Abstract
Objective
To elucidate the relationship between the triggering receptor expressed on myeloid cells 2 (TREM2) risk variant, neuropathological lesions, alterations in gene and protein expression and severity of neuroinflammation.
Methods
Genetic association study of the R47H TREM2 variant with Alzheimer’s disease, neuropathology and changes in TREM2 and TYRO protein tyrosine kinase-binding protein (TYROBP) gene and protein expression and neuroinflammatory markers.
Results
The TREM2 variant is associated with: (i) Alzheimer’s disease (odds ratio: 4.76; P = 0.014); (ii) increased density of amyloid plaques and neurofibrillary tangles in multiple brain regions; (iii) increased TREM2 (P = 0.041) and TYROBP (P = 0.006) gene expression; (iv) decreased TREM2 protein levels (P = 0.016); and (v) upregulation of proinflammatory cytokines (RANTES and IFN-gamma) (P = 0.003) and nominal downregulation of protective markers (α2 macroglobulin, IL-4 and ApoA1) (P = 0.018).
Conclusions
These findings link the TREM2 missense mutation with specific molecular abnormalities and increases in neuropathological lesions in the human brain.
1. Introduction
Over the last year, a series of independent studies have reported a strong association of the triggering receptor expressed on myeloid cells 2 (TREM2) gene with Alzheimer’s disease (AD) [1–7]. For one particular nonsynonymous variant, rs75932628 (encoding R47H), the association reached genome-wide significance [1,2]. TREM2 does not possess a signaling motif, but it forms a receptor-signaling complex with TYRO protein tyrosine kinase-binding protein (TYROBP) and thereby regulates the activation of dendritic cells, macrophages, osteoclasts and microglial cells. An independent study that examined gene-regulatory networks identified TYROBP as a key regulator for AD [8].
Additional evidence for a functional role of the R47H comes from a brain imaging volumetric study, where carriers of a risk allele in close proxy with R47H, lost brain volume at a rate that was significantly faster than noncarriers [9]. Carriers of the R47H variant between the ages of 80 and 100 years without AD had poorer cognitive function than noncarriers [2]. In AD animal models, such as TgCRND8 [1] and aged APP23 [10] mice, TREM2 dysregulation is noted in amyloid plaque-associated microglia. TREM2-TYROBP signaling in microglia has been shown to be critical to the clearance of debris of central nervous system lesions [11]. Therefore, based on findings from animal studies, it has been suggested that TREM2-TYROBP functions to resolve inflammation-induced neuronal damage [12,13] to activate phagocytosis of damaged cells [14], and is likely to be associated with reduced severity of dementia. To our knowledge, no previous study has examined the effect of R47H mutation on AD-associated lesion severity and TREM2-TYROBP signaling in normal and pathological human brain tissue.
We have examined the relationship between the R47H TREM2 variant with AD in a large postmortem cohort of neuropathologically confirmed cases with AD and controls, alterations in gene and protein expression and severity of neuroinflammation and neuropathological lesions. We hypothesized that the R47H TREM2 variant will be significantly associated with AD, more severe AD-associated neuropathology (amyloid plaques and neurofibrillary tangles) and alterations in TREM2 and TYROBP gene and protein expression levels, as well as, markers of inflammatory response.
2. Methods
2.1. Brain tissue samples
Brain tissue specimens were derived from the Icahn School of Medicine at Mount Sinai and the Alzheimer’s Disease Research Center Brain Bank. The precise tissue handling procedures have been described in detail previously [15–18]. All antemortem neuropsychological, diagnostic and autopsy protocols were approved by the Icahn School of Medicine at Mount Sinai and other relevant Institutional Review Boards. Each sample has been extensively characterized, based on clinical and neuropathological criteria in diagnostically relevant [Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) –defined] brain regions [19], including the: (i) clinical dementia rating (CDR) [20,21]; (ii) neuritic plaques (NP) density; and (iii) distribution of neurofibrillary tangle (NFT) pathology using Braak neuropathology staging [22]. CERAD criteria were used to group cases into normal (CERAD-1), definite AD (CERAD-2), probable AD (CERAD-3) or possible AD (CERAD-4). Non-AD-associated neuropathologic features of each brain, such as cerebrovascular disease, were also accessed by CERAD criteria and protocols. For more details see Methods in the Supplement.
2.2. Molecular analysis
DNA extraction and genotyping
Samples of DNA from the postmortem cohort were extracted from the superior temporal gyrus using the Genomic DNA-Tissue MiniPrep kit (Zymo Research, Irvine, California). TREM2 rs75932628 genotyping was performed blind to phenotype measures with a competitive allele-specific PCR system (LGC Genomics, Beverly, MA).
RNA extraction and real-time qPCR
For these analyses, 16 AD carriers of the rs75932628-T allele (ADcarriers) were matched for age, sex, ethnicity, CDR (for AD cases only) and PMI with non-carrier AD cases (ADnoncarriers) and controls (Supplement Table 1). Total RNA was extracted from 50 mg of frozen tissue prepared from the superior temporal gyrus (STG), as described in detail elsewhere [23–25]. Obtained cDNA was pre-amplified (10 cycles) using TaqMan PreAmp Master Mix (Life Technologies, Carlsbad, CA) with the set of pooled Taqman assays used in the analysis under the standard condition (Supplement Table 2). The STG was selected as the region of interest as: (i) it demonstrated the most significant increase in NP in TREM2 ADcarriers compared to ADnoncarriers (see below); (ii) it shows profound transcriptional vulnerability in gene expression microarray studies in AD [15]; and (iii) there is abundant expression of TREM2 [26].
Western Blotting
Protein abundance was measured in the STG from ADcarriers, ADnoncarriers and controls (n=9/group; total n=27) using Western blotting. ADnoncarriers and controls were matched for age, gender, ethnicity, CDR (for AD cases only) and PMI with ADcarriers. Blots were incubated with antibodies: mouse anti-human TREM2 (clone 2B5; 1:1000 v/v dilution, Novus Biologicals; Littleton, CO), rabbit anti-human TYROBP (TYROBP; 1:500 v/v dilution; Aviva System Biology, San Diego, CA). The specificity and equal affinity for wt and mutant TREM2 immunostaining was validated and confirmed with another TREM2 antibody (R&D Biosystems; Minneapolis, MN) that shows equal recognition of mutant TREM2 [27] (Supplement Figure 1). Either rabbit anti-human TUBB (1:20000 v/v dilution) or mouse anti-human TUBB (1:5000 v/v dilution) both from Novus Biologicals (Littleton, CO) were used for normalization purpose in multiplex blotting.
Luminex Milliplex MAP immunoassays
Two protein panels of inflammatory markers based on Luminex xMAP technology were used (Millipore, Billerica, MA) for protein quantification in the STG. The first panel (Milliplex MAP human 21 plex cytokine/chemokine panel) includes eotaxin, G-CSF, GM-CSF, IFN-alpha2, IFN-gamma, IL-12(p40), IL-12(p70) IL-15, IL-1RA, IL-1beta IL-4, IL-6, IL-7, IL-8, IP-10(CXCL10), MCP-1, MIP-1beta, RANTES, TNFalpha, TNFbeta and VEGF. The second panel (human neurodegenerative panel 1) includes alpha 2 macroglobulin, ApoA1, ApoCIII, ApoE, complement Factor H, complement C3 and prealbumin. IL1 beta, IL-12(p70), TNFalpha and TNFbeta were excluded due to below threshold detection signal. For more details in the molecular analysis methods see Methods in the Supplement.
2.3. Statistics
Demographics data were compared among groups using Kruskal Wallis (continuous, non-parametric variables), ANOVA (continuous, parametric variables) or chi-square (categorical variables). Parameters for factor analysis using principal components extraction included Eigenvalues > 1 and factor loadings > 0.5. Pearson correlations were performed to examine the relation of potential confounds (age, gender, postmortem interval (PMI), RNA integrity number (RIN) and batch) with the outcome variables (derived from the qPCR, WB or Luminex assays). Parametric or non-parametric analysis of covariance was used for comparison of the variables of interest among groups. Logistic regression was used to compare the distribution of allelic and genotype frequency in case–control series. All statistics were 2-tailed, and significance was set at p < 0.05. For multiple testing corrections we applied false discovery rate [28] (FDR) at < 0.05. Statistics and graphics were performed using the R package (Version 3.0.2). Genetic analysis was performed with Plink (Version 1.07) [29].
3. Results
3.1. Association of the rs75932628-T allele with AD and CDR
The T allele of the rs75932628 conferred an increased risk of AD using cases with a diagnosis of definite AD by CERAD criteria [19] (N=265) and controls (N=225) (odds ratio [OR]: 4.76; 95% confidence interval [CI]: 1.37–16.54; P=0.014) (Table 1). Overall, 16 of 265 cases with AD (all with definitive AD) and 3 of 225 controls were carriers for the rs75932628-T allele (Supplemental Table 3). The association of the rs75932628-T allele with AD was further examined using controls defined by both clinical (clinical dementia rating [20,21] (CDR)=0; no cognitive impairment) and neuropathological criteria. A higher, nearly double, odds ratio was observed in cases with definitive AD (N=265) compared to controls (N=139); (OR: 8.87; 95%CI: 1.16–67.58; P=0.035), by applying more strict diagnostic criteria. Interestingly, of the 3 neuropathologically unaffected controls carrying the rs75932628-T allele, one had questionable cognitive impairment (CDR=0.5) and one presented with severe dementia (CDR=3) (Supplemental Table 3). The third case was not demented at the time of death. In the more strictly defined cohort (N=404 controls and AD cases), a higher frequency of the rs75932628-T was observed (minor allele frequency (MAF) = 2.10%). Rs75932628-T was found in 6.04% (16/265; MAF = 3.02%) of cases with definitive AD and in 0.72% of the controls with CDR=0 (1/139; MAF = 0.36%). The association became notably weaker when all cases (N=411; including CERAD defined possible, probable and definite AD) and all controls (N=225), including those with questionable and severe dementia, were included (OR: 3.00; 95%CI: 0.86–10.40; P=0.084). Carriers of the rs75932628-T allele had higher CDR, which was not significant (P = 0.171).
Table 1.
Association between the rs75932628-T Variant and Alzheimer ’s disease in Comparison with the Control Group.
| Cases | Controls | OR(95% CI) | P | |||||
|---|---|---|---|---|---|---|---|---|
| No. of T Alleles | No. of Cases | MAF(%) | No. of T Alleles | No. of Controls | MAF(%) | |||
| All Cases - Controls | 16 | 411 | 1.95% | 3 | 225 | 0.67% | 3.00 (0.86–10.40) | 0.084 |
| Definitive Cases - Controls | 16 | 265 | 3.02% | 3 | 225 | 0.67% | 4.76 (1.37–16.54) | 0.014 |
| Definitive Cases - Controls with CDR=0 | 16 | 265 | 3.02% | 1 | 139 | 0.36% | 8.87 (1.16–67.58) | 0.035 |
P values were calculated by logistic regression with the use of PLINK software, version 1.07. In bold are P < 0.05.
3.2. Association with ApoE ε4 and rare APP mutation
As expected, the strong association of ApoE ε4+ variant was replicated in the current cohort (OR: 3.19; 95%CI: 2.08–4.89; P=9.87×10−8; Supplement Table 4). The effect of the ApoE ε4 allele was investigated on the association between the rs75932628-T allele with AD (Supplement Table 5). The difference in the frequency of the rs75932628-T allele in ApoE ε4 carriers compared to ApoE ε4 noncarriers was not statistically significant (p=0.586 in all AD cases; p=0.816 in definitive AD cases). In a logistic regression model, the interaction between the rs75932628 and ApoE ε4 was not significant in any of the comparisons using different criteria to define cases vs. controls (all Ps > 0.9). The same cohort was also genotyped for a rare missense mutation (rs63750847) at the APP gene [30]. This variant was not identified in any of our sample.
3.3. Association of the rs75932628-T allele with NP and NFT density
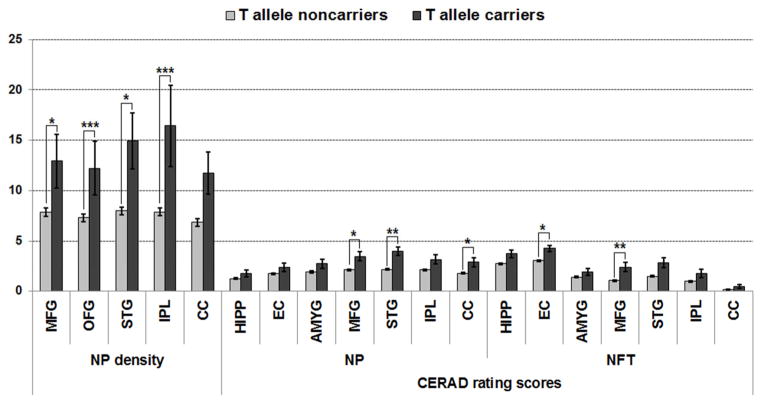
Fifty-four AD cases had secondary neuropathologies ranging from cerebrovascular disease to meningioma (Supplement Table 6), but these neuropathology findings were not significantly associated with the TREM2 allele (all Ps > 0.1). The association of rs75932628-T allele with NP and NFT was examined with analysis of covariance using sex and age as covariates in the whole cohort (N=655 subjects) (Figure 1; Supplement Table 7). Carriers of rs75932628-T allele had increased NP density in all examined brain regions, with more prominent changes in the orbital frontal cortex (P < 0.0001 at FDR = 0.002) and inferior parietal cortex (P = 0.0001 at FDR = 0.001). Similarly, they had increased densities of NFTs; this effect was significant after multiple testing corrections in the middle frontal gyrus (P = 0.0037 at FDR = 0.0149) and entorhinal cortex (P = 0.0161 at FDR = 0.0428). We then examined whether TREM2 is associated with more severe neuropathological lesions in ADcarries compared to ADnoncarriers. ADcarriers had increased NP and NFT density in all examined brain regions and this effect was significant for NP after multiple testing corrections in the superior temporal gyrus (P = 0.0001 at FDR = 0.0029) (Supplement Table 8; Supplement Figure 2).
Figure 1.
Association of the rs75932628-T Variant with NP and NFT. Bars show the standard error of the mean. Abbreviations: neuritic plaques (NP); neurofibrillary tangle (NFT); middle frontal gyrus (MFG; Brodmann area 9), orbital frontal cortex (OFG; Brodmann area 45/47), superior temporal gyrus (STG; Brodmann area 21/22), inferior parietal cortex (IPL; Brodmann area 39) and calcarine cortex (CC; Brodmann area 17); hippocampus (HIPP); entorhinal cortex (EC; Brodmann area 28/34); amygdala (AMYG). * P < 0.05; ** P < 0.005; *** P < 0.0005 and FDR < 0.05.
3.4. Association of the rs75932628-T allele with gene expression alterations
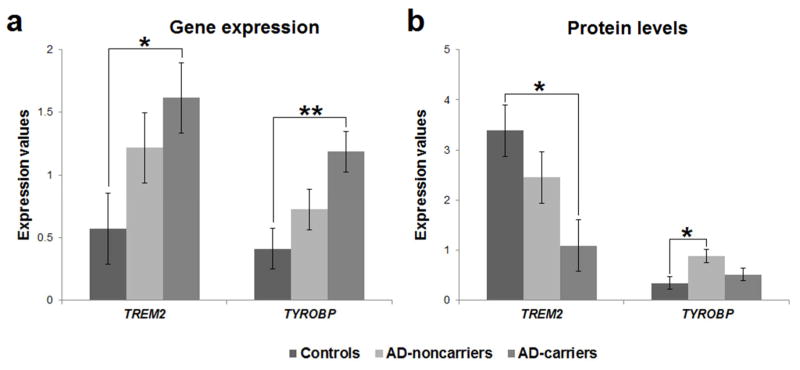
The association of rs75932628-T allele with gene expression was examined in STG with analysis of covariance using sex, age, PMI and RIN as covariates. The ANCOVA revealed a significant main effect of group for TREM2 [F(2,41) = 3.454; P = 0.041; η2 = 0.144] and TYROBP [F(2,41) = 5.767; P = 0.006; η2 = 0.220] gene expression. Bonferroni post hoc analysis showed that ADcarriers have higher TREM2 (P = 0.038) and TYROBP (P = 0.005) gene expression compared to controls (Figure 2a).
Figure 2.
(a) Gene expression and (b) protein level differences among controls, ADnoncarriers and ADcarriers for TREM2 and TYROBP in the superior temporal gyrus. Bars show the standard error of the mean. * P < 0.05; ** P < 0.005.
3.5. Association of the rs75932628-T allele with alterations in protein abundance
The association of rs75932628-T allele with protein levels was examined with analysis of covariance using sex, age and PMI as covariates. The ANCOVA revealed a significant main effect of group for TREM2 [F(2,21) = 5.092; P = 0.016; η2 = 0.327] and TYROBP [F(2,21) = 4.487; P = 0.024; η2 = 0.299] protein levels. Bonferroni post hoc analysis showed that controls have higher TREM2 (P = 0.014) and lower TYROBP (P = 0.024) protein level compared to ADcarriers and ADnoncarriers, respectively (Figure 2b; Supplement Figure 3).
3.6. Association of the rs75932628-T allele with alterations in inflammatory markers
To explore further the role of rs75932628 T allele in AD, we accessed a comprehensive panel of 24 immune markers including markers for inflammation and neurodegeneration in brain specimens from STG of controls, ADnoncarriers and ADcarriers (n=14/group). Cases were matched for age, sex, ethnicity, CDR and PMI. For the sake of data reduction and variable classification we submitted the outcome variables to factor analysis using principal components extraction. The Kaiser–Meyer–Olkin measure of sampling adequacy (0.503) and Bartlett’s test of sphericity (χ2 = 401.641, df =276, P < 0.0001) indicated that the data were appropriate for factor analysis. A total of 24 variables were included in the analysis and eight factors were extracted that accounted for 74.5% of the total variance (Supplement Table 9). The association of rs75932628-T allele with each factor was examined with analysis of covariance using sex, age and PMI as covariates. The ANCOVA revealed a significant main effect of group for factor 4 [F(2,36) = 4.468; P = 0.018; η2 = 0.199; FDR = 0.072] and factor 6 [F(2,36) = 7.002; P = 0.003; η2 = 0.281; FDR = 0.024]. Only the association with factor 6 survived corrections for multiple testing (FDR < 0.05). Factor 6 conformed to a proinflammatory profile and included the markers RANTES and IFN-gamma. Bonferroni post hoc analysis showed that both ADcarriers (P = 0.002) and ADnoncarriers (P = 0.019) have higher factor 6 values compared to controls (Supplement Figure 4; Supplement Table 9). Factor 4 was consistent with a “protective” role and included the markers α2 macroglobulin, IL-4 and ApoA1. Bonferroni post hoc analysis showed that ADcarriers have decreased factor 4 values compared to controls (P = 0.017).
4. Discussion
The nonsynonymous R47H TREM2 variant was significantly more prevalent in autopsy confirmed AD cases than in controls. TREM2 variant increases risk for AD independently of ApoE ε4 allele. Comparison with previously published studies using only clinical criteria for the diagnosis of AD suggested that the estimated allele frequency and odds ratio of TREM2 variant increases, by as much as 2–3 fold, when the phenotype of studied cases is defined using stringent neuropathological diagnostic criteria. This observation underscores the strength and importance of diagnostic fidelity to reduce phenotypic heterogeneity in genetic association studies.
The R47H variant is located within the extracellular immunoglobulin-like domain and recent results support a significant effect on ligand binding affinity as well as structural configuration of TREM2 [31], with possible subsequent signaling dysregulation. Here we demonstrate that the R47H variant is associated with distinct signaling in cases with AD. More specifically, R47H AD carriers demonstrate: (i) upregulation of TREM2 and TYROBP gene expression; (ii) downregulation of TREM2 protein expression; and (iii) upregulation of proinflammatory cytokines (RANTES and IFN-gamma) and downregulation of protective markers (α2 macroglobulin, IL-4 and ApoA1).
Our findings are consistent with previous findings of increased TREM2 and TYROBP gene expression in AD [8,32]. Interestingly, increased TREM2 and TYROBP expression in AD appears to be R47H “dose dependent” since AD non-R47H-carriers demonstrate intermediate changes in TREM2 and TYROBP gene expression and TREM2 protein levels. These results indicate that the TREM2 risk variant leads to further decompensation of molecular markers that are abnormal in AD. On the other hand, TYROBP protein increases only in AD R47H non-carriers. A speculative, albeit parsimonious, interpretation could be that TYROBP gene and protein upregulation represents a compensatory mechanism in AD, which is absent in AD R47H non-carriers. Compelling evidence from genome-wide microarray and animal models studies suggest that the immune/inflammatory–associated pathways of the brain occupy a central role not only in cognitive impairment in AD dementia [8,32–34], but also in protection and preservation of cognitive function [32,35]. Thus, the association of the TREM2 missense mutation with more severe neuropathology and increased inflammation may mark a signaling pathway/network central to the etiology of AD.
While ~40% of the variation in protein concentration can be explained by mRNA abundances additional mechanisms, some acting through miRNAs, such as post-transcriptional, translational and regulation of degradation and stability fine-tune protein abundances [36]. In our study, we did not explore any of the above mechanisms. Therefore, the mechanism that underlies the discrepancy between TREM2 mRNA and protein expression is unclear. Interestingly, a recent study provided evidence that the protein abundance of R47H TREM2 mutant is expressed at lower rates, suggesting that this variant prevents TREM2 maturation, transport to the cell surface, and shedding [27]. Based on the above we can speculate that carriers of the TREM2 mutation present lower protein abundance due to increased immature/mature TREM2 ratio and increased TREM2 degradation with parallel compensatory increased mRNA gene expression.
In macrophages, TREM2 has an anti-inflammatory function. More specifically, it inhibits toll-like receptor (TLR) mediated maturation of dendritic cells, as well as proliferation of antigen-specific T-cells [37–39]. Reduction of TREM2 expression enhances inflammatory cytokine responses by macrophages following stimulation of TLR [40]. In the central nervous system, TREM2 is primarily expressed on microglia [41]. The protective vs damaging roles of microglia in AD continues to be debated: activation of microglia may lead to removal of amyloid and cell debris or promote synaptic remodeling [42]; on the other hand, activated microglia can release proinflammatory cytokines and induces neuroinflammation, potentially leading to neurotoxicity [43]. TREM2 expression correlates positively with amyloid phagocytosis by microglia in a transgenic APP animal model [44]. In the same study, TREM2 was also positively correlated with the ability of microglia to stimulate the proliferation of CD4+ T cells, without secretion of IFN-gamma, which suggested that TREM2-positive microglia is involved in activation of innate immune response [44]. Reduced or increased phagocytosis of apoptotic neurons is observed with silencing or overexpression of TREM2 in microglia, respectively [11]. Therefore, consistent with the present findings, it has been suggested that that TREM2 has a protective role in the pathogenesis of AD and perhaps other neurodegenerative disorders [45–47].
Examination of multiple markers of inflammation and neurodegeneration in the current study identified upregulation of proinflammatory mediators (RANTES and IFNgamma) in AD. The effect was more pronounced in ADcarriers. The directionality of expression of selective immune-related markers is suggestive of ongoing activation of inflammatory signaling, and along with other reports in the literature, inflammatory markers indicative of chronic activation of immune responses in AD which becomes exaggerated in R47H missense carriers. Typically, inflammation is an adaptive response to infection or to tissue injury with its eventual resolution once homeostasis is re-established. Chronic inflammation, on the other hand, seems to be associated with tissue malfunction rather than to host defense or tissue repair [48]. The current findings suggest that TREM2-TYROBP complexes play a role in AD-associated chronic inflammation and encourage the exploration of their roles in mediating neuro-glial interactions.
Consistent with the possible protective role of TREM2 in AD, the TREM2 variant was associated with increased NP and NFT density across all samples or within the AD group. This association might be due to compromised TREM2-mediated phagocytic activity of microglia to remove cellular debris and toxic agents, as shown in in vitro and in a transgenic APP animal model [44,49]. Our finding of TREM2 variant association with increased densities of NFTs validates in neuropathologically well-characterized specimens recent genetic findings, where TREM2 variants showed strong association with the levels of cerebrospinal fluid tau and tau phosphorylated at threonine 181 [50].
In conclusion, the results presented indicate that the TREM2 variant is found more frequently when case and control definitions are based on stringent cognitive compromise and neuropathological diagnostic criteria. While TREM2-TYROBP signaling is compromised in AD, carriers of the missense mutation present an even more prominent dysregulation of TREM2-TYROBP gene/protein expression which is associated with increased inflammation and more severe neuropathology, including increased densities of amyloid plaques and neurofibrillary tangles.
Supplementary Material
Supplement Figure 1. TREM2 Western blot in superior temporal cortex of ADcarriers and ADnoncarriers by using rabbit monoclonal antibody against human TREM2 (Novus Biologicals) and goat polyclonal antibody against human TREM2 (R&D systems). Infrared (IR) fluorescence detection was performed using secondary antibodies: goat anti-rabbit IRDye 680 and donkey anti-goat-800 (Li-Cor Biosciences, Lincoln, NE) under standard conditions. The R&D polyclonal antibody against the N-terminus has been previously validated for equal recognition of mutant TREM2 (Kleinberger et al., 2014). Both TREM2 antibodies detect the same band and show similar changes in the signal intensity.
Supplement Figure 2. Example pathological hallmarks in carriers of R47H TREM2 variant. Shown are pathological findings in an (a) ADcarrier of the R47H TREM2 variant and (b) ADnoncarrier. Silver (modified Bielschowsky) staining was done in sections from the superior temporal gyrus (rostral 1/3 of the gyrus). The ADcarrier had mature plaques (black arrow) and numerous neurofibrillary tangles (red arrow).
Supplement Figure 3. Western blots of TREM2 and TYROBP in AD subjects. Western blotting was performed under reduced conditions and visualized using Li-Cor Odyssey system. TREM2 appeared as a single band ~40kD and TYROBP appeared as a single band ~30kD, likely representing homodimers of TYROBP (~13kD). Multiple freeze/thaw cycles with extended boiling (~20 min) in the presence of 2-mercaptoethanol demonstrated reduction of 30kD band to the ~13kD band. AD carriers marked by T/C and AD noncarriers marked by C/C.
Supplement Figure 4. Quantification of neurodegenerative and inflammatory markers in the superior temporal gyrus of controls, ADnoncarriers and ADcarriers (N=14 subjects/group). * P < 0.05; ** P < 0.005
Supplement Table 1. Cohort characteristics for AD carriers of the rs75932628-T variant and noncarriers cases and controls.
Supplement Table 2. TaqMan gene expression assays used in the study.
Supplement Table 3. Demographic, clinical and neuropathological characteristics of the rs75932628-T carriers
Supplement Table 4. Association between the ApoE ε4+ variant and Alzheimer’s disease in Comparison with the Control Group.
Supplement Table 5. Effect of ApoE ε4 on the association of rs75932628-T with Alzheimer’s disease.
Supplement Table 6. Secondary neuropathology in rs75932628-T carriers and noncarriers.
Supplement Table 7. Association of the rs75932628-T variant with NP and NFT density for all samples.
Supplement Table 8. Association of the rs75932628-T variant with NP and NFT density for AD cases only.
Supplement Table 9. Principal component analysis for markers of neuroinflammation and neurodegeneration. Analysis of covariance was applied to examined the effect of groups (Controls, ADnoncarriers and ADcarriers) on each factor.
Research in Context.
Systematic review: We have evaluated the literature on the association of TREM2 with AD. We identified multiple reports, including two independent studies published at New England Journal of Medicine (Guerreiro et al 2013 and Jonsson et al 2013) implicating R47H TREM2 variant as risk factor for AD.
Interpretation: We genotyped the R47H TREM2 markers in a large postmortem cohort of neuropathologically confirmed cases and controls and performed association analyses for disease status and neuropathological lesions (amyloid plaques and neurofibrillary tangles). For 16 AD cases that were carriers for the risk allele, we also examined changes in TREM2 and TYROBP gene and protein expression and neuroinflammatory markers. Our findings clearly support the association of TREM2 variant with AD pathology and specific molecular abnormalities.
Future directions: Additional mechanistic studies in animal models are required to characterize the molecular mechanisms that are responsible for this association.
Acknowledgments
This work was supported by National Institutes of Health (P01-AG02219, P50-AG05138, MH066392 to VH), Veteran Administration MIRECC, Leir Foundation. This work was supported in part through the computational resources and staff expertise provided by the Department of Scientific Computing at the Icahn School of Medicine at Mount Sinai. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer’s disease. The New England journal of medicine. 2013;368:117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. The New England journal of medicine. 2013;368:107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitez BA, Cooper B, Pastor P, Jin SC, Lorenzo E, Cervantes S, et al. TREM2 is associated with the risk of Alzheimer’s disease in Spanish population. Neurobiology of aging. 2013;34:1711, e15–7. doi: 10.1016/j.neurobiolaging.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz A, Dols-Icardo O, Bullido MJ, Pastor P, Rodriguez-Rodriguez E, Lopez de Munain A, et al. Assessing the role of the TREM2 p. R47H variant as a risk factor for Alzheimer’s disease and frontotemporal dementia. Neurobiology of aging. 2014;35:444, e1–4. doi: 10.1016/j.neurobiolaging.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Engelman CD, Koscik RL, Jonaitis EM, Hermann BP, La Rue A, Sager MA. Investigation of triggering receptor expressed on myeloid cells 2 variant in the Wisconsin Registry for Alzheimer’s Prevention. Neurobiology of aging. 2014;35:1252–4. doi: 10.1016/j.neurobiolaging.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiology of aging. 2013;34:2077, e11–8. doi: 10.1016/j.neurobiolaging.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez Murcia JD, Schmutz C, Munger C, Perkes A, Gustin A, Peterson M, et al. Assessment of TREM2 rs75932628 association with Alzheimer’s disease in a population-based sample: the Cache County Study. Neurobiology of aging. 2013;34:2889, e11–3. doi: 10.1016/j.neurobiolaging.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–20. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajagopalan P, Hibar DP, Thompson PM. TREM2 and neurodegenerative disease. The New England journal of medicine. 2013;369:1565–7. doi: 10.1056/NEJMc1306509#SA3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, et al. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56:1438–47. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. The Journal of experimental medicine. 2005;201:647–57. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang T, Yu JT, Zhu XC, Tan L. TREM2 in Alzheimer’s disease. Molecular neurobiology. 2013;48:180–5. doi: 10.1007/s12035-013-8424-8. [DOI] [PubMed] [Google Scholar]
- 13.Golde TE, Streit WJ, Chakrabarty P. Alzheimer’s disease risk alleles in TREM2 illuminate innate immunity in Alzheimer’s disease. Alzheimer’s research & therapy. 2013;5:24. doi: 10.1186/alzrt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, et al. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. Journal of neurochemistry. 2009;109:1144–56. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haroutunian V, Katsel P, Schmeidler J. Transcriptional vulnerability of brain regions in Alzheimer’s disease and dementia. Neurobiology of aging. 2009;30:561–73. doi: 10.1016/j.neurobiolaging.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, et al. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA: the journal of the American Medical Association. 1999;281:1401–6. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- 17.Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Archives of neurology. 1998;55:1185–91. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 18.Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Archives of neurology. 1999;56:713–8. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- 19.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.Dooneief G, Marder K, Tang MX, Stern Y. The Clinical Dementia Rating scale: community-based validation of “profound’ and “terminal’ stages. Neurology. 1996;46:1746–9. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 23.Roussos P, Katsel P, Davis KL, Bitsios P, Giakoumaki SG, Jogia J, et al. Molecular and genetic evidence for abnormalities in the nodes of Ranvier in schizophrenia. Archives of general psychiatry. 2012;69:7–15. doi: 10.1001/archgenpsychiatry.2011.110. [DOI] [PubMed] [Google Scholar]
- 24.Roussos P, Katsel P, Davis KL, Giakoumaki SG, Siever LJ, Bitsios P, et al. Convergent Findings for Abnormalities of the NF-kappaB Signaling Pathway in Schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roussos P, Katsel P, Davis KL, Siever LJ, Haroutunian V. A system-level transcriptomic analysis of schizophrenia using postmortem brain tissue samples. Archives of general psychiatry. 2012;69:1205–13. doi: 10.1001/archgenpsychiatry.2012.704. [DOI] [PubMed] [Google Scholar]
- 26.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. American journal of human genetics. 2002;71:656–62. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Science translational medicine. 2014;6:243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini YHY. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–9. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 31.Abduljaleel Z, Al-Allaf FA, Khan W, Athar M, Shahzad N, Taher MM, et al. Evidence of trem2 variant associated with triple risk of Alzheimer’s disease. PLoS One. 2014;9:e92648. doi: 10.1371/journal.pone.0092648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsel P, Tan W, Haroutunian V. Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS One. 2009;4:e7642. doi: 10.1371/journal.pone.0007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2173–8. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor perspectives in medicine. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parachikova A, Agadjanyan MG, Cribbs DH, Blurton-Jones M, Perreau V, Rogers J, et al. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiology of aging. 2007;28:1821–33. doi: 10.1016/j.neurobiolaging.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature reviews Genetics. 2012;13:227–32. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito H, Hamerman JA. TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. European journal of immunology. 2012;42:176–85. doi: 10.1002/eji.201141679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–4. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 39.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 40.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nature immunology. 2005;6:579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, et al. Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. The European journal of neuroscience. 2004;20:2617–28. doi: 10.1111/j.1460-9568.2004.03729.x. [DOI] [PubMed] [Google Scholar]
- 42.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s Disease. Frontiers in integrative neuroscience. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melchior B, Garcia AE, Hsiung BK, Lo KM, Doose JM, Thrash JC, et al. Dual induction of TREM2 and tolerance-related transcript, Tmem176b, in amyloid transgenic mice: implications for vaccine-based therapies for Alzheimer’s disease. ASN neuro. 2010;2:e00037. doi: 10.1042/AN20100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, et al. TREM2 in neurodegeneration: evidence for association of the p. R47H variant with frontotemporal dementia and Parkinson’s disease. Molecular neurodegeneration. 2013;8:19. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borroni B, Ferrari F, Galimberti D, Nacmias B, Barone C, Bagnoli S, et al. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiology of aging. 2014;35:934, e7–10. doi: 10.1016/j.neurobiolaging.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Cuyvers E, Bettens K, Philtjens S, Van Langenhove T, Gijselinck I, van der Zee J, et al. Investigating the role of rare heterozygous TREM2 variants in Alzheimer’s disease and frontotemporal dementia. Neurobiology of aging. 2014;35:726, e11–9. doi: 10.1016/j.neurobiolaging.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 49.Sieber MW, Jaenisch N, Brehm M, Guenther M, Linnartz-Gerlach B, Neumann H, et al. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLoS One. 2013;8:e52982. doi: 10.1371/journal.pone.0052982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruchaga C, Kauwe JS, Harari O, Jin SC, Cai Y, Karch CM, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013;78:256–68. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. TREM2 Western blot in superior temporal cortex of ADcarriers and ADnoncarriers by using rabbit monoclonal antibody against human TREM2 (Novus Biologicals) and goat polyclonal antibody against human TREM2 (R&D systems). Infrared (IR) fluorescence detection was performed using secondary antibodies: goat anti-rabbit IRDye 680 and donkey anti-goat-800 (Li-Cor Biosciences, Lincoln, NE) under standard conditions. The R&D polyclonal antibody against the N-terminus has been previously validated for equal recognition of mutant TREM2 (Kleinberger et al., 2014). Both TREM2 antibodies detect the same band and show similar changes in the signal intensity.
Supplement Figure 2. Example pathological hallmarks in carriers of R47H TREM2 variant. Shown are pathological findings in an (a) ADcarrier of the R47H TREM2 variant and (b) ADnoncarrier. Silver (modified Bielschowsky) staining was done in sections from the superior temporal gyrus (rostral 1/3 of the gyrus). The ADcarrier had mature plaques (black arrow) and numerous neurofibrillary tangles (red arrow).
Supplement Figure 3. Western blots of TREM2 and TYROBP in AD subjects. Western blotting was performed under reduced conditions and visualized using Li-Cor Odyssey system. TREM2 appeared as a single band ~40kD and TYROBP appeared as a single band ~30kD, likely representing homodimers of TYROBP (~13kD). Multiple freeze/thaw cycles with extended boiling (~20 min) in the presence of 2-mercaptoethanol demonstrated reduction of 30kD band to the ~13kD band. AD carriers marked by T/C and AD noncarriers marked by C/C.
Supplement Figure 4. Quantification of neurodegenerative and inflammatory markers in the superior temporal gyrus of controls, ADnoncarriers and ADcarriers (N=14 subjects/group). * P < 0.05; ** P < 0.005
Supplement Table 1. Cohort characteristics for AD carriers of the rs75932628-T variant and noncarriers cases and controls.
Supplement Table 2. TaqMan gene expression assays used in the study.
Supplement Table 3. Demographic, clinical and neuropathological characteristics of the rs75932628-T carriers
Supplement Table 4. Association between the ApoE ε4+ variant and Alzheimer’s disease in Comparison with the Control Group.
Supplement Table 5. Effect of ApoE ε4 on the association of rs75932628-T with Alzheimer’s disease.
Supplement Table 6. Secondary neuropathology in rs75932628-T carriers and noncarriers.
Supplement Table 7. Association of the rs75932628-T variant with NP and NFT density for all samples.
Supplement Table 8. Association of the rs75932628-T variant with NP and NFT density for AD cases only.
Supplement Table 9. Principal component analysis for markers of neuroinflammation and neurodegeneration. Analysis of covariance was applied to examined the effect of groups (Controls, ADnoncarriers and ADcarriers) on each factor.