Abstract
Background
Eczema Vaccinatum (EV) is a life threatening complication of smallpox vaccination in patients with atopic dermatitis (AD) characterized by dissemination of vaccinia virus (VV) in the skin and internal organs. Mutations in the filaggrin gene, the most common genetic risk factor for AD, confer a greater risk for eczema herpeticum in AD patients, suggesting that it impairs the response to cutaneous viral infections.
Objective
To determine the effects of filaggrin deficiency on the response of mice to cutaneous VV inoculation.
Methods
VV was inoculated by scarification of unsensitized skin, or skin topically sensitized with ovalbumin (OVA), in filaggrin-deficient, flaky tail (ft/ft) mice or wild-type (WT) controls. The size of primary and satellite skin lesions were measured and H&E staining was performed. VV genome copy number and cytokine mRNA levels were measured by quantitative PCR.
Results
VV inoculation in unsensitized skin of ft/ft mice, independently of the matted hair mutation, resulted in larger primary lesions, more abundant satellite lesions, heavier viral loads in internal organs, greater epidermal thickness, dermal cellular infiltration, and higher local Il17a, Il4, Il13, and Ifng mRNA levels than in WT controls. VV inoculation at sites of topical OVA application amplified all of these features in ft/ft mice, but had no detectable effect in WT controls. The number of satellite lesions and the viral loads in internal organs following cutaneous VV inoculation were significantly reduced in both unsensitized and topically sensitized ft/ftxIl17a−/− mice.
Conclusion
Filaggrin deficiency predisposes to EV. This is mediated primarily through the production of IL-17A.
Clinical Implications
IL-17A blockade may prevent the development of EV.
Capsule summary
Filaggrin deficiency and allergic skin inflammation synergize to promote susceptibility to vaccinia virus dissemination. This susceptibility is mediated primarily by IL-17A, which is highly expressed in the skin of filaggrin deficient mice.
Keywords: Vaccinia virus, filaggrin, IL-17A
Introduction
Atopic dermatitis (AD) is a chronic pruritic inflammatory skin disorder that affects more than 15% of children in the USA 1. AD is characterized by a defect in skin barrier function that results in dry itchy skin, and is aggravated by skin injury inflicted by scratching 2. Patients with AD are prone to Th2-dominated immune responses as well as bacterial and viral infections of the skin 3–5. In particular, they are susceptible to dissemination of viruses such as vaccinia (VV) and herpes (HSV) viruses 6,7, resulting in eczema vaccinatum (EV) and eczema herpeticum (EH), respectively. In EV patients, VV spreads through skin, resulting in large primary lesions surrounded by satellite lesions, and infects internal organs. Although smallpox was declared eradicated in 1979, recent fears that variola virus might be used as a biological weapon, along with concerns regarding the EV susceptibility of unimmunized atopic populations, have prompted the development of new safer vaccines. Predicting the efficacy of such vaccines in the absence of human smallpox depends on understanding the mechanisms of the disease. The reasons why patients with AD are at risk for EV are not known.
Strong genetic associations exist between mutations in the filaggrin (FLG) gene and AD 8–10. The FLG gene encodes for profilaggrin, the major component of keratohyalin granules. Patients with AD who carry FLG mutations have more persistent disease, a higher incidence of skin infections with herpes virus that results in EH 11,12, and a greater risk of multiple allergies including asthma, allergic rhinitis, and peanut allergy 13,14. Whether filaggrin deficiency predisposes to EV has not been studied.
Flaky tail (ft/ft) mice carry a 5303delA frame shift mutation in the FLG gene, which is in linkage disequilibrium with a second mutation at the matted (ma) locus that confers hair matting 15, 16. ft/ft mice have skin barrier dysfunction 17. We, and others, have previously reported that the homozygous ft mutation on an outbred genetic background is associated with spontaneous development of eczematous skin lesions, increased mRNA expression in skin of Il17a, Il4, Il13, and Ifng, and elevated serum IgE levels 17,18. Following topical cutaneous exposure to ovalbumin (OVA), ft/ft mice on a mixed background develop allergic skin inflammation evidenced by epidermal thickening, dermal CD4+ cell infiltration, and exaggerated local and systemic Th17 and Th2 responses 18. In contrast, topical application of OVA elicited no detectable local or systemic immune responses in wild-type (WT) BALB/c and C57BL/6 mice 18. In WT mice, tape stripping, a surrogate for human scratching, is a prerequisite for induction of allergic skin inflammation and systemic immune responses to EC application of antigen. Genetic and environmental factors favoring the induction of a cutaneous Th2 response lead to impaired effector immune responses and ineffective elimination of VV and HSV in mice resulting in phenotypes analogous to human EV and EH 19–22. Animal studies support the hypothesis that skin barrier dysfunction caused by FLG mutations or by mechanical injury secondary to scratching plays a key role in antigen sensitization that leads to the development of AD.
To understand whether filaggrin deficient mice are susceptible to developing EV, we backcrossed the mutant FLG allele of the ft/ft strain onto the Th2-prone BALB/c background. This inbred strain has been well characterized with respect to models of allergic skin inflammation and EV 19,23–28. Transferring the ft/ft genotype into this background permits rigorous analyses of ft/ft phenotypes, and a comparison of the responses of the mutant and WT BALB/c controls. In this study we demonstrate that ft/ft mice inoculated with VV in the skin develop more severe skin lesions, greater viral dissemination, and more intense cutaneous inflammation than WT controls, indicating that filaggrin deficiency impairs VV containment. All these features were exaggerated in ft/ft mice inoculated with VV in skin topically sensitized with OVA, indicating that filaggrin deficiency and allergic inflammation synergize to impair VV containment. IL-17A was shown to mediate the susceptibility of ft/ft mice to VV, as EV features were markedly attenuated in ft/ftxIl17a−/− mice.
Methods
Mice
Flaky tail (ft/ft) mice from Jackson laboratory were backcrossed onto the BALB/c mice from Charles River Laboratories. ft/ft mice on BALB/c background were subsequently crossed with Il17a−/− BALB/c mice to generate ft/ftxIl17a−/− mice. Later in the course of the study, we were able to remove by extensive backcrossing the matted hair mutation (ma) present in the original mixed strain. The mice with the removed ma mutation were designated ft/ft.mawt/wt. The sample size in each group was n = 5. All mice were bred in our animal facility, and kept in a specific pathogen-free environment and fed an OVA-free diet. All procedures performed on the mice were in accordance with the Animal Care and Use Committee of the Boston Children’s Hospital.
Topical skin sensitization and VV inoculation
The dorsal skin of anesthetized six to eight-week-old female mice was shaved and rested for two days for recovery from any injury by shaving, then 100 µg OVA (Grade V; Sigma) in 100 µl of normal saline, or placebo (100 µl of normal saline), were placed on a patch of sterile gauze (1×1 cm), which was secured to dorsal skin with a transparent bio-occlusive dressing (Tegaderm, Westnet Inc.). Each mouse had a total of three one-week exposures to the patch separated by two-week intervals.
Shaved dorsal skin of unsensitized mice or the sensitized site of topically sensitized mice was inoculated with VV Western Reserve strain (VR-1354, ATCC) by means of skin scarification by using 107 plaque forming unit (pfu/mouse). Skin was examined 7 days after inoculation (see Fig. S1). Lesion sizes were analyzed with the National Institutes of Health’s Image software Image J (National Institutes of Health, Bethesda, Md). No mortality was observed following VV inoculation.
Histological analysis
Multiple 4 µm sections of skin were stained with hematoxylin and eosin (H&E). Neutrophils were counted in a blinded fashion in 10–15 high-power fields (HPFs) at a magnification of 400×. Epidermal thickness of 10 different randomly chosen sites was measured in each skin section from each mouse at a magnification of 400×.
Quantitative PCR analysis of VV genomes
Tissue samples were immediately frozen and stored at −80°C. Quantification of VV ge nomes was performed as previously described 20. Cytokine mRNA expression was shown as fold induction relative to uninfected WT skin unless otherwise specified. Viral genome measurements by PCR correlated with viral titer measurements by plaque assay.
Quantitative PCR analysis of cytokines
Total RNA was extracted from homogenized skin tissue or from cultured cells with RNAqueous extraction kit (Ambion Inc) following the manufacturer’s instructions. cDNA was generated with iScript cDNA synthesis kit (Bio-rad Laboratory). Quantitative real-time PCR was done using Taqman Gene Expression Assay, universal PCR master mix and the ABI Prism 7300 sequence detection system with commercial primers and probes, all from Applied Biosystems. Fold induction of target gene expression was calculated using the comparative method for relative quantitation by normalization to the internal control β2-microglobulin, as described previously 29.
In vitro cytokine production and VV specific IgG2a antibody ELISA
These assays were performed as described previously 30.
Statistical analysis
A Mann-Whitney’s U test was used to compare the distribution of each outcome between two groups. A Bonferroni corrected significance level of 0.05 was used to account for pairwise comparisons among three or more groups. All analyses were performed using the Graphpad Prism version 5.0 (Graphpad Software).
Results
ft/ft mice are susceptible to cutaneous VV inoculation
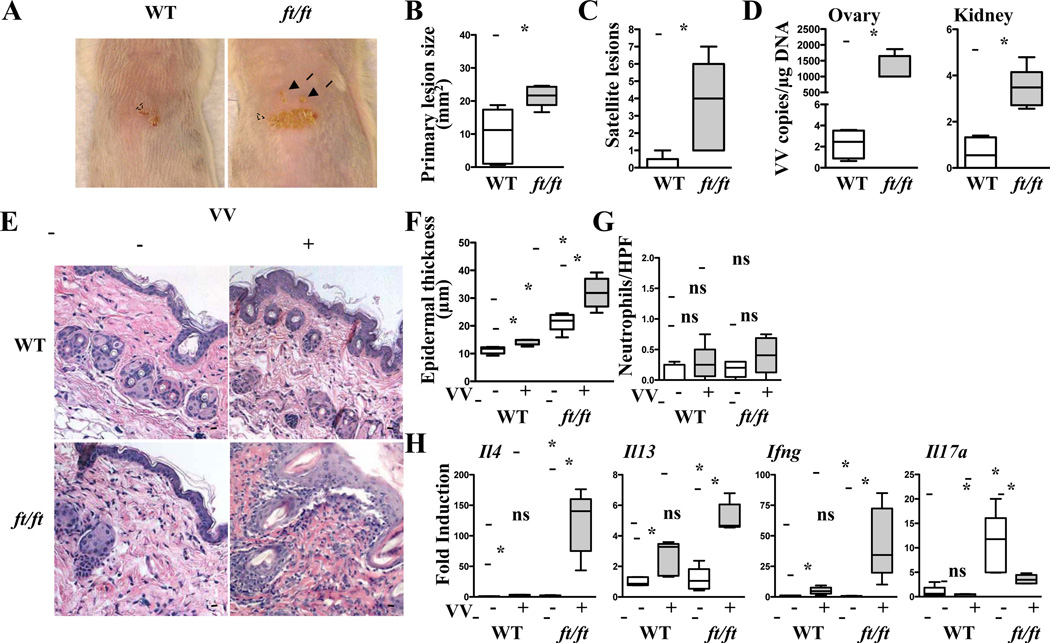
We tested the hypothesis that ft/ft mice are predisposed to the development of EV. Seven days after VV inoculation in the back skin, ft/ft mice developed significantly larger primary lesions and significantly higher numbers of satellite lesions than WT BALB/c controls (Fig. 1A–C). Furthermore, they exhibited significantly higher viral loads in internal organs than WT controls (Fig. 1D). These results suggest that ft/ft mice are more susceptible to developing features of EV following inoculation with VV.
Figure 1. ft/ft mice develop features of EV in response to cutaneous VV inoculation.
A–C. Gross appearance (A), area of primary lesions (B), and number of satellite lesions (C). Dashed circles indicate primary lesions. Arrows indicate satellite lesions. D. Viral load. E–G. H&E stained skin sections (200× magnification) (E), epidermal thickness (F), number of neutrophils (G), cytokine mRNA expression (H). Scale bars: 100 µm. The line in the box indicates median and the whiskers represent from the minimum to maximum (n = 5 per group). *P < .05. ns, not significant.
Inoculation of ft/ft mice with VV resulted in a significant increase in epidermal thickness, cellular infiltration of the dermis by mononuclear cells, as well as skin Il4, Il13, and Ifng mRNA, as compared to uninfected ft/ft mice or VV inoculated WT controls (Fig. 1E,F,H). Inoculation of WT controls with VV resulted in a slight but significant increase in epidermal thickness, skin Il4, Il13, and Ifng mRNA as compared to uninfected WT controls (Fig. 1E,F,H). However, no increase in skin infiltrating neutrophils was observed in any of the experimental groups (Fig. 1E,G). Epidermal thickness and skin Il17a mRNA expression were increased in the skin of uninfected ft/ft mice compared to uninfected WT controls (Fig. 1F,H), as previously reported in ft/ft mice on mixed background 18. The high basal levels of skin Il17a mRNA in ft/ft mice were significantly decreased following VV inoculation, but remained higher than the Il17a mRNA levels in the skin of VV inoculated WT mice (Fig. 1H). VV inoculation had no detectable effect on Il17a mRNA in the skin of WT controls. Splenocytes from ft/ft mice and WT controls inoculated with VV secreted comparable amounts of IL-4, IL-13, IFN-γ, and IL-17A in response to in vitro stimulation with VV (Fig. S2A). Serum levels of VV specific IgG2a were higher in VV inoculated ft/ft mice than WT controls (Fig. S2B). These results demonstrate that ft/ft mice mount exaggerated cutaneous Th2 and Th1 responses to VV infection.
Recently, it has become evident that filaggrin null (Flg−/−) mice differ from ft/ft mice in that they do not develop spontaneous dermatitis and do not have an abnormal transepidermal water loss 31. However, Flg−/− mice, like ft/ft mice mount a Th2 response to topical sensitization of antigen 31. The differences between ft/ft mice and Flg−/− mice raised the possibility that the homozygous ma mutation linked to the ft mutation might contribute to the susceptibility of ft/ft mice to cutaneous inoculation with VV. To address this issue, we backcrossed our ft/ft mice on BALB/c background to generate ft/ft mice that lack the ma mutation, as evidence by the lack of matted hairs and by genomic sequencing for the ma mutation 32(data not shown). These mice were indistinguishable from ft/ft mice that harbor the ma mutation in their susceptibility to VV infection, as measured by the size of the primary lesion, the number of satellite lesions, viral loads in internal organs, epidermal thickening, and local cytokine expression profile (Fig. S3). The differences in aforementioned changes between ft/ft or ft/ft.mawt/wt mice and WT controls, but not between ft/ft and ft/ft.mawt/wt mice, were statistically significant. These results indicate that the ma mutation does not play an important role in the susceptibility of ft/ft mice to VV inoculation.
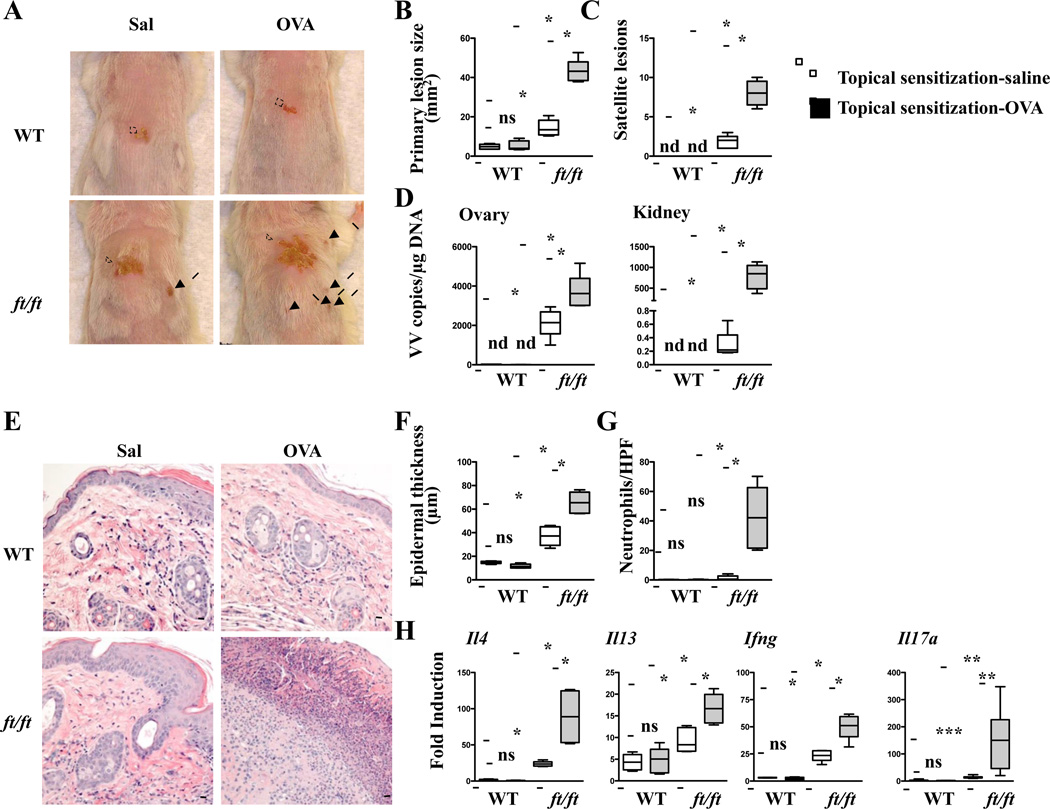
VV inoculation of topically sensitized skin aggravates the features of EV in ft/ft mice
Topical application of OVA to the shaved unstripped back skin elicited local skin inflammation and a systemic immune response in ft/ft mice on BALB/c background (Fig. S4A–D and data not shown), as previously shown in ft/ft mice on mixed background 18. In contrast, topical application of OVA to the shaved unstripped back skin did not result in the development of local skin inflammation or a systemic response in WT controls (Fig. S4A–D and data not shown). We tested the hypothesis that VV inoculation of ft/ft mice at sites of topical OVA application would result in the development of more severe features of EV. Mice were topically sensitized with OVA or saline over a 7-week period, then were inoculated with VV at the site of sensitization and examined 7 days later (Fig. S4E). ft/ft mice inoculated with VV at OVA exposed sites developed significantly larger primary lesions, exhibited significantly higher number of satellite lesions, and had significantly greater VV loads in the ovary and kidney compared to ft/ft mice inoculated with VV at saline exposed sites or WT controls inoculated with VV at OVA exposed site (Fig. 2A–D). They also had significantly greater epidermal thickness, significantly denser dermal infiltration with neutrophils, and significantly higher expression of Il17a, Il4, Il13, and Ifng mRNA (Fig. 2E–H). The size of the primary lesion, epidermal thickness and expression of Il17a, Il4, Il13, and Ifng mRNA were comparable in WT mice inoculated with VV in OVA or saline exposed skin, and significantly reduced compared to those in ft/ft mice inoculated with VV at saline exposed sites (Fig. 2A–C,H). WT mice inoculated with VV in OVA or saline exposed skin had no satellite lesions, no detectable viral loads in the internal organs, and very few neutrophils in the primary lesion (Fig. 2C–E,G). Splenocyte cytokine secretion in response to VV stimulation was similar in ft/ft and WT mice (data not shown). VV specific serum IgG2a levels were higher in ft/ft mice than in WT mice, regardless of whether the skin was sensitized with OVA or saline (Fig. S5A). These results suggest that cutaneous sensitization to topically encountered antigens in ft/ft mice aggravates their susceptibility to EV.
Figure 2. Exaggerated EV features in ft/ft mice inoculated with VV in OVA sensitized skin.
A–C. Gross appearance (A), area of primary lesions (B), and number of satellite lesions (C). Dashed circles indicate primary lesions. Arrows indicate satellite lesions. D. Viral load. E–H. H&E stained skin sections (200× magnification) (E), epidermal thickness (F), number of neutrophils (G), cytokine mRNA expression (H). Scale bars: 100 µm. The line in the box indicates median and the whiskers represent from the minimum to maximum (n = 5 per group). *P < .05, **P < .01 and ***P < .001. ns, not significant. nd, not detectable.
IL-17A contributes to the increased susceptibility of ft/ft mice to cutaneous VV inoculation
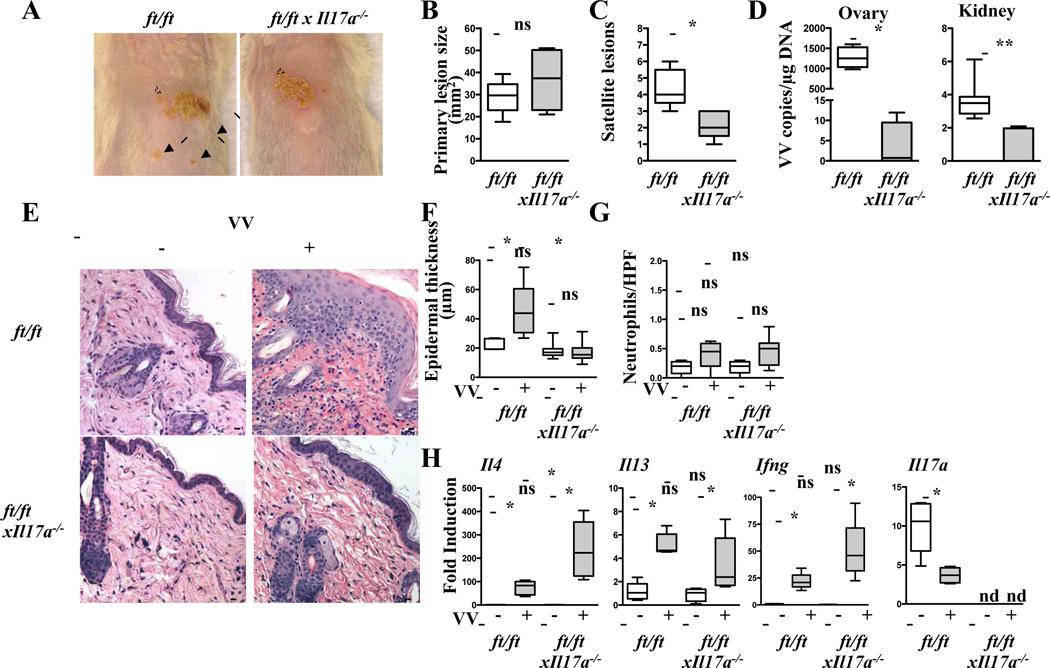
We have previously reported that treatment with anti-IL-17A attenuates EV features in WT mice inoculated with VV in tape stripped OVA sensitized skin 30. Here we tested the hypothesis that increased IL-17A expression in the skin of ft/ft mice contributes to their increased susceptibility to VV inoculation. We bred the ft/ft genotype onto the IL-17A null background and examined responses to VV inoculation.
There was no detectable skin inflammation in 6–8 week old ft/ftxIl17a−/− mice or ft/ft mice. As expected, Il17a mRNA expression was undetectable in ft/ftxIl17a−/− mice. Following VV inoculation in unsensitized skin, ft/ftxIl17a−/− mice developed significantly less satellite lesions and significantly lower viral loads compared to ft/ft controls, but the size of the primary lesion was comparable between the two groups (Fig. 3A–D). VV inoculated ft/ftxIl17a−/− mice had significantly less epidermal thickening than VV inoculated ft/ft controls with no increase in dermal infiltration with neutrophils (Fig. 3E–G). However, VV inoculated ft/ftxIl17a−/− skin showed significantly higher levels of Il4 mRNA expression than VV inoculated ft/ft skin. The levels of Il13 and Ifng mRNA in VV inoculated skin were not significantly different between the two groups (Fig. 3H). VV inoculated ft/ftxIl17a−/− skin exhibited comparable epidermal thickness and dermal infiltration with neutrophils, but significantly higher levels of Il4, Il13, and Ifng mRNA as compared to uninfected ft/ftxIl17a−/− skin (Fig. 3E–H). Epidermal thickness, dermal infiltration with neutrophils, and skin Il4, Il13, and Ifng mRNA levels were comparable between uninfected ft/ft and ft/ftxIl17a−/− mice (Fig. 3E–H). Splenocyte secretion of IL-4, IL-13 and IFN-γ in response to VV stimulation and VV specific serum IgG2a levels were comparable in ft/ftxIl17a−/− and ft/ft mice inoculated with VV (data not shown and Fig. S5B).
Figure 3. Absence of IL-17A attenuates EV features caused by VV inoculation in the unsensitized skin of ft/ft mice.
A–C. Gross appearance (A), area of primary lesions (B), and number of satellite lesions (C). Dashed circles indicate primary lesions. Arrows indicate satellite lesions. D. Viral load. E–H. H&E stained skin sections (200× magnification) (E), epidermal thickness (F), number of neutrophils (G), cytokine mRNA expression (H). Scale bars: 100 µm. The line in the box indicates median and the whiskers represent from the minimum to maximum (n = 5 per group). *P < .05 and **P < .01. ns, not significant. nd, not detectable.
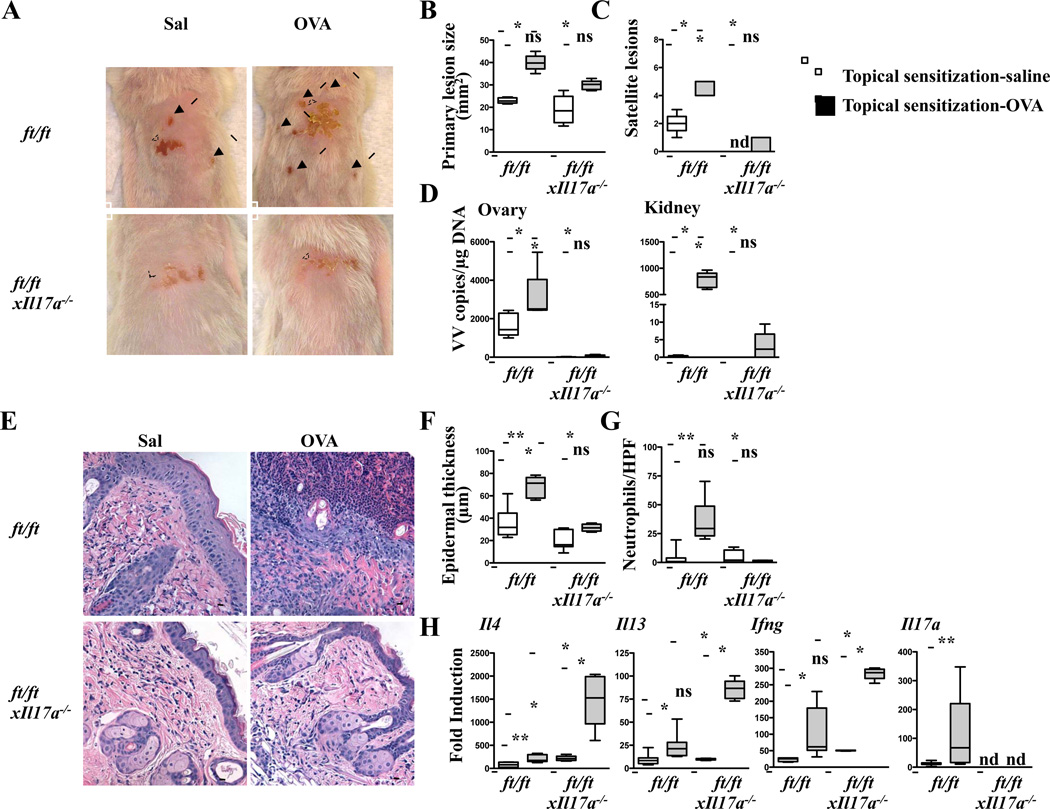
We next examined the response of ft/ftxIl17a−/− mice to VV inoculation at the site of cutaneous sensitization with OVA. Following VV inoculation in skin topically sensitized with OVA, ft/ftxIl17a−/− mice developed significantly smaller primary lesions, significantly less of satellite lesions, and significantly lower viral loads in internal organs compared to similarly sensitized ft/ft controls (Fig. 4A–D). They also developed significantly less epidermal thickening and significantly less neutrophil infiltration in the dermis; however, they expressed significantly more Il4, Il13 and Ifng mRNA in VV infected skin than VV infected OVA-sensitized skin of ft/ft controls or saline exposed ft/ftxIl17a−/− skin (Fig. 4E–H). Splenocyte secretion of IL-4, IL-13 and IFN-γ in response to VV stimulation and VV specific serum IgG2a levels were comparable in topically sensitized or saline exposed ft/ftxIl17a−/− and ft/ft mice inoculated with VV (data not shown and Fig. S5C). The size of primary lesions, number of satellite lesions, viral loads in internal organs, epidermal thickness, and dermal neutrophil infiltration were comparable between topically sensitized or saline exposed ft/ftxIl17a−/− mice inoculated with VV. These results suggest that increased expression of IL-17A in the skin of ft/ft mice contributes to their enhanced susceptibility to cutaneous infection with VV.
Figure 4. Absence of IL-17A attenuates EV features caused by VV inoculation in the OVA sensitized skin of ft/ft mice.
A–C. Gross appearance (A), area of primary lesions (B), and number of satellite lesions (C). Dashed circles indicate primary lesions. Arrows indicate satellite lesions. D. Viral load. E–H, H&E stained skin sections (200× magnification) (E), epidermal thickness (F), number of neutrophils (G), cytokine mRNA expression (H). Scale bars: 100 µm. The line in the box indicates median and the whiskers represent from the minimum to maximum (n = 5 per group). *P < .05 and **P < .01. ns, not significant. nd, not detectable.
Discussion
We demonstrate that filaggrin deficient ft/ft mice are more susceptible to developing features of EV following VV inoculation in the skin, and that filaggrin deficiency and allergic inflammation synergize to promote VV dissemination. The increased susceptibility of ft/ft mice to EV depends on IL-17A.
ft/ft mice, but not WT controls, developed features of EV following VV inoculation in unsensitized skin. These included larger primary lesions at the site of cutaneous VV inoculation, higher numbers of satellite skin lesions and greater viral dissemination to internal organs compared to WT controls. The increased susceptibility of ft/ft mice to VV dissemination, a feature of EV, is in line with the observation that filaggrin mutations predispose patients with AD to eczema herpeticum 11. Taken together, these results suggest that filaggrin plays a critical role in containing viral dissemination following cutaneous infection.
The lesions in ft/ft mice were characterized by epidermal thickening, which was significantly more than in WT controls, and massive upregulation of Th1 and Th2 cytokine expression compared to WT controls. The Th1 cytokine IFN-γ and IgG2a antibody play a protective role in limiting VV dissemination 33. Splenocyte production of IFN-γ in response to VV stimulation was comparable in ft/ft mice and WT controls, while VV specific IgG2a antibody levels were slightly increased in ft/ft mice, ruling out a role for a difference in these systemic immune responses in the increased susceptibility of ft/ft mice to VV. They rather suggest that disrupted barrier function underlies the propensity of ft/ft mice for VV dissemination. The increase in VV specific IgG2a antibody levels in ft/ft mice may be the consequence of their increased antigenic load.
Il17a mRNA expression is significantly elevated in the skin of ft/ft mice raised under specific pathogen free conditions 18. The mechanisms by which filaggrin deficiency predisposes to increased IL-17A skin expression may include increased absorption of microbes from the skin, which drives the production of IL-17A promoting cytokines that include IL-6 and IL-23 18. This is supported by the observation that IL-17A expression in the skin is attenuated in ft/ft mice raised under germ free conditions (data not shown). Paradoxically, Il17a mRNA expression was attenuated following VV inoculation, possibly due to downregulation by the massive increase in Il4 and Ifng expression 34,35. The increased expression of Th1 and Th2 cytokines in VV inoculated skin of ft/ft mice was not simply a reflection of a stronger systemic antigen specific immune response in these mice. In fact, splenocytes from ft/ft mice and WT controls secreted comparable amounts of cytokines in response to VV stimulation. One possible explanation for the local increase in Th1 and Th2 cytokine production in VV inoculated skin of ft/ft mice could be increased cutaneous expression of molecules that promote cytokine production by infiltrating T cells. These could include thymic stromal lymphopoietin, which amplifies Th2 cytokine expression 36 and IL-12, which stimulates IFN-γ expression by Th1 cells 37. Increased dermal cellular infiltration in VV inoculated skin sites of ft/ft mice may also have contributed to the increased cytokine expression at these sites.
Cutaneous antigen sensitization in ft/ft mice mimics skin exposure to environmental antigens in patients with AD whose barrier dysfunction allows cutaneous sensitization upon exposure to antigen 18, 38. Topical sensitization with OVA elicited a vigorous Th2 response in the skin of ft/ft mice on BALB/c background as we had previously shown on mixed background 18. VV inoculation of ft/ft mice in skin sites topically sensitized with OVA exaggerated the EV features that develop in these mice including primary lesion size, number of satellite lesions and viral loads in internal organs, suggesting that filaggrin deficiency and allergic inflammation synergize to promote VV dissemination. It also resulted in more severe skin inflammation characterized by intense dermal neutrophil infiltration and an increase in Il17a expression compared to VV inoculation in saline exposed or unsensitized skin from these mice. Consistent with our previous report 18, topical application of OVA to shaved, unstripped WT skin did not result in the development of skin inflammation, and elicited minimal responses to VV inoculation. In contrast, EC sensitization of WT mice by application of OVA to tape stripped skin, which disrupts the skin barrier, elicits a vigorous Th2 response 28. Moreover, VV inoculation in EC sensitized tape stripped skin of WT mice results in the development of EV features and in massive local neutrophil infiltration and Il17a expression 30. These observations suggest that a preexisting cutaneous inflammatory response to an unrelated antigen promotes the development of EV features and enhances the local IL-17A response when VV is inoculated in the skin.
The increased susceptibility of ft/ft mice to develop satellite lesions and viral dissemination following VV inoculation in unsensitized skin was dependent on IL-17A. The number of satellite lesions and the viral loads in internal organs in ft/ftxIl17a−/− mice inoculated in unsensitized skin, or in skin topically sensitized with OVA, were reduced to the levels in WT controls. Th1 and Th2 cytokine secretion by VV stimulated splenocytes and serum levels of VV specific IgG2a antibody were comparable in ft/ftxIl17a−/− and ft/ft mice, indicating that IL-17A played no detectable role in the systemic Th1 and Th2 responses of ft/ft mice to cutaneous inoculation with VV. However, in the absence of IL-17A, epidermal thickening was reduced and mRNA expression of Il4 was increased in VV inoculated skin of ft/ftxIl17a−/− mice. These findings are consistent with previous observations that IL-17A activates keratinocytes 39–41 and downregulates Th2 cytokine expression 35. Neutrophil infiltration in VV inoculated OVA sensitized skin, which was observed in ft/ft mice, was abolished in ft/ftxIl17a−/− mice. This finding is consistent with the role of IL-17A in driving neutrophil chemotaxis via the induction of CXCL2 expression in target cells 42. It is also in agreement with our previous finding that administration of anti-IL-17A neutralizing antibody inhibits neutrophil accumulation in tape stripped EC sensitized skin of WT mice 30. These findings suggest that IL-17A plays a critical role in the development of EV and in the local neutrophilic infiltration of VV inoculated skin in ft/ft mice.
The vast majority of AD patients with FLG mutations are heterozygous 43. However, mice heterozygous for the ft mutation have no detectable skin abnormalities and do not mount an immune response to topical sensitization with antigen (our unpublished observations). Consistent with their lack of a cutaneous phenotype, ft/+ heterozygous mice had a comparable response to VV inoculation as WT controls (data not shown). Altogether, these findings suggest that genetic and/or environmental factors operate in humans to modulate the effect of heterozygous FLG mutations in humans on skin biology, and thereby susceptibility of VV inoculation.
Patients with AD can develop EV even in the absence of active skin lesions 44. The filaggrin deficient mice we have studied had no grossly visible dermatitis, yet they were susceptible to developing EV features following VV inoculation in the skin. This parallel with the human AD phenotype further supports the validity of using filaggrin deficient mice as a model for the study of EV. The protective effect of IL-17A deficiency in these mice suggests that neutralization of IL-17A might be an effective therapy for EV in filaggrin deficient patients with AD.
Supplementary Material
Acknowledgement
We thank Dr. Hans Oettgen for scientific discussion and reading the manuscript. We thank Dr. Denise Babineau for discussion about statistical analysis of experimental data.
Funding: This work was supported by NIH/NIAID contract HHSN272201000020C (RSG). MKO was supported by the Boston Children's Hospital Faculty Career Development Fellowship and the Children’s Hospital Pediatric Associates Award.
Abbreviations
- AD
atopic dermatitis
- EH
eczema herpeticum
- EV
eczema vaccinatum
- FLG
filaggrin
- ft/ft
flaky tail
- H&E
hematoxylin and eosin
- HPF
high power fields
- HSV
herpes simplex virus
- IL
interleukin
- ma
matted
- OVA
ovalbumin
- pfu
plaque-forming unit
- WT
wild-type
- VV
vaccinia virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. The Journal of allergy and clinical immunology. 2014;134:769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. The Journal of allergy and clinical immunology. 2014;134:792–9. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Kuo IH, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2013;131:266–278. doi: 10.1016/j.jaci.2012.12.1563. [DOI] [PubMed] [Google Scholar]
- 4.Slifka MK, Leung DY, Hammarlund E, Raue HP, Simpson EL, Tofte S, et al. Transcutaneous yellow fever vaccination of subjects with or without atopic dermatitis. The Journal of allergy and clinical immunology. 2014;133:439–447. doi: 10.1016/j.jaci.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. The Journal of allergy and clinical immunology. 2014;133:1041–1047. doi: 10.1016/j.jaci.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Engler RJ, Kenner J, Leung DY. Smallpox vaccination: Risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–365. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 7.Wollenberg A, Wetzel S, Burgdorf WH, Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667–674. doi: 10.1016/j.jaci.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 9.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. The Journal of allergy and clinical immunology. 2006;118:214–219. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Cole C, Kroboth K, Schurch NJ, Sandilands A, Sherstnev A, O'Regan GM, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2014;134:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. The Journal of allergy and clinical immunology. 2009;124:507–513. doi: 10.1016/j.jaci.2009.07.034. 13 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bin L, Edwards MG, Heiser R, Streib JE, Richers B, Hall CF, et al. Identification of novel gene signatures in patients with atopic dermatitis complicated by eczema herpeticum. The Journal of allergy and clinical immunology. 2014;134:848–855. doi: 10.1016/j.jaci.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. The New England journal of medicine. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 14.McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. The Journal of allergy and clinical immunology. 2013;131:280–291. doi: 10.1016/j.jaci.2012.12.668. [DOI] [PubMed] [Google Scholar]
- 15.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nature genetics. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane PW. Two new mutations in linkage group XVI of the house mouse. Flaky tail and varitint-waddler-J. The Journal of heredity. 1972;63:135–140. doi: 10.1093/oxfordjournals.jhered.a108252. [DOI] [PubMed] [Google Scholar]
- 17.Moniaga CS, Egawa G, Kawasaki H, Hara-Chikuma M, Honda T, Tanizaki H, et al. Flaky tail mouse denotes human atopic dermatitis in the steady state and by topical application with Dermatophagoides pteronyssinus extract. The American journal of pathology. 2010;176:2385–2393. doi: 10.2353/ajpath.2010.090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. The Journal of allergy and clinical immunology. 2009;124:485–493. doi: 10.1016/j.jaci.2009.05.042. 93 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott JE, Elkhal A, Freyschmidt EJ, Macarthur DH, McDonald D, Howell MD, et al. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol. 2007;120:1382–1388. doi: 10.1016/j.jaci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Freyschmidt EJ, Mathias CB, Macarthur DH, Laouar A, Narasimhaswamy M, Weih F, et al. Skin inflammation in RelB(−/−) mice leads to defective immunity and impaired clearance of vaccinia virus. J Allergy Clin Immunol. 2007;119:671–679. doi: 10.1016/j.jaci.2006.12.645. [DOI] [PubMed] [Google Scholar]
- 21.Tian T, Liu L, Freyschmidt EJ, Murphy GF, Kupper TS, Fuhlbrigge RC. Overexpression of IL-1alpha in skin differentially modulates the immune response to scarification with vaccinia virus. The Journal of investigative dermatology. 2009;129:70–78. doi: 10.1038/jid.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–973. doi: 10.1016/j.jaci.2011.02.010. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Kumar L, Mathias C, Zurakowski D, Oettgen H, Gorelik L, et al. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. The Journal of allergy and clinical immunology. 2009;123:875–882. doi: 10.1016/j.jaci.2009.02.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin H, Oyoshi MK, Le Y, Bianchi T, Koduru S, Mathias CB, et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. The Journal of clinical investigation. 2009;119:47–60. doi: 10.1172/JCI32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. The Journal of clinical investigation. 2003;112:1058–1066. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laouini D, Kawamoto S, Yalcindag A, Bryce P, Mizoguchi E, Oettgen H, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. The Journal of allergy and clinical immunology. 2003;112:981–987. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci U S A. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, et al. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A. 2009;106:14954–149549. doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, et al. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. The Journal of allergy and clinical immunology. 2012;129:1538–1546. doi: 10.1016/j.jaci.2012.01.068. e6. [DOI] [PubMed] [Google Scholar]
- 32.Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. The Journal of allergy and clinical immunology. 2013;132:1121–1129. doi: 10.1016/j.jaci.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, et al. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med. 2004;199:1585–1593. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooney LA, Fox DA. Regulation of Th17 maturation by interleukin 4. Critical reviews in immunology. 2013;33:379–387. doi: 10.1615/critrevimmunol.2013007096. [DOI] [PubMed] [Google Scholar]
- 35.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. The Journal of allergy and clinical immunology. 2012;130:845–852. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunological reviews. 2013;252:89–103. doi: 10.1111/imr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. The Journal of allergy and clinical immunology. 2009;124:496–506. doi: 10.1016/j.jaci.2009.06.046. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. The Journal of investigative dermatology. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. The Journal of allergy and clinical immunology. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. e4. [DOI] [PubMed] [Google Scholar]
- 41.Kanda N, Koike S, Watanabe S. IL-17 suppresses TNF-alpha-induced CCL27 production through induction of COX-2 in human keratinocytes. J Allergy Clin Immunol. 2005;116:1144–1150. doi: 10.1016/j.jaci.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. The Journal of investigative dermatology. 2006;126:1200–1202. doi: 10.1038/sj.jid.5700365. [DOI] [PubMed] [Google Scholar]
- 44.Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:832–840. doi: 10.1093/cid/cir952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.