Abstract
This study sought to investigate the effect of calorie restriction (CR) on skeletal muscle sphingolipid metabolism and its contribution to improved insulin action in rats after a 6 month feeding study. Twenty nine (29) male Fischer-344 rats were randomized to an ad libitum (AL) diet or 30% CR. Dietary intake, body weight, and insulin sensitivity were monitored. After 6 months, skeletal muscle (vastus lateralis) was obtained for insulin signaling and lipid profiling. Calorie restriction significantly decreased insulin and glucose levels and also altered the expression and activity of proteins involved in sphingolipid formation and metabolism. The quantities of ceramides significantly increased in CR animals (p<0.05; n=14–15), while ceramide metabolism products (i.e glycosphingolipids: hexosylceramides and lactosylceramides) significantly decreased (p<0.05; n=14–15). Ceramide phosphates, sphingomyelins, sphingosine and sphingosine phosphate were not significantly different between AL and CR groups (p=ns; n=14–15). Lactosylceramide quantities correlated significantly with surrogate markers of insulin resistance (HOMA-IR) (r=0.7, p<0.005). Products of ceramide metabolism (glycosphingolipids), known to interfere with insulin signaling at elevated levels were significantly reduced in the skeletal muscle of CR animals. The increase in insulin sensitivity is associated with glycosphingolipid levels.
Keywords: Calorie restriction, Lipids, Sphingolipids, Glycosphingolipids, insulin sensitivity
Introduction
Obesity is strongly associated with, and is a major contributor to insulin resistance. Both in vitro and in vivo studies have demonstrated that exposure of skeletal muscle to excessive lipids leads to accumulation of fatty acid derived metabolites such as triacylglercols (TAGs), diacylglycerols (DAGs), and the sphingolipid ceramide [1–5]. The addition of palmitate in cell cultures and infusions of palmitate in rodents lead to the accumulation of ceramide in insulin sensitive tissues and an attenuation of insulin action [4, 5]. Elevated ceramide concentrations have been observed in skeletal muscle of obese insulin-resistant individuals. However, it is not just ceramide itself but rather its metabolites that are instrumental in the development of insulin resistance. While sphingolipids are a relatively minor component of the lipid milieu in most tissues, they are among the most pathogenic lipids in the onset of metabolic disorders associated with excess adiposity [5].
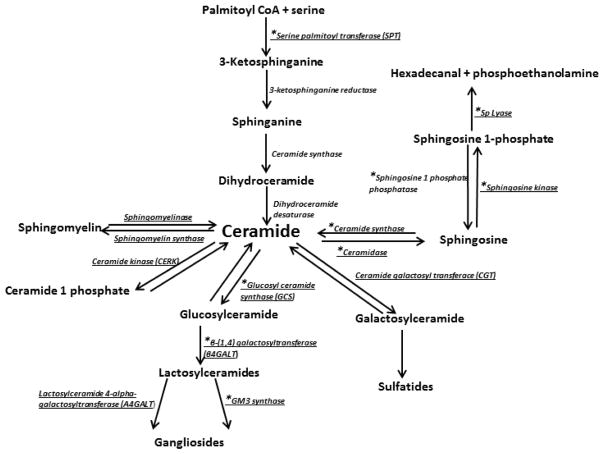
Ceramides maybe formed through the de novo pathway from palmitate or dietary lipids or through recycling of the free sphingosine and sphingomyelin hydrolysis [6] (Figure 1). Ceramides formed through these three pathways have different effects on insulin signaling with ceramides from the de novo pathway being deleterious to insulin signaling while those formed from the salvage pathway have no effect on insulin signalling [6]. Ceramide provides the platform for the synthesis of complex sphingolipids (ceramide1-phosphate, sphingomyelin and the glycosphingolipids (glucosylceramides, galactosylceramides, lactosylceramides, sulfatides and gangliosides) and undergoes catabolism to form sphingosine, sphingosine1-phosphate, [1, 2] (Figure 1). In cell membranes, sphingolipids form functional microdomains (lipid rafts) which affect insulin signal transduction. Sphingolipids are also considered a molecular link between inflammation and insulin resistance [3].
Figure 1. Overview of formation and the major metabolic pathways of ceramides.
Enzymes are shown in italics. Enzymes whose expression in skeletal muscle was determined in this study are underlined. *Denotes enzymes whose expression was significantly different between AL and CR groups.
An improvement in insulin sensitivity is one of the most consistent features of calorie restriction (CR), as observed in rodent and nonhuman primate models [7–10]. Although CR has been associated with reduction of protein glycation, scavenging of reactive oxygen species, modulation of thermogenesis, DNA repair, and altering oncogene expression and protein degradation [7–11], the cellular and molecular mechanisms by which CR attenuates progression of metabolic diseases and enhances insulin action is not precisely known. Another mechanism considered for CR is the effect on tissue lipid content, but, there are reports that suggest that moderate weight loss, by diet alone or in combination with exercise do not alter muscle lipid depots, despite significant improvements in insulin sensitivity [11]. It is also known that lipid metabolites such as sphingolipids constitute a very small percentage of lipid depots but have great metabolic importance. In this regard, inhibitors of sphingolipid synthesis show enormous therapeutic potential as they influence a broad spectrum of metabolic diseases in rodents [12,13].
A comprehensive evaluation of the mechanisms by which CR enhances insulin action would require investigation in multiple tissues in vivo because coordinated mechanisms from liver, adipocytes, and skeletal muscle all contribute to whole-body insulin action. In this study, we focused on skeletal muscle because it is the site of the majority of insulin-stimulated glucose disposal and would allow investigation into the role of skeletal muscle lipids. We hypothesized that CR improves insulin sensitivity through sphingolipid homeostasis in skeletal muscle and we evaluated insulin signaling and changes in expression of proteins involved in sphingolipid metabolism. Using liquid chromatography coupled with mass spectrometry (LC-MS/MS) we determined changes in sphingolipid levels in a rodent model subjected to chronic CR. We also assessed expression and activity of enzymes involved in the formation of ceramides and the glycosphingolipids: glucosylceramides, lactosylceramides and gangliosides and overall reduction in the quantities of these metabolites in skeletal muscle. Our experimental parameters were then correlated with surrogate markers of insulin action.
RESEARCH DESIGN AND METHODS
Animals
Animal experiments conformed to a protocol approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Male Fischer 344 x Brown Norway F1 hybrid rats (n=29) were obtained from the contract colony maintained by the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). On arrival at 11 months of age, rats were singly housed in plastic cages in a vivarium maintained at 22±2°C, 70±10% humidity. Throughout the study the rats were provided fortified NIH-31 diet (Catalog #7017 Harlan Teklad Madison, WI) and water using a Hyrodopac system (Seaford, DE). At 12 months they were randomly divided into two groups. Half were maintained ad libitum (AL) on this diet, while half were maintained on CR by providing the same diet at 70% of the level of food intake by the AL control group, which was adjusted as needed. Mean food intake of the AL group was monitored weekly. The CR group was then provided 70% of this estimate the following week. The CR study section lasted 6 months.
Body weight and blood chemistry
Body weight was measured weekly. Fasting serum glucose level was measured monthly by a colorimetric hexokinase glucose assay (Sigma Diagnostics, St. Louis, MO). Serum insulin was assessed monthly by an insulin ELISA kit (Millipore, Billerica, MA). At 6 months age, after an overnight fast, rats were injected in the peritoneal cavity with 1U/kg insulin. After 10 minutes, blood samples were collected by heart puncture in rats under anesthesia (Ketamine/Xylazine at 75/10 mg/kg IP) as a terminal procedure. At the end of the blood draw, rats were immediately euthanized by guillotine under isoflourane. Tissues were collected in liquid nitrogen and stored at −80 °C.
Western blotting for Insulin signaling and Sphingolipid metabolism enzymes
Frozen muscle tissue (20 mg) was homogenized in buffer A (25 mM HEPES, pH 7.4, 1% Nonidet P-40 (NP-40), 137 mM NaCl, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml pepstatin, 5 μg/ml leupeptin). The samples were centrifuged at 14,000 g for 20 minutes at 4°C and protein concentrations of the supernatants determined by Bio-Rad protein assay kit (Bio-Rad laboratories, Inc. Hercules, CA). Aliquot lysates (50 μg) were subjected to standard western blotting procedures. For insulin signaling specific antibodies used were anti IRS 1 and 2, anti AKt-1 and 2, AKt-1 phos Ser 473 (Millipore, Temecula, CA), anti AKt-2 phos Ser 474 (Bioworld, St Louis MN). For sphingolipid metabolism enzymes specific antibodies used were anti serine palmitoyl tranferase SPT1 and SPT2, anti-ceramide galactosyl transferace (CGT), anti β-1,4-galactosyltransferases (β4GALT) and anti lactosylceramide 4-alpha-galactosyltransferase (A4GALT), anti-glucosylceramide synthase (GCS) anti acid ceramidase, neutral ceramidase, ceramide kinase, shingomyelin synthase 1 (SMS1), neutral sphingomyelinase (NSMASE), Acid sphingomyelinase (ASMASE), ganglioside monosialosyllactosylceramide (GM3) synthase and SP Lyase all from Santa Cruz Biotechnology (Dallas, TX). Others were SPK1 and SPK2 from Biovision (Milipitas, CA). Western blot results were verified in three separate experiments. All the proteins were normalized to β-actin.
Quantification of Lipid metabolites
Frozen muscle tissue (100 mg) from each animal was homogenized in deionized water and protein was determined by Bio-Rad protein assay kit (Bio-Rad laboratories, Inc. Hercules, CA). After addition of extraction standards (unnatural derivatives) C17:0 ceramide, dC17:1 sphingosine and C17:0 sphingomyelin, each sample was subjected to a double extraction for lipids using the Folch extraction under acidified conditions. Liquid chromatography-electrospray ionization tandem-mass spectrometry (LC-MSMS) was used to measure intracellular levels of sphingolipids on a Waters Acquity UPLC. The detector was a Waters Aquity Xevo triple quadruple MS/MS with ion source ESI operated in the positive mode. According to the retention times of standards, common product ion and ions reflecting fatty acid substituents, all target compounds were quantified as shown in Obanda et al. [4]. Quantities of ceramides, hexosylceramides, lactosylceramides, sphingomyelins and ceramide 1 phosphates (C1P) in each sample was determined. Ceramide catabolism products quantified were sphingosine and sphingosine 1 phosphate (S1P). All sphingolipid values were standardized by protein level in the muscle. Metabolites quantified and proteins (enzymes) measured are shown in Figure 1. Another 100mg of tissue was used to quantify triglycerides and diglycerides by gas chromatography coupled with flame ionization detection (GC-FID) as shown in Obanda et al. [14].
Enzyme activities
The activity of SPT in skeleton muscle was analyzed by the method described by Williams et al. [15] with minor modification. Briefly, 50mg tissue were homogenized in buffer (50mM N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid), 5mM dithiothreitol (DTT), 10mM EDTA, and 0.25M sucrose, pH 7.4), and centrifuged (12000rpm for 30 min). Aliquots of 50 ug protein of the supernatant were used for analysis of SPT activity, using [3H]-serine (Perkin Elmer, Waltham MA), and palmitoyl-CoA (Sigma Aldrich, St. Louis, MO) as substrates and activity determined as ng/min/mg protein of 3-Ketosphinganine formed.
The activity of ceramide synthase was determined using d17:0 sphinganine as described in Mullen et al.[16] with modifications. Reaction buffer was 20 mM Hepes, pH 7.4, 25 mM KCl, 2 mM MgCl2, 0.5 mM DTT, 0.1% (w/v) fatty acid-free BSA, 10 μM 17:0 sphinganine, and 50 μM fatty acid-CoA. The 100 μl reactions were started with the addition of 50 μg homogenate protein and incubated with shaking at 35°C for 30–120 min. Reactions were stopped with the addition of 250 μl chloroform/methanol (2:1). The tubes were vortexed, centrifuged at 5000rpm for 5 min to separate the phases, and the lower organic phase used to determine dihydroceramide formed by LC/MS/MS.
Activity of both acid and neutral sphingomyelinases (SMASE) in tissue homogenates was determined by a sphingomyelinase fluorometric assay Kit (Cayman chemical, Ann Arbor, MI) according to manufactures instructions. Activity of glucosylceramide synthase (GCS) was determined using the method described by Marks et al [17] using C6-NBD-ceramide. Skeletal muscle 100ug was homogenized in ice-cold buffer as shown above. Homogenate equivalent to 50ug protein were used to determine GCS activity as a conversion ratio (% conversion) from C6-NBD-ceramide to C6-NBD-glucosylceramide.
Statistical analysis
A non-paired two tailed Student’s t test was used to test for statistical significance when comparing group means between AL and CR groups. Data were expressed as means ± S.E.M. P values < 0.05 were considered statistically significant.
RESULTS
Body weight and Blood glucose and insulin levels
As expected the CR group had significantly lower body weight, serum glucose, serum insulin levels and improved insulin resistance (HOMA-IR) (Figure 2). Akt 2 protein abundance and Akt phosphorylation, IRS1 and IRS2 protein abundance were significantly increased in the CR group. No change in Akt 1 expression was observed between the groups. Serine phosphorylation of IRS1 at Ser307 was significantly reduced in CR group (data not shown).
Figure 2. Body weight, serum insulin, serum glucose and, muscle insulin signaling proteins of 18 month old rats fed ad libitum and calorically restricted.
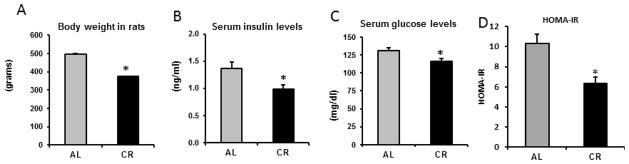
A. Body weight at the end of the study. Rats were 18 months at this stage.
B. Fasting serum glucose level was measured by colorimetric hexokinase glucose assay
C. Serum insulin was assessed using a rat insulin ELISA kit
D. HOMA–IR was calculated from glucose and insulin levels as [(Glucose*Insulin)/405].
Quantification proteins involved in sphingolipid metabolism and Quantification of Lipid metabolites
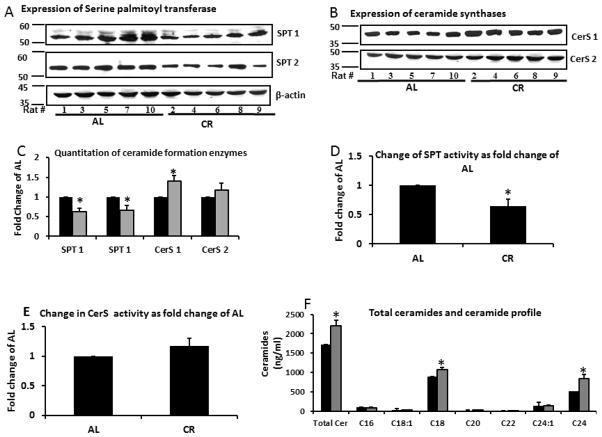
Total triglycerides and diglycerides in the skeletal muscle were not significantly different between the two groups (data not shown). Expression of SPT 1 and 2, the proteins involved in de novo synthesis of ceramides from palmitoyl CoA and serine was down regulated in the CR group. SPT activity was also significantly lower in CR animals LC-MS/MS showed that ceramide levels were increased in the CR group (Figure 3). Further analysis showed that CR group had higher expression of ceramide synthase CerS1 and CerS2. We analyzed CerS1 because it is mainly expressed in skeletal muscle while CerS2 is the most ubiquitously expressed of all the CerS isoforms. Enzyme activity showed a significant reduction in SPT activity and increase in CerS activity in CR (Figure 3). The most prominent ceramide species was C18 followed by C24. CR resulted in significant increases in C24 with a 63% increase (p<0.05) followed by C18 with a 19.7% increase (p<0.05) (Figure 3; Table 1).
Figure 3. Expression of Serine palmitoyl transferace (SPT) was downregulated, while that of ceramide synthases (CerS) was upregulated Ceramides quantities were increased.
Skeletal muscle tissue (20 mg) from rats fed either AL or CR was homogenized and expression of A: SPT1 and 2 and B: CerS1 and 2 examined by standard western blotting.
C. Quantitation of western blot gels. All proteins standardized by β-actin.
D. Activity of SPT was assayed using [3H]-serine and palmitoyl-CoA as substrates and activity determined by amount of 3-Ketosphinganine formed.
E. Activity of CerS as determined by amount of dihydroceramide formed from 17:0 sphinganine and fatty acid-CoA as substrates.
F. Total ceramides and the ceramide profile in 100 mg muscle tissue were quantified by liquid chromatography-electrospray ionization tandem-mass spectrometry (LC-MS/MS) and standardized per mg of protein. Data are presented as mean ±SEM. *P<0.05; n=14–15, significant difference between the AL and CR groups according to a non-paired students t-test.
Table 1.
Summary of changes in sphingolipid quantities between AL in CR groups
| Sphingolipid | % Change in CR compared to AL | Statistical significance (p value) |
|---|---|---|
| Ceramides | ||
| • Cer C16 | −7.0 | 0.27 |
| • Cer C18:1 | 2.5 | 0.79 |
| • Cer C18 | 19.7 | *0.006 |
| • Cer C20 | 12.0 | 0.14 |
| • Cer C22 | 17.7 | 0.07 |
| • Cer C24:1 | 1.2 | 0.88 |
| • Cer C24 | 63.1 | *0.011 |
| Total ceramides | 29.3 | *0.008 |
| Sphingomyelins | ||
|---|---|---|
| • Sphingomyelin C16 | 20.1 | 0.21 |
| • Sphingomyelin C18:1 | 19.0 | 0.41 |
| • Sphingomyelin C18 | 6.7 | 0.29 |
| • Sphingomyelin C24:1 | 9.5 | 0.38 |
| • Sphingomyelin C24 | 6.7 | 0.59 |
| Total Sphingomyelins | 11.9 | 0.38 |
| Ceramide 1 Phosphates | ||
|---|---|---|
| • Cer phos C16 | −39.1 | 0.09 |
| • Cer phos C18:1 | −57.9 | *0.0005 |
| • Cer phos C18 | 0.95 | 0.95 |
| • Cer phos C24:1 | 23.7 | 0.36 |
| • Cer phos C24 | −58.6 | *0.0009 |
| Total Ceramide Phosphates | 1.6 | 0.9 |
| Hexosylceramides (Glucosylceramides & galactosylceramides) | ||
|---|---|---|
| • HexCer C16 | −45.3 | *0.032 |
| • HexCer C18:1 | 23.2 | 0.31 |
| • HexCer C18 | −54.0 | *0.035 |
| • HexCer C24:1 | −45.4 | *0.022 |
| • HexCer C24 | −67.7 | *0.025 |
| Total Hexosylceramides | 47.3 | *0.032 |
| Lactoylceramides | ||
|---|---|---|
| • LacCer C16 | −44.5 | *0.00034 |
| • LacCer C18:1 | −40.1 | *0.00062 |
| • LacCer C18 | −48.2 | *0.0002 |
| • LacCer C24:1 | −53.1 | *0.009 |
| • LacCer C24 | −5.0 | 0.83 |
| Total Lactosylceramides | −46.0 | *0.00022 |
| Sphingosine | −11.8 | 0.08 |
| Sphingosine 1 phosphate | −7.9 | 0.26 |
Indicates statistically significant difference between AL and CR groups (P<0.05)
Expression of the sphingomyelin synthases SMS1 and SMS2 was higher in CR but not significantly different from AL group (p=ns). The expression of sphingomyelinases (SMASE) and total sphingomyelins were also not significantly different (Table 1). Sphingomyelinase activity was not different between AL and CR groups and the most prominent sphingomyelin species was SM C16 and SM C24:1 with no significant changes in the profile between the two groups (data not shown). Expression of ceramide kinase (CERK), the enzyme that phosphorylates ceramides, was not different (p=ns). The quantity of total ceramide phosphates between AL and CR groups was also not significantly different (Table 1). The most prominent ceramide phosphate species was C18 making up about 90% of all ceramide phosphates in both AL and CR (data not shown). Table 1 shows a significant decrease in ceramide phosphate C18:1 and C24. However these species represent a very small portion of the total ceramide phosphates in muscle (1.7% and 3.8% respectively) and therefore the effect is likely insignificant.
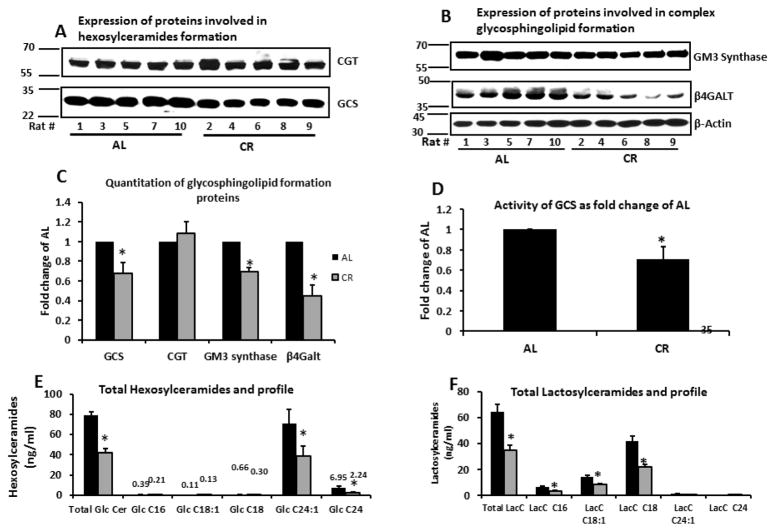
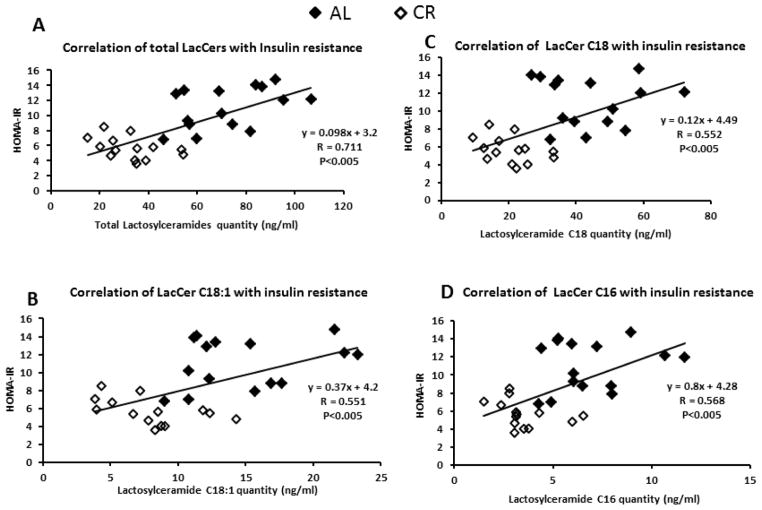
The most notable change in sphingolipid composition was observed with the glycosphingolipids. A significant decrease in total hexosylceramides was observed in the CR group (47% reduction p<0.05) (Figure 4; Table 1). Western blotting revealed a significant reduction in the expression of glucosylceramide synthase (GCS) but not ceramide galactosyl transferase CGT in the CR group (Figure 4). GCS activity was also significantly lower in CR. The most prominent hexosylceramides were C24:1 and C24 and we observed a significant 45.4% and 67.7% reduction respectively in the CR group (p<0.05). Similarly a significant decrease in total lactosylceramides (LacCer) was observed in the CR group (46% reduction; p<0.05) (Figure 4; Table 1). The most prominent LacCer species were C18 and C18:1. Both were significantly reduced in CR by 48.2% and 53.1% respectively (p<0.05). Western blotting revealed a significant reduction in the expression of β-(1,4) galactosyltransferase (β4GALT) and GM3 synthase in the CR group. Both enzymes are involved in synthesis of complex glycosphingolipids from LacCer. Total LacCers and individual species C16, C18 and C18:1 correlated significantly with insulin resistance index (HOMA-IR) (P<0.005) as shown in Figure 5. Because of limitations in our lipidomics capabilities we were not able to quantify gangliosides.
Figure 4. Expression of proteins involved in glycosphingolipid formation and quantities of glycosphingolipids in skeletal muscle.
A. Skeletal muscle tissue (20 mg) from rats fed either AL or CR was homogenized and expression of GCS and CGT examined by standard western blotting.
B. Expression of GM3 synthase and β4Galt. All proteins standardized by β-actin.
C. Quantitation of western blot gels. All proteins standardized by β-actin.
D. Activity of GCS as determined by percent conversion of C6-NBD-ceramide to C6-NBD-glucosylceramide.
E. Total Hexosylceramides and profile in 100mg muscle tissue were quantified by LC-MS/MS and standardized per mg of protein. Data are presented as mean ±SEM.
F. Total Lactosylceramides and profile in 100mg muscle tissue were quantified by LC-MS/MS standardized per mg of protein. Data are presented as mean ±SEM. *P<0.05; n=14–15, significant difference between the AL and CR groups according to a non-paired students t-test.
Figure 5. Lactosylceramide quantities significantly correlated with insulin resistance as measured by the index HOMA-IR.
A. Correlation of Total lactosylceramides with insulin resistance.
B. Correlation of lactosylceramide C18:1 with insulin resistance.
C. Correlation of lactosylceramide C18 with insulin resistance.
D. Correlation of lactosylceramide C16 with insulin resistance.
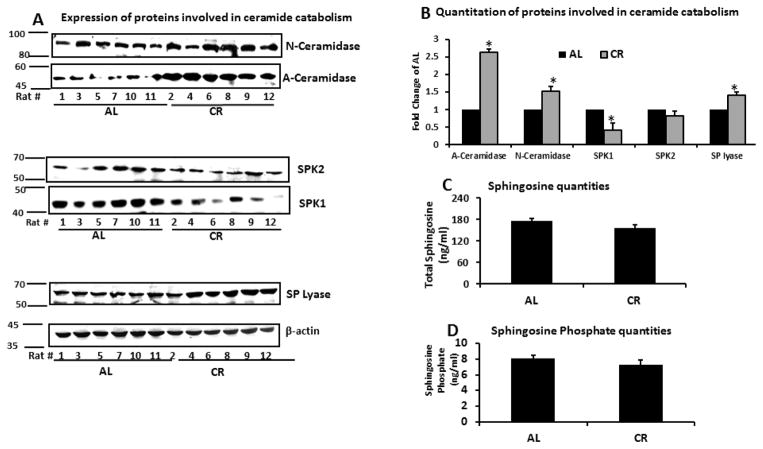
Sphingosine and sphingosine 1 phosphate (SIP) quantities were not significantly different between AL and CR groups (p=ns) (Figure 6; Table 1). However in the CR group, western blotting consistently showed a significantly higher expression of acid and neutral ceramidase the enzymes responsible for breaking ceramide into the sphingosine and free fatty acid. Additionally the CR group had a much lower expression of sphingosine kinase1 (SPK1) but not SPK2 enzymes responsible phosphorylating the free sphingosine. Finally, SP lyase the enzyme that breaks up SIP into hexadecanal and phosphoethanolamine was significantly more highly expressed in the CR group (Figure 6).
Figure 6. Expression of proteins involved in ceramide catabolism and levels of the free sphingosine and sphingosine phosphate in skeletal muscle.
A. Skeletal muscle tissue (20 mg) from rats fed either AL or CR was homogenized and expression of ceramidase, sphingosine kinase 1, sphingosine kinase 2 and SP lyase examined by standard western blotting.
B. Quantitation of western blots. All proteins standardized by β-actin.
C. Sphingosine quantities in 100 mg skeletal muscle tissue were quantified by LC-MS/MS and standardized per mg of protein.
D. Sphingosine phosphate quantities in 100 mg muscle tissue were quantified by LC-MS/MS and standardized per mg of protein.
Data are presented as mean ±SEM. *P<0.05; n=14–15, significant difference between the AL and CR groups according to a non-paired students t-test.
DISCUSSION
In agreement with other studies [7–10] our data supports the hypothesis that CR enhances insulin signaling with lower levels of glucose and insulin in serum albeit with no significant change in muscle lipid depots; no significant change was observed in triglycerides and diglycerides. Our novel finding is that the increase in insulin sensitivity observed in CR was associated with a reduction in complex sphingolipids, i.e. glycosphingolipids, in skeletal muscle tissue. It is known that insulin sensitizing agents lower muscle ceramide and glycoshingolipid levels in both humans and rodents [2, 5, 18–20,21]. Sphingolipids constitute a relatively minor component of total lipid content in skeletal muscle and most other tissues. Thus, the implications may be that although CR does not significantly reduce lipid deposition in skeletal muscle, it has a significant effect on sphingolipid metabolism thus significantly impacting insulin sensitivity.
Sphingolipids consist of a dynamic network of several bioactive signaling molecules. The, strength of the study was assessing the sphingolipid profile rather than one single metabolite. Ceramide provides the platform for synthesis of complex sphingolipids. Unexpectedly, the CR group had significantly higher levels of total ceramides. The most notable change in CR was the downregulation of expression and activity of enzymes involved in glycosphingolipid formation and the overall significant reduction in glycosphingolipids (glucosylceramides and lactosylceramides) quantities. This is an important finding as the metabolites have been shown to directly attenuate insulin signaling by dephosphorylating Akt protein [2,4,5]. No significant changes in sphingomyelins, ceramide phosphates, sphingosine and sphingosine phosphate were observed.
The increased ceramides in the CR group was unexpected since this group had a lower expression of SPT1 and SPT2 the enzymes involved in the rate limiting step of de novo synthesis of ceramides (Figure 3). Additionally, serine and palmitoyl CoA the two substrates for de novo ceramide synthesis are more abundant with more calorie intake. Other studies have also shown that caloric restriction decreases sphingolipid synthesis by lowering SPT activity [11,12]. This suggested that the increased ceramides were likely not from de novo synthesis. They were also unlikely to be from the sphingomyelin hydrolysis pathway because the expression of sphingomyelin synthesis and hydrolysis enzymes was not different between the AL and CR groups. In addition sphingomyelinase activity was also not different.. CerS isoforms are involved in both de novo synthesis and salvage pathway (see Figure 1). Six mammalian CerS isoforms with a distinct, but overlapping acyl CoA preference participate in the remodeling of ceramide resulting in different ceramide species [6, 16,22]. Studies investigating the roles of CerS in the regulation of sphingolipids have relied on plasmid-mediated overexpression of CerS proteins or selected knockdown using RNAi to show increased or reduced ceramide synthesis as a gain of function from these proteins [6,16]. Little is known about the regulation and inter-regulation of these proteins with regard to maintaining overall sphingolipid homeostasis. In determining the effect of CR on these proteins we focused on CerS1 and CerS2 because CerS1 is the predominant isoform in skeletal muscle while CerS2 is ubiquitously expressed in most tissues. Our study showed that CR resulted in a higher expression of CerS1 and CerS2 isoforms. CerS1 has a preference for C18 acyl chain (Stearoyl CoA) and mainly produces C18 ceramide while CerS2 has a preference for long acyl chains and results overall elevation of ceramides C22, C24 and C24:1. In agreement with reported substrate specificities of the different CerS isoforms, we observed a 63% elevation of C24, a 17.7% increase in C22 and a 19% increase in C18 in the CR group and no change in C24:1 (Figure 3, Table 1). CerS5 and CerS6 (not determined in this study) prefer palmitoyl CoA as substrates and generate predominantly C16 ceramide [5,16, 22,23]. The 7% decrease in ceramide C16 in CR was not statistically significant (p=ns). Frangioudakis et al [6] showed that ceramides generated through the de novo pathway have a different effect on insulin signaling to those generated through the salvage pathway. Specifically overexpression of CerS1 and CerS6 increased specific ceramides while promoting insulin action and overexpression of CerS2, CerS3, CerS4 and CerS5 increased ceramide levels without accompanying inhibitory insulin effects. We concluded that the overexpression of ceramide CerS1 and increase in CerS activity explain the increased insulin sensitivity in the CR group despite total higher ceramide levels. This observation underscores the importance of ceramides in cell function.
CR had no significant effect on the expression of proteins involved in formation of phosphosphingolipids (ceramide phosphates and sphingomyelins) the major constituent of lipid rafts in cell membranes (Table 1). Sphingomyelins the most abundant complex sphingolipids in mammalian tissues are produced by the action of sphingomyelin synthases SMS1 and SMS2 through the transfer of a phosphocholine head group to ceramide. Breakdown of sphingomyelin through hydrolysis of the phosphocholine head groups by sphingomyelinases (SMASE) produces ceramide and free phosphocholine [24]. We observed no significant differences in the expression of SMASEs between the AL and CR groups. Sphingomyelin quantities were also not different with SM 16 and SM 24:1 being the most abundant species (Data not shown). We therefore concluded that sphingomyelin hydrolysis did not contribute to the increased ceramide levels in CR. Ceramide is phosphorylated to produce ceramide-1-phosphate (C1P) by ceramide kinase (CERK) [25]. Our study revealed an enrichment of C1P species containing acyl chain length C18 in both AL and CR groups with no significant difference in levels of total CIP or C18 or CERK expression (Table 1).
The most notable change between the CR and AL groups was observed among the glycosphingolipids (Figure 4, Table 1). Glucosylceramide is synthesized in the cis-golgi from ceramide and UDP-glucose by the enzyme GCS. Glucosylceramide is the precursor for the majority of glycosphingolipids which play an essential role in lipid raft formation and thus insulin signaling and glucose homeostasis. Expression of GCS and its activity was significantly lower in the CR group. Furthermore quantification of total hexosylceramides (glucosylceramides and galactosylceramides) showed a highly significant 48% reduction in CR compared with AL group (Figure 4; Table 1). In vivo studies in rodents have shown that inhibitors of GCS have an array of beneficial effects on metabolism and reverse many complications associated with obesity and diabetes [2,18–20]. The expression of β-(1,4) galactosyltransferase (β4GALT) the enzyme involved in formation of lactosylceramide from glucosylceramide was downregulated in CR (Figure 5). Lactosylceramides were primarily comprised of C16 and C18 fatty acid isoforms and were significantly reduced by 46% in CR. Lactosylceramides were the only metabolite that showed a significant correlation with insulin resistance as assessed with HOMA-IR (Figure 5). Although we did not quantify gangliosides and sulfatides in this study, protein expression showed a significant reduction in expression of GM3 synthase which catalyzes the transfer of α-sialic acid to the terminal galactose of lactosylceramide to form the simplest ganglioside GM3. It is the first step in the formation of almost all other gangliosides. It has been shown that inhibition of GM3 synthesis reverses TNF-α induced insulin resistance in 3T3-L1 adipocytes and, mice lacking the GM3-synthase gene display lower fasting glucose levels and improved glucose tolerance [20,21]. CR did not reduce the expression of alpha-(1,4) galactosyltransferase (A4GALT) the enzyme that catalyzes the transfer of galactose to lactosylceramide to form the ganglioside globotriaosylceramide (data not shown). Because of the robust improvement in insulin action observed with reduction in glycosphingolipids in several studies, we concluded that the improvement in insulin sensitivity associated with CR is most likely due to the reduction in glycosphingolipid accumulation.
Sphingolipids are eventually catabolized to sphingosine through the family of ceramidases. Although we observed significant increase in expression of ceramidases in CR, sphingosine levels were not significantly different between the AL and CR groups (Figure 6; Table 1). Conversion of sphingosine to sphingosine phosphate (SIP) is achieved through sphingosine kinases. In the final step of sphingolipid breakdown, SIP is degraded by sphingosine-1-phosphate lyase (SP lyase) in the ER to produce hexadecanal and phosphoethanolamine. We observed a significantly lower expression of sphingosine kinases (SPK1 and SPK2) and a significantly higher expression of SP lyase in CR group. The actual levels of SIP were not significantly different between the two groups. Overexpression of acid ceramidase has been shown to be insulin sensitizing in vitro [26,27] because it lowers the quantity of ceramides formed from the de novo pathway and results in formation of the downstream product, S1P, which has antagonistic effects to ceramide [27]. In contrast to ceramide, S1P promotes nutrient uptake, increases basal and insulin-stimulated glucose uptake and activates Akt/PKB in a wide variety of cell types [25, 27]. However in this study we observed no increase in free sphingosine or SIP. It is therefore likely that CR does not improve insulin sensitivity through the actions of SIP in skeletal muscle.
Conclusion
Our data demonstrates that CR results in changes in pathways involved in ceramide formation and metabolism. The data supports the finding that a contributing mechanism by which CR enhances insulin action in vivo is secondary to modulation of glycosphingolipid accumulation in skeletal muscle.
Acknowledgments
FUNDING
DO was supported in part, and the study utilized resources provided by NIH Grant P50 AT002776. The study also utilized resources of grant NIH Grant 1U54GM104940.
We thank Dr. Donald Ingram of Pennington Biomedical Research center for providing the animals. We thank Dr Jacqueline Stephens for constructive review and editing of the manuscript.
Abbreviation’s and Accronyms
- AL
Ad libitum
- CR
Calorie Restriction
- SPT
Serine palmitoyl transferace
- CerS
Ceramide synthase
- GCS
Glucosylceramide synthase
- CGT
Ceramide galactosyl transferace
- AC
Acid ceramidase
- NC
Neutral ceramidase
- CERK
Ceramide Kinase
- SMS1
Sphingomyelin synthase 1
- SMS2
Sphingomyelin synthase 2
- NSMase
Neutral sphingomyelinase
- ASMase
Acid sphingomyelinase
- SPK1
Sphingosine kinase 1
- SPK2
Sphingosine kinase 1
- (β4GALT)
β-(1,4) galactosyltransferase
- A4GALT
Lactosylceramide 4-alpha-galactosyltransferase
- GM3
Ganglioside monosialosyllactosylceramide
Footnotes
DISCLOSURE STATEMENT
All authors have no conflict of interest.
COMPETING INTERESTS
All the authors have no competing interests.
AUTHOR CONTRIBUTIONS
D. Obanda-Performed LC/MS-MS analysis of sphingolipids, western blotting and prepared and edited manuscript.
Y. Yu-Performed western blotting experiments.
Z. Wang- Study design and plasma measurements.
W. Cefalu-Study design and edited manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole body metabolism. J Clin Invest. 2013;121(11):4222–30. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst van Ejik AM, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–49. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikonen E, Vainio S. Lipid Microdomains and Insulin Resistance: Is there a connection? Sci STKE. 2005;(268):pe3. doi: 10.1126/stke.2682005pe3. [DOI] [PubMed] [Google Scholar]
- 4.Obanda DN, Hernandez A, Ribnicky D, et al. Bioactives of Artemisia dracunculus L. mitigate the role of ceramides in attenuating insulin signaling in rat skeletal muscle cells. Diabetes. 2012;61(3):597–605. doi: 10.2337/db11-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summers SA. Sphingolipids and insulin resistance. The five Ws. Current opinion in Lipidology. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 6.Frangioudakis G, Diakanastasis B, Liao BM, et al. Ceramide accumulation in L6 skeletal muscle cells due to increased activity of ceramide synthase isoforms has opposing effects on insulin action to those caused by palmitate treatment. Diabetologia. 2013;56(12):2697–701. doi: 10.1007/s00125-013-3035-5. [DOI] [PubMed] [Google Scholar]
- 7.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, β-Cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res. 2004;12:761–69. doi: 10.1038/oby.2004.92. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZQ, Floyd E, Qin J, et al. Modulation of skeletal muscle insulin signaling with chronic caloric restriction in cynomolgus monkeys. Diabetes. 2009;58:1488–98. doi: 10.2337/db08-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cefalu WT, Wang ZQ, Bell-Farrow AD, et al. Caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): metabolic, physiologic, and atherosclerotic measures from a 4-year intervention trial. J Gerontol A Biol Sci Med Sci. 2004;59:1007–14. doi: 10.1093/gerona/59.10.b1007. [DOI] [PubMed] [Google Scholar]
- 11.Malenfant P, Tremblay A, Doucet E, et al. Elevated intramyocellular lipid concentration in obese subjects is not reduced after diet and exercise training. Am J Physiol. 2001;280:E632–E639. doi: 10.1152/ajpendo.2001.280.4.E632. [DOI] [PubMed] [Google Scholar]
- 12.Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in Lipid Research. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mechanisms of Ageing and Development. 2001;122:895–908. doi: 10.1016/s0047-6374(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 14.Obanda DN, Cefalu W. Modulation of Cellular Insulin signaling and PTP1B effects by lipid metabolites in skeletal muscle cells. J Nutr Biochem. 2013;24(8):1529–1537. doi: 10.1016/j.jnutbio.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams RD, Wang E, Merrill AH., Jr Enzymology of long-chain base synthesis by liver: Characterization of serine palmitoyltransferase in rat liver microsomes. Archives of Biochemistry and Biophysics. 1984;228(1):282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 16.Mullen TD, Spassieva S, Jenkins RW, Kitatani K, Bielawski J, et al. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J Lipid Res. 2011;52(1):68–77. doi: 10.1194/jlr.M009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks DL, Paul P, Kamisaka Y, Pagano RE. Methods for studying glucosylceramide synthase. Methods Enzymol. 2000;311:50–59. doi: 10.1016/s0076-6879(00)11066-3. [DOI] [PubMed] [Google Scholar]
- 18.Bietrix F, Lombardo E, van Roomen CP, et al. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2010;30(5):931–7. doi: 10.1161/ATVBAHA.109.201673. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Przybylska M, Wu IH, et al. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 2009;50(1):85–93. doi: 10.1002/hep.22970. [DOI] [PubMed] [Google Scholar]
- 20.Tagami S, Inokuchi J, Kabayama K, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the center of sphingolipid metabolism and biology. Biochem J. 2012;441(3):789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–71. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsutake S, Date T, Yokota H, et al. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett. 2012;7–586(9):1300–5. doi: 10.1016/j.febslet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Chavez JA, Holland WL, Bar J, et al. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280(20):20148–53. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 27.Merrill AH. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]