Abstract
Heterologous immunity refers to the phenomenon whereby a history of an immune response against one pathogen can provide a level of immunity to a second unrelated pathogen. Previous investigations have shown that heterologous immunity is not necessarily reciprocal, such as in the case of vaccinia virus (VACV). Replication of VACV is reduced in mice immune to a variety of pathogens, while VACV fails to induce immunity to several of the same pathogens, including lymphocytic choriomeningitis virus (LCMV). Here we examine the lack of reciprocity of heterologous immunity between VACV and LCMV and find that they induce qualitatively different memory CD8 T cells. However, depending on the repertoire of an individual host, VACV can provide protection against LCMV simply by experimentally amplifying the quantity of T cells cross-reactive with the two viruses. Thus, one cause for lack of reciprocity is differences in the frequencies of cross-reactive T cells in immune hosts.
Keywords: CD8 T cell, cytotoxicity, heterologous immunity, interferon gamma, lymphocytic choriomeningitis virus, mouse, private specificity, vaccinia virus
Introduction
A history of infection with one pathogen can alter the host’s immune response to a second unrelated pathogen in either a beneficial or detrimental manner. This heterologous immunity can result in either a substantial reduction or else an increase in pathogen titer, often with alterations in pathology and disease course. The mechanisms behind heterologous immunity vary, but it often involves T cells, stimulated either by epitopes cross-reactive between viruses or else altered by a change in environmental cytokines. Of note is that the protection conferred by heterologous immunity is not necessarily reciprocal. Immunity to pathogen A being protective against pathogen B does not guarantee that the immunity to pathogen B will protect against pathogen A. For example, a history of infection of mice with lymphocytic choriomeningitis virus (LCMV), Pichinde virus (PICV), murine cytomegalovirus (MCMV), influenza A virus (IAV), or Bacillus Calmette-Guerin (BCG) provides a certain level of heterologous protective immunity against vaccinia virus (VACV) in the form of reduced organ viral titer by one to two log PFU early (day 3–4) after infection (Chen et al., 2003; Mathurin et al., 2009; Selin et al., 1998). However, a prior history of VACV infection does not protect against subsequent challenge with LCMV, MCMV or PICV (Selin et al., 1998).
Protection against VACV in LCMV-immune mice is mediated by both CD8 and CD4 T cells through IFNγ. IFNγ-producing CD8 and CD4 T cells are recruited in LCMV-immune mice into the site of VACV infection (Chen et al., 2001; Selin et al., 1998), and anti-IFNγ-treated or IFNγ receptor (R) knock-out (KO) LCMV-immune mice are not protected against VACV (Selin et al., 1998). Adoptive transfers of LCMV-immune splenocytes protect against VACV infection, and this protection is reduced by depleting either the CD8 or the CD4 T cells in the donor cell population (Selin et al., 1998). In vivo cytokine assays show that, however, most of the IFNγ-producing T cells in LCMV-immune mice early after VACV challenge are CD8 T cells (Mathurin et al., 2009), and that LCMV epitope-specific T cells in adoptively transferred populations selectively proliferate in response to VACV infection (Kim et al., 2002; 2005).
Cross-reactive T cells are thought to be involved in immune protection against VACV in this system. T cells specific for the LCMV epitopes NP205–212, GP34–41, and GP118–125 may proliferate after VACV challenge, with the specificity of the responding T cells depending on the individual mouse (Kim et al., 2005). Subsets of T cells specific to each of these three LCMV epitopes cross-react with a single VACV epitope, A11R198–205, and A11R198–205-specific T cell lines from LCMV-immune mice can bind to both VACV A11R198–205 and LCMV GP118–125 or GP34–41 tetramers (Cornberg et al., 2010). Structural studies defining the nature of cross-reactivity have been done between the LCMV GP34–41 and the VACV A11R198–205 epitopes (Z. T. Shen et al., 2013), and GP34–41/A11R198–205 cross-reactive cell lines proliferate in response to VACV infection in vivo and provide protective immunity (Cornberg et al., 2010). It should be pointed out, however, that this type of cross-reactive response is not seen in all mice. Because of variations in the private specificity of the LCMV-immune CD8 T cell memory pool, some mice preferentially utilize cross-reactive responses against the NP205–212 or GP118–125 epitopes, and sometimes cross-reactivity is not seen against any of those epitopes, thereby demonstrating the complexity of heterologous immunity (Kim et al., 2005).
Despite this demonstration of cross-reactive T cells, a history of a VACV infection did not provide protective heterologous immunity to LCMV or to other tested viruses, yet four different viruses and BCG each provided protective immunity against VACV. We question here whether the protective immunity in this system is purely dependent on T cell cross-reactivity or whether other factors are involved – factors that may explain the lack of reciprocity in protective immunity. There are substantial biological differences between the VACV and LCMV infections. VACV replicates preferentially in the peripheral organs while LCMV replicates primarily in the lymphoid organs. IFNγ very effectively inhibits VACV replication in mice (Harris et al., 1995; Karupiah et al., 1993; Liu et al., 2004), and frequencies of IFNγ-producing memory CD8 T cells can correlate directly with protection against VACV (Moutaftsi et al., 2009). LCMV is not as sensitive to IFNγ (van den Broek et al., 1995); rather, LCMV is controlled mostly by contact-dependent perforin-mediated cytotoxicity without a need for IFNγ, yet perforin or Fas cytotoxicity plays little role in the clearance of VACV (Kägi et al., 1995; Walsh et al., 1994). Further, the number of cytolytic CD8 T cells correlates directly with target killing and the control of infection in the LCMV system (Ganusov et al., 2011).
In some systems heterologous immunity has been suggested to be due solely to the non-specific activation of memory T cells by pathogen-elicited cytokines, which induce the memory cells to make IFNγ (Yager et al., 2009) or express the receptor NKG2D (Chu et al., 2013), which enables them to kill stress ligand-expressing cells. Perhaps VACV might be better at activating and being susceptible to such mechanisms than other viruses, rendering it very susceptible to heterologous immunity.
In this report we question why a history of VACV infection does not protect against LCMV and ask whether the properties or the number of their memory cells can explain this lack of reciprocity in heterologous immunity. The hypothesis to be tested was that the non-reciprocal nature of heterologous immunity was due either to qualitative or quantitative differences in the memory T cell populations. We conclude that this is mostly a consequence of the number and private specificity of the memory CD8 T cell population in VACV-infected mice.
Materials and Methods
Mice and viruses
C57BL/6 male mice between 5–6 weeks of age were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice received the first inoculum when they reached at least 6–7 weeks of age. LCMV, Armstrong strain, was propagated in baby hamster kidney BHK21 cells (Welsh et al., 1976; Welsh and Seedhom, 2008). VACV, Western Reserve strain, was propagated in L929 cells (Yang et al., 1989). Recombinant VACV expressing LCMV glycoprotein (VACV-GP) was kindly provided by Dr. J. Lindsay Whitton (Whitton et al., 1988). Mice were infected with 5×104 PFU LCMV, 106 PFU wild type VACV, or 5×106 PFU VACV-GP intraperitoneally, and were considered immune after 6 weeks post infection. A higher inoculum of VACV-GP was used than of wild type VACV because the recombinant virus was less virulent. Experiments were done in compliance with the Animal Welfare Act and the National Institutes of Health guidelines for the ethical care and use of animals in biomedical research according to protocols approved by the Institutional Animal Care and Use Committee of UMMS.
Cell lines
Vero cells (African green monkey kidney epithelial cell line; ATCC) were maintained in minimum essential medium (MEM; Life Technologies) supplemented with 10% fetal calf serum (FCS; Sigma), 2 mM Penicillin/Streptomycin (Pen/Strep; Gibco) and 2 mM L-glutamine (L-glu; Gibco). DC2.4 cells (Z. Shen et al., 1997) are a dendritic cell line maintained in RPMI-1640 (Gibco) supplemented with 10% FCS, 2 mM Pen/Strep, 2 mM L-glu, non-essential amino acid (Gibco) and 10 mM HEPES (Gibco).
Dendritic cell (DC) immunization
Cultured immortalized dendritic cells DC2.4 were split 1:4 on the day before immunization. Trypsin-dispersed DC2.4 cells (at 107 cells/ml) were pulsed with 5mM filter-sterilized A11R198–205 peptide in FCS in a 37°C water bath for 40 min with tapping every 10 min. Pulsed DC2.4 cells were washed 4 times with 50 ml of Hank’s Balanced Salt Solution (HBSS) and counted. Twenty-five million A11R198–205-pulsed DC2.4 cells were administered subcutaneously on the upper right abdomen. HBSS was used as a control for the injection.
Plaque assay
Harvested splenocytes were homogenized in 1 ml of media, spun at 2000 rpm for 20 min at 4°C, and the supernatants were stored at −80 °C. Monolayers of Vero cells were set up overnight in 6-well plates by seeding 2×105 cells/well. Organ homogenates (100 µl) and 10-fold dilutions were gently mixed onto and incubated with the monolayer in 1 ml of media for 1.5 hrs at 37 °C, 5% CO2. Plates were then overlaid with 4 ml of a 1:1 mixture of 1% SeaKem-ME agarose in distilled water and 2X EMEM supplemented with 6% FCS, 5 mM Pen/Strep, 5 mM L-glu, and 1% Fungizone (Gibco). After 4 days, plates were stained with 0.02% neutral red in the agarose mixture above. Plaques were counted the following day.
Surface staining
Splenocytes in suspension were washed in staining buffer (1% FCS in PBS), blocked with anti-CD16/32 (clone 2.4G2; Fc block), and stained with combinations of anti-CD4 (clone RM4–5), anti-CD8α (clone 53–6.7), anti-CD44 (clone IM7), anti-CD62L0 (clone MEL-14), anti-CD127 (clone A7R34), anti-KLRG1 (clone 2F1), anti-CD27 (clone LG.3A10), anti-CD43 (clone 1B11), anti-Vα2 (clone B20.1), anti-Ly5.1 (clone A20), or anti-Thy1.1 (clone HIS51) for 20 min at 4°C. Stained cells were fixed with CytoFix (BD Biosciences) for 5 min at 4°C, washed, and stored at 4°C until data collection by LSRII (BD Biosciences). Data were analyzed by FlowJo software (TreeStar).
Intracellular cytokine staining (ICS)
Splenocytes were stimulated on 96-well U-bottomed plates with 1 µg/ml (1 µM) synthetic peptide GP33–41 (KAVYNFATC), GP34–41 (AVYNFATC), GP118–125 (ISHNFCNL), GP276–286 (SGVENPGGYCL), NP396–404 (FQPQNGQFI), NP205–212 (YTVKYPNL) (Kotturi et al., 2007), or A11R198–205 (AIVNYANL) (Kim et al., 2005), 10 U/ml human recombinant IL-2 (BD Biosciences) and 1 µl/ml of GolgiPlug (BD Biosciences) for 5 hrs at 37 °C, 5% CO2 as described (Brehm et al., 2002). Anti-CD3ε (250ng/ml; clone 145–2C11) was used in place of peptides as positive controls. After Fc-block and surface staining as described above, cells were fixed and permeabilized with CytoFix/CytoPerm (BD Biosciences) for 20 min at 4°C. Cells were then washed twice with 1X Perm/Wash buffer and stained with anti-IFNγ (clone XMG1.2), anti-TNF (clone MP6-XT22) and anti-IL-2 (clone JES6-5H4) for 25 min at 4°C. Washed samples were analyzed on an LSRII within 5 days. To assess the accumulation of lytic granules, samples were stimulated with GP33–41 peptide for 4–6 days, and stained with anti-human Granzyme B (clone GB12) after fixation and permeabilization.
Proliferation assay with CFSE
Splenocytes were labeled with 2 µM carboxyfluorescein succinimidyl ester (CFSE) in warm HBSS at 2×107 cells/ml for 15 min at 37°C with tapping every 5 min. Labeled splenocytes were washed in HBSS and counted. The Ly5.1 P14 CD8 T cell frequency of each mouse was determined by flow cytometry. Equal numbers of P14 memory CD8 T cells and supplementary naïve splenocytes were stimulated with GP33–41 on a 96-well plate for 4–6 days. Proliferated populations were surface-stained and analyzed by LSRII.
Statistical analysis
Student’s t test was calculated using Excel or Prism. Data were presented with mean and standard error of the mean (SEM).
Results
Memory CD8 T cells from VACV-GP-immune mice display phenotypes of stronger proliferative recall potential
Memory T cells of different qualities can be generated by different infections or vaccinations due to differences in cytokines environments and antigen abundance or persistence (Mueller et al., 2010; Pillai et al., 2011; Tewari et al., 2004). If these memory T cells were to be re-activated during a subsequent infection with a heterologous agent, they may influence the host response and disease outcome differently. To see if memory CD8 T cells generated from LCMV and VACV infections were of different qualities, P14 TCR transgenic mouse splenocytes containing 1–3×104 CD8 T cells were adoptively transferred into C57BL/6 mice, which were then inoculated i.p. with either 5×104 PFU LCMV strain Armstrong or 5×106 PFU recombinant VACV-GP on the same day. Six weeks or more later, P14 memory CD8 T cells were analyzed from the spleen using their congenic marker Thy1.1 in phenotypic and functional assays so that similar transgenic T cells activated in the context of two different infections were examined.
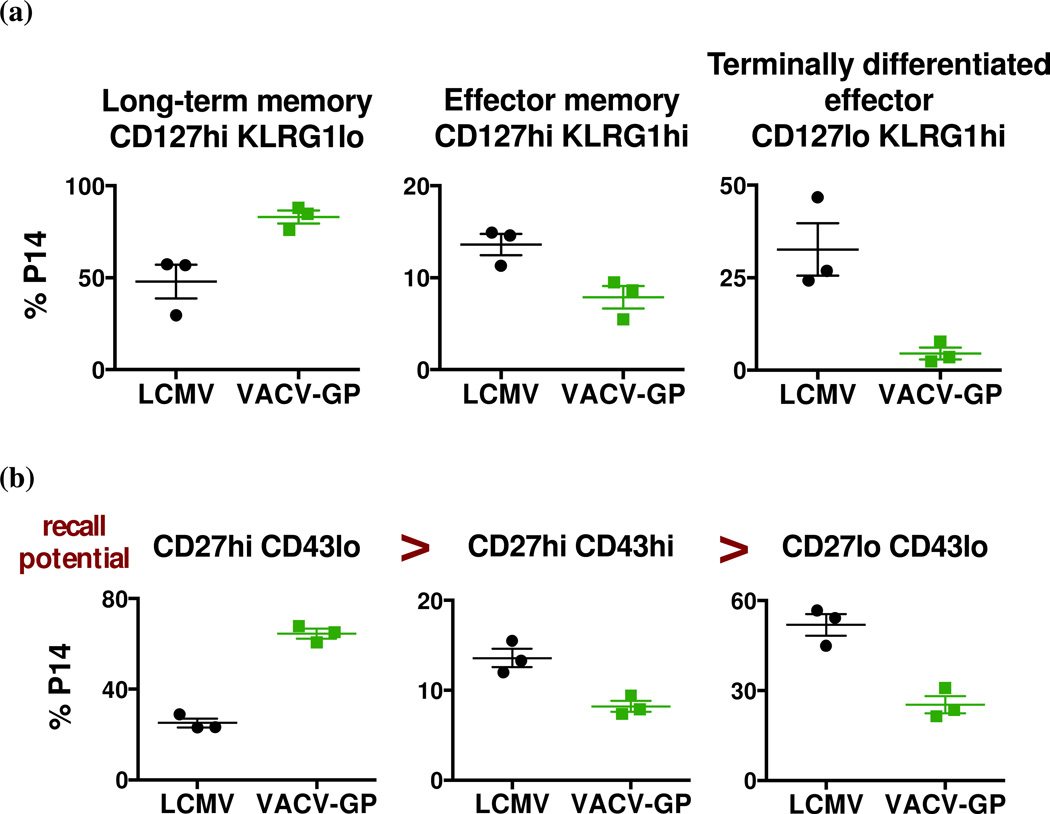
Killer cell lectin-like receptor G1 (KLRG1) signaling in CD8 T cells impairs proliferation, and its expression correlates with proliferative incapacity of antigen-experienced CD8 T cells (Gründemann et al., 2006; Voehringer et al., 2001). CD127, the IL-7 receptor, is important for homeostatic proliferation of memory CD8 T cells (Schluns et al., 2000). The combination of KLRG1 and CD127 has been used to classify memory CD8 T cells: CD127hi KLRG1lo as long-term memory T cells, CD127hi KLRG1hi as effector memory T cells, and CD127lo KLRG1hi as terminally differentiated effectors (Belz and Kallies, 2010). In both LCMV- and VACV-GP-immune hosts, over 50% of the memory P14 CD8 T cells expressed a long-term memory phenotype (CD127hi KLRG1lo) at 7 weeks post infection (Fig. 1a). However, a higher proportion of P14 memory CD8 T cells from LCMV-immune mice was KLRG1hi (both CD127hi and CD127lo), suggesting that LCMV-immune memory T cells may have a lower proliferative potential. The phenotype of the memory population changed over time, and over 90% of P14 memory CD8 T cells from both immune groups displayed the long-term memory phenotype at 23 weeks post infection (data not shown).
Figure 1. LCMV and VACV-GP infections generate memory CD8 T cells of different phenotype compositions.
C57BL/6 mice that received Thy1.1 P14 splenocytes were infected i.p. with LCMV or recombinant VACV-GP. Splenocytes were analyzed for (a) CD127 and KLRG1 expression at week 7 post infection (p values = 0.0234, 0.0272 and 0.0183 respectively), and for (b) CD27 and CD43 expression at week 6 post infection (p values = 0.0002, 0.0106 and 0.0044 respectively). Data are representative of at least two separate experiments at similar time points.
The pattern of activation markers on memory CD8 T cells has been shown to correlate with recall response potential in terms of accumulation at the site of infection and IL-2 production (Hikono et al., 2007), and the phenotypes of cells exhibiting recall response potential from high to low are as follows: CD27hi CD43lo > CD27hi CD43hi > CD27lo CD43lo. In LCMV-immune mice, there was a lower frequency of P14 memory CD8 T cells that expressed the higher recall potential phenotype (CD27hi CD43lo), and about 50% expressed the lower recall potential phenotype (CD27lo CD43lo) at 6 weeks post infection (Fig. 1b). On the other hand, about 60% of P14 memory CD8 T cells from VACV-GP-immune mice expressed the higher recall potential phenotype and relatively small percentage of cells were of the lower recall potential phenotype. Both groups had a relatively low percentage of CD27hi CD43hi P14 memory CD8 T cells, but over time the frequency of these cells gradually increased while the frequency of lower recall potential P14 memory CD8 T cells declined.
The P14 memory CD8 T cells from LCMV- and VACV-GP-immune hosts were therefore phenotypically different, but in ways that did not seem to explain the lack of reciprocity in the heterologous immunity. The analysis by surface markers suggested that memory T cells from VACV-GP-immune mice were more capable of recall responses and should theoretically have the capacity to protect well against subsequent LCMV infections. On the other hand, it is possible that partially activated effector cells with poorer proliferative potential were of greater significance to heterologous immunity to VACV.
More memory CD8 T cells from LCMV-immune mice are multiple-cytokine producers
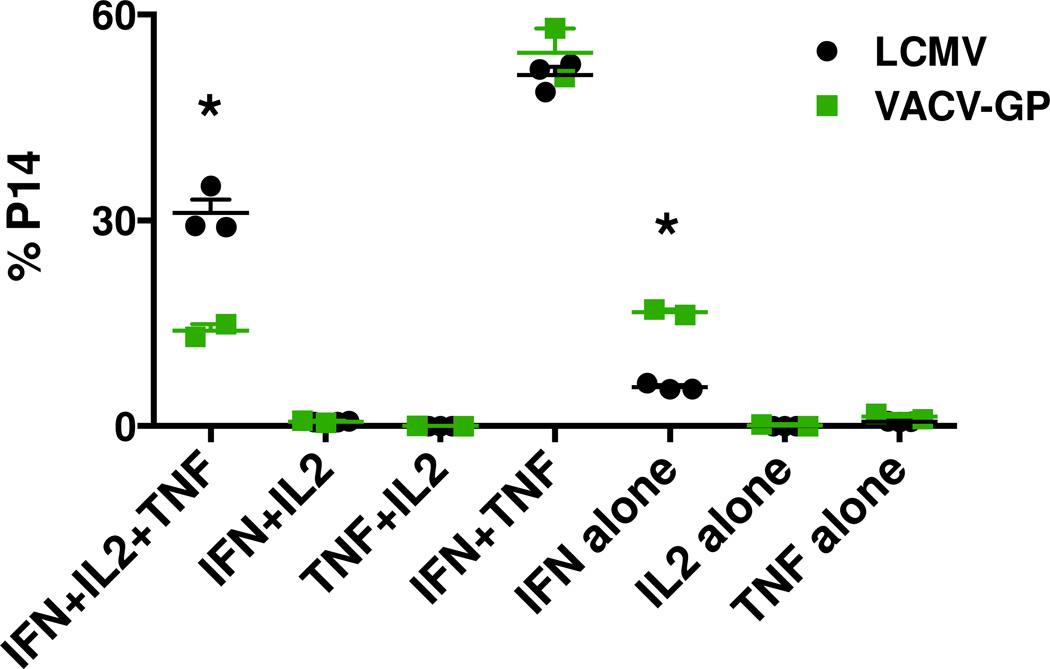
To evaluate the effector functions of the memory CD8 T cells, splenocytes from LCMV- and VACV-GP-immune mice were examined by ICS after in vitro stimulation with GP33–41 synthetic peptide. The majority of Thy1.1 P14 memory CD8 T cells from both immune groups were able to produce IFNγ and TNF upon peptide stimulation (Fig. 2). A greater proportion of P14 memory CD8 T cells from LCMV-immune mice than those from VACV-GP-immune mice produced three cytokines, IFNγ, TNF and IL-2, in the same cells. More P14 memory CD8 T cells from VACV-GP-immune mice produced IFNγ alone when stimulated. The cytokine production profile revealed that LCMV infection generated a subset of memory CD8 T cells of more versatile effector functions than that generated by VACV infection. We thus questioned whether this ability to produce IL-2 might have given the LCMV-induced memory T cell population a proliferative advantage on challenge.
Figure 2. Greater proportion of memory CD8 T cells from LCMV infection is capable of producing multiple cytokines upon re-stimulation.
C57BL/6 mice that received Thy1.1 P14 splenocytes were infected i.p. with LCMV or recombinant VACV-GP. Splenocytes harvested at day 46 (weeks 6.5) post infection were analyzed for cytokine production by ICS after GP33–41 peptide stimulation for 5 hr. Data are representative of three separate experiments. (*) denotes statistical significance (p < 0.05) between groups.
Memory CD8 T cells from both immune groups proliferate and produce granzyme B upon stimulation in vitro
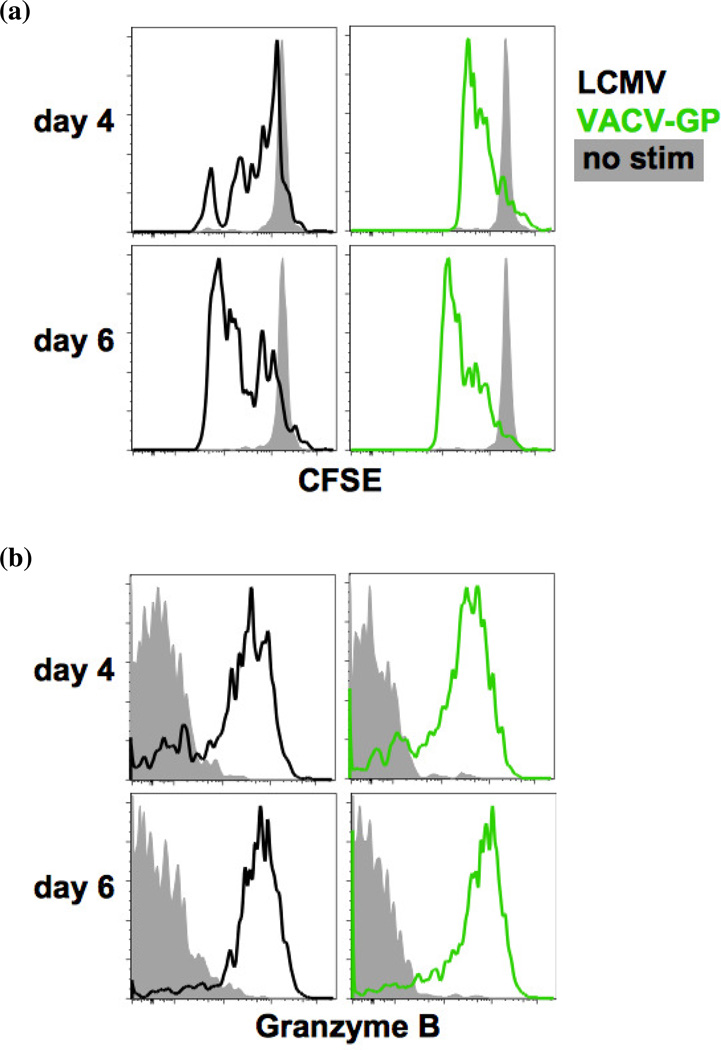
To compare the proliferative responses to re-stimulation in vitro, CFSE-labeled splenocytes from LCMV- and VACV-GP-immune mice were stimulated with GP33–41 peptide in a 96-well plate for 4–6 days. The frequency of Ly5.1 P14 memory CD8 T cells was higher in the LCMV-immune host than in the VACV-GP-immune host. Therefore, using the frequency determined for each mouse prior to CFSE-labeling, the P14 memory CD8 T cells were plated in equal numbers along with supplementary naïve splenocytes to make up equal total number of cells in each well. After peptide stimulation, P14 memory CD8 T cells from both LCMV- and VACV-GP-immune mice proliferated, and the frequencies of CFSE-diluted P14 memory CD8 T cells in both immune groups were above 80% by day 6 of in vitro stimulation (Fig. 3a).
Figure 3. P14 memory CD8 T cells from LCMV- and VACV-GP-immune mice proliferate and accumulate granzyme B uponin vitro stimulation.
C57BL/6 mice that received Ly5.1 P14 splenocytes were infected i.p. with LCMV or recombinant VACV-GP. Splenocytes harvested at day 85 (week 12) post infection were labeled with CFSE and stimulated with GP33–41 peptide for 4–6 days. The numbers of Ly5.1 P14 memory CD8 T cells were equalized for each well using supplementary naïve splenocytes. Cells were analyzed for (a) proliferation and (b) granzyme B accumulation. Data are representative of two separate experiments.
Upon re-stimulation, memory cells produce granzyme B in vesicles in preparation for target cell killing. The accumulation of lytic granules in memory CD8 T cells was evaluated along with the in vitro proliferation experiment. Peptide stimulation caused similar proportions of P14 memory CD8 T cells from the LCMV- and the VACV-GP-immune mice to produce granzyme B after 4 days (Fig. 3b). At day 6 post stimulation, over 80% of P14 memory CD8 T cells in both groups have accumulated granzyme B.
Thus, despite some differences in marker phenotypes and cytokine profiles, P14 memory CD8 T cells from LCMV- and VACV-GP-immune hosts responded to re-stimulation with similar levels of proliferation and granzyme B accumulation in vitro.
Greater number of potentially cross-reactive memory CD8 T cells in LCMV-immune mice
The magnitude of a T cell response is influenced by the number of antigen-specific T cells present at the time of infection. If cross-reactive memory CD8 T cells mediate heterologous immunoprotection between LCMV and VACV, the number of potentially cross-reactive memory CD8 T cells in the immune mice may be critical. On average, there were about 1.2×104 A11R198–205-specific memory CD8 T cells in a VACV-immune mouse per spleen (Table 1), and we know from previous studies that not all of these would be cross-reactive to one of the three cross-reactive LCMV epitopes (Cornberg et al., 2010; Kim et al., 2005; Z. T. Shen et al., 2013). The directly tested A11R198–205 epitope specific T cell frequency in mice immune only to LCMV is low and variable, averaging about 0.1% (supplementary Fig. 1A). Nevertheless, the virus-specific memory CD8 T cell frequency potentially cross-reactive was higher in the LCMV-immune than in VACV-immune mice. Combining the CD8 T cell populations that recognize the three cross-reactive epitopes GP34–41, GP118–125 and NP205–212, there are approximately 1.2×105 potentially cross-reactive CD8 T cells per LCMV-immune spleen. However, due to the phenomenon of private specificity, only a fraction of these potentially cross-reactive cells may actually cross-react and respond during heterologous infection. Nevertheless, there are still 10 times more memory CD8 T cells that may potentially cross-react with VACV in the LCMV-immune mice. The lower number of potentially cross-reactive memory CD8 T cells in VACV-immune mice may be one of the reasons why a history of VACV infection is not protective during subsequent LCMV infection.
Table 1. LCMV-immune mice possess a greater number and frequency of memory CD8 T cells that are specific for the cross-reactive epitopes between LCMV and VACV.
C57BL/6 mice were inoculated with 5×104 PFU LCMV Armstrong or 106 PFU VACV. The frequencies of epitope-specific memory CD8 T cells were determined in the spleen of the immune mice after at least 8 weeks post infection by ICS.
| VACV-immune | LCMV-immune | |||
|---|---|---|---|---|
| Specificity: | A11R198–205 | GP34–41 | GP118–125 | NP205–212 |
| 17795 (0.09%) | 147362 (1.0%) | 22956 (0.15%) | 30442 (0.20%) | |
| 20258 (0.16%) | 45323 (0.49%) | 10277 (0.11%) | 14194 (0.15%) | |
| 5164 (0.06%) | 50350 (0.5%) | 25229 (0.26%) | 35223 (0.35%) | |
| 8019 (0.09%) | 30518 (0.77%) | 16436 (0.41%) | 5712 (0.14%) | |
| 8199 (0.09%) | 93536 (1.2%) | 38985 (0.50%) | 22293 (0.29%) | |
| Average: | 11887 (0.1%) | 73418 (0.8%) | 22776 (0.3%) | 21573 (0.2%) |
DC(A11R)-immunization boosts A11R–specific memory and confers heterologous immunoprotection
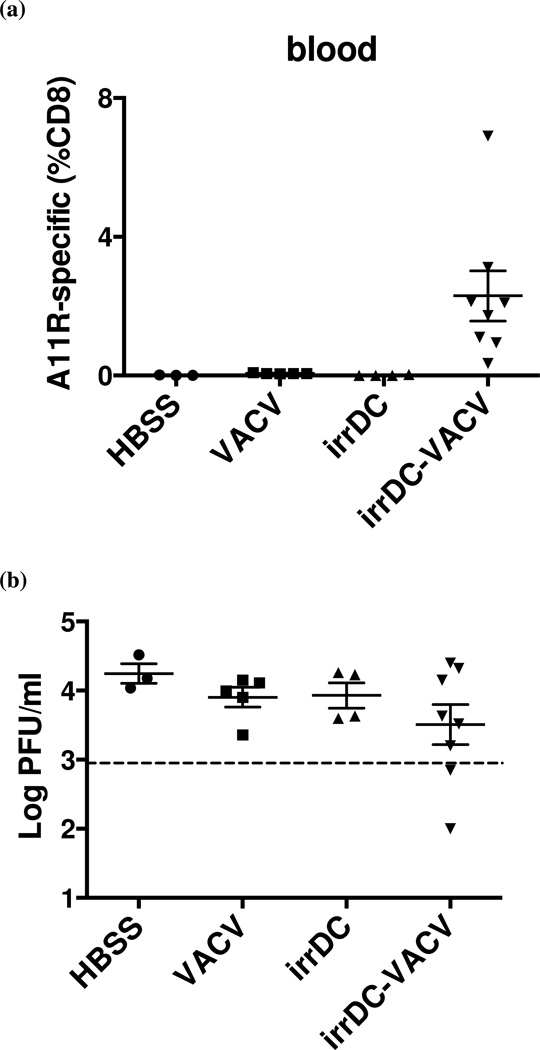
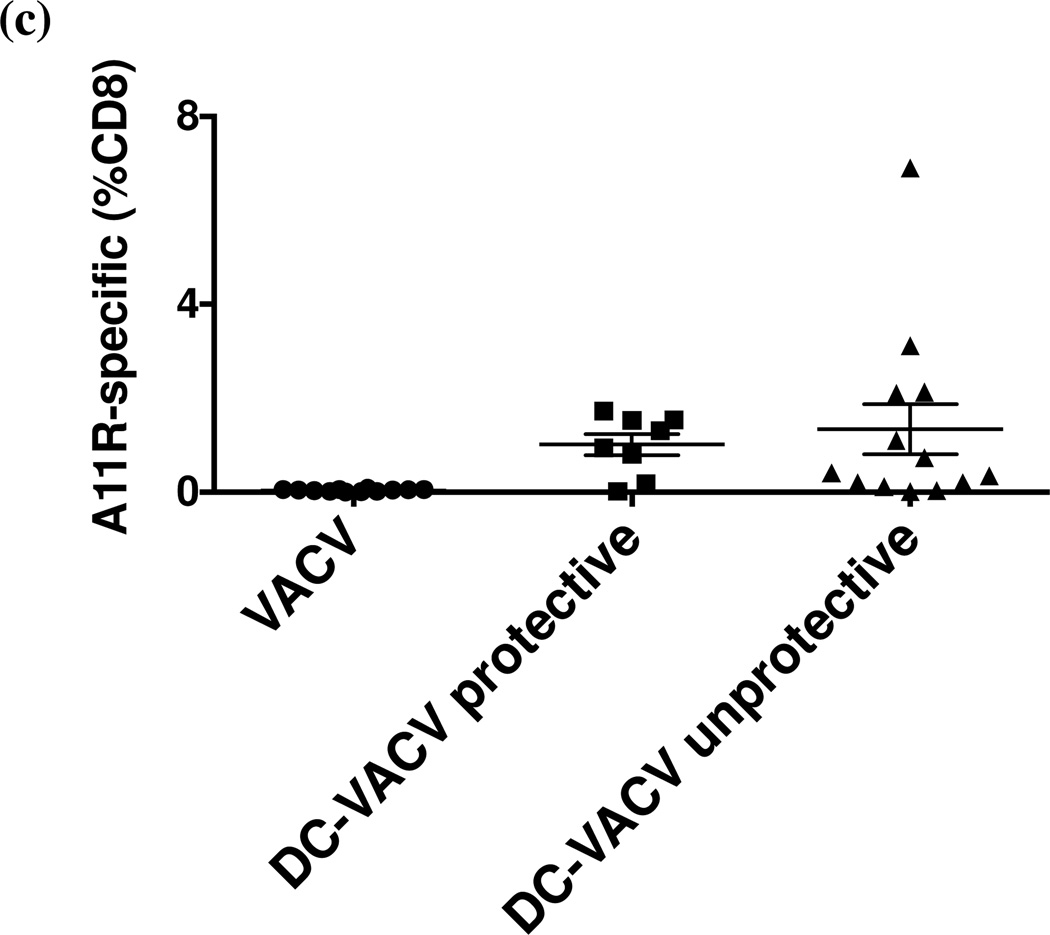
To examine whether the number of potentially cross-reactive memory CD8 T cells is critical for protection during heterologous infection between LCMV and VACV, we increased the number of A11R198–205-specific memory CD8 T cells in a VACV-immune mice by dendritic cell immunization and tested whether the higher number of cross-reactive memory CD8 T cells may confer protection against LCMV. Immortalized DC2.4 cells were pulsed with A11R198–205 peptide, irradiated, and administered to mice subcutaneously (s.c). Seven days later, pulsed DC-immunized and mock-immunized (HBSS-injected) mice were infected i.p. with 106 PFU wild type VACV. After 6 weeks, mice were screened for A11R198–205-specific memory CD8 T cell frequency in the blood. Peptide-pulsed DC-priming greatly increased the frequency of A11R198–205-specific memory CD8 T cells in the VACV-immune mice to 1.2% on average, with the highest reaching 6.9% of CD8 T cells in one of the experiments (Fig. 4a). The frequency of A11R198–205-specific memory CD8 T cells in control VACV-immune mice was less than 0.1%, and DC-immunization without VACV infection did not generate an appreciable number of A11R198–205-specific memory CD8 T cells. A similar increase of the frequency of A11R198–205-specific memory CD8 T cells (to an average about 1.3%) was observed in spleens of DC-immunized VACV-immune mice in a separate experiment. Those mice were sacrificed before the removal of the spleens and, therefore, not challenged with LCMV. The phenotype of A11R198–205-specific memory CD8 T cells from the spleens was analyzed in one of the experiments, and variations of phenotypic marker (CD27, CD43, CD62L, CD127, KLRG-1) expression and cytokine (IFNγ , TNF and IL-2) production were found even within the same group. However, the overall differences in phenotypes between DC-VACV and VACV alone groups were not statistically significant.
Figure 4. Immunization with irradiated A11R198–205-pulsed DC2.4 increases the frequency of A11R198–205-specific CD8 T cells in VACV-immune mice and correlates with protection of some mice during heterologous LCMV challenge.
C57BL/6 mice were immunized s.c. with A11R198–205-pulsed irradiated DC2.4 cells and infected i.p. with 106 PFU VACV after 7 days. (a) At week 6 post VACV infection, the frequency of A11R198–205-specific (CD44hi IFNγ+) memory CD8 T cells was measured in blood by ICS before i.p. challenge with 5×104 PFU LCMV Armstrong. (b) LCMV titer was determined in the spleen at day 6 post heterologous challenge. Mice with LCMV titers lower than three times the standard deviation and at least half a log below the geometric mean LCMV titer in the VACV-immune control group (dotted line) were considered protected. (c) Combined data from four similar experiments comparing LCMV titer at day 6 post infection to the frequency of A11R198–205-specific memory CD8 T cells in blood prior to LCMV challenge suggested that high pre-bleed A11R198–205-specific CD8 T cell frequency could not guarantee immune protection during heterologous LCMV challenge.
Immune mice were subsequently challenged i.p. with 5×104 PFU LCMV Armstrong. Viral titer and virus-specific T cell response were evaluated at 6 days post LCMV infection. Protection was determined for each experiment separately. Mice were considered protected when their LCMV titers in the spleen at day 6 post infection were lower than three times the standard deviation and at least half a log below the geometric mean LCMV titer (i.e. the arithmetic average of the log 10 titers) in the VACV-immune control group. Fig. 4b shows an individual representative experiment, where 25% of the mice in the DC-immunized VACV-immune group were protected and had lower LCMV titers at day 6 post infection.
The same experiment depicted in Fig. 4a and b was repeated several times, and the comprehensive analysis of all the experiments showed that among the DC-immunized VACV-immune mice that achieved a higher A11R198–205-specific memory CD8 T cell frequency prior to challenge, about 38% had reduced LCMV titer after challenge (Fig. 4c). The elevated A11R198–205-specific memory CD8 T cell frequency prior to challenge may have contributed to the protection against LCMV infection because none of the control VACV-immune mice, whose A11R198–205-specific memory CD8 T cell frequencies were low (<0.05% in the blood), had reduced LCMV titers after challenge. However, a high pre-bleed frequency did not guarantee immune protection from LCMV challenge. The phenomenon of private specificity probably plays an important role in governing which immune T cell population can respond to the cross-reactive LCMV epitopes and protect against heterologous LCMV infection.
Virus-specific CD8 T cell response correlates with protection against LCMV
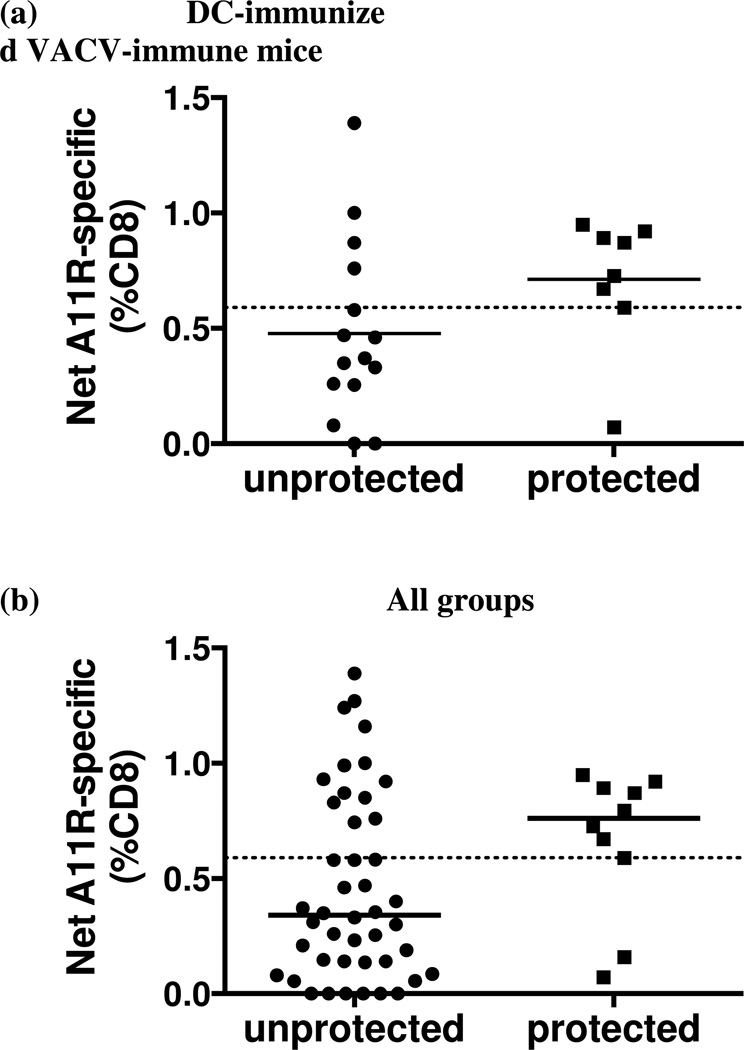
Given the complexities of heterologous immunity where only a subset of T cells specific to one epitope cross-reacts with another epitope, and where different mice have different patterns of cross-reactivity due to the private specificity of their T cell repertoire (Cornberg et al., 2010; Kim et al., 2005), a different approach was needed to analyze these data. We therefore used, the Fisher’s exact test, a classic human epidemiological tool to analyze risk, or, in this case, protection from infection. We compared, within the DC-immunized VACV-immune groups, the net A11R198–205-specific memory CD8 T cell frequency (after background subtraction) at day 6 post LCMV challenge between mice that were protected during LCMV infection and those that were not. We found that mice with 0.59% or more of their CD8 T cells specific to A11R198–205 were significantly more likely to be protected against LCMV infection than those with less than 0.59% (Fig. 5a). The segregation was more obvious when all mice regardless of their history of infection were included in the analysis (Fig. 5b). The animals that were protected during LCMV infection had 0.59% or higher frequency of CD8 T cells that were A11R198–205-specific at day 6 post LCMV infection.
Figure 5. Epitope-specific CD8 T cell frequency at day 6 post LCMV infection correlates with protection.
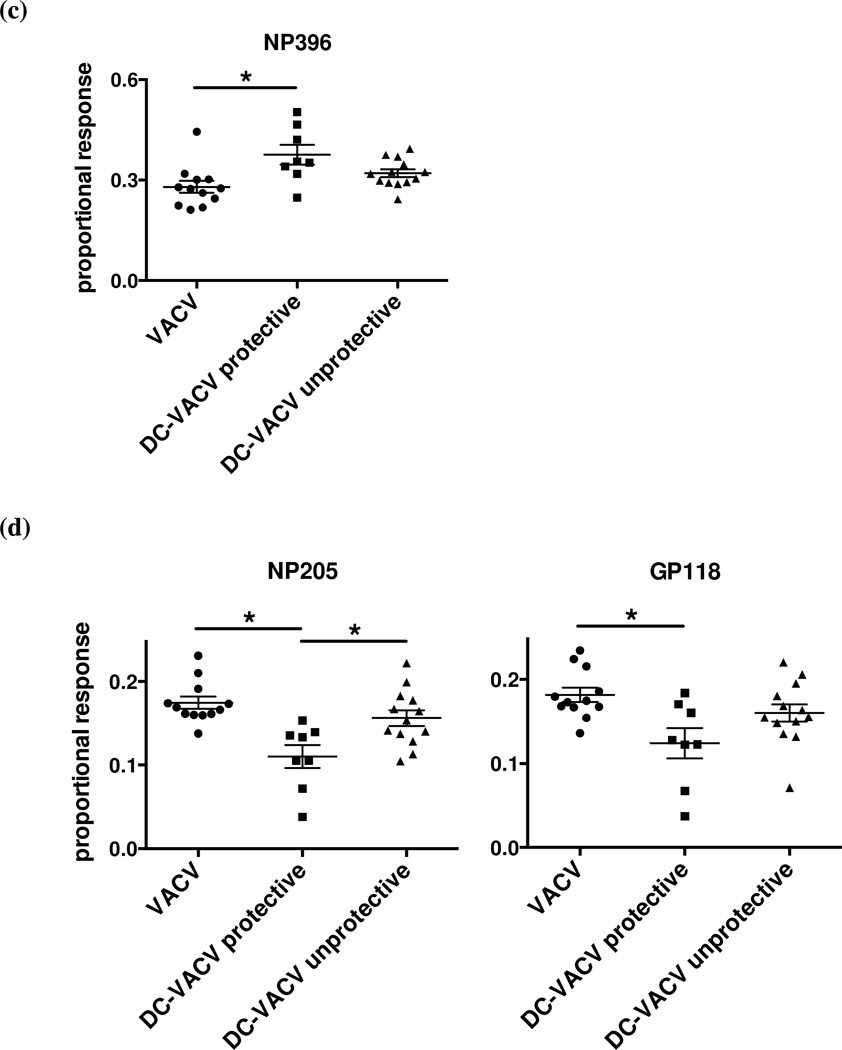
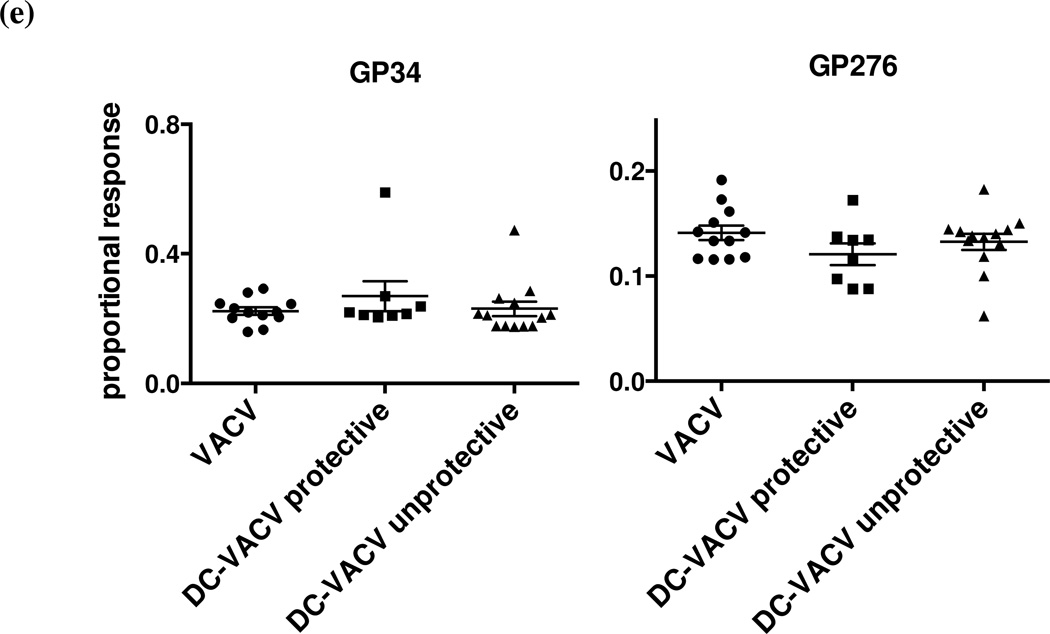
DC-immunized VACV-immune mice and controls were infected i.p. with LCMV. LCMV viral titer in the spleen and A11R198–205-specific CD8 T cell frequency were evaluated at 6 days post LCMV infection. Mice with LCMV titers lower than three times the standard deviation and at least half a log below the geometric mean LCMV titer in the VACV-immune control group were considered protected. Net A11R198–205-specific CD8 T cell frequency in the spleen between the protected and the unprotected animals in (a) DC-immunized VACV-immune group (p = 0.0272) and (b) all groups (p = 0.0107) were analyzed by Fisher’s exact test. Dotted lines in each graph marked the 0.59% threshold frequency. (c-e) Proportional responses to LCMV epitopes were calculated from CD8 T cells responding to GP34–41, GP118–125, NP205–212, NP396–404 and GP276–286 at day 6 post LCMV infection by dividing the percentage of a specific response by the sum of the major specific responses. DC-immunized VACV-immune mice protected from LCMV infection displayed a significantly higher proportional response to NP396–404 (c) but lower proportional responses to NP205–212 and GP118–125 (d). Proportional responses to GP34–41 and GP276–286 were not significantly different between groups (e). (*) denotes statistical significance (p < 0.05) between groups.
We questioned whether f the production of IL-2 may play a part in protection. Therefore, IL-2 analysis of the T cell response was included at day 6 post LCMV infection in some of the experiments. We found that, however, the overall frequency of IL-2+ A11R198–205-specific CD8 T cells was lower in the protected animals (average 1.0%) than in the unprotected ones (average 1.6%), which were similarly DC-immunized and VACV-infected. The frequency of IL-2+ A11R198–205-specific CD8 T cells may reflect the ongoing CD8 T cell response to the LCMV infection but does not explain protection because mice protected by the DC-VACV regimen showed similar frequency of IL-2+ A11R198–205-specific CD8 T cells as the unprotected HBSS controls.
We next questioned how this boosting of the A11R198–205-specific response in a VACV infection would affect the hierarchy of the T cell responses to LCMV epitopes in the VACV-immunized mice protected or not protected against LCMV infection. The responses to the major LCMV-specific epitopes GP34–41, GP118–125, NP205–212, NP396–404 and GP276–286 at day 6 post LCMV infection were evaluated. Proportional response was calculated by dividing the percentage of a specific response by the sum of all the major epitope-specific responses. DC-immunized VACV-immune mice that were protected during LCMV infection surprisingly mounted a significantly higher proportional response not to the cross-reactive epitopes but instead to the highly immunodominant NP396–404 epitope (Fig. 5c). The mice displayed a significantly lower proportional response to NP205–212 and GP118–125 (Fig. 5d), and the proportional responses to GP34–41 and GP276–286 were not significantly different between groups (Fig. 5e). In this regard, the response of the DC-immunized VACV-immune mice that were not protected during LCMV infection resembled that of the control VACV-immune mice, which were similarly not protected during LCMV infection.
Discussion
The purpose of this study was to find out why heterologous immunity, the immunity that can be developed to one pathogen after a host had been exposed to a different unrelated pathogen, is not necessarily reciprocal. In this case, considering VACV and LCMV, heterologous immunity could be boosted simply by increasing the number of potentially cross-reactive T cells, as increasing the number of A11R198–205-specific memory CD8 T cells by peptide-pulsed DC immunization rendered at least some of the VACV-immune mice more resistant to LCMV infection. This is consistent with studies examining heterologous immunity between LCMV and the distantly related arenavirus, PICV. These two viruses have highly cross-reactive Kb-restricted NP205–212 epitopes that share 6 of 8 amino acids in common (Brehm et al., 2002). LCMV induces better protective immunity against PICV that does PICV against LCMV (Selin et al., 1998), but there are more NP205–212-specific T cells in the LCMV memory pool than in the PICV memory pool (Brehm et al., 2002), perhaps contributing to this dichotomy. One of course cannot rule out the differences in TCR repertoires and affinities in these immune populations (Cornberg et al., 2010; 2006), but that was beyond the scope of the present study.
Sometimes the simplest explanation is the correct explanation, as the above results seem to indicate. However, one can easily hypothesize other mechanisms for a lack of reciprocity in heterologous immunity, and these might be important in other viral combinations. There certainly is reason to believe that the quality of memory cells rather than just quantity could be very different in host immune responses to different viruses. In fact, we showed here that the GP33–41 -specific P14 transgenic memory CD8 T cells generated from LCMV and LCMV GP-expressing recombinant VACV (VACV-GP) infections were phenotypically different, but these differences could not be readily connected to the differences in heterologous immunity. The phenotypes of the memory CD8 T cells generated from LCMV- and VACV-GP-infected mice were compared using adoptively transferred P14 CD8 T cells with a congenic marker so that the memory generated in cells with identical T cell receptors in two different infection environments might be compared. The observation that LCMV infection generated more memory CD8 T cells that produce multiple cytokines, including IL-2, may provide a partial explanation why LCMV-specific CD8 T cells are efficient in eliminating VACV-infected targets. However, the memory CD8 T cells generated by VACV-GP infection displayed a phenotype of higher recall potential (CD27hi CD43lo) and less proliferative incapacity (KLRG1lo), seemingly at odds with their inability to reduce LCMV viral titer. Further experiments showed that the memory CD8 T cells from both LCMV- and VACV-GP-immune hosts could proliferate well and produce granzyme B upon in vitro re-stimulation, ultimately arguing against a relative inability to function properly. Of note is that the phenotypes of the VACV immunodominant epitope B8R20–27-specific memory CD8 T cells generated by VACV-GP and WT VACV infections were similar, with comparable frequencies of multiple cytokine-producing cells and similar composition of cells of different recall potential phenotypes, as indicated by their expressions of CD27 and CD43 (data not shown). We also questioned whether the memory cells induced after DC priming were qualitatively different in protected animals than in non-protected animals. We found that the overall frequency of IL-2+ A11R198–205-specific CD8 T cells was slightly lower in the protected animals (average 1.0%) than in the unprotected ones (average 1.6%), but this likely just means that the presence of higher levels of antigen was continuing to stimulate the T cells, rather than reflecting on any intrinsic T cell deficiency.
Resident effector memory T cells have been reported to be important for protection against VACV in parabiotic studies (Jiang et al., 2012), and the enhanced effector function of LCMV-immune T cells resembles that of effector memory cells. This would potentially make cross-reactive LCMV-specific memory T cells better at mediating heterologous immunity as compared to the cross-reactive VACV-specific T cells with a more resting central memory cell phenotype.
There certainly could be other reasons for the lack of reciprocity in heterologous immunity. For example, it may be that a large virus, such as VACV, which encodes more than 200 proteins and perhaps many hundreds of epitopes, would more likely encode an epitope that could engage some T cells in a memory pool than would a small virus, such as LCMV, which encodes only four proteins. We doubt that we have exhausted the identification of all cross-reactive epitopes between LCMV and VACV and are, in fact, somewhat surprised that to date we have identified 3 cross-reactive epitopes encoded by the small virus and only one by the large complex virus. We would still argue that it might be easier for a small virus to escape the restraints of heterologous immunity, and we suggest that heterologous immunity may have put selective pressure that contributed to large viruses encoding so many proteins to interfere with antigen processing and T cell surveillance. Nevertheless, in the case mentioned above, the lack of reciprocity in heterologous immunity could still be due to the frequencies of cross-reactive epitopes. Other explanations for the lack of reciprocity in heterologous immunity may relate to the direct properties of viruses to induce or be sensitive to anti-viral cytokines. VACV is more sensitive than LCMV to IFNγ (van den Broek et al., 1995), and LCMV is relatively poor at inducing IL-12, which induces IFNγ (Orange and Biron, 1996). LCMV is regulated more by perforin-dependent cytotoxicity (Kägi et al., 1995; Walsh et al., 1994). Indeed, we have hypothesized that the IFNγ-sensitive VACV may induce IL-12 that enhances the environmental levels of IFNγ, thereby effectively controlling the VACV infection. In fact some reports have suggested that certain cytokines may non-specifically activate memory T cells to produce IFNγ without a need for T cell cross-reactivity (Yager et al., 2009), and in this manner they could provide some resistance to a virus like VACV. We have not ruled out that this may contribute some component to the ability of LCMV immunity to protect against VACV, but in this report we focused on why VACV did not protect against LCMV.
It has been shown that the cross-reactive T cells from LCMV-immune mice can proliferate in vivo during acute VACV infection (Kim et al., 2005), but LCMV only minimally elicits proliferation of VACV-specific memory T cells at day 6 post infection (Kim et al., 2002). However, a more specific focus on the A11R198–205-specific CD8 T cells revealed their proliferative response upon LCMV infection. When CFSE-labeled whole splenocytes from VACV-immune mice were adoptively transferred into naïve congenic hosts that were subsequently infected with LCMV, only 0.5% of the donor splenocytes were A11R198–205-specific by ICS at day 6 post LCMV infection, but all of the A11R198–205-specific CD8 T cells were CFSE low (data not shown), suggesting that the A11R198–205-specific memory CD8 T cells that could still be detected at that time had proliferated during LCMV infection. Moreover, the expanded cross-reactive T cells could be detected when the T cells returned to homeostasis. In VACV-LCMV double immune mice, the number of cross-reactive A11R198–205-specific memory CD8 T cells at 9 weeks post LCMV infection was on average 10 times higher than the number in VACV-immune mice (Supplemental Fig. 1).
This study shows that the number of potentially cross-reactive memory CD8 T cells can influence the outcome of heterologous infection, but for proper evaluation of the data one must take into consideration the profound influence of T cell private specificity on the generation of protective heterologous responses. This is because even though two epitopes may elicit cross-reactive T cell responses, only a subset of the epitope-specific T cells will be cross-reactive, and the proportion of the response that is cross-reactive will vary between individuals (Kim et al., 2005). A11R198–205-pulsed DC-immunization greatly increased the frequency of A11R198–205-specific CD8 T cells in VACV-immune mice. Although the frequency of A11R198–205-specific CD8 T cells before LCMV challenge did not definitively predict protection, the net frequency of A11R198–205-specific CD8 T cells generated by day 6 post LCMV infection correlates with protection. This is consistent with the phenomenon of private specificity, where not every T cell population recognizing a cross-reactive epitope in one virus will react with the cross-reactive epitope in the heterologous virus. Therefore, a high frequency of A11R198–205-specific memory CD8 T cells before LCMV challenge may not necessarily be protective. It has been shown that a certain number of T cells is required to eliminate the infected targets and control viral spread. Calculation from a previous study suggested that 104 LCMV-specific effector CD8 T cells could reduce LCMV titer by 1.5 log and that 105 cells could eliminate the virus in the first 20 hours post inoculation (Ehl et al., 1997). Our calculated cutoff of 0.59% CD8 T cells of A11R198–205-specificity that correlated with LCMV titer reduction falls within the range of that previous calculation. Therefore, both private specificity and the number of cross-reactive memory CD8 T cells determine the outcome of heterologous infection between LCMV and VACV.
The A11R198–205-pulsed DC-immunized VACV-immune mice that were protected from infection rather surprisingly had elevated levels of T cells specific to the immunodominant NP396–404 epitope of LCMV and not to the subdominant cross-reactive epitopes. Previous studies have shown that adoptively transferred NP396–404-specific CD8 T cells are the most effective, in comparison to GP276–286 and GP33–41-specific CD8 T cells, in clearing LCMV infection in mice (Gallimore et al., 1998). Thus, a situation that favored the generation of a higher NP396–404 response may well lead to more rapid clearance of LCMV. It is not clear why immunization with a cross-reactive epitope should stimulate a T cell response against a non-cross-reactive epitope. Though NP396–404 is certainly not as cross-reactive as NP205–212, GP34–41 or GP118–125 in LCMV-immune mice after VACV challenge (Kim et al., 2005), NP396–404-specific memory CD8 T cell frequency is often higher in the LCMV-immune mice challenged with VACV and in VACV-immune mice challenged with LCMV (Supplemental Fig. 2), suggesting that there could be low levels of cross-reactivity. An alternative explanation, however, is that the high NP396–404 response is a consequence of better immune protection early on rather than the cause of it. High viral loads eliminate NP396–404-specific CD8 T cells by way of apoptosis (Richter et al., 2012; Wherry et al., 2003; Zajac et al., 1998), so better immune protection early in infection would lead to antigen levels too low to delete the NP396–404-specific T cells, whose frequencies would thus increase compared to the non-protected controls. Interpretation of these data is difficult, because the prolonged presence of antigen can both continue to stimulate antigen-specific T cell proliferation as well as drive T cells into exhaustion and apoptosis. Nevertheless, despite all the possibilities for different mechanisms regulating the non-reciprocal nature of heterologous immunity and variations in the anti-viral T cell responses, our data support the idea that the simplest mechanism, i.e., the numbers of potentially cross-reactive T cells in the memory pool, can best explain heterologous immunity between LCMV and VACV.
Supplementary Material
Acknowledgements
We thank Michael Brehm and Mina Seedhom for scientific discussions, and Keith Daniels for technical assistance.
This work was funded by U.S. National Institutes of Health Training grant T32 AI007349, and research grants R37 AI017672, PO1 AI046620, AI081675 and AI-46578. The views expressed are those of the authors and not necessarily those of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belz GT, Kallies A. Effector and memory CD8+ T cell differentiation: toward a molecular understanding of fate determination. Current Opinion in Immunology. 2010;22:279–285. doi: 10.1016/j.coi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003;163:1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-Activated Memory CD8 T Cells Control Early Pathogen Load in an Innate-like, NKG2D–Dependent Manner. CellReports. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim S-K, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM, Selin LK. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006;116:1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim S-K, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. CD8 T Cell Cross-Reactivity Networks Mediate Heterologous Immunity in Human EBV and Murine Vaccinia Virus Infections. The Journal of Immunology. 2010;184:2825–2838. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehl S, Klenerman P, Aichele P, Hengartner H, Zinkernagel RM. A functional and kinetic comparison of antiviral effector and memory cytotoxic T lymphocyte populationsin vivo andin vitro. Eur. J. Immunol. 1997;27:3404–3413. doi: 10.1002/eji.1830271240. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee H-G. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganusov VV, Barber DL, De Boer RJ. Killing of Targets by CD8+ T Cells in the Mouse Spleen Follows the Law of Mass Action. PLoS ONE. 2011;6:e15959. doi: 10.1371/journal.pone.0015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründemann C, Bauer M, Schweier O, Oppen von N, Lässing U, Saudan P, Becker K-F, Karp K, Hanke T, Bachmann MF, Pircher H. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- Harris N, Buller RML, Karupiah G. Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. Journal of Virology. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G, Xie Q-W, Buller RML, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Kägi D, Seiler P, Pavlovic J, Ledermann B, Bürki K, Zinkernagel RM, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur. J. Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Kim S-K, Brehm MA, Welsh RM, Selin LK. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol. 2002;169:90–98. doi: 10.4049/jimmunol.169.1.90. [DOI] [PubMed] [Google Scholar]
- Kim S-K, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005;201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotturi MF, Peters B, Buendia-Laysa F, Sidney J, Oseroff C, Botten J, Grey H, Buchmeier MJ, Sette A. The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. Journal of Virology. 2007;81:4928–4940. doi: 10.1128/JVI.02632-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhai Q, Schaffner DJ, Wu A, Yohannes A, Robinson TM, Maland M, Wells J, Voss TG, Bailey C, Alibek K. Prevention of lethal respiratory vaccinia infections in mice with interferon-alpha and interferon-gamma. FEMS Immunol Med Microbiol. 2004;40:201–206. doi: 10.1016/S0928-8244(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Mathurin K, Martens G, Kornfeld H, Welsh R. CD4 T cell-mediated heterologous immunity between mycobacteria and poxviruses. Journal of Virology. 2009 doi: 10.1128/JVI.02393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutaftsi M, Salek-Ardakani S, Croft M, Peters B, Sidney J, Grey H, Sette A. Correlates of protection efficacy induced by vaccinia virus-specific CD8 +T-cell epitopes in the murine intranasal challenge model. Eur. J. Immunol. 2009;39:717–722. doi: 10.1002/eji.200838815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Langley WA, Li G, García-Sastre A, Webby RJ, Ahmed R. Qualitatively Different Memory CD8+ T Cells Are Generated after Lymphocytic Choriomeningitis Virus and Influenza Virus Infections. J Immunol. 2010;185:2182–2190. doi: 10.4049/jimmunol.1001142. [DOI] [PubMed] [Google Scholar]
- Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- Pillai VKB, Kannanganat S, Penaloza-MacMaster P, Chennareddi L, Robinson HL, Blackwell J, Amara RR. Different patterns of expansion, contraction and memory differentiation of HIV-1 Gag-specific CD8 T cells elicited by adenovirus type 5 and modified vaccinia Ankara vaccines. Vaccine. 2011;29:5399–5406. doi: 10.1016/j.vaccine.2011.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8+T-cell exhaustion during chronic viral infection irrespective of the type of antigen resenting cell. Eur. J. Immunol. 2012;42:2290–2304. doi: 10.1002/eji.201142275. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- Shen ZT, Nguyen TT, Daniels KA, Welsh RM, Stern LJ. Disparate Epitopes Mediating Protective Heterologous Immunity to Unrelated Viruses Share Peptide-MHC Structural Features Recognized by Cross-Reactive T Cells. The Journal of Immunology. 2013;191:5139–5152. doi: 10.4049/jimmunol.1300852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K, Sacha J, Gao X, Suresh M. Effect of chronic viral infection on epitope selection, cytokine production, and surface phenotype of CD8 T cells and the role of IFN-gamma receptor in immune regulation. J Immunol. 2004;172:1491–1500. doi: 10.4049/jimmunol.172.3.1491. [DOI] [PubMed] [Google Scholar]
- van den Broek MR, Müller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- Walsh CM, Matloubian M, Liu C-C, Ueda R, Kurahara CG, Christensen JL, Huang MTF, Young JD-E, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Lampert PW, Burner PA, Oldstone MBA. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology. 1976;73:59–71. doi: 10.1016/0042-6822(76)90060-x. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Seedhom MO. Lymphocytic Choriomeningitis Virus (LCMV): Propagation, Quantitation, and Storage. Curr Protoc Microbiol Unit 15A.1. 2008 doi: 10.1002/9780471729259.mc15a01s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of Virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton JL, Southern PJ, Oldstone MBA. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988;162:321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, Blackman MA. gamma-Herpesvirus-induced protection against bacterial infection is transient. Viral Immunol. 2009;22:67–72. doi: 10.1089/vim.2008.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.