Abstract
Mitogen inducible gene 6 (Mig-6) is an important mediator of progesterone (P4) signaling to inhibit estrogen (E2) signaling in the uterus. Ablation of Mig-6 in the murine uterus leads to the development of endometrial hyperplasia and E2-induced endometrial cancer. To identify the molecular pathways regulated by Mig-6, we performed microarray analysis on the uterus of ovariectomized Mig-6f/f and PGRcre/+ Mig-6f/f (Mig-6d/d) mice treated with vehicle or P4 for 6 hours. The results revealed that 772 transcripts were significantly regulated in the Mig-6d/d uterus treated with vehicle as compared with Mig-6f/f mice. The pathway analysis showed that Mig-6 suppressed the expression of gene-related cell cycle regulation in the absence of ovarian steroid hormone. The epithelium of Mig-6d/d mice showed a significant increase in the number of proliferative cells compared to Mig-6f/f mice. This microarray analysis also revealed that 324 genes are regulated by P4 as well as Mig-6. Cited2, the developmentally important transcription factor, was identified as being regulated by the P4-Mig-6 axis. To determine the role of Cited2 in the uterus, we used the mice with Cited2 that were conditionally ablated in progesterone receptor-positive cells (PGRcre/+ Cited2f/f; Cited2d/d). Ablation of Cited2 in the uterus resulted in a significant reduction in the ability of the uterus to undergo a hormonally induced decidual reaction. Identification and analysis of these responsive genes will help define the role of P4 as well as Mig-6 in regulating uterine biology.
Keywords: Progesterone, Uterus, Progesterone, Mig-6, Cited2
Introduction
The uterus is an important hormone-responsive reproductive organ in mammals. The ovarian steroid hormones progesterone (P4) and estrogen (E2) are essential mediators of reproductive events associated with the establishment and maintenance of pregnancy [1,2]. E2 stimulates the proliferation of both uterine luminal and glandular epithelium [3]. In contrast, P4 inhibits E2-mediated proliferation of the luminal and glandular epithelium [4,5]. P4 is a critical regulator of reproductive events associated with the embryo implantation, decidualization of the endometrial stromal cells and maintenance of pregnancy [2,6]. The physiological effects of P4 are mediated through its cognate receptor, the progesterone receptor (PGR) [7]. The fertility defects exhibited by the progesterone receptor knockout (PRKO) mice unequivocally demonstrated the critical importance of P4 and its receptor in the establishment and maintenance of pregnancy [2,8].
Progestin is a synthetic progesterone. Progestin has been used in the conservative endocrine treatment of early endometrial cancer patients in order to preserve their fertility, as well as in palliative treatment of advanced-stage patients [9,10]. Interruption of P4 signaling, occurring from the loss of the PGR itself or through the loss of its interacting partners or downstream effectors, leads to a physiological state of P4 resistance [11]. P4 resistance is seen in a wide variety of diseases. P4 resistance is a hallmark of endometriosis [12,13]. P4 resistance is also seen in the endometrium of women with polycystic ovary syndrome (PCOS) [14,15]. Expression of the progesterone receptor (PGR) was known to be positively correlated with a good prognosis and response to progestin treatment [16]. However, more than 30% of patients with progestin treatment did not respond to progestin due to de novo or acquired progestin resistance [9,17,18]. The mechanism of progestin resistance is still unknown. Understanding the precise mechanism of P4 regulation in the endometrium is of critical importance in developing therapeutic approaches to alleviate this women’s health crisis.
Mig-6 has been shown to be critical for uterine functioning because conditional ablation of Mig-6 (PGRcre/+ Mig-6f/f; Mig-6d/d) in the mouse uterus results in infertility due to a defect of embryo implantation [19,20]. Additionally, Mig-6d/d mice leads to the development of animals with epithelial hyperplasia, adenoma and adenocarcinomas in organs, such as the uterus, lung, gallbladder and bile duct [19,21,22,23]. Endometrial tumorigenesis is accelerated by double ablation of Mig-6 and Pten compared to single ablation of Mig-6 or Pten [20]. However, the precise mechanism of Mig-6 in endometrial cancer remains poorly understood.
Here, we identified Mig-6 regulated uterine genes using Mig-6d/d mice in combination with high density DNA microarray analysis. This analysis indicates that Mig-6 plays an important role for uterine functioning by modulating the regulation of cell cycle related genes and the ability of P4 to regulate specific genes. The results of our investigation provide significant insights into our understanding of the importance of steroid hormone regulation in female reproduction and endometrial cancer.
MATERIALS AND METHODS
Animals and tissue collection
Mig-6 “floxed” (Mig-6f/f) and PGRcre/+ Mig-6f/f (Mig-6d/d) mice [22,24] were maintained in the designated animal care facility according to the Michigan State University institutional guidelines. All animal procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University. Eighteen Mig-6f/f and 18 Mig-6d/d mice were ovariectomized at 6 weeks of age. After 2 weeks of rest, vehicle (sesame oil) or P4 (in sesame oil; Sigma-Aldrich, St. Louis, MO; 1 mg/mouse in 100 µl) was administered into ovariectomized mice via s.c. injection. At 6 hours following the P4 or vehicle injection, mice were euthanized for tissue collection. Cited2 f/f mice [25] were bred with PGR-Cre mice [24] to generate PGRcre/+ Cited2 f/f (Cited2d/d) mice. Uterine tissues were immediately frozen at the time of dissection and stored at −80°C for RNA extraction or fixed with 4% (v/v) paraformaldehyde for immunohistochemistry.
RNA isolation and microarray Analysis
Total RNA was extracted from the uterine tissues using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). RNA was pooled from the uteri of 3 mice per genotype and treatment. All RNA samples were analyzed with a Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE) before microarray hybridization. Microarray data analysis was performed as previously described [26]. To adjust arrays to a common baseline using invariant set normalization, DNA-Chip Analyzer dChip was used and the perfect match (PM) model described by Li and Wong [27] was used to estimate expression. We selected differentially expressed genes within each treatment in the Mig-6f/f and Mig-6d/d mice using a two-sample comparison according to the following criteria: lower boundary of 90% confidence interval of fold change greater than 1.2 and an absolute value of difference between group means greater than 80. Differentially expressed genes were classified according to canonical pathway analyzed by Ingenuity System Software (Ingenuity Systems Inc., Redwood City, CA).
Quantitative real-time PCR Analysis
Total RNA was extracted from the uterine tissues using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). The expression levels of mRNA were measured by real-time PCR TaqMan analysis using an Applied Biosystems StepOnePlusTM system (Applied Biosystems, Foster City, CA) and real-time PCR SYBR Green detection system (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. mRNA quantities were normalized against the housekeeping gene, 18S RNA. Primer sequences used in these studies are shown in Supplementary Table 1.
Immunohistochemistry
Immunohistochemistry analysis was performed as previously described [20]. Uterine sections from paraffin-embedded tissue were preincubated with 10% normal serum in phosphate-buffered saline (PBS) and incubated with anti-KI67 (ab15580; Abcam, Cambridge, MA) antibody in 10% normal serum in PBS. On the following day, sections were washed in PBS and incubated with a secondary antibody (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Immunoreactivity was detected using the Vectastain Elite DAB kit (Vector Laboratories).
Induction of decidualization
The hormonally induced decidual response has been previously described [28]. The ovariectomized Cited2f/f and Cited2d/d female mice were subjected to the following hormonal regimen: 100 ng of E2 per day for three days; two days rest; then three daily injections of 1 mg of P4 plus 6.7 ng of E2. At 6 hours following the third P4 and E2 injection, the left uterine horn was mechanically stimulated by scratching the full length of the anti-mesometrial side with a burred needle. The other horn was left unstimulated as a control. Daily injections of P4 (1 mg/mouse) plus E2 (6.7 ng/mouse) were continued for 5 days to maximize the decidual response. The mice were sacrificed on day 5. The uteri were then excised, weighed and fixed in 4% paraformaldehyde for histological analysis.
Statistical analysis
For data with two groups, Student’s t test was used. For data containing more than two groups, one way ANOVA was used, followed by Tukey’s post hoc multiple range. All data are p resented as means ± SEM. p < 0.05 was considered statistically significant. All statistical analyses were performed using the Instat package from GraphPad (San Diego, CA, USA).
RESULTS
Mig-6 suppresses cell cycle progression
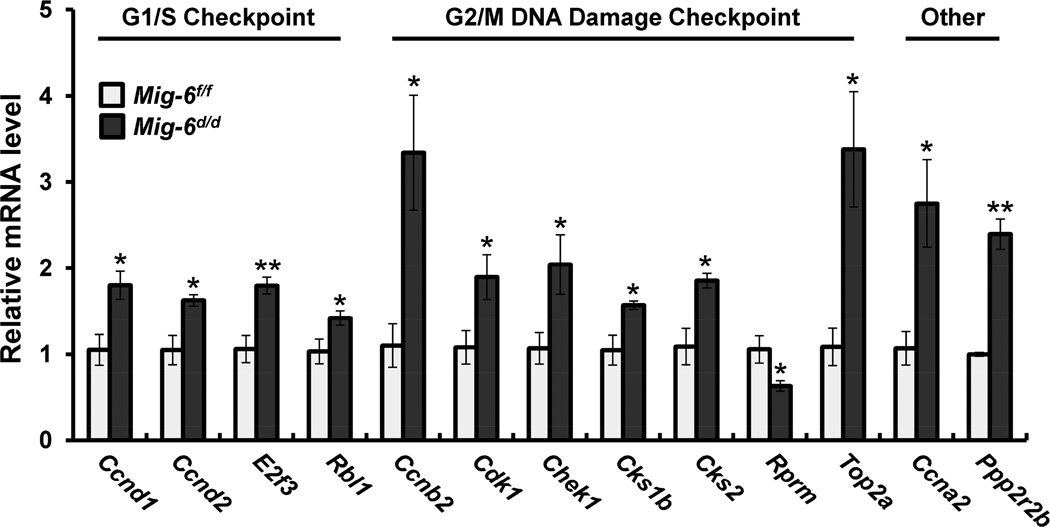
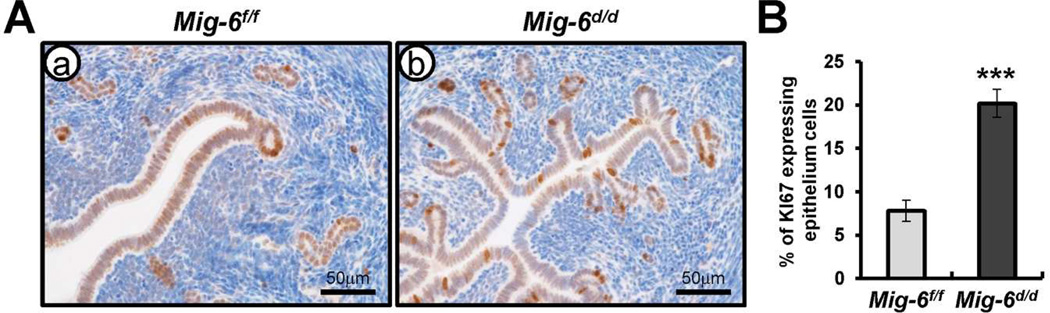
Previously, Mig-6d/d mice are infertile due to an inability of the uterus to undergo embryo implantation [19,20]. To identify the molecular pathways regulated by Mig-6 in a steroid-hormone independent and dependent manner, we performed high-density DNA microarray analysis on the uteri of ovariectomized Mig-6f/f and Mig-6d/d mice treated with vehicle or progesterone (P4) for 6 hours. The 772 genes were identified as differentially expressed when comparing the nonhormone-stimulated Mig-6f/f to Mig-6d/d uterus (Supplementary Table 2). This difference could be accounted for by the impact of Mig-6 in the uterus. To determine which pathways are regulated by Mig-6 ablation, we performed pathway analysis using Ingenuity Systems Software. The altered pathways included G1/S checkpoint regulation, G2/M DNA damage checkpoint regulation, cell cycle regulation, ILK signaling, p53 signaling, HIF1α signaling, and Wnt/β-catenin signaling (Supplementary Table 3). Among these, we observed an increase in genes necessary for cell cycle progression in the Mig-6d/d uterus compared to Mig-6f/f. To validate our microarray analysis, we determined the transcript levels on uteri of Mig-6f/f and Mig-6d/d mice treated with vehicle for 6 hours. The mRNA expression level of Ccnd1, Ccnd2, E2f3, and Rbl1, cell cycle G1/S checkpoint regulation genes, Ccnb2, Cdk1, Chek1, Cks1b, Cks2, and Top2a, cell cycle G2/M DNA damage checkpoint regulation genes, and Ccna2 and Ppp2r2b, cell-cycle-related genes were significantly increased and Rprm was significantly decreased in the Mig-6d/d uteri as compared with Mig-6f/f (Fig. 1). Immunohistochemical staining of KI67 as a proliferation marker showed that proliferation was significantly increased in the epithelium of Mig-6d/d mice compared with Mig-6f/f mice (Fig. 2A and B). These results suggest that Mig-6 has a negative regulator for cell cycle progression and epithelial cell proliferation.
Fig. 1.
Regulation of cell cycle genes and epithelial proliferation by Mig-6. Validation of Mig-6 regulated genes in the murine uterus. Real-time RT-PCR analysis were performed on uteri of Mig-6f/f and Mig-6d/d mice treated with vehicle for 6 hours. The results represent the mean ± SEM. * p<0.05 and ** p<0.01.
Fig. 2.
A) Immunohistochemical analysis of KI67 as a proliferation marker in uteri of Mig-6f/f (a) and Mig-6d/d (b) mice treated with vehicle for 6 hours. b) Quantification of KI67 positive cells in epithelium cells. The results represent the mean ± SEM. *** p<0.001.
Identification of P4- and Mig-6-regulated genes
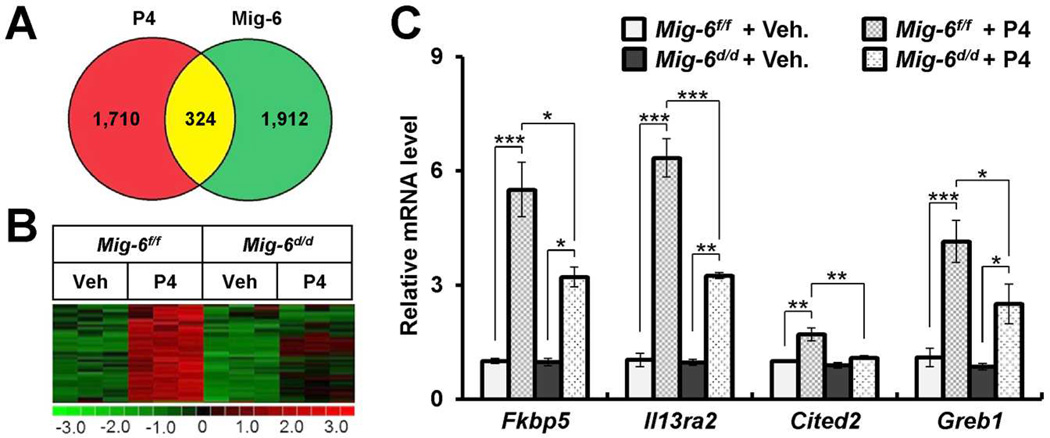
Previously, we have shown that Mig-6-ablated mice uteri increased estrogen signaling in the presence of P4, which typically antagonizes ESR activity [19,20]. The overall goal of this investigation was to identify genes that are regulated by the P4-Mig-6 axis. A total of 324 genes were common in both vehicle-treated vs. P4-treated control Mig-6f/f mice and P4-treated Mig-6d/d vs. Mig-6f/f mice (Fig. 3A). Of the 324 genes, 107 genes were more highly expressed in the P4 treated control uteri and 217 genes decreased. Of the 107 genes, 39 genes were markedly decreased in the P4-treated Mig-6d/d mice compare to P4-treated Mig-6f/f mice (Fig. 3B). A complete list is presented in Supplementary Table 4. To validate our microarray analysis, we performed qPCR analysis on the uteri of Mig-6f/f and Mig-6d/d mice treated with vehicle or P4 for 6 hours. The transcript levels of FK506 binding protein 5 (Fkbp5), interleukin 13 receptor, alpha 2 (Il13ra2), Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 (Cited2), and genes regulated by estrogen in breast cancer protein (Greb1) were significantly increased in the uteri of Mig-6f/f mice treated with P4 compared to vehicle. However, these inductions were significantly decreased in the Mig-6d/d mice treated with P4 compared to Mig-6f/f mice (Fig. 3C).
Fig. 3.
Validation of Mig-6 and progesterone regulated genes in the mouse uterus. A) Venn diagrams demonstrating the relationship between genes modulated in the Mig-6f/f and Mig-6d/d uterus in response to acute treatment with P4. Red circles indicate genes selected by vehicle-treated Mig-6f/f vs P4-treated Mig-6f/f; Green, by P4-treated Mig-6f/f vs P4-treated Mig-6d/d. The numbers, displayed within the intersections of the circles indicate the common genes by two comparisons. B) Clustering analysis of Mig-6 dependent induced genes by P4 (p<0.05, 1.2-fold change) in uteri. The extent of gene expression changes is represented by a green-red color scale (green: low expression and red: high expression). C) Quantitative real time PCR analysis of transcript levels of Mig-6 dependent induced genes by P4 in Mig-6f/f and Mig-6d/d mice treated with vehicle or P4 for 6 hours. The results represent the mean ± SEM. * p<0.05, ** p<0.01, and *** p<0.001.
Cited2 is required for uterine decidualization
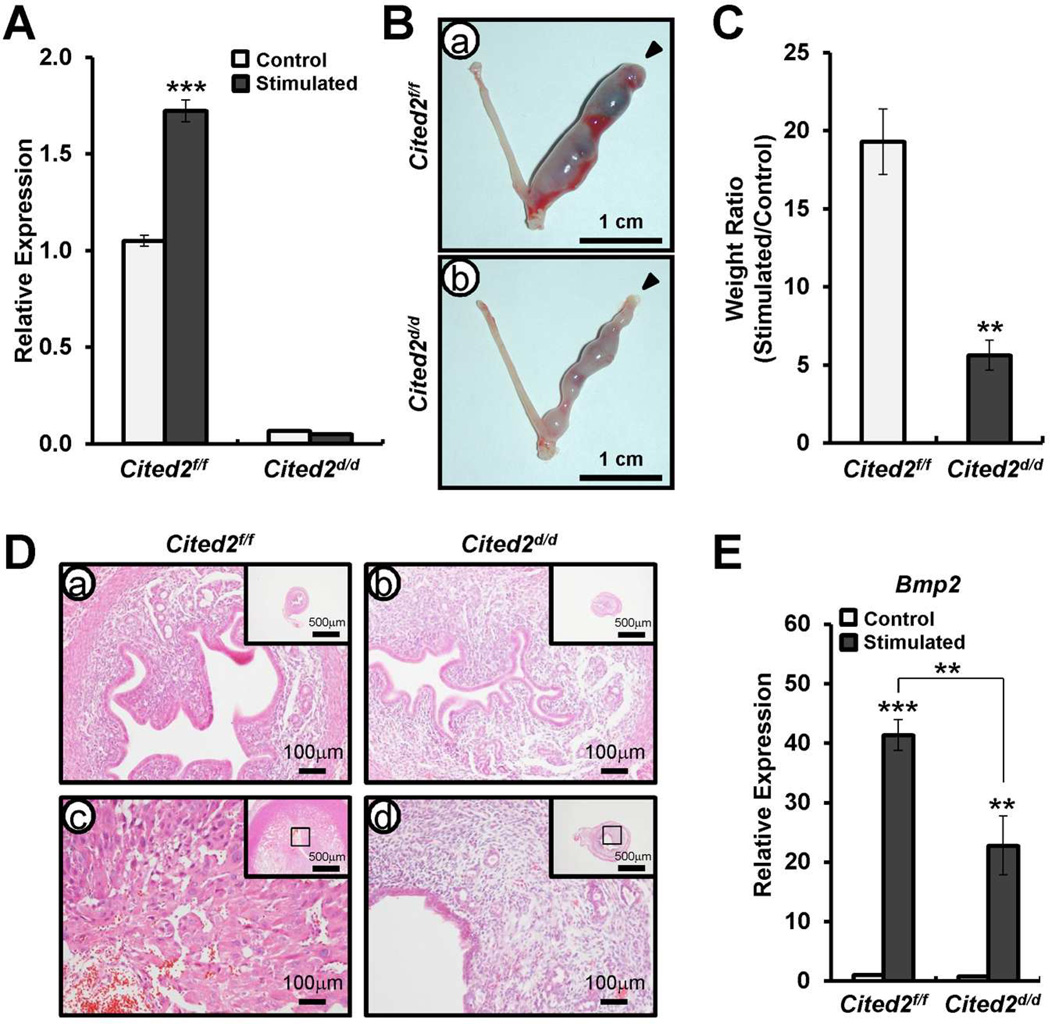
Cited2 is a member of the Cited transcription coactivator family that binds the p300/corticosterone-binding protein coactivator and a P4- target gene. To determine the role of Cited2 in the uterus, we generated the mice with Cited2 conditionally ablated in progesterone receptor-positive cells (PGRcre/+ Cited2f/f; Cited2d/d) [24,25]. P4 signaling is crucial for decidualization of the endometrial stromal cells for successful pregnancy [29]. To determine whether decidualization of the uterus is affected by Cited2 ablation, we examined the ability of Cited2d/d mice to undergo a well-characterized induced decidualization after artificial hormonal induction. Ovariectomized female Cited2f/f and Cited2d/d mice were provided with E2+P4, and the uterus mechanically stimulated to mimic the presence of an implanting embryo and induce decidualization. First, we checked the transcript levels of Cited2 in uteri from Cited2f/f and Cited2d/d mice after artificially induced decidualization by real-time PCR Analysis. Cited2 mRNA expression was significantly lower in Cited2d/d mice compared with Cited2f/f mice in the uterine horn with/without stimuli (Fig. 4A). Cited2f/f mice showed a decidual uterine horn that responded well to stimuli for artificial induction. However, Cited2d/d mice displayed remarkable reduction in the decidual response (Fig. 4B). Weight ratio of stimulated to control horn was significantly decreased in Cited2d/d mice compared to Cited2f/f control mice (Fig. 4C). Histological analysis confirmed that well-developed decidual cells were detected in the decidual uterine horn of Cited2f/f control mice, but differentiated decidual stromal cells were decreased in the decidual uterine horn of Cited2d/d mice (Fig. 4D). In addition, the expression of the known decidualization marker, bone morphogenetic protein 2 (Bmp2), was reduced in the decidual uterine horn of the Cited2d/d mice compared with the decidual uterine horn of Cited2f/f control mice (Fig. 4E). Collectively, the uteri of Cited2d/d mice were impaired to undergo a hormonally induced decidual reaction. These results suggest that Cited2 plays an important role for decidualization as a P4 and Mig-6 target gene.
FIG. 4.
Decidual defect of Cited2d/d mice. A) Quantitative real time PCR analysis of transcript levels of Cited2 in uteri from Cited2f/f and Cited2d/d mice after artificially induced decidualization. Ovariectomized mice were primed with E2 plus P4, and one uterine horn was mechanically stimulated to mimic the presence of an implanting embryo and induce decidualization. The other horn was unstimulated and served as a control. The results represent the mean ± SEM. *** p<0.001. B) Decidualization response of Cited2d/d mice. Gross morphology of the uteri of Cited2f/f (a) and Cited2d/d (b) mice after artificially induced decidualization. C) Ratio between the weight of stimulated and control horn collected from Cited2f/f and Cited2d/d mice. Results represent means ± SEM of 3 animals per group. ** p<0.01. D) Histology of uteri was investigated by H&E staining. E) Quantitative real time PCR analysis of Bmp2 as a decidual differentiation marker in uteri of Cited2f/f and Cited2d/d mice after artificially induced decidualization. The results represent the mean ± SEM. ** p<0.01, and *** p<0.001.
DISCUSSION
The ablation of Mig-6 increased proliferation of endometrial epithelial cells [19,30]. However, the molecular mechanism of Mig-6 has not been studied in uterine biology. In this study, we have identified Mig-6- and P4-regulated uterine genes using the Mig-6d/d mouse and high-density DNA microarray analysis. The design of our microarray analysis was to determine the role of Mig-6 in the uterus. This analysis identified P4-dependent as well as -independent genes whose expression was altered by Mig-6 ablation. The choice of conducting the microarray analysis on uteri isolated for 6 hours after a single dose of P4 was for the purpose of identifying direct targets of P4 activity. Previously, we showed that Mig-6 is a P4 signaling mediator that suppresses E2 signaling in the uterus [19,20,31]. However, the majority of genes are altered in ovariectomized Mig-6d/d mice treated with vehicle (772 genes). Our microarray analysis showed an increase in genes necessary for cell cycle progression, such as cell cycle G1/S checkpoint regulation genes, cell cycle G2/M DNA damage checkpoint regulation genes, and cell-cycle-related genes in the Mig-6d/d uterus compared to Mig-6f/f. Furthermore, proliferation of luminal epithelium was significantly increased in the uterus of Mig-6d/d mice treated with vehicle. These results showed that Mig-6 has an intrinsic hormone-independent tumor suppressor role in cell cycle progression and epithelial cell proliferation.
This microarray analysis identified 324 genes in which the ablation of Mig-6 impacted the P4 regulation of genes in ovariectomized mice given a pharmacological dose of P4. We compared and analyzed direct P4-regulated genes and P4 regulated genes in Mig-6 response to identify the P4-responsive transcriptome that require Mig-6. The genes whose regulation by P4 is altered by Mig-6 ablation reflect metabolic proteins, cell cycle, structural proteins, transcription factors, and transport protein. Investigation of the expression pattern of these genes during natural pregnancy combined with functional analysis of the role of these genes in the establishment and maintenance of pregnancy will determine the significance of the Mig-6 regulation of these genes during pregnancy. The 39 of 324 genes showed Mig-6 dependent P4-regulation in the uterus. As validation of our microarray analysis, the transcriptional regulation of several P4 responsive genes such as Fkbp5Il13ra2Cited2, and Greb1 [31,32] are significantly attenuated in the uterus of the Mig-6d/d mice compare to Mig-6f/f . Therfore, the identification of the precise mechanism of P4-PGR regulation in the endometrium is crucial for understanding the causes of impairments in proper uterine functioning.
Cited2 is a member of the Cited (CBP/p300-Interacting Transactivators with glutamic acid (E)/aspartic acid (D)-rich C-terminal domain) transcription coactivator family. CITED2 can bind to DNA binding proteins, such as LHX2, TFAP2, PPARα, PPARγ, and Smads, recruit the histone acetyltransferases CBP/p300, and act as a transcriptional regulator [33,34,35,36]. Cited2 is induced by the P4-PGR signaling and expressed in the uterus during early pregnancy [31]. Cited2 is identified as a Mig-6 target gene in our microarray analysis. However, the function of Cited2 in reproductive biology is elusive. To explore the function of Cited2 in the uterus, we generated uterine specific Cited2 ablation mice since targeted deletion of Cited2 results in embryonic lethality [35]. We show that Cited2 d/d mice show a decrease in the uterine response to P4 during the decidual reaction. Our results suggest that Cited2 plays an important role for P4-dependent decidualization.
In summary, the Mig-6-dependently regulated target genes have been identified by microarray analysis. Epithelial cell proliferation of the ovarian steroid hormone independently increased in Mig-6d/d mice. The uteri of Mig-6d/d mice showed an increase of gene expression including cell cycle G1/S checkpoint regulation genes, cell cycle G2/M DNA damage checkpoint regulation genes, and cell-cycle-related genes. The alteration of P4-responsive genes in Mig-6d/d mice suggest a dual Mig-6 function as a positive regulator as well as negative regulator for P4 functioning. The results of our investigation provide significant insights into our understanding of the importance of Mig-6 in pregnancy and female reproduction.
Supplementary Material
We identify Mig-6- and P4-regulated uterine genes by microarray analysis.
Mig-6 suppresses cell cycle progression and epithelial cell proliferation in uterus.
We identify the Mig-6 dependent induced genes by P4
Cited2 plays an important role for decidualization as a P4 and Mig-6 target gene.
Acknowledgments
We would also like to thank John P. Lydon, Ph.D. and Francesco J. DeMayo, Ph.D. (Baylor College of Medicine, Houston, TX) for PGRcre/+ mice and Amanda Sterling for manuscript preparation. This work was supported by a NIH Grant R01HD057873 and an American Cancer Society Research Scholar Grant RSG-12-084-01-TBG (to J.W.J.), and an Australian Research Council grant DP0346729 and a National Health and Medical Research Senior Research Fellowship ID1042002 (to S.LD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11:266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 2.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 3.Quarmby VE, Korach KS. The influence of 17 beta-estradiol on patterns of cell division in the uterus. Endocrinology. 1984;114:694–702. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- 4.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 5.Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 7.DeMayo FJ, Zhao B, Takamoto N, Tsai SY. Mechanisms of action of estrogen and progesterone. Ann N Y Acad Sci. 2002;955:48–59. doi: 10.1111/j.1749-6632.2002.tb02765.x. discussion 86-48, 396-406. [DOI] [PubMed] [Google Scholar]
- 8.Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 9.Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, Lim JY, Kwon YS, Lee IH, Lim KT, Lee KH, Shim JU, Mok JE, Kim TJ. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19:1068–1073. doi: 10.1111/IGC.0b013e3181aae1fb. [DOI] [PubMed] [Google Scholar]
- 10.Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikura H, Shozu M, Isaka K. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–1958. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 11.Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, DeMayo FJ. Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol. 2012;26:1428–1442. doi: 10.1210/me.2011-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–111. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- 14.Shen ZQ, Zhu HT, Lin JF. Reverse of progestin-resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstet Gynecol. 2008;112:465–467. doi: 10.1097/AOG.0b013e3181719b92. [DOI] [PubMed] [Google Scholar]
- 15.Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE, Giudice LC, Lessey BA. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab. 2011;96:1737–1746. doi: 10.1210/jc.2010-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 17.Hoekstra AV, Kim JJ, Keh P, Schink JC. Absence of progesterone receptors in a failed case of fertility-sparing treatment in early endometrial cancer: a case report. J Reprod Med. 2008;53:869–873. [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, Vande Woude GF, Giudice LC, Young SL, Lessey BA, Tsai SY, Lydon JP, DeMayo FJ. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci U S A. 2009;106:8677–8682. doi: 10.1073/pnas.0903632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor-1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol Reprod. 2010;82:706–713. doi: 10.1095/biolreprod.109.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B, Resau J, Sigler R, Bronson R, Vande Woude GF. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene. 2007;26:269–276. doi: 10.1038/sj.onc.1209790. [DOI] [PubMed] [Google Scholar]
- 22.Jin N, Gilbert JL, Broaddus RR, Demayo FJ, Jeong JW. Generation of a Mig-6 conditional null allele. Genesis. 2007;45:716–721. doi: 10.1002/dvg.20348. [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Lee DK, Cho SN, Orvis GD, Behringer RR, Lydon JP, Ku BJ, McCampbell AS, Broaddus RR, Jeong JW. Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer. Cancer Res. 2013;73:5090–5099. doi: 10.1158/0008-5472.CAN-13-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 25.Preis JI, Wise N, Solloway MJ, Harvey RP, Sparrow DB, Dunwoodie SL. Generation of conditional Cited2 null alleles. Genesis. 2006;44:579–583. doi: 10.1002/dvg.20251. [DOI] [PubMed] [Google Scholar]
- 26.Franco HL, Lee KY, Broaddus RR, White LD, Lanske B, Lydon JP, Jeong JW, DeMayo FJ. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod. 2010;82:783–790. doi: 10.1095/biolreprod.109.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7:82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- 29.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP, Jeong JW. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene. 2010;29:3770–3780. doi: 10.1038/onc.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–3505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- 32.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 33.Chou YT, Yang YC. Post-transcriptional control of Cited2 by transforming growth factor beta. Regulation via Smads and Cited2 coding region. J Biol Chem. 2006;281:18451–18462. doi: 10.1074/jbc.M601720200. [DOI] [PubMed] [Google Scholar]
- 34.Tien ES, Hannon DB, Thompson JT, Vanden Heuvel JP. Examination of Ligand-Dependent Coactivator Recruitment by Peroxisome Proliferator-Activated Receptor-alpha (PPARalpha) PPAR Res. 2006;2006:69612. doi: 10.1155/PPAR/2006/69612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bamforth SD, Braganca J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- 36.Bai L, Merchant JL. A role for CITED2, a CBP/p300 interacting protein, in colon cancer cell invasion. FEBS Lett. 2007;581:5904–5910. doi: 10.1016/j.febslet.2007.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.