Abstract
Background
Conventional multi-session genetic counseling is currently recommended when disclosing APOE genotype for risk of Alzheimer’s disease (AD) in cognitively normal individuals.
Objective
To evaluate the safety of brief disclosure protocols for disclosing APOE genotype for risk of Alzheimer’s disease (AD).
Methods
A randomized, multicenter non-inferiority trial was conducted at 4 sites. Participants were asymptomatic adults having a first-degree relative with AD. A standard disclosure protocol by genetic counselors (SP-GC) was compared to condensed protocols, with disclosures by genetic counselors (CP-GC) and by physicians (CP-MD). Pre-planned co-primary outcomes were anxiety and depression scales 12 months after disclosure.
Results
343 adults (mean age 58.3, range 33–86 years, 71% female, 23% African American) were randomly assigned to the SP-GC protocol (n= 115), CP-GC protocol (n=116) or CP-MD protocol (n=112). Mean post-disclosure scores on all outcomes were well below cut-offs for clinical concern across protocols. Comparing CP-GC to SP-GC, the 97.5% upper confidence limits at 12 months after disclosure on co-primary outcomes of anxiety and depression ranged from a difference of 1.2 to 2.0 in means (all p<0.001 on non-inferiority tests), establishing non-inferiority for condensed protocols. Results were similar between European Americans and African Americans.
Conclusions
These data support the safety of condensed protocols for APOE disclosure for those free of severe anxiety or depression who are actively seeking such information.
Keywords: Alzheimer, APOE, genetics, genomics, risk assessment, personalized medicine
INTRODUCTION
The ε4 allele of APOE is a common and robust risk factor for Alzheimer’s disease (AD), carried by approximately 25% of the population. In the Risk Evaluation and Education for Alzheimer’s disease (REVEAL) study, we have utilized the model of disclosing APOE genotype for risk of AD to explore translational questions associated with genetic risk disclosure. In a previous randomized controlled trial, we demonstrated that disclosing APOE genotypes with an extended counseling protocol was not associated with increased anxiety, depression or distress.1 The pre-disclosure counseling in that trial followed what were later published as official recommendations for genetic risk assessment of AD, and that were based upon Huntington Disease (HD) Society of America’s Guidelines for Genetic Testing for Huntington Disease,3 a protocol that the recommendations called the “gold standard for genetic testing for adult onset conditions”.2 Briefly, this protocol includes two pre-test and one or more post-test genetic counseling sessions conducted in person and incorporates both neurologic and psychiatric evaluations. Sessions address the physical, psychological, social, and family history factors that may influence the decision-making process to ensure informed decision-making about testing while minimizing the risks of adverse psychological outcomes.2
In this report, we describe a separate trial in which all subjects received APOE disclosure, but were randomized into one protocol that followed the gold standard above, or into one of two protocols with highly condensed pre-testing education and counseling. We hypothesized that subjects receiving the condensed protocols with disclosure from a genetic counselor would show no greater anxiety or depression than subjects receiving the standard protocol one year after disclosure.
METHODS
Study Population and Instruments
We recruited cognitively normal adult first-degree relatives (FDRs) of patients with AD through mailings to research registries, referrals from collaborating physicians, advertisements in local newspapers and community outreach at senior centers and nursing homes. We excluded individuals with two or more affected FDRs and individuals from families where the average AD onset age was under 60. We screened out individuals who demonstrated potential memory problems by scoring below an education-adjusted 87 on the Modified Mini-Mental State Examination4 and individuals with very severe anxiety and depression, as defined below. We selected European-American or African-American for enrollment because we had sufficient data to create ethnicity-specific risk models for these groups that incorporated APOE genotype.5 Given ambiguous data about the relationship between APOE and AD for other ethnicities,6,7 however, we excluded other populations.
The co-primary outcomes were validated self-report scales of anxiety and depression at 12 months after disclosure. We measured anxiety using the 21-item Beck Anxiety Inventory (BAI)8 and depression using the 20-item Center for Epidemiological Studies-Depression Scale (CES-D).9 BAI scores can range from 0 to 63, with scores above 15 indicating moderate anxiety and scores above 25 indicating severe anxiety. CES-D scores can range from 0 to 60, with scores 16 or above indicating moderate depression and scores above 26 indicating severe depression.10 Test-related distress at 12 months after disclosure served as a secondary outcome, measured using the Impact of Event Scale (IES),11 a 15-item self-report instrument commonly used in genetic disclosure research.12 The IES assessed the frequency of intrusive and avoidance thoughts related to the genetic risk assessment over the past week, with scores of 0–5 on individual items summed to create an overall score (range 0–75, scores 20 or above indicating significant distress). Because the IES measures distress specific to genetic risk disclosure, it was administered only after testing. We also evaluated secondary outcomes of BAI, CES-D and IES scores at 6 weeks and 6 months after the disclosure of genetic risk information.
Study Design
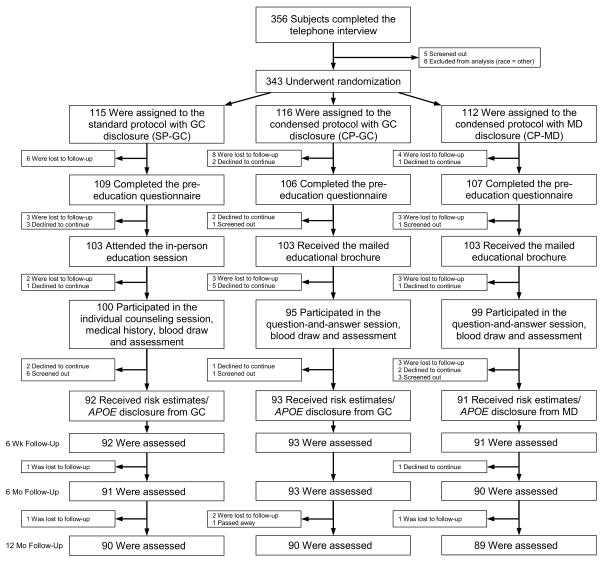
As described more fully in prior publications,1,13 the multidisciplinary REVEAL Study group designed the study protocol and risk disclosure procedures, including, for this trial, specific risk curves for African American subjects.5 The study was designed as a non-inferiority trial, despite inherent limitations of this approach,14 because the goal of the study was develop a protocol that markedly reduced clinical service demands rather than one that improved outcomes that had already been shown to be safe.1 The study was conducted at sites in academic medical centers in Boston, Cleveland, New York and Washington, DC. An independent external Ethics and Safety Board (ESB), as well as institutional review boards at each study site, oversaw the protocol and consent development. Subjects provided informed consent by telephone at the time of study enrollment, then again in writing prior to the blood draw for genotyping. The overall design of the study is shown in Figure 1.
Figure 1.
Flowchart for Enrollment
Following an initial phone interview, subjects were block randomized equally into one of three treatment arms, within strata defined by site, age (<60 vs ≥60), race, and gender. In the reference protocol, pre-test education and counseling took place with a genetic counselor (the SP-GC arm).3 Participants attended a semi-structured 35 minute in-person education session with a genetic counselor that included: a formal definition of AD, an overview of risk factors for AD (e.g., age, family history) and the level of risk in the general population; an explanation of APOE and its implications for risk of AD; a description of procedures involved in APOE testing; a preview of what would be provided in their risk assessment (e.g., risk figures and their format); and a summary of known benefits, risks, and limitations of APOE testing. At the blood-draw visit, a genetic counselor collected and reviewed the subject’s family history of dementia and personal medical information, and proactively addressed psychosocial aspects of testing. In the two condensed protocols the in-person education session was replaced with a mailed brochure (see Supplemental Figure 1), and subjects provided family history and medical information on mailed forms. When blood was drawn in the condensed protocols, genetic counselors reviewed the family history and medical information subjects mailed back and responded to participant questions rather than proactively addressing psychosocial aspects of testing. The two condensed protocols differed only in who was doing the disclosure. APOE was genotyped at Athena Diagnostics, a CLIA-certified facility.
Approximately one month after the blood draw, subjects received their APOE genotypes and numerical AD risk assessments as previously described.1,5,15 In brief, all subjects were shown a single graph with gender and race-specific risk curves and were told their APOE genotype and numeric estimates of their cumulative lifetime (potential range: 13–77%) and remaining risk for AD (cumulative incidence from current age to the age of 85 years). A genetic counselor disclosed results to subjects in the SP-GC arm and in one condensed protocol arm (CP-GC), while a study physician disclosed results in the other condensed protocol arm (CP-MD). The four physicians doing the disclosure were specialists in dementia, but had received no formal training in genetic counseling.
Study staff administered the BAI and CES-D prior to the blood draw (baseline) and at all follow-up time points. The IES was administered only at follow-up time points. The ESB reviewed the protocol, monitored study progress and established criteria for adverse event reporting. An immediate interview was planned for any subjects whose BAI or CES-D scores exceeded 26 or increased by more than 15 points from baseline at any point in the study. Cases of concern to the clinical teams were discussed in monthly phone calls. The chair of the ESB reviewed aggregated results annually. This trial was registered with clinicaltrials.gov identifier NCT00089882.
Statistical Analysis
We used ANOVA and chi-square testing to compare demographic features of the randomized groups. We compared discontinuation rates and subject variables associated with discontinuation across protocols using t-tests and chi-square tests. ANOVA was used to compare session lengths across protocols.
In estimating power for the primary analysis, we followed recommendations16 for defining non-inferiority as occurring if the upper limits of 1-sided 97.5% confidence intervals (equivalent to upper bounds of 2-sided 95% CIs) for mean differences between protocols were less than a pre-specified margin of 5 points on each of the outcome scales, the same intervals used in analyses for the initial REVEAL Study trial.1 In comparing co-primary outcomes of BAI and CES-D scores in the SP-GC vs CP-GC arms at 12 months, we estimated that we had more than 90% power at alpha=0.05/2 (for the 2 co-primary outcomes) = 0.025 to confirm non-inferiority within this margin.
To test the primary hypothesis of non-inferiority between SP-GC to CP-GC, post-disclosure levels of the two co-primary outcomes (BAI and CES-D) were evaluated at 12 months for non-inferiority first using linear models, with no adjustment for potential confounders; and second using linear models adjusting for age, gender, education, baseline scores and APOE genotype. Because these measures were skewed with a floor effect at zero, we also conducted pairwise Wilcoxon Rank Sum tests with no adjustment for covariates, as well as with Tobit models adjusting for the same covariates as the linear regression models. Secondary analyses comparing the non-inferiority of the CP-MD protocol to the SP-GC and CP-MD protocols mirrored these analyses. P values for these analyses were calculated from one-sided non-inferiority tests assuming that scores on a condensed protocol were not more than 5 points higher than the comparison protocol.
In addition to assessing co-primary outcomes at 12 months, we conducted secondary analyses to examine the outcomes at the baseline visit (post-education pre-disclosure) and at the 6 week and 6 month post-disclosure visits. Both condensed protocols were identical through the baseline visit, so data in these two arms were combined on multiple linear regression analyses of pre-disclosure outcomes, adjusting for age, gender, race and education. We conducted both intention-to-treat (ITT) and per-protocol analyses on pre-disclosure data since ITT analyses can bias interpretation in non-inferiority studies.16,17 Only per-protocol analyses were conducted and reported on post-disclosure data since we could not reliably impute APOE genotypes. P values for comparisons of baseline, pre-disclosure scores were calculated from tests that mean scores for the condensed protocols were not equivalent to mean scores for the standard protocol. P values for post-disclosure analyses were calculated from one-sided non-inferiority tests replicating the 12-month analyses that scores on a condensed protocol were not more than 5 points higher than a comparison protocol.
Interactions between randomization arm and APOE genotype were omitted from final models because they failed to reach significance at p≤0.05. For both pre- and post-disclosure analyses, missing values were imputed with the Markov chain Monte Carlo method of multiple imputation using PROC MI statistical software, version 9.3 (SAS Institute). Variables to calculate joint probabilities for multiple imputation were selected using an inclusive strategy, and included all variables used in analyses as well as additional variables whose sole purpose in these analyses were to improve performance of the imputation models.18 These additional variables were collected through self-report in the phone interview, pre-education, and follow-up questionnaires, and included income, AD risk perceptions, and less proven measures of test-related affect.19 We also evaluated the CP-MD protocol and the CP-GC protocol on all outcomes, using the procedures described above and controlling for baseline scores where applicable.
RESULTS
Of the 356 subjects who completed the introductory telephone interview, 5 subjects were screened out because upon further review, their family history of AD did not meet eligibility requirements and 8 were excluded because they self-identified as other than European-American or African-American and were told their numeric risk estimates could not be estimated accurately. Ultimately, 96% were randomized and analyzed (Figure 1). Of 343 subjects who were randomized, 20 (5.8%) subjects declined to continue in the study for the following non-exclusive reasons: study demands (9), concerns about anticipated emotional responses to test results (7), or potential discrimination (3), limitations of test information (3), lack of interest (2), lack of AD prevention options (1), and personal health problems (1). Thirty-five (10.2%) others discontinued without explanation (were lost to follow-up) prior to disclosure. We also screened out the following during the trial, but before genetic risk disclosure: two individuals whose family history of AD did not meet eligibility requirements after further review by genetic counselors; one participant who suggested that testing might influence a future decision to pursue suicide; three subjects with cognitive scores below eligibility criteria; and six subjects with depression scores above our pre-specified threshold. Demographic characteristics for participants included in the ITT analysis did not vary by randomization arm (Table 1) and were similar to those of the prior trial1 except for the higher percentage of African Americans in this trial. Ultimately, 276 (80.5%) of the subjects initially randomized received AD risk assessments with APOE genotype disclosure.
Table 1.
Characteristics of participants in ITT analyses.* P values represent a test that randomization arm differed.
| Characteristic | Standard Protocol, GC Disclosure (n=115) | Condensed Protocol, GC Disclosure (n=116) | Condensed Protocol, MD Disclosure (n=112) | p |
|---|---|---|---|---|
| Age: yrs | 0.94 | |||
| Mean | 58.1±10.1 | 58.2±10.9 | 58.6±11.0 | |
| Range | 36–78 | 33–86 | 36–86 | |
| Female sex: n (%) | 79 (69) | 84 (72) | 82 (73) | 0.72 |
| African-American race: n (%)† | 27 (23) | 28 (24) | 24 (21) | 0.88 |
| Education: yrs | 0.13 | |||
| Mean | 16.1±2.6 | 16.2±2.7 | 15.5±2.8 | |
| Range | 9–20 | 3–20 | 5–20 | |
| Currently married: n (%) | 65 (57) | 62 (53) | 68 (61) | 0.54 |
| Site: n (%) | 1.00 | |||
| Boston | 38 (33) | 38 (33) | 37 (33) | |
| Cleveland | 25 (22) | 25 (22) | 22 (20) | |
| Washington, DC | 23 (20) | 24 (21) | 21 (19) | |
| New York | 29 (25) | 29 (25) | 32 (29) | |
| Self-referred to study: n (%) | 81 (70) | 69 (59) | 70 (63) | 0.20 |
| More than 1 relative with AD: n (%)‡ | 48 (42) | 57 (49) | 49 (44) | 0.50 |
Plus-minus values are means ± standard deviations
Race was self-reported
Including non-first degree relatives (e.g., grandparent or cousin).
Whether or not a subject received their pre-test education through a genetic counselor (SP-GC arm) or through a brochure (CP-GC and CP-MD arms) did not affect the likelihood that the subject would drop out of the protocol (p=0.88). However, African American ethnicity (p<0.01) and lower education (p<0.01) were significantly associated with a greater likelihood of dropout prior to disclosure. At the pre-disclosure assessment, subjects in all arms scored well below cut-offs for clinical concern on the three outcomes.
Pre-disclosure education sessions were structured to last approximately 35 minutes in length within the SP-GC arm and did not occur in the CP arms where a brochure was sent instead. In the SP-GC arm the blood draw visit, including counseling, averaged 20.3 minutes in length, while the blood draw visits with question-and-answer only averaged 13.2 minutes across the CP arms (p<0.001). Genetic risk disclosure sessions averaged 22.4 minutes in the SP-GC arm, 23.2 minutes in the CP-GC arm, and 18.7 minutes in length in the CP-MD arm (p<0.001). At the pre-disclosure (blood draw) visit where anxiety and depression scales were administered for the first time, the ITT analysis of difference in means between subjects in the standard and condensed protocols was 0.1 (95% CI −1.2 to 1.0, p=0.87) on the BAI, and 0.7 (95% CI −0.9 to 2.3, p=0.40) on the CES-D. Non-ITT analyses were similar (see Supplementary Table 1).
Table 2 summarizes the unadjusted analysis of primary and secondary study outcomes (adjusted analyses are presented in Supplementary Table 2). All scores were well below standard cutoffs for clinical concern, regardless of disclosure protocol. Two-sided 95% confidence intervals for the mean difference between the SP-GC and both the CP-GC and CP-MD arms at 12 months after risk estimation and APOE genotype disclosure were below the predefined 5-point margin of non-inferiority for all scales. Secondary analyses also showed non-inferiority of both condensed protocols at earlier time points on anxiety and depression, as well as for the CP-GC protocol on test-related distress 12 months post-disclosure compared to the SP-GC. However, non-inferiority could not be demonstrated on test-related distress six weeks and six months post-disclosure for the CP-MD protocol. Similarly sub-analyses supported non-inferiority of the CP-MD protocol compared to the CP-GC protocol on anxiety and depression measures, but higher test-related distress scores were noted in the CP-MD protocol at the 6-week (Δ=2.8, 95%CI = 0.4 to 5.1, non-inferiority p=0.03) and 6-month (Δ=3.0, 95%CI = 0.5 to 5.4, non-inferiority p=0.05) post-disclosure time points (see Supplementary Table 2). Pairwise Wilcoxon Rank Sum tests comparing the CP-GC to the SP-GC, and comparing the CP-MD to the SP-GC, with no adjustment for covariates, as well as with Tobit models adjusting for the same covariates as the linear regression models, were conducted and the results were consistent with the linear regression models (data not shown).
Table 2.
Unadjusted anxiety, depression and test-related distress scores by randomization arm, stratified by outcome and time after APOE genotype disclosure. P values represent a one-sided non-inferiority test, using linear models, that scores on a specific condensed protocol are not more than 5 points higher than the control standard protocols.*
| SP-GC (n=92) | CP-GC (n=93) | CP-MD (n=91) | CP-GC vs SP-GC (95% CI) | p | CP-MD vs SP-GC (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| 12 month outcomes | |||||||
| BAI† | 3.0±0.5 | 3.7±0.5 | 3.9±0.5 | 0.7 (−0.7 to 2.0) | <0.001 | 0.9 (−0.5 to 2.2) | <0.001 |
| CES-D‡ | 6.2±0.6 | 5.6±0.6 | 6.9±0.6 | −0.6 (−2.4 to 1.2) | <0.001 | 0.6 (−1.1 to 2.4) | <0.001 |
| IESΨ | 3.4±0.8 | 3.3±0.8 | 5.5±0.8 | −0.1 (−2.2 to 2.1) | <0.001 | 2.0 (−0.1 to 4.2) | 0.007 |
| 6 month outcomes | |||||||
| BAI | 3.2±0.5 | 3.1±0.5 | 4.4±0.5 | −0.2 (−1.6 to 1.2) | <0.001 | 1.2 (−0.3 to 2.6) | <0.001 |
| CES-D | 6.3±0.7 | 5.8±0.7 | 8.1±0.7 | −0.5 (−2.4 to 1.5) | <0.001 | 1.8 (−0.1 to 3.8) | 0.002 |
| IES | 3.9±0.9 | 4.0±0.9 | 7.0±0.9 | 0.1 (−2.4 to 2.6) | <0.001 | 3.1 (0.6 to 5.6) | 0.136 |
| 6 week outcomes | |||||||
| BAI | 2.6±0.5 | 3.6±0.5 | 4.3±0.5 | 0.9 (−0.4 to 2.3) | <0.001 | 1.7 (0.3 to 3.0) | <0.001 |
| CES-D | 5.7±0.7 | 5.8±0.7 | 8.1±0.7 | 0.1 (−1.9 to 2.0) | <0.001 | 2.4 (0.4 to 4.3) | 0.008 |
| IES | 2.8±0.9 | 5.1±0.9 | 8.2±0.9 | 2.3 (−0.1 to 4.8) | 0.033 | 5.4 (3.0 to 7.9) | 0.724 |
Plus-minus values are means ± standard errors. CI are two-sided 95% confidence intervals.
Scores on the Beck Anxiety Inventory (BAI) range from 0 to 63, with higher scores indicating greater anxiety.
Scores on the Center for Epidemiological Studies Depression Scale (CES-D) range from 0–60, with higher scores indicating greater depression.
Scores on the Impact of Event Scale (IES) range from 0 to 75, with higher scores indicating greater distress.
Overall, 26% of study subjects reported moderate anxiety (BAI≥16), depression (CES-D≥16), or test-related distress (IES≥20) at one or more follow-up time points, with no differences by randomization arm (p=0.23). Secondary analyses did not show significant interaction by race, APOE status or randomization arms on BAI and CES-D scores at 12 month (p≥0.27). Secondary analyses were also conducted to compare ε4-positive and negative subjects as shown in Table 3. As previously described in the initial REVEAL Study trial,1 we also found in this trial that ε4-positive subjects showed no more symptoms of general anxiety or depression than ε4-negative subjects, but did show greater test-specific distress at all follow-up time points that was clinically trivial, but statistically significant (IES Δ=4.9 at 6 weeks, 3.0 at 6 months, and 2.4 at 12 months, all p≤0.01).
Table 3.
Differences on study outcomes between APOE ε4-positive and ε4-negative subjects, stratified by outcome and time after APOE genotype and AD risk disclosure. Positive numbers indicate greater anxiety, depression, or test-related distress among ε4-positive subjects than ε4-negative subjects. P values represent a post-hoc test, using linear models, that scores differ by APOE result after adjusting for randomization, age, sex, race, and education.*
| Time point | Anxiety (BAI) Δ (95% CI) |
p | Depression (CES-D) Δ (95% CI) |
p | Test-related Distress (IES) Δ (95% CI) |
p |
|---|---|---|---|---|---|---|
| 12 mo | 0.2 (−0.9 to 1.4) | 0.69 | −0.3 (−1.8 to 1.3) | 0.74 | 2.4 (0.6 to 4.2) | 0.01 |
| 6 mo | 0.6 (−0.6 to 1.8) | 0.32 | 0.1 (−1.6 to 1.8) | 0.89 | 3.0 (0.9 to 5.1) | <0.01 |
| 6 wk | 0.4 (−0.8 to 1.5) | 0.55 | 0.5 (−1.2 to 2.2) | 0.55 | 4.9 (2.9 to 6.9) | <0.01 |
Plus-minus values are means ± standard errors. CI are confidence intervals. Scores were adjusted for age, education, sex, and race.
DISCUSSION
This trial compares the impact of different disclosure protocols for APOE genotype. In comparisons between the standard and condensed protocols where both were delivered by genetic counselors, volunteer subjects randomized to receive a condensed protocol did not experience greater anxiety or depression symptoms, nor greater test-related distress, 12 months after disclosure. Non-inferiority could not be demonstrated for the secondary outcome of test-related distress at earlier time points, but these differences were still minor. Our findings, in conjunction with prior analyses showing no decreases in knowledge or information recall after receiving the condensed protocols,20 add weight to suggestions that genetic susceptibility test providers may be able to streamline protocols for persons volunteering for such information without compromising their wellbeing, at least when results are disclosed by a genetic counselor. The condensed protocols we used required one less in-person appointment and saved considerable clinician time, substantially reducing the demands of testing on providers and test-recipients alike. In fact, blood draw sessions were shorter in the condensed protocols despite the omission of an opportunity for subjects to address concerns during an in-person education session. The time savings was attributable primarily to having subjects mail family history and personal medical information in advance rather providing this information for the first time during the blood draw session. These findings are encouraging, given how medical providers may expect escalating requests for genetic testing in the near future. Findings of non-inferiority may be explained by prior work showing that motivations for testing are myriad.21,22 Our condensed protocol was less scripted, and may have provided more opportunities for addressing individual goals rather than the generalized concerns that may be of less relevance to specific test recipients. If so, test recipients may benefit from the incorporation of a decision aid into the educational brochure that helps them set realistic expectations about the ability of testing to satisfy those outcomes.23 Alternatively, genetic susceptibility testing may pose lower psychological risks to volunteer populations than often speculated. Other randomized trials of genetic testing disclosure have shown no incremental risk to psychological wellbeing through group education24 or telephone disclosure,25 but minor increases in anxiety using computer education rather than in-person counseling.26
This study also compares disclosure protocols administered by genetic counselors to those administered by non-geneticist physicians. While none of the outcomes in this comparison suggested that genetic information was harmful, scores on scales of test-related distress were not consistently within the margin for non-inferiority when results were disclosed through a non-geneticist physician rather than a genetic counselor. Inferences from this comparison are limited because there was such a small number of genetic counselors and non-geneticist physicians. Moreover, the genetic counselors were female, had each served as study coordinators at their respective sites and spent more time on average in the disclosure session; whereas the physicians were all male and spent less time on average in the disclosure session. Nevertheless, the differences observed between the CP-GC and CP-MD protocols suggest that GCs might be more effective in relieving short-term emotional distress than physicians providing disclosure through the same protocol. Analyses of cases where genetic testing was ordered without a genetics specialist and surveys of genetic counselors suggest that nonspecialists often provide insufficient genetic counseling prior to testing.27,28 The physicians in our study did not have formal training in medical genetics but they were well versed in explaining the probabilistic nature of APOE findings, and therefore were not typical of practicing physicians.
Our study has limitations because we excluded individuals with low cognitive testing scores as well as those with very severe anxiety and depression; and the volunteers who participated tended to be well educated, and (by virtue of their participation) positively inclined toward genetic testing. While we did not specifically track the characteristics of persons who were offered and declined participation, we followed the same recruitment practices as we did in our earlier trials where enrollees were found to be younger and better educated than persons who declined enrollment.29 Thus individuals who might be less motivated to learn these results, who were experiencing mild cognitive symptoms, who had higher levels of baseline distress or who were older or less well educated might not show the same results. For individuals receiving genetic risk results for other common complex conditions such as diabetes or heart disease, a different set of outcomes (involving appropriate interpretation and subsequent behaviors) will likely be more important than distress and our study does not address this. The physicians in our study were familiar with communicating genetic risk information and may not be representative of other physicians lacking formal training in genetics. Lastly, non-inferiority trials may introduce greater subjectivity and allow fewer protections against bias than superiority trials.14 Nonetheless, our data challenge the existing recommendations for disclosure of APOE for risk of Alzheimer’s disease,2 and add evidence that suggests that a condensed pre-test educational protocol for disclosure of potentially distressing genetic risk information about a frightening and untreatable common disease to willing recipients can be safe.
Supplementary Material
Supplementary Figure 1. The 4 page educational brochure that replaced in-person education in the condensed protocols.
Supplementary Table 1. Mean baseline (pre-disclosure) anxiety (BAI) and depression (CES-D) scores analyzed by pre-test education experience (i.e. Standard vs Condensed arms). Analyzes control for age, education, sex, and race. P values are reported for the test, using multiple linear regression, that mean scores for the combined condensed protocols are not equivalent to mean scores for the standard protocol.
Supplemental Table 2. Anxiety, depression and test-related distress scores by randomization arm, stratified by outcome and time after APOE genotype disclosure, in models that include baseline scores as covariates where applicable (i.e., BAI and CES-D models). P values represent a one-sided non-inferiority test, using linear models, that scores on a specific condensed protocol are not more than 5 points higher than the reference protocol after adjusting for APOE result, age, sex, race, education and baseline score, where applicable. *
RESEARCH IN CONTEXT.
Systematic review: APOE genotyping in asymptomatic individuals for risk of Alzheimer’s disease (AD) has been controversial for some time, both within the AD community and as a paradigm for common complex risk assessment in the medical genetics community. We have searched PubMed and other sources for over 10 years for published research and opinions in this arena.
Interpretation: Over the past decade, there has been a reluctant appreciation that some individuals wish to know their APOE genotypes for AD risk assessment. Current expert-based recommendations for such disclosures emphasize conventional, time-intensive genetic counseling. To our knowledge, our research provides the only empirical data on more condensed protocols for APOE genotype disclosure.
Future directions: Larger scale studies on the impact of disclosing APOE genotype may more definitively answer the question of safety and benefit of this information.
Acknowledgments
This work was supported by NIH grants HG002213, HG09029, AG13846, HG006993, TR000433, RR00533 and RR10284.
Additional members of the REVEAL Study group are as follows: G. Annas, Boston University School of Medicine, Boston; D. Bhatt, VA Boston Healthcare System, Brigham and Women’s Hospital, and Harvard Medical School, Boston; B. Biesecker, National Human Genome Research Institute, Bethesda; D. Blacker, Harvard School of Public Health, Boston; C. Chen, Boston University School of Public Health, Boston; E. Cox, Weill Cornell Medical College, New York; J.G. Davis, Weill Cornell Medical College, New York; L. Farrer, Boston University School of Medicine and Boston University School of Public Health, Boston; P. Griffith, Morehouse School of Medicine, Atlanta; K. Harkins, Perelman School of Medicine, Philadelphia; S. Hiraki, Albert Einstein College of Medicine, Bronx; M. Johnson, Howard University, Washington, DC; S. Johnson, Howard University, Washington, DC; E. Juengst, University of North Carolina School of Medicine, Chapel Hill; J. Karlawish, Perelman School of Medicine, Philadelphia; L. Le, University of Michigan School of Public Health, Ann Arbor; T. Marteau, Kings College, London; E, McCarty Wood, Perelman School of Medicine, Philadelphia; T. Obisesan, Howard University, Washington, DC; R. Petersen, Mayo Alzheimer’s Disease Research Center, Rochester, MN; S. Post, Stony Brook University, Stony Brook; K. Quaid, Indiana University School of Medicine, Indianapolis; L. Ravdin, Weill Cornell Medical College, New York; D. Roter, Johns Hopkins Bloomberg School of Public Health, Baltimore; R. Stern, Boston University School of Medicine, Boston; A. Sadovnick, University of British Columbia, Vancouver; S. Sami, Case Western Reserve University, Cleveland; P. Sankar, Perelman School of Medicine, Philadelphia; E. Topol, Scripps Research Institute, La Jolla; W. Uhlmann, University of Michigan, Ann Arbor; L. Waterston, Brigham and Women’s Hospital, Boston; L. Wright, Medical College of Georgia, Athens. No compensation was received by these individuals in exchange for their participation beyond the NIH funding cited above.
Footnotes
None of the authors report conflicts of interest relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361(3):245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman JS, Hahn SE, Catania JW, et al. Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13(6):597–605. doi: 10.1097/GIM.0b013e31821d69b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Huntington Association and the World Federation of Neurology Research Group on Huntington’s Chorea. Guidelines for the molecular genetics predictive test in Huntington’s disease. J Med Genet. 1994;31(7):555–559. doi: 10.1136/jmg.31.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 5.Christensen KD, Roberts JS, Royal CDM, et al. Incorporating ethnicity into genetic risk assessment for Alzheimer’s disease: The REVEAL Study experience. Genet Med. 2008;10(3):207–214. doi: 10.1097/GIM.0b013e318164e4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crean S, Ward A, Mercaldi CJ, et al. Apolipoprotein E epsilon4 prevalence in Alzheimer’s disease patients varies across global populations: a systematic literature review and meta-analysis. Dementia and geriatric cognitive disorders. 2011;31(1):20–30. doi: 10.1159/000321984. [DOI] [PubMed] [Google Scholar]
- 7.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 8.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 9.Radloff LS. The CES-D Scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 10.Santor DA, Zuroff DC, Ramsay JO, Cervantes P, Palacios J. Examining scale discriminability in the BDI and CES-D as a function of depressive severity. Psychol Assess. 1995;7:131–139. [Google Scholar]
- 11.Horowitz M, Wilner N, Alvarez MA. Impact of event scale: A measure of subjective distress. Psychosom Med. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Payne K, Nicholls S, McAllister M, Macleod R, Donnai D, Davies LM. Outcome measurement in clinical genetics services: A systematic review of validated measures. Value Health. 2008;11(3):497–508. doi: 10.1111/j.1524-4733.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 13.Roberts J, Cupples L, Relkin N, Whitehouse P, Green RC. Genetic risk assessment for adult children of people with Alzheimer’s disease: The Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study. J Geriatr Psychiatr Neurol. 2005;18(4):250–255. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- 14.Snapinn SM. Noninferiority trials. Curr Control Trials Cardiovasc Med. 2000;1(1):19–21. doi: 10.1186/cvm-1-1-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cupples LA, Farrer L, Sadovnick D, Relkin N, Whitehouse P, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: The REVEAL Study. Genet Med. 2004;6(4):192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- 16.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SW. Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. JAMA. 2006;295(10):1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 17.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: The importance of rigorous methods. Br Med J. 1996;313(7048):36–39. doi: 10.1136/bmj.313.7048.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins LM, Schafer JL, Kam C-M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6(4):330–351. [PubMed] [Google Scholar]
- 19.Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(1):50–56. doi: 10.1097/wad.0b013e318188429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts JS, Chen CA, Uhlmann WR, Green RC. Effectiveness of a condensed protocol for disclosing APOE genotype and providing risk education for Alzheimer’s disease. Genet Med. 2012;14(8):742–748. doi: 10.1038/gim.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen K, Roberts J, Uhlmann W, Green RC. Changes to perceptions of the pros and cons of genetic susceptibility testing after APOE genotyping for Alzheimer disease risk. Genet Med. 2011;13(5):409–414. doi: 10.1097/GIM.0b013e3182076bf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts JS, LaRusse SA, Katzen H, et al. Reasons for seeking genetic susceptibility testing among first-degree relatives of people with Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2003;17(2):86–93. doi: 10.1097/00002093-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Edwards A, Gray J, Clarke A, et al. Interventions to improve risk communication in clinical genetics: Systematic review. Patient Educ Couns. 2008;71(1):4–25. doi: 10.1016/j.pec.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Calzone KA, Prindiville SA, Jourkiv O, et al. Randomized comparison of group versus individual genetic education and counseling for familial breast and/or ovarian cancer. J Clin Oncol. 2005;23(15):3455–3464. doi: 10.1200/JCO.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins J, Calzone KA, Dimond E, et al. Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med. 2007;9(8):487–495. doi: 10.1097/gim.0b013e31812e6220. [DOI] [PubMed] [Google Scholar]
- 26.Green MJ, Petersen SK, Baker M, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: A randomized controlled trial. JAMA. 2004;292(4):442–452. doi: 10.1001/jama.292.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48–63. doi: 10.1007/s10897-013-9605-3. [DOI] [PubMed] [Google Scholar]
- 28.Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413–423. [PubMed] [Google Scholar]
- 29.Roberts JS, Barber M, Brown TM, et al. Who seeks genetic susceptibility testing for Alzheimer’s disease? Findings from a multisite, randomized clinical trial. Genet Med. 2004;6(4):197–203. doi: 10.1097/01.gim.0000132688.55591.77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The 4 page educational brochure that replaced in-person education in the condensed protocols.
Supplementary Table 1. Mean baseline (pre-disclosure) anxiety (BAI) and depression (CES-D) scores analyzed by pre-test education experience (i.e. Standard vs Condensed arms). Analyzes control for age, education, sex, and race. P values are reported for the test, using multiple linear regression, that mean scores for the combined condensed protocols are not equivalent to mean scores for the standard protocol.
Supplemental Table 2. Anxiety, depression and test-related distress scores by randomization arm, stratified by outcome and time after APOE genotype disclosure, in models that include baseline scores as covariates where applicable (i.e., BAI and CES-D models). P values represent a one-sided non-inferiority test, using linear models, that scores on a specific condensed protocol are not more than 5 points higher than the reference protocol after adjusting for APOE result, age, sex, race, education and baseline score, where applicable. *