Abstract
Since the internal carotid artery supplies blood to both the eye and the brain, ocular microvascular hemodynamics can be altered due to ischemic stroke. The purpose of the current study was to establish the feasibility of conjunctival microcirculation imaging for detection of inter-ocular differences in microvascular hemodynamics in subjects with unilateral ischemic stroke. Conjunctival microcirculation imaging was performed in both eyes of 15 healthy control subjects and 12 subjects following unilateral ischemic stroke. Diameter and axial blood velocity were measured in multiple conjunctival venules of each eye. A two-way repeated measures analysis of variance was performed to determine the effects of stroke (control vs. stroke) and side of stroke (ipsilateral vs. contralateral) on conjunctival diameter and axial blood velocity. There was not a significant main effect of stroke on conjunctival diameter (P = 0.7) or conjunctival axial blood velocity (P = 0.9). There was not a significant main effect of side of stroke on conjunctival diameter (P = 0.8), but there was a significant main effect of side of stroke on conjunctival axial blood velocity (P = 0.02). There was a significant interaction effect between stroke and side of stroke (P = 0.04), indicating conjunctival axial blood velocity was lower in ipsilateral eyes than in contralateral eyes of stroke subjects. Conjunctival axial blood velocity and internal carotid artery blood velocity were correlated in stroke subjects (r = 0.75, P = 0.01, N = 10). Conjunctival microcirculation imaging is a feasible method to detect inter-ocular differences in microvascular hemodynamics in subjects with unilateral ischemic stroke.
Keywords: Conjunctiva, Microvasculature, Hemodynamics, Microcirculation, Ischemic stroke
Introduction
Stroke is the third leading cause of death and the leading cause of long-term disability in the United States. A common cause of ischemic stroke is insufficient blood supply from the internal carotid artery (ICA) to one side of the brain. Currently, vascular imaging techniques are available for evaluation of ICA stenosis and blood velocity in subjects with ischemic stroke (Bleeker et al., 2012; Gough, 2011; Marquering et al., 2012; Rodriguez et al., 2011; Zachrisson et al., 2012). Since oxygenated blood is supplied to the eye and brain by the ICA, assessment of the ocular microcirculation may provide useful information about the cerebral blood supply. Previous studies have established relationships between conjunctival and cerebral blood flow in dogs (Ohtani, 1996) and in humans during aortic arch surgery (Schaser et al., 2003). Furthermore, alterations in conjunctival blood flow due to ICA occlusion have also been reported (Pavlou and Wolff, 1959). Recently, several studies have demonstrated retinal microvascular changes to be associated with, or predictive of stroke (De Silva et al., 2011; Doubal et al., 2009; Ikram et al., 2006; McGeechan et al., 2009; Ong et al., 2013; Wang et al., 2007; Wieberdink et al., 2010; Wong et al., 2001). Since the retina and conjunctiva have a common source of blood from the ophthalmic artery and analogous alterations in the retinal and conjunctival microvasculature have been reported in hypertension and diabetes (To et al., 2013; To et al., 2011), it is likely that the conjunctival microcirculation is also affected by stroke. The purpose of the current research study was to evaluate the feasibility of our conjunctival microcirculation imaging method (Gaynes et al., 2012; Kord Valeshabad et al., 2014; Shahidi et al., 2010; Wanek et al., 2013) for detection of inter-ocular hemodynamics differences due to unilateral ischemic stroke.
Materials and Methods
Subjects
The research study was approved by an Institutional Review Board at the University of Illinois at Chicago. Prior to enrollment, the research study was explained to the subjects and informed consents were obtained according to the tenets of the Declaration of Helsinki. Fifteen healthy control subjects (male 7, female 8) without a history of cerebrovascular, hypertension or ocular diseases and 12 subjects (male 7, female 5) with a clinical diagnosis of unilateral ischemic stroke participated in this study. The median time interval between the occurrence of stroke and conjunctival microcirculation imaging was 30 days (range: 2 - 233 days). Exclusion criteria were inability to consent, hemorrhagic stroke, hypertension (blood pressure ≥ 140/90 mmHg), intracranial aneurysms that required surgery, sickle cell disease, history of eye diseases, ocular surface conditions, eye drop treatment, or use of local sympathomimetic or para-sympatholytic medications prior to conjunctival imaging. The diagnosis of stroke was confirmed by computed tomography (CT) and/or magnetic resonance imaging (MRI). Transthoracic and transesophageal echocardiogram reports, telemetry evaluations and ICA narrowing, as determined by Doppler ultrasound, MRA, CTA or digital subtraction angiography, were recorded from clinical charts. Bilateral ICA blood velocity measurements using Doppler ultrasound were available in 5 of 12 stroke subjects. The median time interval between Doppler ultrasound and conjunctival microcirculation imaging in these 5 subjects was 44 days (range: 4 - 232 days). The mechanism of stroke was determined based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria (Adams et al., 1993) by one of the manuscript authors (FDT) who was masked to the conjunctival imaging data. Systolic and diastolic blood pressure (SBP, DBP) and heart rate (HR) were measured at the time of conjunctival microcirculation imaging and 3 repeated measurements were averaged. Demographic and clinical data were compiled in all subjects.
Image Acquisition
Conjunctival microcirculation imaging was performed with the use of our optical imaging system (EyeFlowTM) as previously described (Shahidi et al., 2010; Wanek et al., 2013). In brief, a slit lamp biomicroscope was utilized to visualize the conjunctival microcirculation, while subjects were seated with their head secured by the chin and forehead support, and presented with an external fixation target to minimize eye movement during imaging. A narrow band filter with a transmission wavelength of 540 ± 5 nm, which corresponds to a hemoglobin absorption peak, was placed in the path of the slit lamp illumination light to improve contrast of the microvasculature and visualization of red blood cells. Several 2-second image sequences, displaying red blood cell movement within the conjunctival microcirculation, were captured by a digital charged coupled device camera attached to the slit lamp biomicroscope. The frame rate of the camera was 30 Hz, thereby 60 images were acquired in each image sequence. In order to minimize the potential effects of variable light exposure and heating of the conjunctival surface on hemodynamics measurements, image acquisition was standardized in all subjects. The light levels were kept constant by using identical settings on the slit lamp illumination, placing a heat absorbing filter (ThorLabs Inc, Newton, New Jersey, USA) in the light illumination path, and restricting the light exposure time to approximately 15 minutes. In all subjects, images were obtained from both eyes at multiple locations that were temporal to the limbus. In stroke subjects, fellow eyes were designated as ipsilateral (IPSI) or contralateral (CONTRA) according to the side of stroke. The individuals who performed conjunctival imaging and image analysis were masked to the side of the stroke in the subjects.
Image Analysis
The image analysis method for deriving measurements of conjunctival diameter and axial blood velocity has been previously described and validated (Kord Valeshabad et al., 2014; Shahidi et al., 2010). In each conjunctival microcirculation image sequence, 10 or more consecutive image frames were manually selected for analysis based on image quality and the absences of blinks and large eye movements. Image registration was then performed on these selected frames to correct for eye motion using a semi-automated, area-based search technique that employed control points, as previously described (Shahidi et al., 2010). From the registered conjunctival microcirculation images, a venule of interest was identified and the centerline was automatically extracted by selecting the end points of the vessel segment and using distance transform to obtain a line between the points. Conjunctival diameter was derived as the full width at half maximum of intensity profiles generated perpendicular to the vessel centerline, averaged along the vessel length. Along the venule centerline, motion of aggregated red bloods cells (or plasma gaps) was tracked in consecutive frames of the registered image sequence to generate a spatial-temporal image that displayed the intensity variation along the length of the vessel as a function of time. Conjunctival axial blood velocity was derived by determining the slope of the prominent bands in the spatial-temporal using 1D cross-correlation between intensity data in the columns of the space time image. The spatial-temporal images were indirectly calibrated based on the known image acquisition frame rate and the spatial resolution of the imaging system (Shahidi et al., 2010). These values defined the x and y-axis of the spatial-temporal images, such that the determination of the slope in the image yielded an accurate measure of conjunctival axial blood velocity.
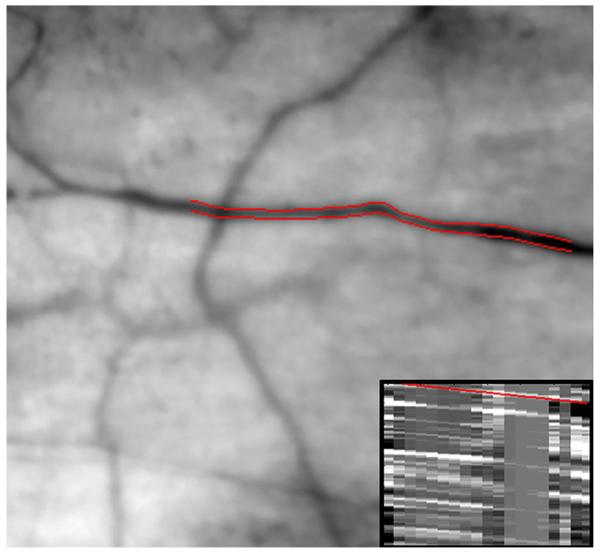
Figure 1 displays an example of conjunctival microcirculation image and spatial-temporal image (insert) obtained in one eye of a subject with unilateral ischemic stroke. Conjunctival axial blood velocity was measured from the spatial-temporal image as the slope of the overlaid red line which was equal to the slope of all prominent bands. In this example, conjunctival diameter and axial blood velocity in the selected venule was 17.6 μm and 0.33 mm/s, respectively. Diameter and axial blood velocity measurements were obtained in 4 or more venules in each eye and an averaged value was then calculated. The median number of venules that were analyzed in each eye was 11 venules (range: 4 -20). Venules were distinguished from arterioles by visualizing the motion of red blood cells within the vessel and determining whether blood drained into another vessel (venule) or diverged into vessel branches (arteriole). Conjunctival venules were selected for hemodynamic measurements since they are more numerous, are visualized with higher contrast as compared to arterioles, and have minimal pulsatility (Koutsiaris et al., 2007; Koutsiaris et al., 2010).
Fig. 1.
Example of conjunctival microcirculation image obtained in one eye of a subject with unilateral ischemic stroke. The outlined edges of the selected conjunctival venule (red lines) were automatically identified from diameter measurements. A spatial-temporal image (insert) was derived from this vessel based on the movement of aggregated red blood cells or plasma gaps along the vessel length. Conjunctival axial blood velocity was measured as the slope of the overlaid red line which was equal to the slope of the prominent bands within the image.
Statistical analysis
Intra-eye variability of conjunctival diameter and axial blood velocity was determined by the standard deviation (SD) of measurements obtained in each eye and averaged in each group of eyes (right or left eyes of control subjects and IPSI or CONTRA eyes of stroke subjects). Kolmogorov-Smirnov test confirmed normal distributions of continuous variables. Systemic variables were compared between control and stroke subjects using unpaired t-tests. Gender ratio was compared between control and stroke subjects using Pearson chi-square test. A two-way repeated measures analysis of variance (ANOVA) was performed to determine the effects of stroke (control vs. stroke) and side of stroke (IPSI vs. CONTRA) on conjunctival diameter and axial blood velocity. In control subjects, IPSI and CONTRA were defined as right and left, respectively. Pearson correlation coefficients were computed to assess the correlation between conjunctival axial blood velocity and ICA blood velocity, and also between conjunctival axial blood velocity and the time interval between the stroke occurrence and conjunctival imaging. Statistical analysis was performed using SPSS version 22 (SPSS Inc, Chicago, IL, USA). Statistical significance was accepted at P < 0.05.
Results
Demographic and clinical data of control and stroke subjects are listed in Table 1. Mean age and the male to female ratio were not statistically different between control (N = 15) and stroke (N = 12) subjects (P ≥ 0.1). Likewise, SBP, DBP, and HR were similar between control (N = 14) and stroke (N = 12) subjects (P ≥ 0.4). The location and mechanism (based on the TOAST criteria) of stroke are provided in Table 2. The mechanism of stroke was cardioembolic (N = 5), large artery occlusive disease (N = 3), cryptogenic (N = 3) or small vessel occlusive disease (N = 1). In 5 of 12 stroke subjects, significant stenosis was found in large vessels. Of these 5 subjects, 4 had significant narrowing of large vessels in the IPSI side.
Table 1.
Demographics and systemic conditions of control (N =15) and stroke (N = 12) subjects. Heart rate and blood pressure data were available in 14 of 15 subjects in the control group. Data are presented as mean ± standard deviation.
| Variable | Control | Stroke | P Value |
|---|---|---|---|
| Age (years) | 56 ± 7 | 63 ± 14 | 0.1 |
| Systolic Blood Pressure (mm Hg) | 122 ± 10 | 126 ± 13 | 0.4 |
| Diastolic Blood Pressure (mm Hg) | 75 ± 8 | 74 ± 10 | 0.8 |
| Heart Rate (beats/min) | 74 ± 17 | 73 ± 11 | 0.8 |
| Male/Female Ratio | 7/8 | 7/5 | 0.5 |
Table 2.
Mechanism and location of stroke, and patency of large vessel. (R: right; L: left; PCA: posterior cerebral artery; MCA: middle cerebral artery; SCA, superior cerebellar artery; ICA: internal carotid artery; BA, basilar artery; VA, vertebral artery).
| Subject | Mechanism | Location | Patency of large vessels |
|---|---|---|---|
| 1 | Large Vessel | R Frontal Lobe | 100% narrowing of R MCA a |
| 2 | Small Vessel | L Pontomedullary | 100% narrowing of R VA and R MCA a |
| 3 | Large Vessel | R PCA | 100% narrowing of L VA and BA a |
| 4 | Cryptogenic | R MCA | No significant narrowing b, c |
| 5 | Cryptogenic | R Cerebellar, R SCA | No significant narrowing a |
| 6 | Large Vessel | L Centrum Semiovale | 99% narrowing of L ICA b, c, e |
| 7 | Cardioembolic | L Occipital | No significant narrowing b, c |
| 8 | Cardioembolic | R PCA | No significant narrowing b, c |
| 9 | Cardioembolic | L Thalamus and Selenium |
No significant narrowing a, b, d |
| 10 | Cardioembolic | R Temporal, Occipital and Parietal Lobes |
No significant narrowing a, b |
| 11 | Cardioembolic | R Basal Ganglion | 100% narrowing of R M1 segment a, f |
| 12 | Cryptogenic | R SCA | No significant narrowing a |
Conventional angiogram
Magnetic resonance angiogram of the head/neck
Carotid ultrasonography
Computed tomography angiography of the head/neck
Vessel was recanalized by endarterectomy
Vessel was recanalized by mechanical thrombectomy
Diameter and axial blood velocity measurements were obtained in 192 and 195 conjunctival venules of right and left eyes of control subjects, respectively. Diameter and axial blood velocity measurements were obtained in 125 and 134 conjunctival venules of CONTRA and IPSI eyes of stroke subjects, respectively. Intra-eye variability in conjunctival diameter measurements were on average 5.3 μm and 5.2 μm in right and left eyes of control subjects, respectively, and 6.4 μm and 5.6 μm in CONTRA and IPSI eyes of stroke subjects, respectively. Intra-eye variability in conjunctival axial blood velocity measurements were on average 0.19 mm/s and 0.24 mm/s in right and left eyes of control subjects, respectively. Intra-eye variability in conjunctival axial blood velocity measurements were on average 0.27 mm/s and 0.20 mm/s in CONTRA and IPSI eyes of stroke subjects, respectively.
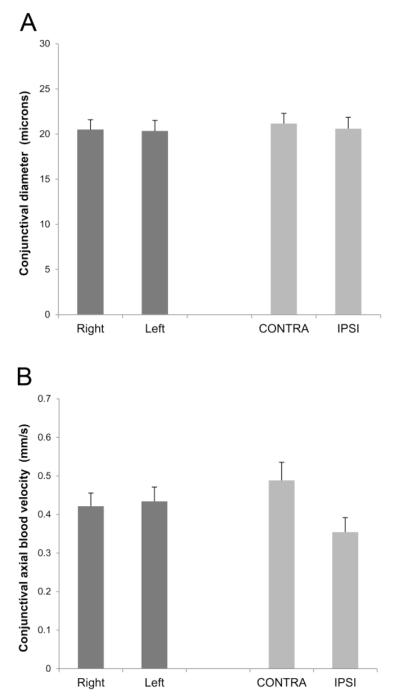
Mean conjunctival diameter measurements in control and stroke subjects are shown in Figure 2A. In control subjects, conjunctival diameter measurements were 20.5 ± 4.2 μm and 20.3 ± 4.5 μm (mean ± SD; N = 15) in right and left eyes, respectively. In stroke subjects, conjunctival diameter measurements were 21.2 ± 3.9 μm and 20.6 ± 4.4 μm (N = 12) in the CONTRA and IPSI eyes, respectively. There was not a significant main effect of stroke (P = 0.7) or side of stroke (P = 0.8) on conjunctival diameter. There was not a significant interaction effect between stroke and side of stroke (P = 0.7).
Fig. 2.
Mean conjunctival diameter (A) and axial blood velocity (B) in fellow eyes of control subjects (N = 15) and contralateral (CONTRA) and ipsilateral (IPSI) eyes of subjects with unilateral stroke (N = 12). Error bars denote standard errors of the means.
Mean conjunctival axial blood velocity measurements in control and stroke subjects are shown in Figure 2B. In control subjects, conjunctival axial blood velocity measurements were 0.42 ± 0.13 mm/s and 0.43 ± 0.14 mm/s in right and left eyes, respectively. In stroke subjects, conjunctival axial blood velocity measurements were 0.49 ± 0.16 mm/s and 0.35 ± 0.13 mm/s in the CONTRA and IPSI eyes, respectively. There was not a significant main effect of stroke on conjunctival axial blood velocity (P = 0.9). However, there was a significant main effect of side of stroke on conjunctival axial blood velocity (P = 0.02). There was a significant interaction effect between stroke and side of stroke (P = 0.04), indicating conjunctival axial blood velocity was lower in IPSI eyes than in CONTRA eyes of stroke subjects, but similar between fellow eyes of control subjects. The inter-ocular difference in conjunctival axial blood velocity was 0.01 ± 0.14 mm/s and 0.13 ± 0.16 mm/s in control and stroke subjects, respectively. Conjunctival axial blood velocity was not significantly correlated with the time interval between the occurrence of the stroke and conjunctival imaging in IPSI or CONTRA eyes of stroke subjects (P ≥ 0.3; N = 12).
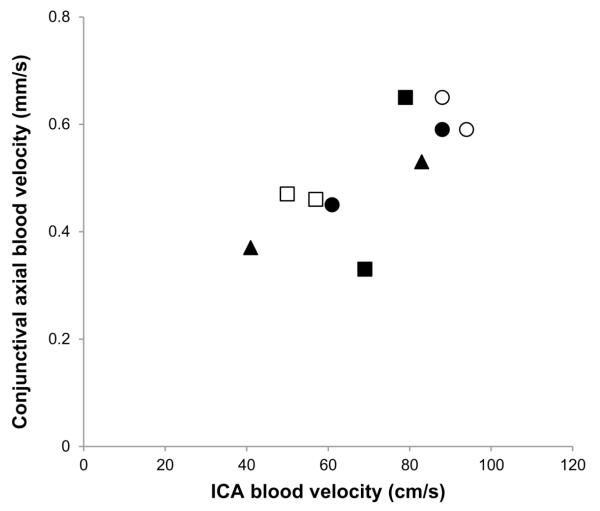
Figure 3 displays conjunctival axial blood velocity plotted as a function of ICA blood velocity with data obtained from both CONTRA and IPSI sides of 5 stroke subjects, yielding 10 data points. Conjunctival axial blood velocity and ICA blood velocity were significantly correlated (r = 0.75, P = 0.01, N = 10).
Fig 3.
Conjunctival axial blood velocity plotted as a function of ICA blood velocity in both CONTRA and IPSI sides of 5 stroke subjects. Identical symbols represent data in the same subject.
Discussion
Since conjunctival and cerebral tissues share similar vascular origins, inter-ocular differences in conjunctival hemodynamics may be present in subjects with unilateral ischemic stroke. The present study established the feasibility of conjunctival microcirculation imaging for detecting reduced conjunctival axial blood velocity in ipsilateral eyes following unilateral ischemic stroke.
In this pilot study, reduced conjunctival axial blood velocity in the IPSI eyes of subjects with unilateral ischemic stroke was reported. Previous studies have reported reduced ICA blood velocity in stroke subjects at both acute and chronic stable phase, without documenting the side of stroke (Bai et al., 2007; Fukuhara et al., 2005). Since the ICA supplies blood to both the conjunctiva and brain, it is plausible that reduced ICA blood velocity can analogously affect the conjunctival and cerebral microcirculation. Furthermore, reduced blood flow in the IPSI middle cerebral artery (MCA) as compared to CONTRA MCA has been reported in unilateral MCA occlusive disease (Kuroda et al., 2012). Moreover, the finding of a correlation between conjunctiva and ICA blood velocity measurements in the current study is consistent with previous studies which reported alterations in conjunctival hemodynamics due to compete ICA occlusion (Pavlou and Wolff, 1959), and following carotid endarterectomy (Schaser et al., 2003). Lastly, the finding of reduced axial blood velocity in conjunctival venules of stroke subjects is in agreement with previously reported reduced intracranial venular blood flow in an animal model of unilateral MCA occlusion (Zhu et al., 2012). Future studies are needed to directly correlate conjunctival and MCA/ICA blood velocity measurements in subjects at the time of stroke.
Conjunctival diameter measurements were similar between fellow eyes of control and stroke subjects. This finding is in contrast to previously reported alterations in retinal vascular caliber due to stroke, cerebral small vessel disease and cardiovascular disease (De Silva et al., 2011; Doubal et al., 2009; Hiroki et al., 2003; Ikram et al., 2006; McGeechan et al., 2009; Ong et al., 2013; Wang et al., 2007; Wieberdink et al., 2010; Wong et al., 2001). Analogous alterations in conjunctival and retinal microvasculatures are expected, since they are both supplied by the ophthalmic artery. The difference in findings in the conjunctiva and retina may be attributed to either the small sample size in our study or higher metabolic demand of the retinal tissue, such that compensatory vasodilation occurs in response to ischemia.
In the current study, the time interval between occurrence of stroke and conjunctival microcirculation imaging varied among subjects. However, since conjunctival axial blood velocity was not correlated with the time interval between stroke and conjunctival imaging, this variable time interval did not likely affect the findings. Additionally, ICA blood velocity was reduced in stroke subjects at chronic stable phase, even beyond 6 months following stroke (Bai et al., 2007; Fukuhara et al., 2005), supporting our finding of reduced conjunctival axial blood velocity within the time intervals investigated in the present study. Nevertheless, conjunctival microcirculation imaging at the time of stroke is needed to provide information about acute microvascular hemodynamic changes and allow direct correlation of findings with clinical imaging.
Conjunctival hemodynamics was assessed in stroke subjects regardless of co-existing systemic diseases and medication use. Although these factors may affect conjunctival hemodynamics, comparison of measurements between fellow eyes circumvented their potential effects on the reported results. The age difference between control and stroke subjects was not statistically significant, though the control subjects were on average 7 years younger than the stroke subjects. Since retinal blood velocity is reduced with age (Tamura et al., 2013), conjunctival axial blood velocity may be expected to be lower in age-matched control subjects than in the control subjects in the present study. Nevertheless, data in a larger population of control subjects are needed to evaluate if there is an association between conjunctival hemodynamics and age.
The limitations of the study were the small sample size and potential inaccuracy in conjunctival axial blood velocity measurements. Using conjunctival axial blood velocity measurements in fellow eyes of stroke subjects and the sample size of the present study, the statistical power was calculated to be 0.89 at a significance level of 0.05, indicating sufficient sample size to detect inter-ocular differences. The accuracy of conjunctival axial blood velocity measurements may have been reduced due to noise in the generated spatial-temporal images. However, this type of error would have similarly contributed to measurement variability in both eyes and thus not likely affected the finding of reduced conjunctival axial blood velocity in the IPSI eyes of the stroke subjects.
Future studies are warranted to evaluate the sensitivity of conjunctival hemodynamic imaging to measure cerebral circulation alterations by comparison with gold standard cerebrovascular imaging techniques. Overall, conjunctival microcirculation imaging is a feasible method to detect inter-ocular differences in microvascular hemodynamics in subjects with unilateral ischemic stroke.
Highlights.
Conjunctival microvascular hemodynamics was assessed in unilateral ischemic stroke.
Conjunctival venular diameter and axial blood velocity were measured.
Conjunctival blood velocity was lower in ipsilateral than in contralateral eyes.
Acknowledgments
Supported by NIH grants EY001792, Senior Scientific Investigator award (MS) and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contribution
AK designed the study, collected data, performed statistical analysis and prepared the manuscript; JW collected and analyzed data, wrote and reviewed the manuscript; FM and RZ collected data; FDT referred subjects, provided clinical data, and reviewed the manuscript; MS designed the study, wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of interest
MS has a patent for the EyeFlow™ technology. All other authors declare that they have no conflicts of interest.
References
- Adams HP, Jr., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Bai CH, et al. Lower blood flow velocity, higher resistance index, and larger diameter of extracranial carotid arteries are associated with ischemic stroke independently of carotid atherosclerosis and cardiovascular risk factors. J Clin Ultrasound. 2007;35:322–30. doi: 10.1002/jcu.20351. [DOI] [PubMed] [Google Scholar]
- Bleeker L, et al. Semi-automatic quantitative measurements of intracranial internal carotid artery stenosis and calcification using CT angiography. Neuroradiology. 2012;54:919–27. doi: 10.1007/s00234-011-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DA, et al. Retinal microvascular changes and subsequent vascular events after ischemic stroke. Neurology. 2011;77:896–903. doi: 10.1212/WNL.0b013e31822c623b. [DOI] [PubMed] [Google Scholar]
- Doubal FN, et al. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry. 2009;80:158–65. doi: 10.1136/jnnp.2008.153460. [DOI] [PubMed] [Google Scholar]
- Drakou AA, et al. The importance of ophthalmic artery hemodynamics in patients with atheromatous carotid artery disease. Int Angiol. 2011;30:547–54. [PubMed] [Google Scholar]
- Fukuhara T, et al. Evaluation of extracranial carotid artery duplex ultrasound scanning parameters in cerebral ischemic or nonischemic patients without significant cervical carotid artery stenosis. J Stroke Cerebrovasc Dis. 2005;14:12–6. doi: 10.1016/j.jstrokecerebrovasdis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gaynes B, et al. Feasibility of conjunctival hemodynamic measurements in rabbits: reproducibility, validity, and response to acute hypotension. Microcirculation. 2012;19:521–9. doi: 10.1111/j.1549-8719.2012.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough MJ. Preprocedural imaging strategies in symptomatic carotid artery stenosis. J Vasc Surg. 2011;54:1215–8. doi: 10.1016/j.jvs.2011.05.101. [DOI] [PubMed] [Google Scholar]
- Hiroki M, et al. Central retinal artery Doppler flow parameters reflect the severity of cerebral small-vessel disease. Stroke. 2003;34:e92–4. doi: 10.1161/01.STR.0000075768.91709.E4. [DOI] [PubMed] [Google Scholar]
- Ikram MK, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66:1339–43. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- Kord Valeshabad A, et al. Conjunctival Microvascular Hemodynamics in Sickle Cell Retinopathy Acta Ophthalmologica. 2014. [In Press] [DOI] [PMC free article] [PubMed]
- Koutsiaris AG, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–86. [PubMed] [Google Scholar]
- Koutsiaris AG, et al. Blood velocity pulse quantification in the human conjunctival pre-capillary arterioles. Microvasc Res. 2010;80:202–8. doi: 10.1016/j.mvr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kuroda H, et al. Accuracy of central benzodiazepine receptor binding potential/cerebral blood flow SPECT imaging for detecting misery perfusion in patients with unilateral major cerebral artery occlusive diseases: comparison with cerebrovascular reactivity to acetazolamide and cerebral blood flow SPECT imaging. Clin Nucl Med. 2012;37:235–40. doi: 10.1097/RLU.0b013e31823ea69f. [DOI] [PubMed] [Google Scholar]
- Marquering HA, et al. Performance of semiautomatic assessment of carotid artery stenosis on CT angiography: clarification of differences with manual assessment. AJNR Am J Neuroradiol. 2012;33:747–54. doi: 10.3174/ajnr.A2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeechan K, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–32. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N. Laser Doppler flowmetry of the bulbar conjunctiva as a monitor of the cerebral blood flow. Nihon Kyobu Geka Gakkai Zasshi. 1996;44:1721–8. [PubMed] [Google Scholar]
- Ong YT, et al. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. 2013;44:2121–7. doi: 10.1161/STROKEAHA.113.001741. [DOI] [PubMed] [Google Scholar]
- Pavlou AT, Wolff HG. The bulbar conjunctival vessels in occlusion of the internal carotid artery. AMA Arch Intern Med. 1959;104:53–60. doi: 10.1001/archinte.1959.00270070055007. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, et al. Correlation between Doppler velocities and duplex ultrasound carotid cross-sectional percent stenosis. Acad Radiol. 2011;18:1485–91. doi: 10.1016/j.acra.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Schaser KD, et al. Noninvasive analysis of conjunctival microcirculation during carotid artery surgery reveals microvascular evidence of collateral compensation and stenosis-dependent adaptation. J Vasc Surg. 2003;37:789–97. doi: 10.1067/mva.2003.139. [DOI] [PubMed] [Google Scholar]
- Shahidi M, et al. Quantitative assessment of conjunctival microvascular circulation of the human eye. Microvasc Res. 2010;79:109–13. doi: 10.1016/j.mvr.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, et al. Association between age and chorioretinal hemodynamics in normal volunteers examined with laser speckle flowgraphy] Nihon Ganka Gakkai Zasshi. 2013;117:110–6. [PubMed] [Google Scholar]
- To WJ, et al. Real-time studies of hypertension using non-mydriatic fundus photography and computer-assisted intravital microscopy. Clin Hemorheol Microcirc. 2013;53:267–79. doi: 10.3233/CH-2012-1567. [DOI] [PubMed] [Google Scholar]
- To WJ, et al. Correlation of conjunctival microangiopathy with retinopathy in type-2 diabetes mellitus (T2DM) patients. Clin Hemorheol Microcirc. 2011;47:131–41. doi: 10.3233/CH-2010-1374. [DOI] [PubMed] [Google Scholar]
- Wanek J, et al. Human bulbar conjunctival hemodynamics in hemoglobin SS and SC disease. Am J Hematol. 2013;88:661–4. doi: 10.1002/ajh.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28:1984–92. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- Wieberdink RG, et al. Retinal vascular calibers and the risk of intracerebral hemorrhage and cerebral infarction: the Rotterdam Study. Stroke. 2010;41:2757–61. doi: 10.1161/STROKEAHA.110.599084. [DOI] [PubMed] [Google Scholar]
- Wong TY, et al. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- Zachrisson H, et al. Functional assessment of high-grade ICA stenosis with duplex ultrasound and transcranial Doppler. Clin Physiol Funct Imaging. 2012;32:241–6. doi: 10.1111/j.1475-097X.2011.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, et al. Imaging the early cerebral blood flow changes in rat middle cerebral artery occlusion stroke model. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:2655–8. doi: 10.1109/EMBC.2012.6346510. [DOI] [PubMed] [Google Scholar]