Abstract
Free radical oxidation of cholesterol and its precursors contribute significantly to the pathophysiology of a number of human diseases. This review intends to summarize recent developments and provide a perspective on the reactivities of sterols toward free radical oxidation, the free radical reaction mechanism, and the biological consequences of oxysterols derived from the highly oxidizable cholesterol precursor, 7-dehydrocholesterol. We propose that the rigid structures, additional substituents on the double bonds, and the well-aligned reactive C–H bonds in sterols make them more prone to free radical oxidation than their acyclic analogs found in unsaturated fatty acids. The mechanism of sterol peroxidation follows some well-established reaction pathways found in the free radical peroxidation of polyunsaturated fatty acids, but sterols also undergo some reactions that are unique to these compounds. Peroxidation of 7-dehydrocholesterol gives arguably the most diverse set of oxysterol products that have been observed to date. The metabolism of these oxysterols in cells and the biological consequences of their formation will be discussed in the context of the pathophysiology of the human disease Smith–Lemli–Opitz syndrome. Considering the high reactivity of sterols, we propose that a number of other cholesterol biosynthesis disorders may be associated with oxidative stress.
Keywords: peroxidation, autoxidation, oxysterol, 7-dehydrocholesterol, Smith–Lemli–Opitz syndrome
Introduction
Cholesterol is abundant in mammalian cells and tissues, and plays important roles in maintaining plasma membrane integrity [1,2], lipid-raft-mediated cell signaling [3,4], activation of the hedgehog pathway during embryonic development [5,6], and myelin formation [7]. Free radical oxidation of cholesterol has been implicated in a number of human diseases such as atherosclerosis [8], Alzheimer’s disease [9], retinal degeneration [10], age-related macular degeneration [11], cataract [12,13], and Niemann–Pick C1 disease [14]. Recently, peroxidation of a cholesterol precursor, 7-dehydrocholesterol (7-DHC), was found to contribute to the pathophysiology of cholesterol biosynthesis disorder, Smith–Lemli–Opitz syndrome (SLOS) [15–24].
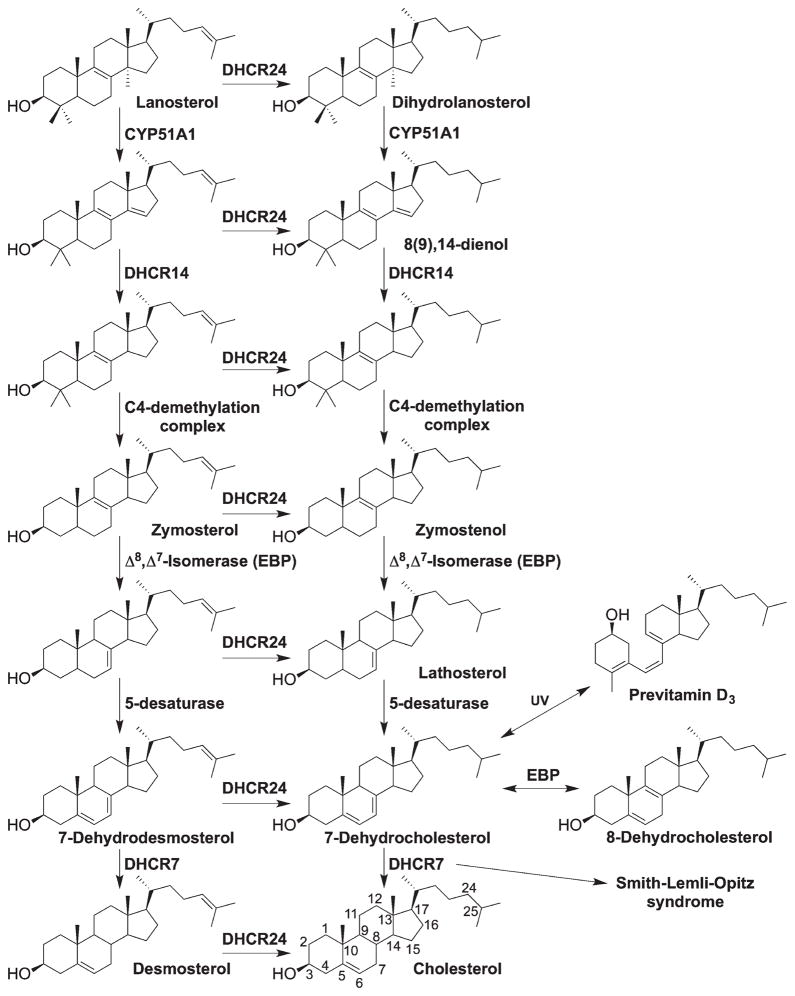
The mechanism of free radical oxidation of cholesterol has been extensively studied and many oxidation products, that is, oxysterols, have been identified [8,25–29]. Major efforts have also been devoted to study the biological activities of these oxysterols [30–33]. However, until recently little was known about the relative reactivity of cholesterol and other oxidizable lipids [15]. Significantly, the cholesterol precursors, 7-DHC and 8-DHC, were found to be among the most reactive lipid hydrogen atom donors to peroxyl radicals, thus making them highly oxidizable [15,16]. 7-DHC accumulates in tissues and fluids (particularly high in the brain) of individuals affected with SLOS, an autosomal recessive disorder that is caused by mutations in the gene encoding 3β-hydroxysterol-Δ7-reductase (DHCR7; EC 1.3.1.21) (Scheme 1) [34–39]. The level of 8-DHC is also elevated in SLOS patients, comparable to that of 7-DHC [36,37,40], due to the functioning of 3β-hydroxysterol- Δ8, Δ7 -isomerase (Ebp; EC 5.3.3.5). Ebp catalyzes the equilibration between the Δ8- and the Δ7-double bond (e.g., zymostenol to lathosterol), and the equilibrium normally favors the Δ7-sterol as it can be subsequently converted to downstream products. However, when 7-DHC accumulates due to the defective 3β-hydroxysterol-Δ7-reductase (DHCR7), 8-DHC can be observed in significant amount as a result of the equilibrium. In fact, defects in each step of cholesterol biosynthesis causes a disorder, which results in accumulation of specific cholesterol precursors [34]. In the postsqualene cholesterol biosynthesis pathway, cyclization of squalene-2,3-epoxide gives the first sterol, lanosterol, which is followed by multistep transformations, leading to the ultimate product cholesterol (Scheme 1). Depending on whether the C24 double bond is reduced early or later by 3β-hydroxysterol-Δ24 -reductase (DHCR24; EC 1.3.1.72), the pathway has been defined as the Kandutsch–Russell pathway or Bloch pathway, respectively [41,42]. Importantly, 7-DHC also serves as the biosynthesis precursor to vitamin D3 in human skin, where ring-B is opened upon UV irradiation [43].
Scheme 1.
Postsqualene cholesterol biosynthesis pathway. Sequence on the left shows the Bloch pathway and the one on the right shows the Kandutsch–Russell pathway.
Oxidation of 7-DHC gives a complex mixture of oxysterols, which led us to re-examine the reactions involved in sterol free radical oxidation [16,17,19]. These oxysterols exert a variety of biological actions, such as reducing cell proliferation, inducing cell differentiation, modulating gene expression, forming adducts with proteins, etc. [18,21,44]. The mechanism of formation and the biological activities of cholesterol-derived oxysterols have been reviewed several times previously [30–33,45–49]. This review will focus on three different aspects: (1) rationalizing the reactivities of cholesterol and its precursors toward free radical oxidation; (2) reaction mechanisms involved in sterol oxidation and the effect of α-tocopherol on product distribution; and; (3) the biological consequences of the novel oxysterols derived from the cholesterol precursor, 7-DHC, and their role in SLOS.
Reactivities of cholesterol and its biosynthetic precursors toward free radical oxidation
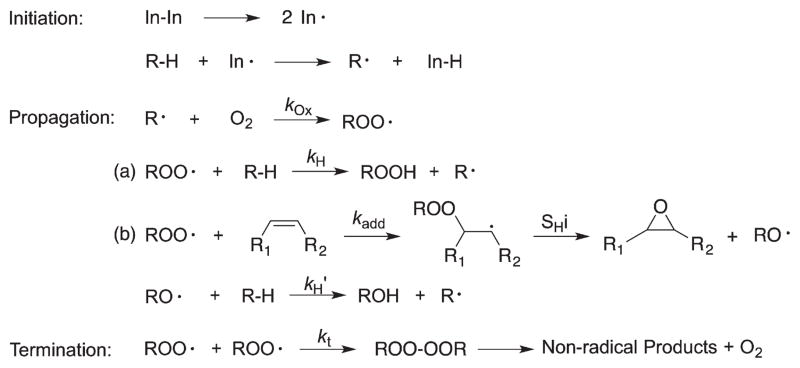
The rate-determining step in the free radical chain oxidation of lipids is the propagation reaction of the peroxyl radical, where generally two types of processes occur (Scheme 2) [48]: (a) a hydrogen atom is transferred from a donor to the chain-carrying peroxyl radical (hydrogen atom transfer); (b) a peroxyl radical adds to a double bond (peroxyl radical addition). We will discuss these two types of reactions separately below.
Scheme 2.
Typical sequence involved in free radical chain oxidation reactions.
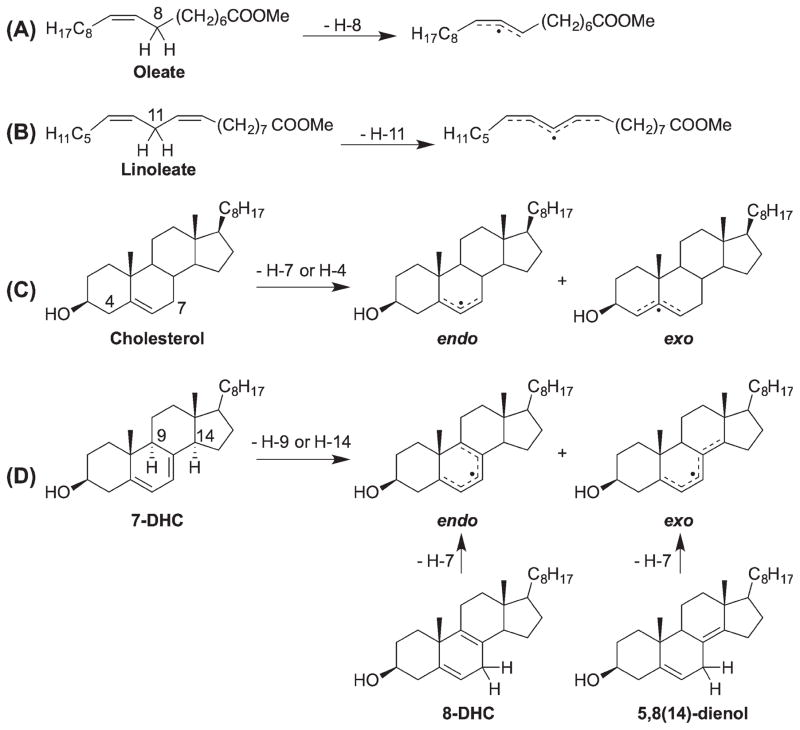
Hydrogen atom transfer
Utilizing a peroxyl radical clock, we have determined the hydrogen atom transfer rate constants (kH) of polyunsaturated fatty acids (PUFAs) and sterols to linoleate peroxyl radicals [15]. We found that cholesterol is a moderately oxidizable lipid with a kH of 11 M−1s−1 at 37°C, about one-sixth of the rate constant for linoleate (62 M−1s−1) [50], but 10 times that of the acyclic monounsaturated oleate (0.88 M−1s−1) [50]. 7-DHC, the immediate cholesterol precursor with one additional double bond at C7, gives a rate constant of 2260 M−1s−1, the largest rate constant known for a lipid molecule. Mechanistic and product analysis suggest that H9 and H14 are the reactive hydrogen atoms [17], which make the kH of 7-DHC 1130 M−1s−1 per hydrogen atom, a rate constant that is more than 35 times that of bis-allylic hydrogen atoms found in PUFAs (for linoleate, kH = 31 M−1s−1/H-atom). This was a surprising finding at the time because 7-DHC only has mono-allylic positions and in the peroxidation of PUFAs, the bis-allylic C–H bonds are much more reactive than the mono-allylic C–Hs (reflecting their respective bond dissociation enthalpies) [51–53].
More recently, we determined the hydrogen atom transfer rate constants of four additional cholestadienols, including cholesta-6,8(9)-dienol [6,8(9)-dienol], 8-dehydrocholesterol (8-DHC or 5,8-dienol), cholesta-5,8(14)-dienol [5,8(14)-dienol], and cholesta-6,8(14)-dienol [6,8(14)-dienol], to be 1370, 994, 911, and 412 M−1s−1, respectively[16]. There is an apparent trend that the reactivity of the unsaturated sterols are better hydrogen atom donors than their acyclic fatty acid analogs and their more flexible cyclic analogs, such as cyclohexene or cyclohexadienes (Table I) [50,54].
Table I.
Summary of hydrogen atom transfer rate constants of sterols in comparison with flexible molecules and the dihedral angles involving the reactive C–Hs and the double bonds.
| Substrate | kH (M−1s−1)a | # of Subb | φHCc | φCCd | endo/exoe | References |
|---|---|---|---|---|---|---|
 Cholesterol |
11 | 3 | H4α-C4.5,6: −7.9° (82.1°) H4β-C4.5,6: 109.1° (19.1°) H7α -C5,6,7: −106.2° (16.2°) H7β-C5,6,7: 137.7° (47.7) |
– | endo/exo | [15] |
 7-Dehydrocholesterol |
2260 | 5 | H9-C7,8,9: −92.3° (2.3°) H14-C7,8,14: 99.4° (9.4°) |
5.7° | endo/exo | [15] |
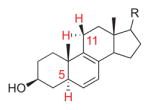 6,8(9)-Dienol |
1370 | 5 | H5-C5,6,7: 79.2° (10.8°) H11α-C8,9,11: 126.2° (36.2°) H11β-C8,9,11: −117.7° (27.7°) |
9.3° | endo/exo | [16] |
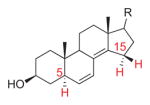 6,8(14)-Dienol |
412 | 5 | H5-C5,6,7: 91.7° (1.7°) H15α-C8,14,15: 51.8° (38.2°) H15β-C8,14,15: −69.9° (20.1°) |
4.6° | exo | [16] |
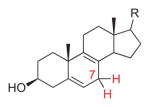 8-Dehydrocholesterol |
994 | 5 | H7α-C5,6,7: −133.5° (43.5°) H7β-C5,6,7: 108.6° (18.6°) H7α-C7,8,9: 129.8° (39.8°) H7β-C7,8,9: −111.5° (21.5°) |
3.2° f | endo | [16] |
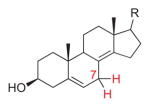 5,8(14)-Dienol |
911 | 5 | H7α-C5,6,7: −114.3° (24.3°) H7β-C5,6,7: 128.5° (38.5°) H7α-C7,8,9: −87.5° (2.5°) H7β-C7,8,9: 31.4° (58.6°) |
21.5° g | exo | [16] |
| Oleate | 0.88 (0.22) | 2 | – | – | – | [50] |
| Conjugated linoleate | 14 (3.5)h | 2 | – | – | – | [53]h |
| Linoleate | 62 (31) | 2 | – | – | – | [50] |
| Cyclohexene | 6 (1.5) | 2 | – | – | endo | [50] |
| 1,3-Cyclohexadiene | 220 (55) | 2 | – | – | endo | [50] |
| 1,4-Cyclohexadiene | 265 (66) | 2 | – | – | endo | [54] |
kH per H-atom is shown in the parentheses.
Number of substituents on the delocalized radical intermediates.
Dihedral angles between the reactive C–H and the double bond plane and the values in the parenthesis show the difference from 90°.
Dihedral angles between the planes containing individual double bond.
endo: radical delocalize within the same ring; exo: radical delocalize across rings.
The angle between the planes C5,6,7 and C7,8,9.
The angle between C5,6,7 and C7,8,14.
value was obtained by extrapolating the rate constants of oleate and linoleate using their computed bond dissociation enthalpy [53].
We suggest that three factors may collectively contribute to the high reactivity of sterols toward hydrogen atom abstraction:
Sterols normally have more alkyl substituents on double bond(s) than those in fatty acids. Substituents can stabilize the transition state and the resulting radical intermediate via hyperconjugation, thus lowering the activation energy of the hydrogen atom transfer process. For example, the allylic radicals formed from cholesterol have three substituents while oleate only has two; the pentadienyl radicals derived from 7-DHC have five substituents while the one derived from linoleate has two (Scheme 3).
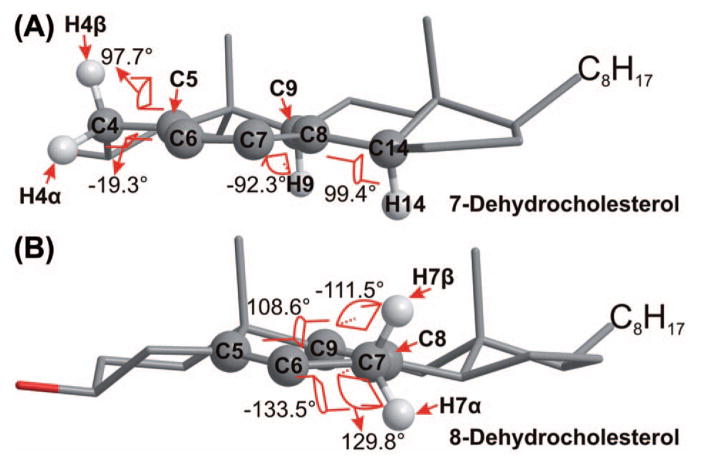
The dihedral angles between the reactive C–H bond and the adjacent double bond and the planarity the two double bonds (if applicable) (Table I and Figure 1). In 7-DHC, molecular mechanics modeling suggests that the two double bonds are close to being planar (the C5–C6–C7–C8 dihedral angle = 5.7°) and the dihedral angles between the reactive C–H bonds (at C9 and C14) and the double bond plane are 92.3 and 99.4°, respectively, both being close to the perpendicular geometry [16] (Figure 1). The planarity of the double bonds and the orthogonality of the C–H bonds make the reactant to resemble the transition state for H atom removal at C9 or C14, where maximum overlapping between the π-orbitals and the reactive C-H bond is expected. Thus, a minimum amount of molecular reorientation is required to reach the transition state, that is, there is less entropy demand. On the other hand, for an acyclic system such as linoleate, all the σ-bonds around the double bonds can freely rotate and a significant amount of entropy is lost in order to reach the transition state. For the same number of double bonds and reactive C–H bonds, the less molecular reorientation required, the more reactive the molecule. This could partially account for the reactivity trend of 7-DHC >6,8(9)-dienol >8-DHC as seen in Table I, all being dienes on ring-B but all being much more reactive than the acyclic linoleate. In 8-DHC, while the two double bonds are close to planar, the bis-allylic C–H bonds are distorted from the perpendicular with H7β being the relatively more orthogonal one than H7α. This rationale, along with the substituent factor discussed above, could also account for the reactivity difference between cholesterol and oleate, both being monounsaturated but cholesterol is 10 times more reactive.
Dienes that adopt cisoid conformations tend to be more reactive than those adopting trannll soid conformations. It is known that the cisoid conformation of a conjugated diene has higher enthalpy than the transoid conformation [55], which would imply smaller activation energy of hydrogen atom transfer from the allylic positions of the cisoid conformation. The high reactivity of cisoid could provide a reasonable explanation for the reactivity trend of the conjugated dienols: 5,7-dienol (7-DHC) >6,8(9)-dienol ≫ 6,8(14)-dienol.
Scheme 3.
Radical intermediates formed from sterols and fatty acids.
Figure 1.
Structures of 7-dehydrocholesterol (A) and 8-dehydrocholesterol (B) (optimized by MM2 in ChemBio3D) showing the dihedral angles between the planes containing the allylic C–H bonds and the adjacent planes containing the double bonds.
Overall, the cholestadienols leading to an endo radical (within the same sterol ring) tend to be more reactive than those that give an exo radical (spanning multiple rings), which could largely be rationalized by factors 2 and 3 [16].
Peroxyl radical addition
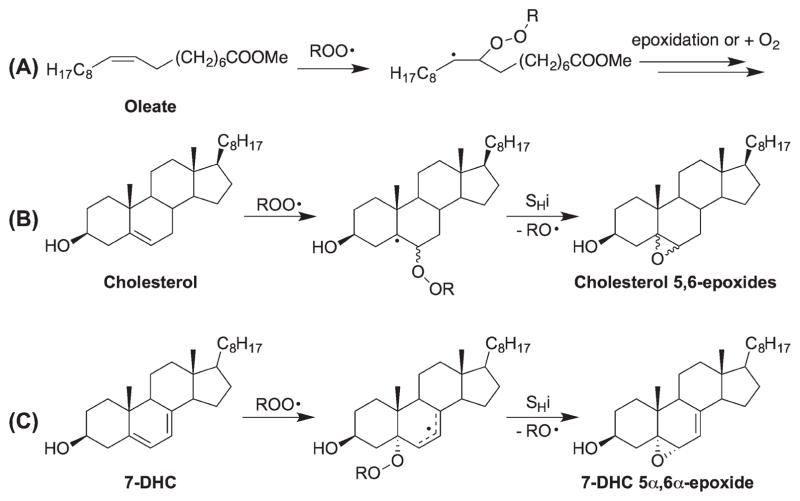
Addition of a peroxyl radical to a double bond, followed by an intramolecular homolytic substitution (SHi), generally gives an epoxide as the main product (addition of another oxygen molecule could compete with the epoxidation, particularly under high oxygen tension) [48]. Analogous to the carbon radical addition reaction [56] (although carbon radical is more nucleophilic), the reactivity of a double bond toward peroxyl radical addition largely depends on the stability of the resulting radical (β-effect) and the steric effect at the carbon center of the reaction (α-effect). As such, three general guidelines for understanding addition reactions can be derived: (a) a double bond more substituted at the center remote from the site of addition would be more reactive than a less substituted structure as the stability of the resulting radical would follow the order of tertiary > secondary primary (e.g., cholesterol > oleate); (b) a conjugated diene would be more reactive than a non-conjugated diene since a stabilized allylic radical would be formed from the former (e.g., 7-DHC > 8-DHC and linoleate) (Scheme 4) and; (c) if similar product radicals are formed, a peroxyl radical would preferentially add to the less hindered carbon center. Thus, it is reasonable to suggest that the unsaturated sterols would be more reactive toward peroxyl radical addition than their acyclic counterparts in fatty acids because the resulting radicals are generally more stable (with more substituents). In particular, the conjugated dienyl cholesterol precursors, such as 7-DHC and the 4,4-dimethylcholesta-8(9), 14-dien-3β-ol [8(9),14-dienol], would be prone to undergo addition reactions.
Scheme 4.
Peroxyl radical addition to sterols and fatty acids.
In the peroxyl radical addition reaction of 7-DHC, steric factors play a major role in the product selectivity, only the 5α,6α-epoxide was observed. Addition to the β-face of the sterol is relatively hindered by the axial methyl groups while the α axial H-9 and H-14 atoms effectively block the peroxyl addition at the α-face of C8. For the addition to C5, again, the top face is shielded by the axial methyl group (C19), leaving the α-face at C5 the default site of attack. These factors control the site and face of addition. In fact, an oxysterol derived from 5α,6α-epoxide of 7-DHC has been found to be a major peroxidation biomarker in cell and animal models of SLOS (vide infra) [19].
Based on the above-proposed principles governing the reactivities of sterols toward hydrogen atom transfer and peroxyl radical addition, other cholesterol precursors that might be prone to free radical peroxidation are the 8(9),14-dienol, zymostenol, and lathosterol (and their counterparts in the Bloch pathway with an additional C24 double bond). Addition at C15 of the 8(9),14-dienol would be kinetically favorable since that carbon is only monosubstituted while the allyl radical formed upon addition would be an endo radical that bears five alkyl substituents. For zymostenol and lathosterol, the allylic C–Hs at C14 (zymostenol) and at both C9 and C14 (lathosterol) are axially positioned and stable allyl radicals would be formed after hydrogen atom transfer (Figure 2).
Figure 2.
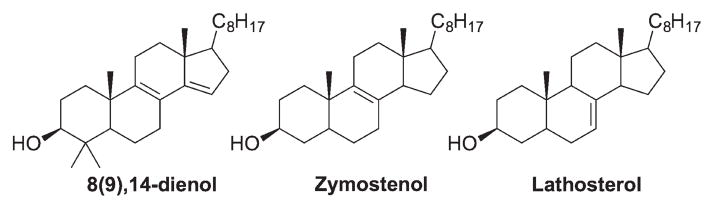
Other potentially reactive cholesterol biosynthesis precursors toward free radical oxidation.
Mechanisms of sterol oxidation and the effect of α-tocopherol on product distribution
Oxygen addition to radical intermediates
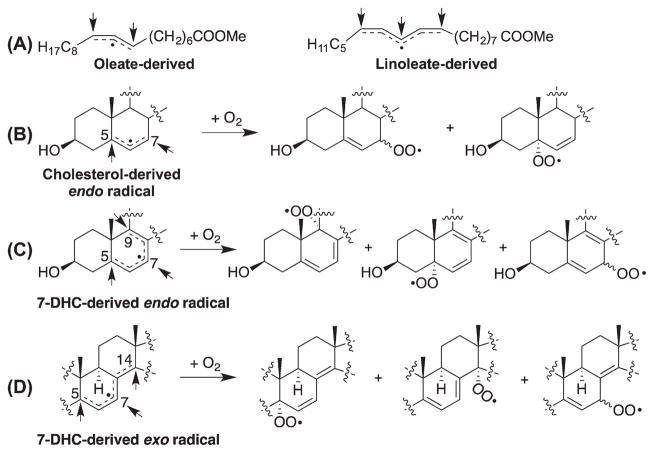
Allylic or pentadienyl radicals are formed from cholesterol or its precursors upon loss of a hydrogen atom (Schemes 3). Oxygen addition to similar radicals derived from oleate or linoleate has been well established [54,57,58], and the knowledge gained from those studies can be applied to understand the reactions of the sterol-derived radicals (Scheme 5). For the pentadienyl radicals derived from 7-DHC, 8-DHC, and the other sterol dienes shown in Table I, oxygen can potentially add to three positions, but the bis-allylic peroxyl radical resulting from the addition at the middle carbon will undergo fragmentation rapidly, giving back to the pentadienyl radical (i.e., β-fragmentation; kβ= 2.6 × 106 s−1 for the linoleate- derived bis-allylic peroxyl radical) [54]. Only in the presence of an excellent hydrogen atom donor that can compete with the β-fragmentation (e.g., α-tocopherol with a kH = 3.5 × 106 M−1s−1) [58–60] can products derived from the bis-allylic oxygen addition be observed. The same has been observed in the oxidation of 7-DHC (discussed below). For the allylic radical derived from oleate, the two potential addition sites lead to allylic products that differ only by the geometry of the double bond [57]. On the other hand, the major radical derived from cholesterol (endo radical formed from loss of H-7) [26] should give peroxyl radicals derived from oxygen addition at C5 and C7. But the C5 hydroperoxide products have not been observed from free radical reactions although they are formed from photooxidation. It seems likely that the rearrangement of the peroxyl radical from the C5 position to C7 is fast since the C5 is in a more stabilized position (with more substituents) [61]. One can speculate that a good hydrogen donor such as tert-butyl hydroperoxide should trap the kinetic products from cholesterol peroxidation, just as the kinetic products are trapped by this reagent in the peroxidation of oleate [57].
Scheme 5.
Addition of oxygen to radical intermediates formed during free radical oxidation.
Intramolecular homolytic substitution (SHi) and peroxyl radical cyclization
SHi typically occurs when a peroxyl radical adds to a double bond as illustrated in Scheme 4, leading to formation of epoxides [48]. This reaction is particularly common for sterols for the above-outlined reasons.
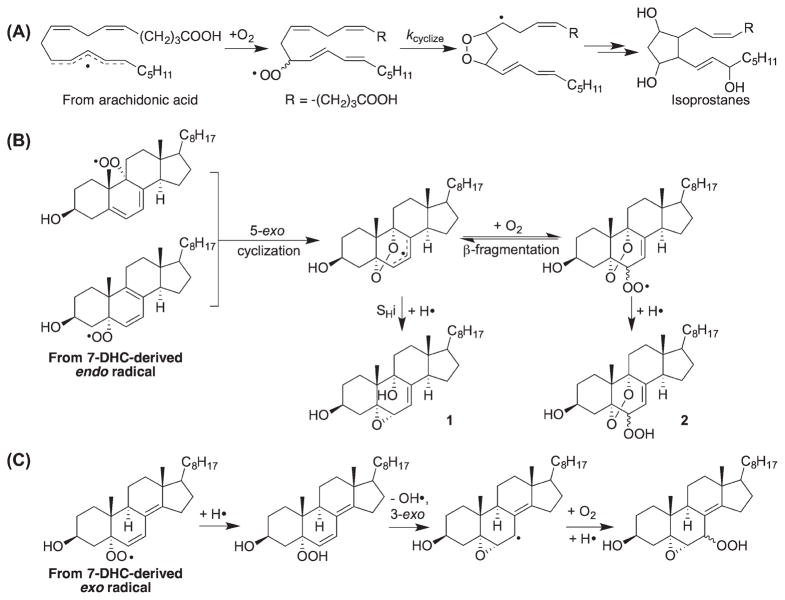
5-Exo peroxyl radical cyclization is an important transformation observed in the free radical oxidation of PUFAs with three or more double bonds [62 – 64]. These types of reactions account for the formation of numerous prostaglandin-like compounds, for example, isoprostanes or neuroprostanes, from the oxidation of arachidonic acid and docosahexaenoic acid, respectively [65–67]. In the oxidation of 7-DHC, two peroxyl radical intermediates formed are well positioned for a 5-exo cyclization (Scheme 6), giving the same cyclic peroxide product [17]. Subsequent SHi on the peroxide or addition of another oxygen leads to some of the major products found in the peroxidation of 7-DHC. The peroxyl radical derived from loss of H14 is not well positioned for 5-exo cyclization, but the hydroperoxide products can undergo homolytic peroxyl bond cleavage, 3-exo cyclization, and addition of another oxygen, to give the observed products [10].
Scheme 6.
Peroxyl radical cyclization and intramolecular homolytic substitution (SHi) reactions involved in peroxidation of fatty acids and sterols.
Effect of α-tocopherol on product distribution
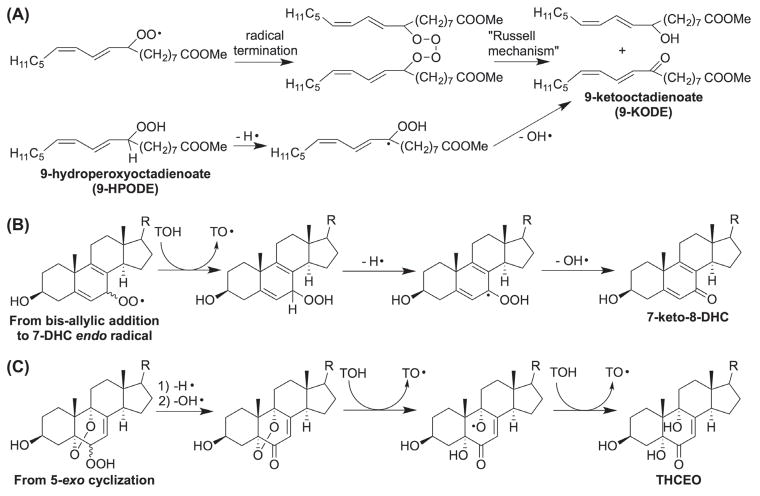
Peroxidation in the presence of α-tocopherol significantly changes the profile of product derived from the cholestadienols [16]. Thus, peroxyl radical addition to the double bond is suppressed when α-tocopherol is present because H-atom transfer from the antioxidant to propagating peroxyl radicals is faster than the addition of those radicals to the diene. Products derived from bis-allylic oxygen addition are found to be the major products because of the rapid trapping of the bis-allylic peroxyl radical by α-tocopherol (competing with β-fragmentation). It is also noteworthy that products containing the “enone” moiety are preferentially formed in dienol peroxidations carried out in the presence of α-tocopherol (Scheme 7).
Scheme 7.
Proposed mechanisms for the formation of enones in peroxidation of fatty acids and sterols. TOH = α-tocopherol.
Although ketone formation has been observed in radical termination reactions in the oxidation of fatty acids (Scheme 7A) [68–70], the formation of ketone products seems more common in sterol free radical oxidation [16]. We have suggested that the key step to the formation of sterol ketones is loss of the remaining bis-allylic or allylic hydrogen atom at the α-position of the hydroperoxide, followed by elimination of a hydroxyl radical. The bis-allylic addition to the 7-DHC-derived endo radical eventually leads to the formation of 7-oxo-5,8-dien-3β-ol (7-keto-8-DHC) (Scheme 7B). On the other hand, the endoperoxyenone (from addition at C5 or C9) formed in Scheme 7C was found to be unstable and was further reduced by α-tocopherol to give 3β,5α,9α-trihydroxycholest-7-en-6-one (THCEO), which can further dehydrate at C9 and C11 to give 3β,5α-dihydroxycholesta-7,9(11)-dien-6-one (DHCDO). Notably, all three enones discussed here have been identified in the brain of a mouse model for SLOS [23,24].
Tocopherol-mediated peroxidation (TMP) can occur under the conditions used in the above studies on 7-DHC, where the radical initiation rate is low and the concentration of α-tocopherol is high [16]. TMP has been suggested to play an important role in the oxidation of LDL [71,72]. Recently, large kinetic isotope effects (KIE; >20) have been observed during TMP of PUFAs and 7-DHC [73], suggesting that tunneling is involved in the step of hydrogen atom transfer to the chain-carrying tocopheryl radical. This would be particularly important for SLOS pathophysiology as the plasma of SLOS patients is enriched with the highly oxidizable 7-DHC.
Oxidation of sterols by other oxidants
Cholesterol reacts with singlet oxygen (1O2) via an “ene” type reaction, leading to 5α-hydroperoxy-6-en-3β-ol (5α-OOH-Chol) as the major product, and 6α- and 6β-hydroperoxy-4-en-3β-ol as the minor products (Scheme 8A) [25,74,75]. 7-DHC undergoes a similar “ene”-type reaction as well as a [4 + 2] cycloaddition, giving 7-hydroperoxy-5-,8-dien-3β-ol (7-OOH-8-DHC) and 5,8-endoperoxy-6-en-3β-ol [EnP(5,8)], respectively, in a ratio of 1:3 (Scheme 8B) [76,77]. Interestingly, the hydroperoxides formed from photooxidation are actually the kinetic products found in the free radical oxidation of cholesterol and 7-DHC. These hydroperoxides rearrange to the thermodynamic products [61] and/or initiate free radical processes upon thermal or transition metal-catalyzed decomposition. Therefore, caution has to be exerted to avoid photooxidation and subsequent transformations that may perturb the endogenous product profile of biological samples [20], where there may be an abundance of chromophores present that can act as photosensitizers.
Scheme 8.
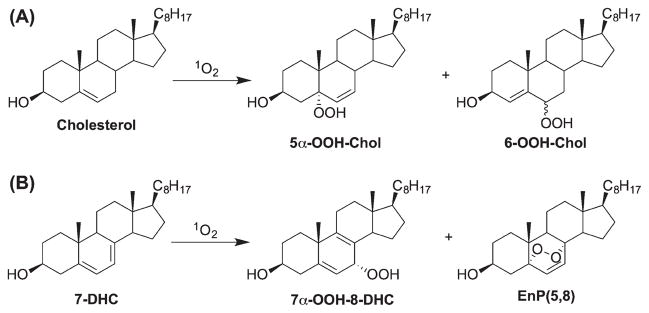
1O2 oxidation of cholesterol and 7-DHC.
Ozonolysis of cholesterol has been studied extensively as it has been closely linked to airway inflammation [78,79]. Major ozonolysis products of cholesterol are the reactive electrophiles 5,6-secosterol and its cyclized product via aldol condensation (Scheme 9) [78–82], which can form adducts with proteins, modulating protein structures and functions [83,84]. However, in lung surfactant, cholesterol 5β,6β-epoxide was found to be the major product, instead of the ring-opening products [78]. Notably, the photooxidation and free radical oxidation product, 5α-OOH-Chol, can also serve as a precursor to the 5,6-secosterol via acid-catalyzed Hock fragmentation [85] (Scheme 9).
Scheme 9.
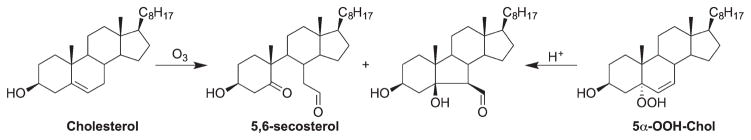
Ozonolysis of cholesterol and Hock fragmentation of 5α-OOH-Chol.
The additional double bond at C7 of 7-DHC also makes it an unusual substrate of cytochrome P450 (CYP) 7A1, leading to the formation of 7-ketocholesterol [86], an oxysterol that was normally formed from cholesterol oxidation at C7 (See Scheme 5B). Indeed, elevated levels of 7-ketocholesterol has been observed in tissues and/or fluids of the rat model of SLOS and human patients [20,87,88], suggesting that 7-DHC is the predominant precursor to this oxysterol in these samples since the level of cholesterol is low. Furthermore, 7-DHC was also found to be a good substrate of CYP 46A1, the nervous-system-specific enzyme, leading to the unusual 25-hydroxy-7-DHC, in addition to the expected 24-hydroxy-7-DHC [20,89].
Biological consequences of 7-DHC-derived oxysterols and their role in SLOS
SLOS displays a broad spectrum of phenotypes including multiple congenital malformations, neurological defects, mental retardation, autism-like behavior, and photosensitivity [34,90,91]. Even before the rate constants of 7-DHC and 8-DHC were determined [15,16], the high reactivity of 7-DHC and/or the oxidative stress in SLOS have been suggested in a number of studies. Porter and coworkers reported over 30 years ago that 7-DHC acted as an excellent hydrogen donor to linoleate peroxyl radicals in oxidations carried out in liposomes [92], which led to our recent measurement of the kH of this sterol [15]. In 1996, De Fabiani et al. reported the identification of cholesta-5,7,9(1l)-trien-3β-ol in plasma of SLOS patients, which was suggested to be formed from the decomposition of 7-OOH-8-DHC (see Scheme 8) [93]. However, these plasma samples were not processed under protected conditions (from oxygen and light), and it was recently demonstrated that 7-OOH-8-DHC and EnP(5,8) (also identified in that study) can be formed from ex vivo photooxidation of 7-DHC [20]. Nevertheless, this study did support the high reactivity of 7-DHC toward oxidation in a biological environment. In 2006, Fliesler and coworkers suggested that retinal degeneration in a rat model for SLOS was caused by elevated levels of lipid peroxides, likely cytotoxic oxysterols derived from 7-DHC, which was intensified by light [94,95]. They also found that supplementation of an antioxidant, dimethylthiourea, protected retina from light damage in this model [94]. In the same year, Kochevar and coworkers reported that 7-DHC enhanced ultraviolet A-induced oxidative stress in keratinocytes [96,97], also consistent with the high oxidizability of 7-DHC.
A decade before the free radical oxidation products of 7-DHC were fully elucidated, Gaoua et al. found that products generated from photooxidation of 7-DHC induced growth retardation of cultured rat embryos [98] and upon our recent identification of individual 7-DHC-derived oxysterols [17,19], systematic studies of their biological activities have been carried out. Korade et al. reported that these oxysterols exerted differential cytotoxicity to the Neuro2aneuroblastoma cells and primary neurons, with oxysterols possessing the endoperoxide moiety being the most toxic ones (e.g., compounds 2 shown in Scheme 6B) [18]. The cytotoxicity of the oxysterols is likely due to reduced cell proliferation, as suggested by the downregulation of proliferation-related genes, and induced differentiation, as indicated by the changes in cell morphology [18]. The oxysterols were also found to affect expression of gene transcripts related to lipid biosynthesis and cell growth [18], accelerate differentiation and arborization of neuronal cells [21], and induce retinal degeneration in the rat model of SLOS [87]. In the following sections, we will focus on the metabolic fate of the primary oxysterols formed from 7-DHC peroxidation: (a) metabolism of the primary oxysterols to more stable oxysterols in cells and (b) adduction of some electrophilic oxysterols with proteins.
Metabolism of primary peroxidation oxysterols derived from 7-DHC
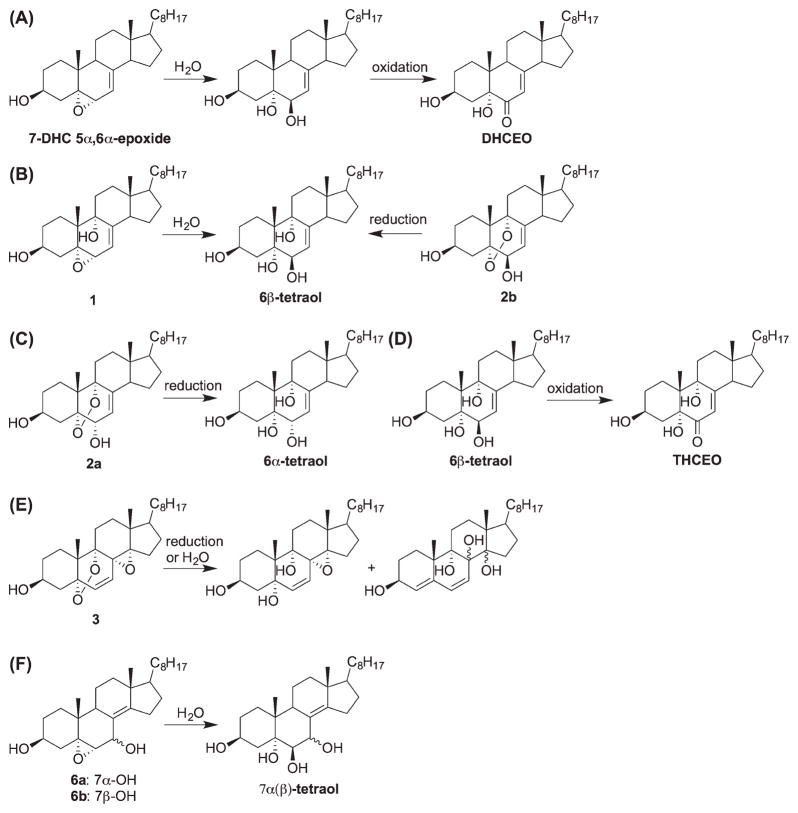
We define the oxysterols formed from free radical oxidation of 7-DHC in solution as the primary oxysterols since they are not metabolized in a biological environment [23]. Upon analysis of samples from cell and animal models for SLOS, the oxysterol profiles in the SLOS samples are distinctly different from the profile found in solution oxidations [19,20,22]. This prompted a study to investigate the metabolism of the primary oxysterols in cells [23]. Thus, Neuro2a and human fibroblast cells were exposed to the primary oxysterols and the metabolites were analyzed by high-performance liquid chromatography–mass spectrometry. The metabolites of the primary 7-DHC oxysterols were found to be identical to the major oxysterols observed in the SLOS cells and tissues. Typical metabolic transformations include reduction of peroxides to alcohols, ring opening of epoxides to give diols, and oxidation of allylic alcohols to ketones, leading to α,β-enone moieties (Scheme 10) [23]. The structures for the metabolites of the primary oxysterols other than 1, 2, and the 5α,6α-epoxide were proposed based on their masses and elution order on normal phase chromatographic separation. Note that some allylic alcohols such as 6α-tetraol and 7α(β)-tetraol were not oxidized to their corresponding ketones. Whether or not an enzyme is involved in the allylic oxidation remains to be elucidated. Among the metabolites, 3bita,5alpha-dihydroxycholesta-7,9(11)-en-6-one (DHCEO) and THCEO have been established as two major biomarkers for the peroxidation of 7-DHC in vivo [19,23].
Scheme 10.
Proposed mechanisms for the metabolism of the primary oxysterols of 7-DHC in cells.
As discussed earlier, THCEO can also be formed from free radical oxidation of 7-DHC when α-tocopherol is present, along with 7-keto-8-DHC and DHCDO[16]. However, the formation of the precursor of DHCEO, 7-DHC 5α,6α-epoxide, would be completely suppressed in the presence of α-tocopherol (vide supra) [16]. Therefore, the presence of both DHCEO and 7-keto-8-DHC in the brain of the SLOS mouse model suggests that both mechanisms (oxidation with or without α-tocopherol) operate in vivo, which collectively contribute to the endogenous oxysterol profile.
Adduction of electrophilic oxysterols with proteins
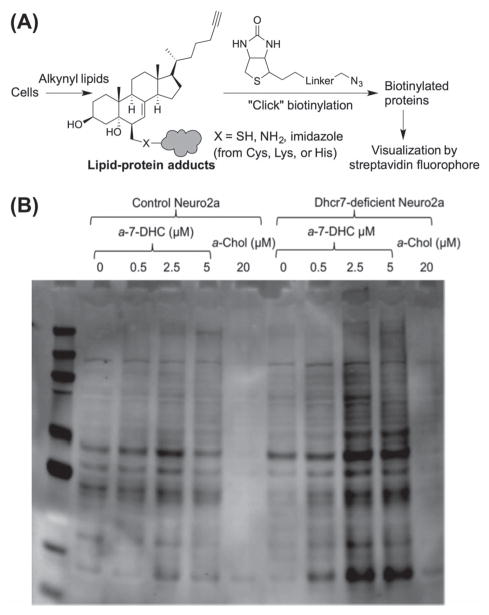
Lipid electrophiles, such as 4-hydroxynonenal, are formed during lipid peroxidation [48,99], and adduction of lipid electrophiles with proteins play important roles in cell signaling under physiological or pathological conditions [100]. Protein adducts from the ozonolysis products of cholesterol have been suggested to lead to protein misfolding [83,84]. Some of the oxysterols formed from 7-DHC are good electrophiles judging from their structures, such as the 5α,6α-epoxide and those oxysterols containing the α,β-enone moiety. When Dhcr7-deficient Neuro2a cells (which cannot effectively convert 7-DHC to cholesterol) were exposed to an alkynylated 7-DHC, a significant amount of protein adduction was observed after the adducted proteins were covalently linked to biotin by “click” chemistry and visualized by streptavidin fluorophore [44] (Figure 3). When the same experiments were carried out in control Neuro2a cells (with intact cholesterol biosynthesis machinery), significantly less adducts were formed. Importantly, exposure to 7-DHC 5α,6α-epoxide alone gave more adducts than was formed from the incubation with the same concentration of 7-DHC. It is notable that adduction by 7-DHC and its epoxide was found to be more extensive than adduction from PUFAs in the same cells and under the same conditions [44].
Figure 3.
(A) Illustration of the strategy for detecting endogenously formed lipid–protein adducts using alkynylated lipids. (B) Adapted from Figure 7 in ref [44]: comparison of protein adducts of metabolites of 25-alkynyl-7-DHC (a-7-DHC) in control Neuro2a versus in Dhcr7-deficient Neuro2a cells (a-Chol = alkynyl cholesterol). This research was originally published in the Journal of Lipid Research (ref [44]). © the American Society for Biochemistry and Molecular Biology.
Conclusions and perspective
Here we proposed that the more rigid and more substituted sterol structure makes cholesterol and its precursors more reactive toward free radical oxidation than the acyclic structures found in fatty acids. Sterols tend to be more reactive than PUFAs containing the same number of double bonds and this reactivity is found for either hydrogen atom transfer or peroxyl radical addition mechanisms. Thus, even a small perturbation on the levels of the reactive cholesterol precursors could result in a significant increase in oxidative stress and a shift of the oxidation product profile (see Table II for an illustration using the levels of different lipids found in human plasma [29,36,101,102]). The free radical oxidation of sterols follows mechanisms that are well established in the oxidation of PUFAs, but there are some unique features such as unusually fast 5-exo cyclizations, SHi on a cyclic peroxide structure, and enone formation in the presence of α-tocopherol that is not common in the PUFA systems. The most reactive sterol found, 7-DHC, was closely associated with the pathophysiology of the human disease SLOS. A number of 7-DHC oxysterols identified in vivo were found to originate from free radical oxidation although enzymatic oxidation also contributes to the oxysterol profile. Continued investigation of the biological activities of the 7-DHC-derived oxysterols may ultimately lead to new therapies that may counter the effect of these oxysterols. Based on our understanding on the reactivities of sterols toward free radical oxidation, a number of other cholesterol precursors, such as the 8(9),14-dienol, lathosterol, and zymostenol, may also serve as good free radical peroxidation substrates, which suggest that oxidative stress may be associated with other cholesterol biosynthesis disorders.
Table II.
Comparison of reactivity of unsaturated lipids toward free radical oxidation in human plasma.a.
Acknowledgments
We thank collaborators and colleagues for their contribution and insights on related projects, including Drs. Zeljka Korade (Psychiatry, Vanderbilt), Karoly Mirnics (Psychiatry, Vanderbilt), Steven J. Fliesler (ophthalmology, SUNY-Buffalo), Wei Liu, Katherine Windsor, and Thiago C. Genaro-Mattos.
Footnotes
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by grants from the National Institute of Health (K99HD073270 (L.X.) and R01HD064727 (N.A.P.)) and National Science Foundation (CHE-1057500 (N.A.P.)). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Shrivastava S, Paila YD, Dutta A, Chattopadhyay A. Differential effects of cholesterol and its immediate biosynthetic precursors on membrane organization. Biochemistry. 2008;47:5668–5677. doi: 10.1021/bi8001677. [DOI] [PubMed] [Google Scholar]
- 2.Rog T, Vattulainen I, Jansen M, Ikonen E, Karttunen M. Comparison of cholesterol and its direct precursors along the biosynthetic pathway: effects of cholesterol, desmosterol and 7-dehydrocholesterol on saturated and unsaturated lipid bilayers. J Chem Phys. 2008;129:154508. doi: 10.1063/1.2996296. [DOI] [PubMed] [Google Scholar]
- 3.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–1273. doi: 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 6.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 7.Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem. 1995;64:895–901. doi: 10.1046/j.1471-4159.1995.64020895.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 9.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez IR, Fliesler SJ. Photodamage generates 7-keto- and 7-hydroxycholesterol in the rat retina via a free radical-mediated mechanism. Photochem Photobiol. 2009;85:1116–1125. doi: 10.1111/j.1751-1097.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez IR, Larrayoz IM. Cholesterol oxidation in the retina: Implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J Lipid Res. 2010;51:2847–2862. doi: 10.1194/jlr.R004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girao H, Mota MC, Ramalho J, Pereira P. Cholesterol oxides accumulate in human cataracts. Exp Eye Res. 1998;66:645–652. doi: 10.1006/exer.1998.0465. [DOI] [PubMed] [Google Scholar]
- 13.Vejux A, Samadi M, Lizard G. Contribution of cholesterol and oxysterols in the physiopathology of cataract: Implication for the development of pharmacological treatments. J Ophthalmol. 2011;2011:471947. doi: 10.1155/2011/471947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter FD, Scherrer DE, Lanier MH, Langmade SJ, Molugu V, Gale SE, et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med. 2010;2:56ra81. doi: 10.1126/scitranslmed.3001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Davis TA, Porter NA. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J Am Chem Soc. 2009;131:13037–13044. doi: 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Porter NA. Reactivities and products of free radical oxidation of cholestadienols. J Am Chem Soc. 2014;136:5443–5450. doi: 10.1021/ja5011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Korade Z, Porter NA. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: Product and mechanistic studies. J Am Chem Soc. 2010;132:2222–2232. doi: 10.1021/ja9080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korade Z, Xu L, Shelton R, Porter NA. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J Lipid Res. 2010;51:3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Korade Z, Rosado DA, Liu W, Lamberson CR, Porter NA. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011;52:1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Liu W, Sheflin LG, Fliesler SJ, Porter NA. Novel oxysterols observed in tissues and fluids of AY9944-treated rats – a model for Smith-Lemli-Opitz Syndrome. J Lipid Res. 2011;52:1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Mirnics K, Bowman AB, Liu W, Da J, Porter NA, Korade Z. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol Dis. 2012;45:923–929. doi: 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korade Z, Xu L, Mirnics K, Porter NA. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2013;36:113–122. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Korade Z, Rosado DA, Jr, Mirnics K, Porter NA. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J Lipid Res. 2013;54:1135–1143. doi: 10.1194/jlr.M035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korade Z, Xu L, Harrison FE, Ahsen R, Hart SE, Folkes OM, et al. Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol Psychiatry. 2014;75:215–222. doi: 10.1016/j.biopsych.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LL. Cholesterol autoxidation 1981–1986. Chem Phys Lipids. 1987;44:87–125. doi: 10.1016/0009-3084(87)90046-6. [DOI] [PubMed] [Google Scholar]
- 26.Sevilla C, Becker D, Sevilla M. An electron spin resonance investigation of radical intermediates in cholesterol and related compounds: relation to solid-state autoxidation. J Phys Chem. 1986;90:2963–2968. [Google Scholar]
- 27.Chisolm GM, Ma G, Irwin KC, Martin LL, Gunderson KG, Linberg LF, et al. 7 beta-hydroperoxycholest-5-en-3 beta-ol, a component of human atherosclerotic lesions, is the primary cytotoxin of oxidized human low density lipoprotein. Proc Natl Acad Sci U S A. 1994;91:11452–11456. doi: 10.1073/pnas.91.24.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown AJ, Leong SL, Dean RT, Jessup W. 7-Hydroperoxy-cholesterol and its products in oxidized low density lipoprotein and human atherosclerotic plaque. J Lipid Res. 1997;38:1730–1745. [PubMed] [Google Scholar]
- 29.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith LL, Johnson BH. Biological activities of oxysterols. Free Radic Biol Med. 1989;7:285–332. doi: 10.1016/0891-5849(89)90136-6. [DOI] [PubMed] [Google Scholar]
- 31.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 32.Javitt NB. Oxysteroids: a new class of steroids with autocrine and paracrine functions. Trends Endocrinol Metab. 2004;15:393–397. doi: 10.1016/j.tem.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Javitt NB. Oxysterols: novel biologic roles for the 21st century. Steroids. 2008;73:149–157. doi: 10.1016/j.steroids.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 36.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 37.Tint GS, Seller M, Hughes-Benzie R, Batta AK, Shefer S, Genest D, et al. Markedly increased tissue concentrations of 7-dehydrocholesterol combined with low levels of cholesterol are characteristic of the Smith-Lemli-Opitz syndrome. J Lipid Res. 1995;36:89–95. [PubMed] [Google Scholar]
- 38.Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krakowiak PA, Nwokoro NA, Wassif CA, Battaile KP, Nowaczyk MJ, Connor WE, et al. Mutation analysis and description of sixteen RSH/Smith-Lemli-Opitz syndrome patients: polymerase chain reaction-based assays to simplify genotyping. Am J Med Genet. 2000;94:214–227. [PubMed] [Google Scholar]
- 40.Honda A, Tint GS, Salen G, Batta AK, Chen TS, Shefer S. Defective conversion of 7-dehydrocholesterol to cholesterol in cultured skin fibroblasts from Smith-Lemli-Opitz syndrome homozygotes. J Lipid Res. 1995;36:1595–1601. [PubMed] [Google Scholar]
- 41.Kandutsch AA, Russell AE. Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol. J Biol Chem. 1960;235:2256–2261. [PubMed] [Google Scholar]
- 42.Bloch K. Sterol molecule: structure, biosynthesis, and function. Steroids. 1992;57:378–383. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- 43.Holick MF, Frommer JE, McNeill SC, Richtand NM, Henley JW, Potts JT., Jr Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun. 1977;76:107–114. doi: 10.1016/0006-291x(77)91674-6. [DOI] [PubMed] [Google Scholar]
- 44.Windsor K, Genaro-Mattos TC, Kim HY, Liu W, Tallman KA, Miyamoto S, et al. Probing lipid-protein adduction with alkynyl surrogates: application to Smith-Lemli-Opitz syndrome. J Lipid Res. 2013;54:2842–2850. doi: 10.1194/jlr.M041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjorkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol. 2002;22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 46.Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009;30:153–170. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Brown AJ, Jessup W. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 49.Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem Phys Lipids. 2011;164:457–468. doi: 10.1016/j.chemphyslip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Howard JA, Ingold JA. Absolute rate constants for hydrocarbon autoxidation.6. Alkyl aromatic and olefinic hydrocarbons. Can J Chem. 1967;45:793–802. [Google Scholar]
- 51.Trenwith AB. Dissociation of 3-methylpenta-1, 4-diene and the resonance energy of the pentadienyl radical. J Chem Soc Faraday Trans I. 1982;78:3131–3136. [Google Scholar]
- 52.Berkowitz J, Ellison GB, Gutman D. Three methods to measure RH bond energies. J Phys Chem. 1994;98:2744–2765. [Google Scholar]
- 53.Pratt DA, Mills JH, Porter NA. Theoretical calculations of carbon-oxygen bond dissociation enthalpies of peroxyl radicals formed in the autoxidation of lipids. J Am Chem Soc. 2003;125:5801–5810. doi: 10.1021/ja034182j. [DOI] [PubMed] [Google Scholar]
- 54.Roschek B, Jr, Tallman KA, Rector CL, Gillmore JG, Pratt DA, Punta C, Porter NA. Peroxyl radical clocks. J Org Chem. 2006;71:3527–3532. doi: 10.1021/jo0601462. [DOI] [PubMed] [Google Scholar]
- 55.Carreira LA. Determination of the torsional potential function of 1,3-butadiene. J Chem Phys. 1975;62:3851–3854. [Google Scholar]
- 56.Giese B. Formation of CC bonds by addition of free radicals to alkenes. Angew Chem Int Ed. 1983;22:753–764. [Google Scholar]
- 57.Porter NA, Mills KA, Carter RL. A mechanistic study of oleate autoxidation: competing peroxyl H-atom abstraction and rearrangement. J Am Chem Soc. 1994;116:6690–6696. [Google Scholar]
- 58.Tallman KA, Pratt DA, Porter NA. Kinetic products of linoleate peroxidation: Rapid beta-fragmentation of nonconjugated peroxyls. J Am Chem Soc. 2001;123:11827–11828. doi: 10.1021/ja0169724. [DOI] [PubMed] [Google Scholar]
- 59.Tallman KA, Roschek B, Jr, Porter NA. Factors influencing the autoxidation of fatty acids: effect of olefin geometry of the nonconjugated diene. J Am Chem Soc. 2004;126:9240–9247. doi: 10.1021/ja049104q. [DOI] [PubMed] [Google Scholar]
- 60.Brash AR. Autoxidation of methyl linoleate: identification of the bis-allylic 11-hydroperoxide. Lipids. 2000;35:947–952. doi: 10.1007/s11745-000-0604-0. [DOI] [PubMed] [Google Scholar]
- 61.Beckwith ALJ, Davies AG, Davison IGE, Maccoll A, Mruzek MH. The mechanisms of the rearrangements of allylic hydroperoxides: 5-alpha-hydroperoxy-3-beta-hydroxycholest-6-ene and 7-alpha-hydroperoxy-3-beta-hydroxycholest-5-ene. J Chem Soc Perkin Trans 2. 1989;7:815–824. [Google Scholar]
- 62.Porter NA, Funk M. Peroxy radical cyclization as a model for prostaglandin biosynthesis. J Org Chem. 1975;40:3614–3615. doi: 10.1021/jo00912a037. [DOI] [PubMed] [Google Scholar]
- 63.Porter NA, Funk MO, Gilmore DW, Isaac R, Nixon J. The formation of cyclic peroxides from unsaturated hydroperoxides: Models for prostaglandin biosynthesis. J Am Chem Soc. 1976;98:6000–6005. doi: 10.1021/ja00435a037. [DOI] [PubMed] [Google Scholar]
- 64.Porter NA, Lehman LS, Weber BA, Smith KJ. Unified mechanism for polyunsaturated fatty acid autoxidation. Competition of peroxy radical hydrogen atom abstraction,. beta. -scission, and cyclization. J Am Chem Soc. 1981;103:6447–6455. [Google Scholar]
- 65.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., II Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ. Isoprostane generation and function. Chem Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts LJ, II, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, et al. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 68.Russell GA. Deuterium-isotope effects in the autoxidation of aralkyl hydrocarbons. mechanism of the interaction of PEroxy radicals. J Am Chem Soc. 1957;79:3871–3877. [Google Scholar]
- 69.Howard J, Ingold K. Self-reaction of sec-butylperoxy radicals. Confirmation of the Russell mechanism. J Am Chem Soc. 1968;90:1056–1058. [Google Scholar]
- 70.Dix TA, Marnett LJ. Conversion of linoleic acid hydroperoxide to hydroxy, keto, epoxyhydroxy, and trihydroxy fatty acids by hematin. J Biol Chem. 1985;260:5351–5357. [PubMed] [Google Scholar]
- 71.Bowry VW, Stocker R. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc. 1993;115:6029–6044. [Google Scholar]
- 72.Ingold KU, Bowry VW, Stocker R, Walling C. Autoxidation of lipids and antioxidation by alpha-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc Natl Acad Sci U S A. 1993;90:45–49. doi: 10.1073/pnas.90.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamberson CR, Xu L, Muchalski H, Montenegro-Burke JR, Shmanai VV, Bekish AV, et al. Unusual kinetic isotope effects of deuterium reinforced polyunsaturated fatty acids in tocopherol-mediated free radical chain oxidations. J Am Chem Soc. 2014;136:838–841. doi: 10.1021/ja410569g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulig MJ, Smith LL. Sterol metabolism. XXV. Cholesterol oxidation by singlet molecular oxygen. J Org Chem. 1973;38:3639–3642. doi: 10.1021/jo00960a050. [DOI] [PubMed] [Google Scholar]
- 75.Girotti AW. Photosensitized oxidation of cholesterol in biological systems: reaction pathways, cytotoxic effects and defense mechanisms. J Photochem Photobiol B. 1992;13:105–118. doi: 10.1016/1011-1344(92)85050-5. [DOI] [PubMed] [Google Scholar]
- 76.Albro PW, Corbett JT, Schroeder JL. Doubly allylic hydroperoxide formed in the reaction between sterol 5,7-dienes and singlet oxygen. Photochem Photobiol. 1994;60:310–315. doi: 10.1111/j.1751-1097.1994.tb05109.x. [DOI] [PubMed] [Google Scholar]
- 77.Albro PW, Bilski P, Corbett JT, Schroeder JL, Chignell CF. Photochemical reactions and phototoxicity of sterols: Novel self-perpetuating mechanism for lipid photooxidation. Photochem Photobiol. 1997;66:316–325. doi: 10.1111/j.1751-1097.1997.tb03154.x. [DOI] [PubMed] [Google Scholar]
- 78.Pulfer MK, Murphy RC. Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant. J Biol Chem. 2004;279:26331–26338. doi: 10.1074/jbc.M403581200. [DOI] [PubMed] [Google Scholar]
- 79.Pulfer MK, Taube C, Gelfand E, Murphy RC. Ozone exposure in vivo and formation of biologically active oxysterols in the lung. J Pharmacol Exp Ther. 2005;312:256–264. doi: 10.1124/jpet.104.073437. [DOI] [PubMed] [Google Scholar]
- 80.Gumulka J, Smith LL. Ozonation of cholesterol. J Am Chem Soc. 1983;105:1972–1979. [Google Scholar]
- 81.Jaworski K, Smith LL. Ozonization of cholesterol in nonparticipating solvents. J Org Chem. 1988;53:545–554. [Google Scholar]
- 82.Wang K, Bermudez E, Pryor WA. The ozonation of cholesterol: separation and identification of 2,4-dinitrophenylhydrazine derivatization products of 3 beta-hydroxy-5-oxo-5,6-secoc-holestan-6-al. Steroids. 1993;58:225–229. doi: 10.1016/0039-128x(93)90023-g. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Q, Powers ET, Nieva J, Huff ME, Dendle MA, Bieschke J, et al. Metabolite-initiated protein misfolding may trigger Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:4752–4757. doi: 10.1073/pnas.0400924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nieva J, Song BD, Rogel JK, Kujawara D, Altobel L, III, Izharrudin A, et al. Cholesterol secosterol aldehydes induce amyloidogenesis and dysfunction of wild-type tumor protein p53. Chem Biol. 2011;18:920–927. doi: 10.1016/j.chembiol.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brinkhorst J, Nara SJ, Pratt DA. Hock cleavage of cholesterol 5alpha-hydroperoxide: An ozone-free pathway to the cholesterol ozonolysis products identified in arterial plaque and brain tissue. J Am Chem Soc. 2008;130:12224–12225. doi: 10.1021/ja804162d. [DOI] [PubMed] [Google Scholar]
- 86.Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu L, Sheflin LG, Porter NA, Fliesler SJ. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim Biophys Acta. 2012;1821:877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W, Xu L, Lamberson CR, Merkens LS, Steiner RD, Elias ER, et al. Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients. J Lipid Res. 2013;54:244–253. doi: 10.1194/jlr.M031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goyal S, Xiao Y, Porter NA, Xu L, Guengerich FP. Oxidation of 7-Dehydrocholesterol and Desmosterol by Human Cytochrome P450 46A1. J Lipid Res. 2014;55:1933–1943. doi: 10.1194/jlr.M051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charman CR, Ryan A, Tyrrell RM, Pearse AD, Arlett CF, Kurwa HA, et al. Photosensitivity associated with the Smith-Lemli-Opitz syndrome. Br J Dermatol. 1998;138:885–888. doi: 10.1046/j.1365-2133.1998.02231.x. [DOI] [PubMed] [Google Scholar]
- 91.Sikora D, Pettit-Kekel K, Penfield J, Merkens L, Steiner R. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet. 2006;140A:1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- 92.Weenen H, Porter NA. Autoxidation of model membrane systems: Cooxidation of polyunsaturated lecithins with steroids, fatty acids, and alpha-tocopherol. J Am Chem Soc. 1982;104:5216–5221. [Google Scholar]
- 93.De Fabiani E, Caruso D, Cavaleri M, Galli Kienle M, Galli G. Cholesta-5,7,9(11)-trien-3 beta-ol found in plasma of patients with Smith-Lemli-Opitz syndrome indicates formation of sterol hydroperoxide. J Lipid Res. 1996;37:2280–2287. [PubMed] [Google Scholar]
- 94.Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:496–504. doi: 10.1016/j.exer.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richards MJ, Nagel BA, Fliesler SJ. Lipid hydroperoxide formation in the retina: correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:538–541. doi: 10.1016/j.exer.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valencia A, Kochevar IE. Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith-Lemli-Opitz syndrome. Free Radic Biol Med. 2006;40:641–650. doi: 10.1016/j.freeradbiomed.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 97.Valencia A, Rajadurai A, Carle AB, Kochevar IE. 7-Dehydrocholesterol enhances ultraviolet A-induced oxidative stress in keratinocytes: Roles of NADPH oxidase, mitochondria, and lipid rafts. Free Radic Biol Med. 2006;41:1704–1718. doi: 10.1016/j.freeradbiomed.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaoua W, Chevy F, Roux C, Wolf C. Oxidized derivatives of 7-dehydrocholesterol induce growth retardation in cultured rat embryos: a model for antenatal growth retardation in the Smith-Lemli-Opitz syndrome. J Lipid Res. 1999;40:456–463. [PubMed] [Google Scholar]
- 99.Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of non-enzymatic transformation of the 9- and 13-hy-droperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 100.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelley RI. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta. 1995;236:45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]
- 102.Haas D, Garbade SF, Vohwinkel C, Muschol N, Trefz FK, Penzien JM, et al. Effects of cholesterol and simvastatin treatment in patients with Smith-Lemli-Opitz syndrome (SLOS) J Inherit Metab Dis. 2007;30:375–387. doi: 10.1007/s10545-007-0537-7. [DOI] [PubMed] [Google Scholar]