motor proteins, microfilament-based myosin VA appears to play an important role in neurofilament transport by delivering them to their microtubule tracks. This process may reduce the duration of pauses and, in turn, enhance the efficiency of neurofilament transport in axons (Alami et al., 2009). The majority of neurofilaments move in anterograde direction with a rate of movement that ranges from 0.14 to 1.4 μm/sec (Roy et al., 2000; Wang and Brown, 2001); an inactivation of anterograde motor proteins (kinesins) results in deficit in axonal transport of neurofilaments and a reduced number of large caliber (sensory) axons (Xia et al., 2003). On the other hand, phosphorylation of neurofilaments may decrease their association with kinesin (Yabe et al., 2000, 1999), thus resulting in the accumulation of neurofilaments and subsequent radial growth of the axon (Nixon et al., 1994; Sánchez et al., 2000). The above observations suggest a role of kinesin-mediated transport of neurofilaments in regulating axonal radial growth. While the present study does not address molecular mechanisms underlying the action of testosterone on axon diameter, our findings suggest that the larger diameter found in males compared with both females and castrated males, as well as the bigger diameter found in sympathetic neuron treated with Mibolerone 10 nM, could be in part a result of the modulatory effect of testosterone on the expression levels of motor proteins and dynamics of cytoskeletal components. Further studies will be necessary to examine this possibility.
It is worth remembering that AR expression varies among distinct neuronal population of the brain with different patterns of expression through the postnatal development period in males and females. This specific context may determine selective susceptibility of specific neuronal populations to androgen actions resulting in a differential impact on axonal transport (Fernández-Guasti et al., 2000; Ravizza et al., 2002; Sheng et al., 2004). In addition, based on the relative ratio of the putative membrane AR and the classical nuclear AR, androgens may result in different effects, regulating the axon transport via distinct and potentially competing signaling pathways.
Overall, these findings support our previous hypothesis of a role of testosterone in regulating axon biology. In particular, testosterone seems to exert its effects at two levels: (1) structural - modulating the cellular processes that affect cytoskeleton and, in turn, radial growth of the axon; (2) molecular - modulating the directionality of axon transport in a time- and dose-dependent manner. The latter phenomenon – especially the rapid switch between testosterone -induced suppression and enhancement of anterograde transport - might represent a mechanism underlying some of the known effects of androgens on behavior and mental health during puberty (Patton and Viner, 2007).
Highlights.
In humans, volume and properties of white matter change during male adolescence
Here we show that axons are thicker in male than female rats
Castration at weaning eliminates this structural sex difference
Synthetic testosterone affects axonal transport in vitro
Acknowledgments
This work was funded by the National Institutes of Health (5R01MH085772). We acknowledge the excellent technical assistance with electron microscopy of Mr. Battista Calvieri and Mr. Steven Doyle from the University of Toronto’s Microscopy Imaging Laboratory. We also thank Celine Bourdon, Yimiao Ou and Mei Huang for advice, technical assistance and help with various experimental procedures.
Footnotes
Conflict of interest: The authors do not have any conflict of interest related to the reported work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alami NH, Jung P, Brown A. Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J Neurosci. 2009;29:6625–34. doi: 10.1523/JNEUROSCI.3829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazany D, Basser PJ, Assaf Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain. 2009;132:1210–20. doi: 10.1093/brain/awp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DM, Carpenter C, Yager C, Golik B, Barry KJ, Shen H, Mikse O, Eggert LS, Schulz DJ, Garcia ML. Variation of the neurofilament medium KSP repeat sub-domain across mammalian species: implications for altering axonal structure. J Exp Biol. 2010;213:128–36. doi: 10.1242/jeb.033787. [DOI] [PubMed] [Google Scholar]
- Baulieu E, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Steroids. 2000;65:605–12. doi: 10.1016/s0039-128x(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Berthold CH, Nilsson I, Rydmark M. Axon diameter and myelin sheath thickness in nerve fibres of the ventral spinal root of the seventh lumbar nerve of the adult and developing cat. J Anat. 1983;136:483–508. [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Carducci F, Piervincenzi C, Battaglia-Mayer A, Confalone G, Visco-Comandini F, Pantano P, Innocenti GM. Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: comparing data from histology and magnetic resonance imaging diffusion tractography. J Neurosci. 2013;33:14501–11. doi: 10.1523/JNEUROSCI.0761-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Melcangi RC. Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J Neuroendocrinol. 2010;22:1137–47. doi: 10.1111/j.1365-2826.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26:1439–47. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzopoulou E, Miguez A, Savvaki M, Levasseur G, Muzerelle A, Muriel MP, Goureau O, Watanabe K, Goutebroze L, Gaspar P, Zalc B, Karagogeos D, Thomas JL. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J Neurosci. 2008;28:7624–36. doi: 10.1523/JNEUROSCI.1103-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci. 2003;23:103–11. doi: 10.1523/JNEUROSCI.23-01-00103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Monteiro MJ, Wong PC, Gill SR, Gearhart JD, Hoffman PN. Involvement of neurofilaments in the radial growth of axons. J Cell Sci Suppl. 1991;15:85–95. doi: 10.1242/jcs.1991.supplement_15.12. [DOI] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pagès M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–34. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, Voskuhl RR, Tiwari-Woodruff SK. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr ND, Goodman BS, Jungmann R, Leschziner AE, Shih WM, Reck-Peterson SL. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. Science. 2012;338:662–5. doi: 10.1126/science.1226734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138:801–7. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Durdiakova J, Ostatnikova D, Celec P. Testosterone and its metabolites--modulators of brain functions. Acta Neurobiol Exp (Wars) 2011;71:434–54. doi: 10.55782/ane-2011-1863. [DOI] [PubMed] [Google Scholar]
- Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci. 2006;119:733–43. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- Eyer J, Peterson A. Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron. 1994;12:389–405. doi: 10.1016/0896-6273(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–35. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Foradori C, Weiser M, Handa R. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005.Non-genomic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Melcangi RC. Steroids and glial cell function. Glia. 2006;54:485–98. doi: 10.1002/glia.20404. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–64. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Hansma DI, Houk JC, Robinson FR. A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res. 1984;298:235–41. doi: 10.1016/0006-8993(84)91423-9. [DOI] [PubMed] [Google Scholar]
- Gillespie MJ, Stein RB. The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res. 1983;259:41–56. doi: 10.1016/0006-8993(83)91065-x. [DOI] [PubMed] [Google Scholar]
- Goldberg JL. How does an axon grow? Genes Dev. 2003;17:941–58. doi: 10.1101/gad.1062303. [DOI] [PubMed] [Google Scholar]
- Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–92. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–38. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hoffman PN. Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J Cell Biol. 1985;101:1332–1340. doi: 10.1083/jcb.101.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PN. Review: The Synthesis, Axonal Transport, and Phosphorylation of Neurofilaments Determine Axonal Caliber in Myelinated Nerve Fibers. Neurosci. 1995;1:76–83. doi: 10.1177/107385849500100204. [DOI] [Google Scholar]
- Hoffman PN, Griffin JW, Price DL. Control of axonal caliber by neurofilament transport. J Cell Biol. 1984;99:705–14. doi: 10.1083/jcb.99.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur ELF, Goldman YE. Coordination of molecular motors: from in vitro assays to intracellular dynamics. Curr Opin Cell Biol. 2010;22:4–13. doi: 10.1016/j.ceb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R, Ghoumari AM, Bielecki B, Steibel J, Boehm N, Liere P, Macklin WB, Kumar N, Habert R, Mhaouty-Kodja S, Tronche F, Sitruk-Ware R, Schumacher M, Ghandour MS. The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain. 2013;136:132–46. doi: 10.1093/brain/aws284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP, Mushynski WE. Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol. 1998;61:1–23. doi: 10.1016/s0079-6603(08)60823-5. [DOI] [PubMed] [Google Scholar]
- Jung C, Lee S, Ortiz D, Zhu Q, Julien JP, Shea TB. The high and middle molecular weight neurofilament subunits regulate the association of neurofilaments with kinesin: inhibition by phosphorylation of the high molecular weight subunit. Brain Res Mol Brain Res. 2005;141:151–5. doi: 10.1016/j.molbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16:1429–31. doi: 10.1096/fj.02-0131fje. [DOI] [PubMed] [Google Scholar]
- Kim JH, Juraska JM. Sex differences in the development of axon number in the splenium of the rat corpus callosum from postnatal day 15 through 60. Brain Res Dev Brain Res. 1997;102:77–85. doi: 10.1016/s0165-3806(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Kim JHY, Ellman A, Juraska JM. A re-examination of sex differences in axon density and number in the splenium of the rat corpus callosum. 1996;740:47–56. doi: 10.1016/s0006-8993(96)00637-3. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Garner JA, Brady ST. Axonal transport of the cytoplasmic matrix. J Cell Biol. 1984;99:212s–221s. doi: 10.1083/jcb.99.1.212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- Lustig R, Hua P, Smith L. An in Vitro Model for the Effects of Androgen on Neurons Employing Androgen Receptor-Transfected PC12 Cells. Mol Cell …. 1994 doi: 10.1006/mcne.1994.1072. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26:245–54. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- Markiewicz L, Gurpide E. Estrogenic and progestagenic activities of physiologic and synthetic androgens, as measured by in vitro bioassays. Methods Find Exp Clin Pharmacol. 1997;19:215–22. [PubMed] [Google Scholar]
- Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Marron TU, Guerini V, Rusmini P, Sau D, Brevini TaL, Martini L, Poletti a. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- Martenson C, Stone K, Reedy M, Sheetz M. Fast axonal transport is required for growth cone advance. Nature. 1993;366:66–9. doi: 10.1038/366066a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Karpas AE, Southworth MB, Dorsa DM, Bremner WJ. Evidence for activation of the central nervous system-pituitary mechanism for gonadotropin secretion at the time of puberty in the male rat. Endocrinology. 1986;119:362–9. doi: 10.1210/endo-119-1-362. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Protein synthesis and axonal transport in goldfish retinal ganglion cells during regeneration accelerated by a conditioning lesion. Brain Res. 1982;251:25–37. doi: 10.1016/0006-8993(82)91270-7. [DOI] [PubMed] [Google Scholar]
- Medori R, Autilio-Gambetti L, Monaco S, Gambetti P. Experimental diabetic neuropathy: impairment of slow transport with changes in axon cross-sectional area. Proc Natl Acad Sci U S A. 1985;82:7716–20. doi: 10.1073/pnas.82.22.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B, Naranjo JR. Mechanisms of Ca(2+)-dependent transcription. Curr Opin Neurobiol. 2001;11:312–9. doi: 10.1016/s0959-4388(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Midorikawa R, Takei Y, Hirokawa N. KIF4 motor regulates activity-dependent neuronal survival by suppressing PARP-1 enzymatic activity. Cell. 2006;125:371–83. doi: 10.1016/j.cell.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE. Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol Ind Health. 1999;15:65–79. doi: 10.1177/074823379901500107. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–93. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y, Weber B, Saleem KS, Augath M, Logothetis NK. Tracing neural circuits in vivo with Mn-enhanced MRI. Magn Reson Imaging. 2006;24:349–58. doi: 10.1016/j.mri.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Nguyen T-VV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–51. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Paskevich PA, Sihag RK, Thayer CY. Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J Cell Biol. 1994;126:1031–46. doi: 10.1083/jcb.126.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares R, Michalland S, Aboitiz F. Cross-species and intraspecies morphometric analysis of the corpus callosum. Brain Behav Evol. 2000;55:37–43. doi: 10.1159/000006640. 6640. [DOI] [PubMed] [Google Scholar]
- Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav Evol. 2001;57:98–105. doi: 10.1159/000047229. 47229. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Raven Press, Inc; New York, NY: 1994. pp. 363–409. [Google Scholar]
- Pajevic S, Basser PJ. An optimum principle predicts the distribution of axon diameters in normal white matter. PLoS One. 2013;8:e54095. doi: 10.1371/journal.pone.0054095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–9. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T, Pesaresi M, French L. White matter as a transport system. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.055. [DOI] [PubMed] [Google Scholar]
- Pelley RP, Chinnakannu K, Murthy S, Strickland FM, Menon M, Dou QP, Barrack ER, Reddy GPV. Calmodulin-androgen receptor (AR) interaction: calcium-dependent, calpain-mediated breakdown of AR in LNCaP prostate cancer cells. Cancer Res. 2006;66:11754–62. doi: 10.1158/0008-5472.CAN-06-2918. [DOI] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Perge Ja, Niven JE, Mugnaini E, Balasubramanian V, Sterling P. Why do axons differ in caliber? J Neurosci. 2012;32:626–38. doi: 10.1523/JNEUROSCI.4254-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Hervé PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–24. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot a, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45:1055–66. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Perrot R, Julien JP. Real-time imaging reveals defects of fast axonal transport induced by disorganization of intermediate filaments. FASEB J. 2009;23:3213–25. doi: 10.1096/fj.09-129585. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, de Webster HF. Fine Structure of the Nervous System: Neurons and Their Supporting Cells. Oxford University Press; USA: 1991. [Google Scholar]
- Ravizza T, Galanopoulou AS, Velísková J, Moshé SL. Sex differences in androgen and estrogen receptor expression in rat substantia nigra during development: an immunohistochemical study. Neuroscience. 2002;115:685–96. doi: 10.1016/s0306-4522(02)00491-8. [DOI] [PubMed] [Google Scholar]
- Reis GF, Yang G, Szpankowski L, Weaver C, Shah SB, Robinson JT, Hays TS, Danuser G, Goldstein LSB. Molecular motor function in axonal transport in vivo probed by genetic and computational analysis in Drosophila. Mol Biol Cell. 2012;23:1700–14. doi: 10.1091/mbc.E11-11-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Coffee P, Smith G, Liem RK, Brady ST, Black MM. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J Neurosci. 2000;20:6849–6861. doi: 10.1523/JNEUROSCI.20-18-06849.2000. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez I, Hassinger L, Sihag RK, Cleveland DW, Mohan P, Nixon Ra. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J Cell Biol. 2000;151:1013–24. doi: 10.1083/jcb.151.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm Behav. 2008;53:753–64. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Sadar MD, Bruchovsky N, Saatcioglu F, Rennie PS, Sato S, Lange PH, Gleave ME. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J Biol Chem. 1997;272:17485–94. doi: 10.1074/jbc.272.28.17485. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Suda K, Thoenen H. Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J Cell Biol. 1979;82:798–810. doi: 10.1083/jcb.82.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JV, Flanagan LA, Janmey PA, Leterrier JF. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol Biol Cell. 2000;11:3495–508. doi: 10.1091/mbc.11.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea TB, Chan WKH. Regulation of neurofilament dynamics by phosphorylation. Eur J Neurosci. 2008;27:1893–901. doi: 10.1111/j.1460-9568.2008.06165.x. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res. 2004;49:185–96. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Dorsa DM. Estrogen and androgen differentially modulate the growth-associated protein GAP-43 (neuromodulin) messenger ribonucleic acid in postnatal rat brain. Endocrinology. 1994;134:1321–8. doi: 10.1210/endo.134.3.8119173. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Starr R, Attema B, DeVries GH, Monteiro MJ. Neurofilament phosphorylation is modulated by myelination. J Neurosci Res. 1996;44:328–37. doi: 10.1002/(SICI)1097-4547(19960515)44:4<328::AID-JNR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Steinsapir J, Socci R, Reinach P. Effects of androgen on intracellular calcium of LNCaP cells. Biochem Biophys Res Commun. 1991;179:90–6. doi: 10.1016/0006-291x(91)91338-d. [DOI] [PubMed] [Google Scholar]
- Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85α, androgen receptor, and Src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- Thorn KS, Ubersax JA, Vale RD. Engineering the processive run length of the kinesin motor. J Cell Biol. 2000;151:1093–100. doi: 10.1083/jcb.151.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi S, Caminiti R, Innocenti GM. Areal differences in diameter and length of corticofugal projections. Cereb Cortex. 2012;22:1463–72. doi: 10.1093/cercor/bhs011. [DOI] [PubMed] [Google Scholar]
- Ullén F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biol. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol. 1999;146:955–66. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron. 2002;34:985–98. doi: 10.1016/s0896-6273(02)00717-1. [DOI] [PubMed] [Google Scholar]
- Vance JE, Campenot RB, Vance DE. The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim Biophys Acta. 2000;1486:84–96. doi: 10.1016/s1388-1981(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Wagner OI, Ascaño J, Tokito M, Leterrier JF, Janmey PA, Holzbaur ELF. The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell. 2004;15:5092–100. doi: 10.1091/mbc.E04-05-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown a. Rapid intermittent movement of axonal neurofilaments observed by fluorescence photobleaching. Mol Biol Cell. 2001;12:3257–67. doi: 10.1091/mbc.12.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Brown A. A hereditary spastic paraplegia mutation in kinesin-1A/KIF5A disrupts neurofilament transport. Mol Neurodegener. 2010;5:52. doi: 10.1186/1750-1326-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SSH, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. J Neurosci. 2008;28:4047–56. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–74. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-H, Roberts EA, Her L-S, Liu X, Williams DS, Cleveland DW, Goldstein LSB. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J Cell Biol. 2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe JT, Jung C, Chan WK, Shea TB. Phospho-dependent association of neurofilament proteins with kinesin in situ. Cell Motil Cytoskeleton. 2000;45:249–62. doi: 10.1002/(SICI)1097-0169(200004)45:4<249::AID-CM1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Yabe JT, Pimenta A, Shea TB. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci. 1999;112(Pt 2):3799–814. doi: 10.1242/jcs.112.21.3799. [DOI] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Veeranna, Nixon Ra. Neurofilaments at a glance. J Cell Sci. 2012;125:3257–63. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, Singer RH, Bassell GJ. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–75. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]