Abstract
Basal cell carcinoma (BCC) is the most common cancer worldwide, and its current treatment options are insufficient and toxic. Surprisingly, unlike several other malignancies, chemopreventive efforts against BCC are almost lacking. Silibinin, a natural agent from milk thistle seeds, has shown strong efficacy against several cancers including ultraviolet radiation-induced skin (squamous) cancer; however, its potential activity against BCC is not yet examined. Herein, for the first time, we report the efficacy of silibinin and its oxidation product 2,3-dehydrosilibinin (DHS) against BCC both in vitro and in vivo using ASZ (p53 mutated) and BSZ (p53 deleted) cell lines derived from murine BCC tumors. Both silibinin and DHS significantly inhibited cell growth and clonogenicity while inducing apoptosis in a dose- and time-dependent manner, with DHS showing higher activity at lower concentrations. Both agents also inhibited the mitogenic signaling by reducing EGFR, ERK1/2, Akt, and STAT3 phosphorylation and suppressed the activation of transcription factors NF-κB and AP-1. More importantly, in an ectopic allograft model, oral administration of silibinin and DHS (200 mg/kg body weight) strongly inhibited the ASZ tumor growth by 44 and 71% (p<0.05), respectively, and decreased the expression of proliferation biomarkers (PCNA and cyclin D1) as well as NF-κB p50 and c-Fos in the tumor tissues. Taken together, these results provide the first evidence for the efficacy and usefulness of silibinin and its derivative DHS against BCC, and suggest the need for additional studies with these agents in pre-clinical and clinical BCC chemoprevention and therapy models.
Keywords: Basal cell carcinoma, chemoprevention, phytochemicals, silibinin, dehydrosilibinin, mitogenic signaling, EGFR
INTRODUCTION
Basal cell carcinoma (BCC) is the most common malignancy worldwide, and BCC incidences are continuously rising due to the aging population, increased exposure to solar ultraviolet (UV) radiation and depletion of the ozone layer [1]. BCC has abnormalities in the hedgehog (Hh) signaling pathway that result in constitutively active Hh signaling. Typically, BCC tumors harbor a mutational inactivation of the tumor suppressor patch (Ptch) gene or activating mutations in smoothened (Smo) [2]. Ptch functions as a negative regulator of Hh signaling and represses the activation of Smo. The binding of Hh ligand to Ptch relieves the repressive effect of Smo [3,4]. Upon release, Smo can send signals through a series of interacting proteins resulting in the activation of the downstream Gli family of transcription factors. There are currently no chemopreventive treatment options available to BCC patients, highlighting the need for better preventive strategies. Several studies have attempted to address this issue showing little to no effect against BCC in the clinical phase [5–8]. Additionally, selective inhibitors against the Hh signaling pathway have severe side effects that constrain patient compliance with those drugs. In particular, with Smo inhibitors, patients develop a drug resistance possibly due to mutations in Smo or amplification of downstream signaling components [9]. The treatment options available to BCC patients are viable if the patient does not have a particularly aggressive or invasive tumor; however, considering the high recurrence rate of BCC tumors and patients with aggressive or invasive tumors, there is an urgent need for better preventive and therapeutic options for BCC patients.
There is a growing body of evidence suggesting crosstalk between Hh and other signaling pathways in the initiation and progression of BCC. Epidermal growth factor receptor (EGFR) has been shown to cooperate with Hh/Gli in oncogenic transformation and is required for Hh/Gli-driven skin cancer [10,11]. In addition to EGFR, signal transducer and activator of transcription (STAT) 3 is also linked to Hh-mediated carcinogenesis. Epidermal ablation of EGFR or STAT3 significantly reduces the tumor number and growth in Smo-driven BCC carcinogenesis [10,12]. Additionally, Gli, the downstream modulator of Smo, is not exclusively activated by Hh signaling but is also modulated by PI3K/AKT, MEK/ERK, protein kinase C, and TGFβ/SMAD signaling pathways [11]. Accordingly, the role of these signaling pathways should also be considered in targeting BCC, while focusing on the Hh signaling pathway that is highly implicated in BCC initiation and development.
There is a significant amount of time (50–55 years) between sun exposure and the development of BCC and squamous cell carcinoma (SCC), offering a unique opportunity to intervene with chemopreventive agents [1]. Whereas extensive efforts have been made in the chemoprevention of SCCs by a wide range of agents [13,14], BCC chemopreventive research has been limited. One such agent that has shown promising effects against UVB radiation induced skin cancer of squamous origin is silibinin (Fig. 1A) [15,16], a flavonoid isolated from the seeds of Silybum marianum (L.) Gaertn., Asteraceae. Silibinin has also shown a strong efficacy as both chemopreventive and anti-cancer agent against a wide range of malignancies [17–19], and in order to establish and characterize a more potent drug, several modifications have been made in the parent silibinin structure [20,21]. One such agent is 2,3-dehydrosilibinin (DHS) (Fig. 1B) – an oxidative derivative of silibinin; although it is also a minor component present in S. marianum and it can be, therefore, considered to be a natural product, too. This compound has considerably stronger antioxidative and cytoprotective activity compared to silibinin [20]. DHS has been found to be a better anti-cancer agent than silibinin in human bladder, colon, and prostate carcinoma cells in culture [22]. We have recently developed a method to synthesize this minority natural component in multigram quantities with high purity, thus allowing its detailed studies in animal models and further exploitation [23]. Taken together, based on the strong efficacy of silibinin and DHS against various malignancies including the established effect of silibinin against squamous skin cancer, herein, for the first time, we assessed the efficacy and associated mechanisms of silibinin and DHS against BCC in both cell culture and animal models.
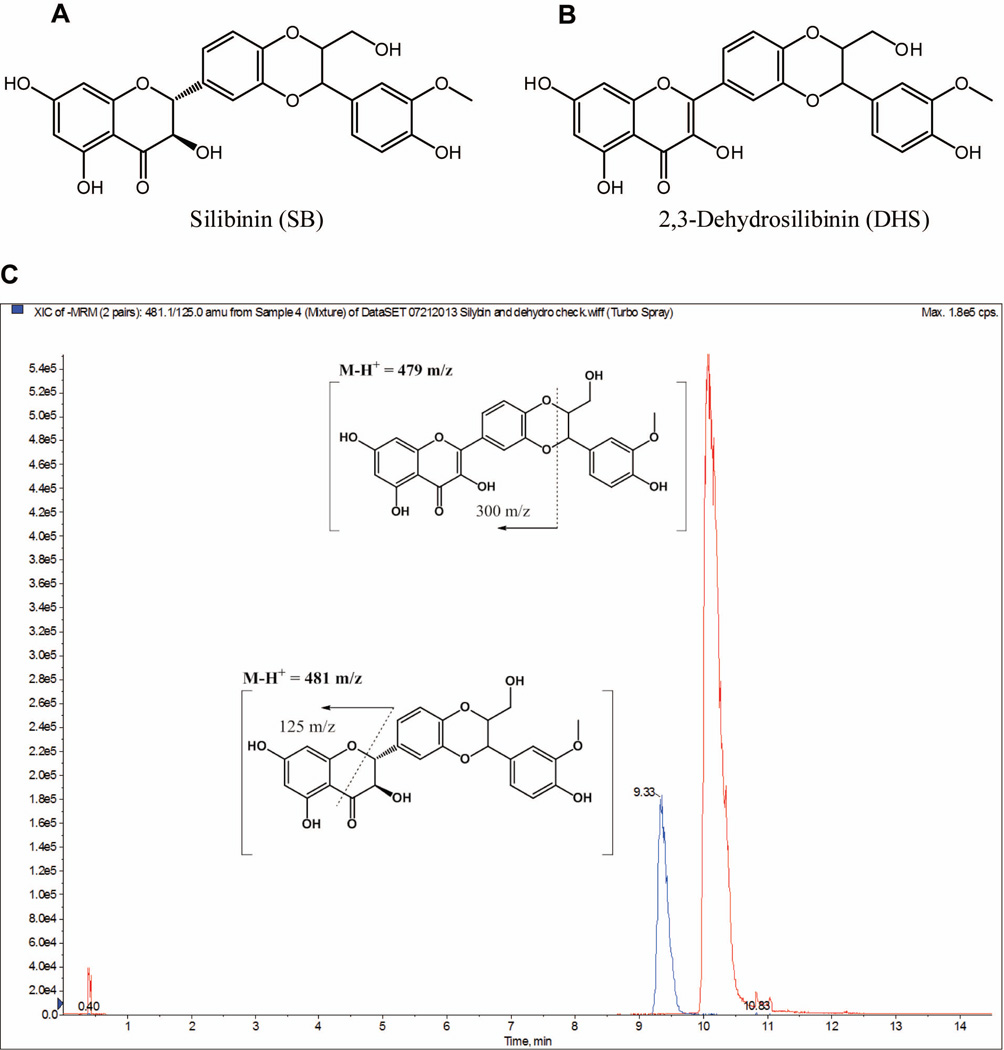
Figure 1.
Chemical structures of (A) silibinin and (B) 2,3-dehydrosilibinin (DHS). (C) A representative HPLC/MS-MS showing both silibinin (retention time: 10.2 min) and DHS (retention time: 9.3 min) and proposed daughter fragmentation.
MATERIALS AND METHODS
Cell lines and reagents
ASZ001 (ASZ) and BSZ cells were received as a gift from Dr. Ervin Epstein of Children’s Hospital and Research Center (Oakland, CA) and cultured in Gibco’s M154F media supplemented with 2% chelexed heat-inactivated fetal bovine serum, 1% penicillin-streptomycin, and 0.05 mM calcium chloride. Silibinin, carboxymethylcellulose (CMC), Harris hematoxylin, dimethyl sulfoxide (DMSO), and trypan blue were obtained from Sigma Aldrich (St. Louis, MO). The antibodies for NF-κB p50, c-Fos, α-tubulin, and EGFR were from Santa Cruz Biotechnology (Santa Cruz, CA). Cyclin D1 antibody was from Neomarkers (Fremont, CA). Phospho-EGFR, phospho-STAT3, STAT3, phospho-ERK1/2, ERK1/2, phospho-AKT, AKT, and secondary anti-rabbit antibodies were from Cell Signaling (Beverly, CA). Proliferating cell nuclear antigen (PCNA) antibody, streptavidin, and biotinylated anti-mouse secondary antibody were from Dako (Carpinteria, CA). The ECL detection system was from GE Healthcare (Buckinghamshire, UK). 3,3’-Diaminobenzidine (DAB) Peroxidase HRP substrate kit was from Vector Laboratories (Burlingame, CA). Phosphate-buffered saline (PBS) was from Roche (Nutley, NJ). Consensus NF-κB and AP-1 specific oligonucleotides and the gel shift assay system were from Promega Corp (Madison, WI). The primary antibodies (used in supershift assay) for c-Jun, c-Fos, p65, and p50 were from Santa Cruz Biotechnology (Santa Cruz, CA). DHS (50 g) was prepared as described previously [23]. Briefly, silybin was refluxed in acetic acid with iodine and potassium acetate and precipitated with water. Acidic hydrolysis and crystallization with ethanol afforded DHS in a good yield and purity.
Cell growth, clonogenic and apoptosis assays
Cells were plated (5 × 104 cells/well) in 6-well plates under standard culture conditions, and after 24 h, treated with DMSO alone (vehicle), silibinin (25–100 µM) or DHS (10–30 µM). The concentrations of silibinin were chosen based on previous studies in other cancer cell lines [22,24,25]. Silibinin and DHS stock solutions were prepared in DMSO. An equal amount of DMSO was present in each treatment, including control; and DMSO concentration did not exceed 0.1% (v/v) in any treatment. At the end of the desired treatment time, cells were trypsinized, collected and counted under the microscope using a hemocytometer. The trypan blue dye exclusion method was used to determine total number of live and dead cells. To assess the effect of silibinin and DHS on clonogenic potential, cells were seeded (1 × 104 cells/well) in 6-well plates, and after 24 h, treated with DMSO alone or various concentrations of silibinin (25–100 µM) or DHS (10–30 µM) in DMSO as above at every 48 h. After the 7th day, cells were washed with PBS and fixed with a mixture of methanol: acetic acid (3:1) for 10 min followed by staining with 0.1% crystal violet for 30 min. The plates were washed with PBS and the number of colonies with ≥ 50 cells was counted under inverted microscope. To quantify silibinin- and DHS-induced apoptotic death, following desired treatments detailed above, cells were stained with Annexin V and propidium iodide using Vybrant Apoptosis Assay kit 2 as per the manufacturer’s protocol (Life Technologies, Grand Island, NY), and the stained cells were analyzed by fluorescent activated cell sorting (FACS) analysis using the core service of the University of Colorado Cancer Center (Aurora, CO).
Western blotting
At the end of the given treatment and respective time points, cell lysates were prepared in nondenaturing lysis buffer [10 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1 % Triton X-100, 1.0 mmol/L EDTA, 1.0 mmol/L EGTA, 0.3 mmol/L phenylmethylsulfonyl fluoride, 0.2 mmol/L sodium orthovanadate, 0.5% NP40, 5 units/ml aprotinin]. Equal amount of protein lysate per sample was used for immunoblot analysis. The membranes were probed with desired primary antibodies followed by peroxidase-conjugated appropriate secondary antibody and visualized by enhanced chemiluminescence detection system. Some blots were multiplexed or stripped and reprobed with different antibodies including those for loading control. The immunoblots were scanned using Adobe Photoshop 6.0 (Adobe system, San Jose, CA) and mean density of bands was determined using Adobe or Scion.
Electrophoretic mobility shift assay (EMSA)
Consensus sequences of double stranded NF-κB oligonucleotide (5’AGT TGA GGG GAC TTT CCC AGG C-3’ and 3’TCA ACT CCC CTG AAA GGG TCC G-5’) or AP-1 oligonucleotide (5’-CGC TTG ATG AGT CAG CCG GAA-3’ and 3’GCG AAC TAC TCA GTC GGC CTT-5’) were end labeled with γ-32P-ATP (3,000 Ci/mmol at 10 m Ci/ml) as per manufacturer's protocol (Promega Corp). Labeled probes were separated from free γ-32P-ATP using G-25 Sephadex column. Nuclear extract were prepared from control, silibinin or DHS treated cells, and these extracts (10 µg protein/sample) along with 5 × gel shift binding buffer were incubated with 1–2 µL (20,000 cpm) of 32P-labeled NF-κB or AP-1 probe for 20 min at 37°C. In super shift and competition assays, nuclear extract was incubated with anti-p65/p50 and c-Jun/c-Fos antibodies for NF-κB and AP-1, respectively, or unlabeled-oligonucleotide before adding labeled NF-κB or AP-1 oligonucleotide. DNA retardation gel (6%) was used to resolve DNA-protein complexes followed by gel drying and autoradiography. The details of nuclear extract preparation and EMSA were those described recently [24,26,27].
Animals and treatments
All the protocols used were approved by the institutional animal care and use committee of the University of Colorado Denver. Five weeks old nude mice (Foxn1nu/nu), obtained from Charles River Laboratories (Wilmington, MA), were injected subcutaneously in the lower flank of each mouse with 1×106 ASZ cells suspended in 0.05 mL of serum-free medium (M154F), mixed with 0.05 ml of matrigel. The following day, mice were randomly divided into 3 groups (n=10 per group) and administered via oral gavage 200 µL vehicle (0.5% CMC), or silibinin (200 mg/kg) or DHS (200 mg/kg) in 200 µL vehicle. The dose of 200 mg/kg of silibinin was chosen based on a previous xenograft study conducted in our laboratory [28]. This is the first study conducted testing the efficacy of DHS in mice. Mice were treated 6 days per week for a total of 7 weeks, and the body weight of each mouse was monitored regularly throughout the study. Once the allograft started growing, the size was measured in two dimensions using a digital vernier caliper. The tumor volume was calculated using the formula 0.5236×L1×(L2)2, where L1 and L2 represent the long and short axis of the tumor measurements, respectively. After final treatments, mice were euthanized at different time points (0–180 min) by inhalation of carbon dioxide followed by cervical dislocation, blood was collected intracardially and placed into heparinized tubes, and plasma was isolated by centrifugation at 5000 rpm for 20 min at 4°C. The plasma was stored at −80 ± 10 °C until thawed, extracted and analyzed by LC/MS-MS methods. Each tumor was also carefully dissected and weighed, and fixed in formalin and processed for immunohistochemistry (IHC) analysis.
IHC staining
Paraffin-embedded sections (5 µm) of the tumor tissues were deparaffinized, rehydrated, and antigen retrieval was performed using sodium citrate buffer (0.01M; pH 6.0) for 20 min in a pressure cooker. Sections were quenched of endogenous peroxidase activity and incubated with primary antibodies: anti-PCNA (1:500 dilution), anti-cyclin D1 (1:100 dilution), anti-NF-κB/p50 (1:50 dilution), anti-c-Fos (1:100 dilution), or PBS (no primary antibody) for the negative control. Sections were then incubated with appropriate secondary biotinylated antibody followed by streptavidin. Color development was achieved by incubation with DAB followed by counter staining with Harris hematoxylin. Microscopic analyses were conducted using Zeiss Axioscope 2 microscope. Photomicrographs were captured using Carl Zeiss AxioCam MrC5 camera with Axiovision Rel 4.5 software. Other details of IHC staining are described in recent studies by us [29,30]. Briefly, quantification of nuclear staining was done by counting brown-positively stained cells and total number of cells at 10 to 12 randomly selected fields at 400× magnification. Immunoreactivity (represented by intensity of brown staining) was scored as 0 (no staining), +1 (very weak but uniform cytoplasmic staining), +2 (weak but uniform cytoplasmic staining), +3 (moderate with peripheral tumor areas with strong patchy cytoplasmic staining) and +4 (strong with both nuclear and cytoplasmic staining). Quantification of IHC data is represented as mean ± SEM of 7–10 samples in each group.
Pharmacokinetic analysis
The plasma samples isolated from the ASZ allograft experiment were used. An Applied Biosystems Sciex 4000® (Applied Biosystems; Foster City, CA) equipped with a Shimadzu HPLC (Shimadzu Scientific Instruments, Inc.; Columbia, MD) and Leap auto-sampler (LEAP Technologies; Carrboro, NC) was used. Liquid chromatography employed an Agilent Technologies, Zorbax extended-C18 50 × 4.6 mm, 5 µm column equipped with a column guard and operated at 40 °C with a flow-rate of 0.4 mL/min. The mobile phase consisted of solvent A: 10 mM ammonium acetate, 0.1% formic acid in water, and solvent B: 50:50 ACN:MeOH. Silibinin and DHS were quantitated via electro-spray ionization negative ion mode (ESI -) using the following conditions: i) an ion-spray voltage of −4200 V; ii) temperature, 450 °C; iii) curtain gas (CUR; set at 10) and Collisionally Activated Dissociation (CAD; set at 12) gas were nitrogen; iv) Ion Source gas one (GS1) and two (GS2) were set at 30; v) entrance potential was set at 10.0 V; vi) quadruple one (Q1) and (Q3) were set on Unit resolution; vii) dwell time was set at 200 msec; and viii) declustering potential (DP), collision energy (CE), and collision cell exit potential (CXP) are voltages (V); silibinin: 479.0 → 298.8 m/z; DP (−70), CE (−24), and CXP (−7); DHS: 481.1 → 125.0 m/z; DP (−70), CE (−38), and CXP (−7). The liquid chromatography used was 20% B held for 0.5 min, ramped to 95% B at 5.0 min and held for 5.5 min, and returned to 20% B at 12.5 min and held for 2.0 min; 14.5 min total run time. Using these conditions, silibinin had a retention time of 10.2 min while DHS had a retention time of 9.3 min (Fig. 1C). Standard curve (SC) and quality control (QC) samples (0.2 – 500 ng/mL) were prepared by mixing stock DMSO solutions (10.0 µL; silibinin or DHS) with control plasma (990 µL). After mixing, serial dilutions (1:1) with control mouse plasma were performed. SC and QC samples were subjected to one freeze thaw cycle (i.e. analogous to the experimental samples) by storing at −80 ± 10 °C (24 h). Plasma samples (125 µL transferred into 1.5 mL Eppendorf tubes) were extracted with two volumes of extraction solution (1:1:1; MeOH:H2O:ACN), vortex mixed (5 s), allowed to sit at room temperature for 10 min, mixed (5 s) again, and then centrifuged at 10,000 rpm (10 min). The supernatants were transferred into a 96-well plate, sealed, placed into the auto-sampler cool stack (7 °C) and all samples (10 µL) were analyzed in triplicate via LC/MS-MS. For both silibinin and DHS, the limit of detections (LOD) and limit of quantitation (LOQ) were 0.24 and 0.48 ng/mL, respectively. Quantification was performed using peak area ratios, and the data was fitted to a 1/x2 weighted linear regression; silibinin, R2 = 0.9997; DHS, R2 = 0.9994.
Statistical analysis
Statistical analysis was performed through SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data was analyzed using analysis of variance (ANOVA) and a statistically significant difference was considered to be at p < 0.05.
RESULTS
Silibinin and DHS inhibit cell growth and the formation of colonies, and induce apoptotic death in BCC cells
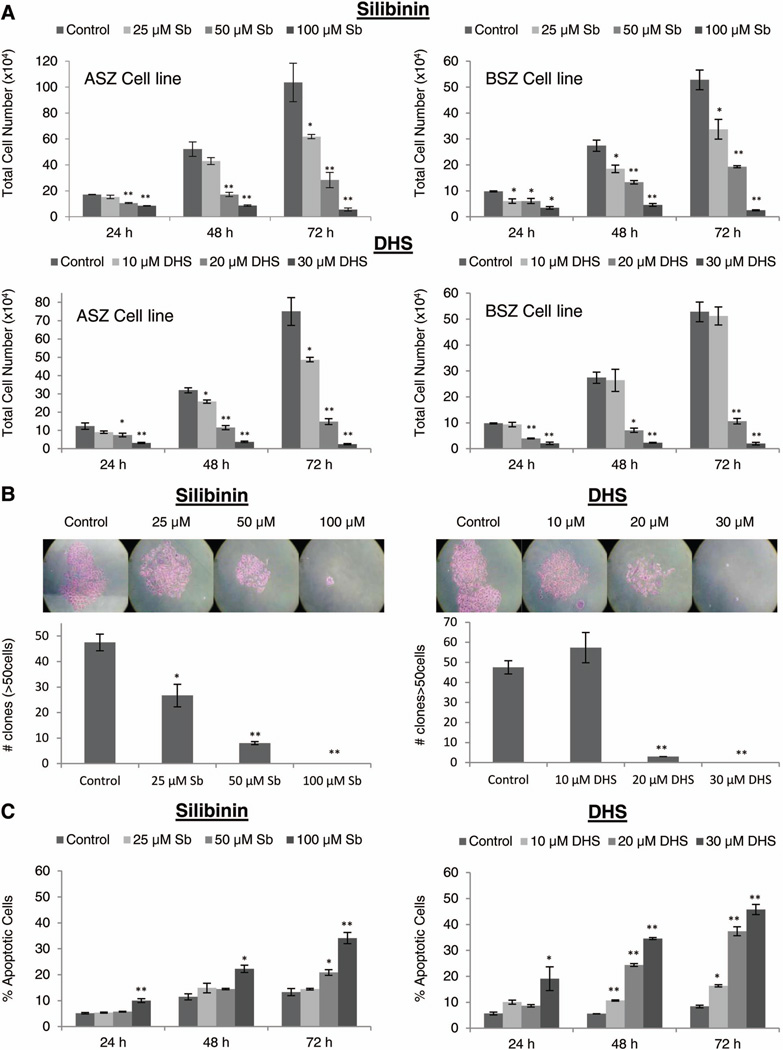
To assess the effects of silibinin and DHS against BCC, initially, we treated murine BCC cells with equal doses of silibinin and DHS (25–100 µM) and analyzed total cell number after 24–72 h. DHS was found to be much more effective than silibinin at these concentrations (data not shown), and therefore, we lowered the doses of DHS to 10–30 µM. Both silibinin and DHS caused a strong cell growth inhibition in a dose- and time-dependent manner in both ASZ and BSZ cells (Fig. 2). Total cell number in ASZ cells decreased by 10–95% (P≤0.05–0.001) and 19–98% (P≤0.05–0.001) following 24–72 h silibinin and DHS treatments, respectively (Fig. 2A). Similarly in BSZ cells, treatment with silibinin and DHS for 24–72 h decreased total cell number by 33–95% (P≤0.05–0.001) and 60–96% (P≤0.05–0.001), respectively (Fig. 2A). Additionally, compared to vehicle controls, both silibinin and DHS also induced cell death in ASZ and BSZ cells; however, in terms of absolute cell death percent, it was not that profound (data not shown). These results clearly showed that both silibinin and DHS exert strong growth inhibitory effects in BCC cells. Since we observed similar treatment responses in both ASZ and BSZ cells, for the rest of the studies, we only employed ASZ cells.
Figure 2.
The effect of silibinin and DHS on cell growth, formation of colonies and apoptotic cell death in BCC cells. (A) ASZ and BSZ cells were treated with either DMSO, silibinin (25–100 µM), or DHS (10–30 µM) for 24–72 h. After given time points, cells were collected and processed as detailed in ‘Materials and Methods’ to analyze silibinin and DHS’s effect on total cell growth in ASZ and BSZ cells. (B) ASZ cells were plated at 1 × 104 cells per well and treated every 48 h with either DMSO, silibinin (25–100 µM), or DHS (10–30 µM). After 7 days, colonies greater than 50 cells were counted and the average of each group plotted. (C) ASZ cells were treated with DMSO, silibinin (25–100 µM), or DHS (10–30 µM) for 24–72 h and analyzed for apoptosis as described in ‘Materials and Methods’. Each bar represents mean ± SEM of three samples for each treatment. *P ≤ 0.05, **P ≤ 0.001, significant with respect to vehicle control.
We next determined the effect of silibinin and DHS treatments on the ability of ASZ cells to form colonies. As shown in Figure 2B, both agents strongly inhibited the formation of colonies by ASZ cells in a dose-dependent manner with 44% to complete (P≤0.05–0.001) and 94% to complete (P≤0.001) inhibition, respectively. To assess whether the observed growth inhibition and cell death are associated with apoptosis, quantitative apoptotic cell death assay was employed following treatment with silibinin or DHS. Both treatments significantly induced apoptotic cell death in a dose-dependent manner, showing 1.6 to 2.6 fold (P≤0.05–0.001) and 1.5 to 6.2 fold (P≤0.05–0.001) percent apoptotic cell population compared to vehicle controls, respectively (Fig. 2C). Together, these results clearly showed that both silibinin and DHS inhibit the colony formation and induce apoptosis in BCC cells.
Silibinin and DHS attenuate mitogenic signaling in ASZ cells
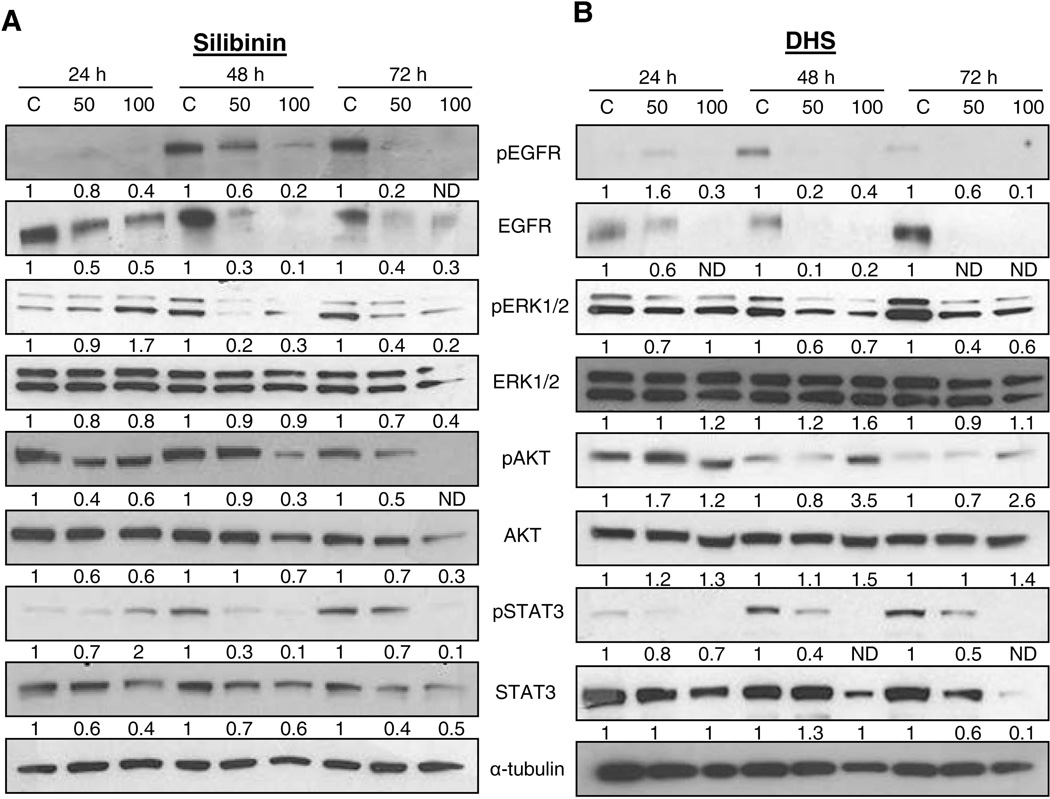
In order to deduce the potential target/s of silibinin and DHS in ASZ cell growth inhibition and apoptotic cell death, we analyzed changes in mitogenic signaling by immunoblotting methods. EGFR was shown to be important in BCC initiation and development in animal models via synergizing with Hh/Gli signaling [10,11]. We, therefore, first sought to analyze the expression levels of phosphorylated and total EGFR in response to silibinin or DHS treatment in ASZ cells. Both silibinin and DHS caused a dose- and time-dependent decrease in phosphorylated and total levels of EGFR (Fig.3A and 3B). Two major down-stream signaling pathways for EGFR are the Ras-Raf-MAPK/ERK and PI3K-AKT that are involved in regulating gene expression, controlling cell proliferation, survival, and inhibition of apoptosis [31], and therefore, the downstream components ERK1/2 and AKT were next analyzed in response to silibinin and DHS treatment. Phosphorylated levels of ERK1/2 and both phosphorylated and total levels of AKT were also down-regulated in response to silibinin treatment. On the other hand, DHS decreased phosphorylated ERK1/2 levels but not phosphorylated AKT (Fig. 3B). Additionally, we also assessed the levels of STAT3, which is linked to Hh-mediated carcinogenesis, and found that both phosphorylated and total levels of STAT3 were also decreased in response to silibinin and DHS treatments in ASZ cells (Fig.3A and 3B). Together, these results provided the first evidence of a potential target of silibinin and DHS in BCC cells, the EGFR, which is involved in various down-stream signaling pathways associated with cell proliferation, survival and induction of apoptosis, and as eluted above, also plays an important role in BCC initiation and development [10,11].
Figure 3.
The effect of silibinin and DHS on mitogenic signaling in ASZ cells. Cells were treated with DMSO, silibinin (25–100 µM), or DHS (10–30 µM) for 24–72 h and total cell lysates were prepared. Lysates were analyzed for p-EGFR, EGFR, p-ERK1/2, ERK1/2, p-AKT, AKT, p-STAT3, and STAT3 in silibinin treated (A) and DHS treated (B) cells. The blots were stripped and re-probed with α-tubulin to confirm equal loading. Densitometry data presented below the bands are ‘fold change’ as compared with control (DMSO treated) after normalization with respective loading control (α-tubulin). ND: Not detectable
Silibinin and DHS inhibit the constitutive activation of NF-κB and AP-1 in ASZ cells
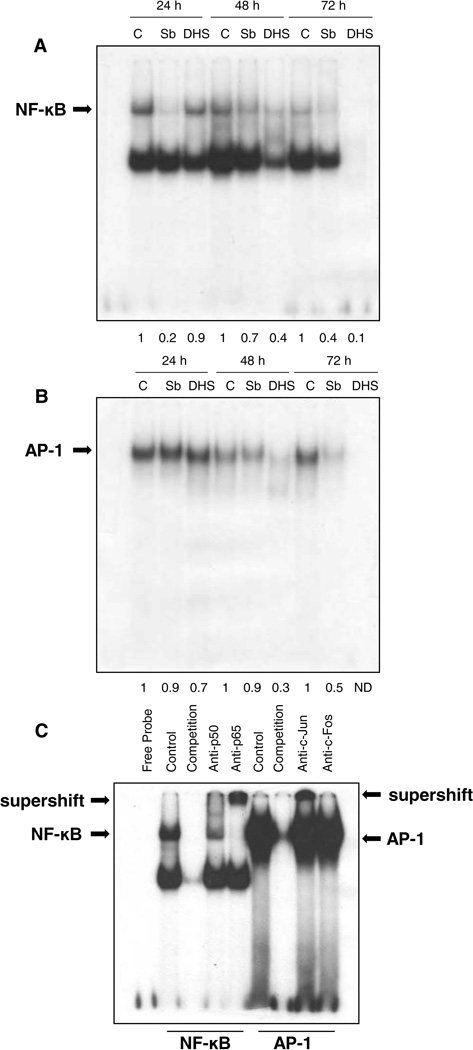
Both NF-κB and AP-1 are the transcription factors involved in various components of carcinogenesis, and are also linked to BCC growth and progression [11,32,33]. Importantly, both these transcription factors are also activated following activation of Ras-Raf-MAPK/ERK and PI3K-AKT pathways [34–36]. Since we observed the strong inhibitory effect of silibinin and DHS on EGFR and its downstream signaling pathways, activation of both NF-κB and AP-1 transcription factors was next assessed in terms of their DNA binding activity in ASZ cells, where both silibinin and DHS treatments inhibited the activation of NF-κB and AP-1 (Fig. 4). Silibinin caused a strong decrease in the DNA binding activity of NF-κB at all-time points (24–72 h) of its treatment (Fig. 4A), while showing a strong inhibitory effect only at 72 h for AP-1 activation (Fig. 4B). On the other hand, DHS caused more of a significant inhibition in the DNA binding activity of both NF-κB and AP-1 at 48 h with complete inhibition by 72 h of its treatment (Fig.4A and 4B). We also performed supershift assay using specific antibodies p50 and p65 for NF-κB and c-Fos and c-Jun for AP-1. We observed a clear supershift for p65 and a partial supershift along with a decrease in gel shift expression for p50 (Fig. 4C), suggesting that the NF-κB complex is composed of p50 and p65 subunits. For c-Fos and c-Jun we did not observe a supershift but a decrease in the gel shift, suggesting the partial presence of c-Fos and c-Jun.
Figure 4.
The effect of silibinin and DHS on DNA binding activity of NF-κB and AP-1 in ASZ cells. ASZ cells were treated with DMSO, silibinin (100 µM), or DHS (30 µM) for 24 to 72 h and the DNA binding activity for NF-κB (A) and AP-1 (B) was determined by EMSA as detailed in ‘Materials and Methods’. (C) Supershift and competition assays were performed using nuclear extract from ASZ cells to confirm the specificity of NF-κB and AP-1 binding. The supershift assay for p50, p65, c-Fos, and c-Jun in ASZ cells is shown. Densitometry data presented below the bands are ‘fold change’ as compared with control (DMSO treated). ND: Not detectable.
Silibinin and DHS inhibit ASZ allografts growth in nude mice without toxicity
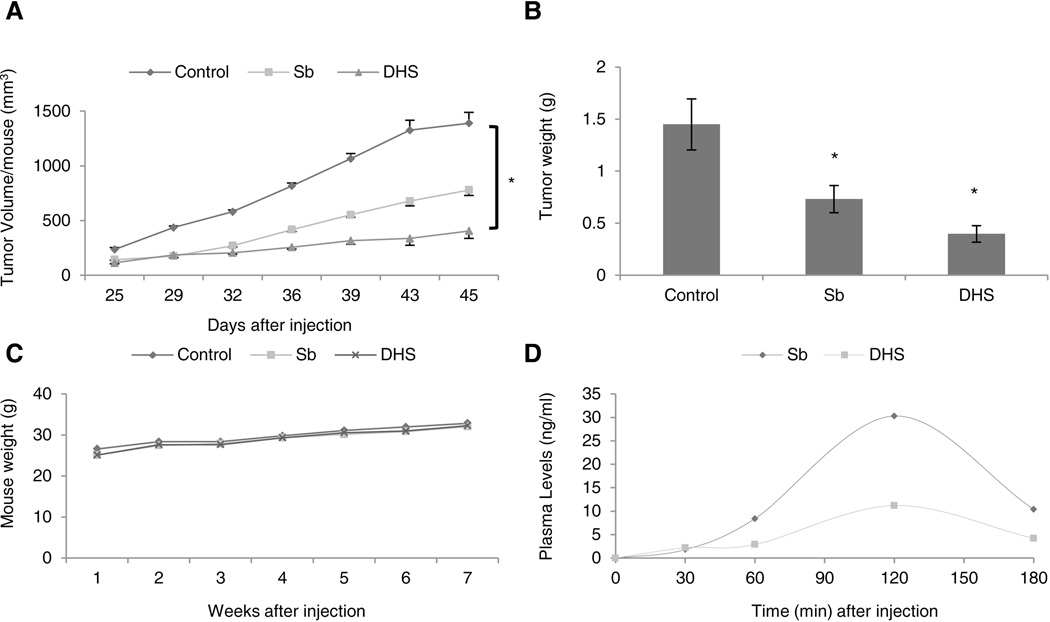
Based on the above summarized cell culture studies showing strong biological effects of both silibinin and DHS in BCC cell lines, we next evaluated their effect on the growth of ASZ cell allografts in nude mice. ASZ cells were injected into nude mice and treated subsequently with vehicle control, silibinin, or DHS. Throughout the study, we observed that both silibinin and DHS treatments strongly inhibit the growth of ASZ allografts in nude mice, and at the end of the study, these two agents showed 44% and 71% (p<0.05) reduction in tumor volume in comparison to control group, respectively (Fig. 5A). The tumor weight was also significantly reduced by 49% and 73% (p<0.05) in silibinin and DHS treated groups, respectively, compared to vehicle control (Fig. 5B). In addition, there was no toxicity of either compound observed, considering that the mouse weight in each treated group remained stable with control group (Fig. 5C). Blood samples were collected from each mouse at various time points and analyzed to demonstrate that silibinin and DHS are orally bioavailable. These studies displayed peak plasma concentration of approximately 30.3 and 11.2 ng/ml (62.86 and 23.33 nM) for silibinin and DHS at 120 min post-last dosing, respectively (Fig. 4D). It is interesting to note that we observed a lower plasma AUC (area under the curve) with DHS yet it displayed higher potency against BCC cells compared to silibinin.
Figure 5.
The effect of silibinin and DHS on ASZ tumor allograft growth in nude mice. ASZ cells were injected into nude mice and treated with vehicle (0.5% CMC), silibinin (200 mg/kg), or DHS (200 mg/kg) via oral gavage and analyzed for (A) tumor volume (twice weekly), (B) tumor weight (at the end of the study), and (C) mouse weight as detailed in ‘Materials and Methods.’ The data shows average tumor volume ± SEM, n = 7–10 mice per group. (D) Blood was collected from mice at various time points (0–180 min) after final treatment to illustrate drug oral bioavailability as detailed in ‘Materials and Methods.’ *P ≤ 0.05, significant with respect to vehicle control.
Silibinin and DHS inhibit in vivo proliferation as well as NF-κB and AP-1 activation
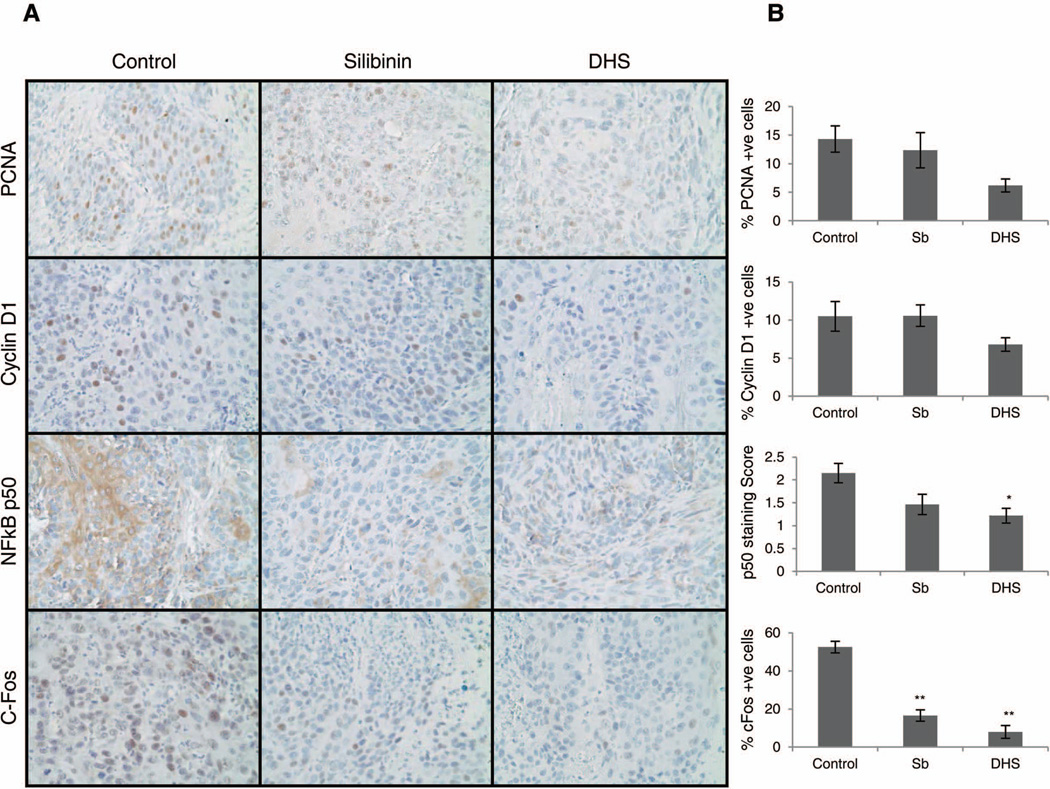
To understand whether the observed molecular effects of silibinin and DHS in cell culture are also translated in vivo and are involved in inhibiting ASZ allografts growth, we fixed and embedded the tumor tissues in paraffin and performed IHC analysis for PCNA, cyclin D1, NF-κB p50, and c-Fos. These analyses were also important to gather evidence in terms of the in vivo effect of these two agents on the proliferation (PCNA and cyclin D1) and inflammation (NF-κB and AP-1/c-Fos) in BCC cells allografts. We observed a decrease in both PCNA and cyclin D1 positive cells in the allografts from silibinin and DHS treated mice compared to vehicle control, with a much stronger effect with DHS (Fig.6A and 6B). Similarly, there was a marked decrease in NF-κB p50 expression in both silibinin and DHS treated allografts with more significant decrease in the DHS group (Fig.6A and 6B). Lastly, we observed a significant lowering in the percentage of c-Fos positive cells with both silibinin and DHS treatments (Fig.6A and 6B).
Figure 6.
The effect of silibinin and DHS on in vivo proliferation, NF-κB p50, and c-Fos activation. (A) ASZ allograft tissues from control and treatment groups were subjected to IHC analysis. Representative photographs are shown for PCNA, cyclin D1, NF-κB p50, and c-Fos. (B) Quantitative data for each biomarker showing the mean ± SEM of 7–10 individual tumor samples from each group. *P ≤ 0.05, **P ≤ 0.001, significant with respect to vehicle control.
DISCUSSION
BCC is a growing problem worldwide due to the rising incidences and lack of effective preventive options. This malignancy causes significant morbidity in patients along with higher instances of tumor recurrence after resection. Silibinin has shown strong protective efficacy against various other cancers including squamous cell carcinoma (SCC), another type of skin cancer [14,16], and several studies have shown silibinin to be a candidate chemopreventive agent in cell culture and animal models of SCC [15,37–39]. Considering that the primary etiologic factor for both BCC and SCC is UV radiation, the use of silibinin against BCC was a prudent question to address. Indeed, our results clearly showed that both silibinin and DHS inhibit cell growth and induce apoptosis by suppressing EGFR, STAT3, NF-κB and AP-1 signaling pathways in murine BCC cells in culture. In our mice allograft study also, both silibinin and DHS showed a strong efficacy against tumor growth that involved the inhibition of proliferation as well as NF-κB and AP-1 activation as observed in the cell culture. Together, these finding are highly significant because this is the first study showing: a) silibinin and DHS efficacy against BCC in both cell culture and mouse models, and b) in vivo anti-cancer efficacy of DHS in any animal model. Importantly, in previous studies, we have shown that silibinin does not affect normal cells including normal human epidermal keratinocytes [42], therefore we consider silibinin to be specifically targeting BCC cells.
Hh pathway inhibitors are clinically used against BCC; however, BCC cells develop resistance towards these inhibitors through mutations in Smo gene and/or amplification of the downstream signaling components [9], suggesting that other signaling pathways, activated in BCC, should also be explored to manage, prevent and treat this most frequent malignancy. Accordingly, our results showing that both silibinin and DHS down-regulated the components of the mitogenic and survival signaling pathways, particularly both phosphorylated and total levels of EGFR, in BCC cells, are important; specifically, because previous studies have shown a high level of crosstalk between EGFR and Hh signaling pathways. For example, in human keratinocytes, sonic hedgehog expression was associated with higher level of phosphorylated EGFR, JNK, and Raf, and that inhibiting EGFR activation blocked matrix infiltration of the cells in raft culture [40]. Notably, EGFR is required in Hh/Gli-driven skin cancer [10]. Also importantly, EGFR is known to activate both Ras-Raf-ERK1/2 and PI3K/AKT pathways [31], and silibinin treatment showed a strong decrease in the phosphorylated levels of ERK1/2 and AKT, as well as total AKT. On the other hand, DHS only showed a strong decrease in phosphorylated ERK1/2 levels that may indicate that DHS exerts its growth inhibitory effects independent of the PI3K/AKT pathway; however, additional investigations should be conducted in future to further support this conclusion. Of interest to note, in murine BCC cells, inhibiting PI3K/AKT signaling also retards tumor development, indicating another potential crosstalk with Hh pathway [41]. Furthermore, STAT3 is also linked to Hh-mediated carcinogenesis, and we found that both silibinin and DHS decreased the phosphorylated and total STAT3 levels in BCC cells. Since STAT3 is activated by EGFR [12], it further explains the observed decrease in both EGFR and STAT3 in BCC cells in response to silibinin and DHS treatments. Overall, silibinin and DHS seem to target multiple signaling pathways in BCC cells. However, in future studies, it will be important to identify the upstream primary target/s of silibinin and DHS in BCC cells that could lead to the modulation of pleotropic signaling pathways. In our ongoing studies, we are analyzing silibinin and DHS effect on hedgehog signaling that is the major pathway involved in BCC development as well as in the modulation of several other signaling pathways.
NF-κB consists of a family of Rel-domain containing proteins that are normally inactive in the cytoplasm, but once activated, they translocate to the nucleus to exert their biological effects. Historically, NF-κB serves as an important regulator in the host immune and inflammatory response [43]. We observed both an inhibition in the DNA binding activity of NF-κB in BCC cells in culture as well as a decrease in NF-κB p50 levels in the ASZ allograft tumor samples following silibinin and DHS treatments. The results of silibinin targeting NF-κB pathway are in line with previous studies showing that silibinin targets NF-κB activation in colorectal and prostate cancer models [24,44]. AP-1 complex consists of homodimers or heterodimers of Jun, Fos, ATF, or MAF protein families [45], and it can be either oncogenic or anti-oncogenic depending on the context, such as cell type, tumor stage or genetic background [46]. c-Fos and Fra1 are known to promote angiogenesis and the invasive growth of cancer by regulating the expression of matrix metalloproteinases (MMPs) and urokinase plasminogen activator system [46]. In addition, AP-1 can regulate genes required for tumor metastasis; c-Fos and c-Jun can induce epithelial to mesenchymal transition (EMT) in cells [46]. Within the tumor allograft model, we found a significant decrease in the expression of c-Fos with both silibinin and DHS treatments in comparison to control. There are conflicting reports about c-Fos expression and its role in BCC; one group reports BCC to have low levels of c-Fos in comparison to normal skin while another group reports an increased expression of c-Fos in BCC [47,48]. These differences may be attributed to the analysis of differing types of BCC tumor samples; thus far, there are five known types of BCC characterized, namely, nodular, infiltrative, micronodular, morpheaform and superficial [49]. The nodular BCC has decreased c-Fos levels in comparison to normal skin whereas the infiltrative type of BCC has increased c-Fos levels. The infiltrative type of BCC is more aggressive and displays a higher rate of recurrence [47]. Since silibinin and DHS were able to significantly decrease the high level of c-Fos observed in ASZ allograft tissues, it seems that both compounds have a potential for their use to treat the more aggressive BCC phenotype.
Comparing the efficacy of silibinin with its derivative DHS, the latter was more effective in both cell culture and animal studies. The cell culture findings in the present study are in line with our recent studies in human bladder, colon, and prostate carcinoma cells showing that DHS is more potent than silibinin in vitro [22]. However, there is no report yet in literature demonstrating the in vivo anti-cancer activity of DHS, and this is the first report showing DHS efficacy against BCC in a nude mouse allograft model without toxicity. Also importantly, DHS showed considerably lower plasma levels compared to silibinin, perhaps due to incomplete absorption or first-pass metabolism [50], but much stronger effect in inhibiting allograft growth. This is also consistent with our cell culture results where DHS caused comparable or stronger effects at almost 1/3rd of silibinin concentrations, although much lower DHS concentration (10 µM) was also not effective in cell culture that might plausibly due to a lack of its activity in targeting critical signaling molecules (EGFR, ERK1/2, Akt, NF-κB and AP-1) at such a lower dose. Overall, clearly, DHS seems a more effective derivative of silibinin; however, more studies investigating the toxicity and tolerability of DHS need to be conducted in future to move this agent forward for additional efficacy studies. On the other hand, there have been over two decades of research on silibinin showing strong anti-cancer and chemopreventive effects without toxicity in several animal models [14,18,38,51], and it is heavily consumed by humans as a dietary supplement for various liver disorders without any known toxicity for decades [52]. Accordingly, it is prudent to still focus on silibinin for future studies defining its anti-cancer and chemopreventive effects against BCC and associated molecular mechanisms. This is also important because, even though DHS has shown better efficacy when fed by oral gavage, we still need to establish its activity in future when applied topically; an applied/real-world treatment protocol that is most practical in the chemoprevention studies for UV radiation-induced skin cancers including BCC and where silibinin has established profound activity.
To summarize, the present study for the first time demonstrated the strong efficacy of both silibinin and DHS against BCC cells in culture and animal models, suggesting the need for additional studies to investigate both chemopreventive and therapeutic efficacy of silibinin and DHS against BCC. Moreover, since both silibinin and DHS abrogated a multitude of signaling pathways in BCC cells, they may be useful agents to combine with Hh inhibitors to clinically manage, prevent and/or treat BCC.
Acknowledgments
Grant Support: This work was supported by NCI R01 grants CA140368, R01 CA140368S NIH/NIAMS SDRC P30 AR057212, CCTSI NCRR/NIH grant 5UL1RR025780 and by Czech-American collaborative project AMVIS LH 13097.
List of Abbreviations
- AP-1
activator protein-1
- ASZ
ASZ001
- BCC
basal cell carcinoma
- CMC
carboxymethylcellulose
- DAB
3’3-diaminobenzidine
- DHS
2,3-dehydrosilibinin
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-related kinase
- Hh
hedgehog
- IHC
immunohistochemistry
- MAPK
mitogen activated protein kinase
- MEK
MAPK/ERK kinase
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor kappa b
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
- Ptch
patch
- Smo
smoothened
- STAT3
signal transducer and activator of transcription 3
- TGFβ
transforming growth factor beta
REFERENCES
- 1.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178(6):890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Exp Dermatol. 2006;15(9):667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 3.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nature reviews Cancer. 2008;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimeault M, Batra SK. Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacol Rev. 2010;62(3):497–524. doi: 10.1124/pr.109.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang JY, Aszterbaum M, Athar M, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/− humans and mice. Cancer Prev Res (Phila) 2010;3(1):25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.So PL, Lee K, Hebert J, et al. Topical tazarotene chemoprevention reduces Basal cell carcinoma number and size in Ptch1+/− mice exposed to ultraviolet or ionizing radiation. Cancer Res. 2004;64(13):4385–4389. doi: 10.1158/0008-5472.CAN-03-1927. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock MA, Bingham SF, Digiovanna JJ, et al. Tretinoin and the prevention of keratinocyte carcinoma (Basal and squamous cell carcinoma of the skin): a veterans affairs randomized chemoprevention trial. J Invest Dermatol. 2012;132(6):1583–1590. doi: 10.1038/jid.2011.483. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Liang X, Ye L, Wang Y. No chemopreventive effect of nonsteroidal anti-inflammatory drugs on nonmelanoma skin cancer: evidence from meta-analysis. PLoS One. 2014;9(5):e96887. doi: 10.1371/journal.pone.0096887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper M, Jaks V, Hohl D, Toftgard R. Basal cell carcinoma - molecular biology and potential new therapies. J Clin Invest. 2012;122(2):455–463. doi: 10.1172/JCI58779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberl M, Klingler S, Mangelberger D, et al. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4(3):218–233. doi: 10.1002/emmm.201100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnidar H, Eberl M, Klingler S, et al. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69(4):1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu D, Fan Q, Zhang X, Xie J. A role for transcription factor STAT3 signaling in oncogene smoothened-driven carcinogenesis. J Biol Chem. 2012;287(45):38356–38366. doi: 10.1074/jbc.M112.377382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bickers DR, Athar M. Novel approaches to chemoprevention of skin cancer. The Journal of dermatology. 2000;27(11):691–695. doi: 10.1111/j.1346-8138.2000.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 14.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41(13):1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25(8):1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 16.Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 2010;12(5):415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raina K, Agarwal R. Combinatorial strategies for cancer eradication by silibinin and cytotoxic agents: efficacy and mechanisms. Acta pharmacologica Sinica. 2007;28(9):1466–1475. doi: 10.1111/j.1745-7254.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin--a promising new treatment for cancer. Anti-cancer agents in medicinal chemistry. 2010;10(3):186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer letters. 2008;269(2):352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazak R, Svobodova A, Psotova J, et al. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorganic & medicinal chemistry. 2004;12(21):5677–5687. doi: 10.1016/j.bmc.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 21.Biedermann D, Vavrikova E, Cvak L, Kren V. Chemistry of silybin. Natural product reports. 2014;31(9):1138–1157. doi: 10.1039/c3np70122k. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal C, Wadhwa R, Deep G, et al. Anti-cancer efficacy of silybin derivatives -- a structure-activity relationship. PLoS One. 2013;8(3):e60074. doi: 10.1371/journal.pone.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gažák R, Trouillas P, Biedermann D, et al. Base-catalyzed oxidation of silybin and isosilybin into 2,3-dehydro derivatives. Tetrahedron Letters. 2013;54(4):315–317. [Google Scholar]
- 24.Raina K, Agarwal C, Agarwal R. Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-kappaB signaling. Molecular carcinogenesis. 2013;52(3):195–206. doi: 10.1002/mc.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deep G, Kumar R, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits fibronectin induced motility, invasiveness and survival in human prostate carcinoma PC3 cells via targeting integrin signaling. Mutat Res Fundam Mol Mech Mutagen. 2014;768:35–46. doi: 10.1016/j.mrfmmm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28(7):1463–1470. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- 27.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells. Molecular cancer therapeutics. 2008;7(7):1817–1826. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deep G, Raina K, Singh RP, Oberlies NH, Kroll DJ, Agarwal R. Isosilibinin inhibits advanced human prostate cancer growth in athymic nude mice: comparison with silymarin and silibinin. Int J Cancer. 2008;123(12):2750–2758. doi: 10.1002/ijc.23879. [DOI] [PubMed] [Google Scholar]
- 29.Velmurugan B, Gangar SC, Kaur M, Tyagi A, Deep G, Agarwal R. Silibinin exerts sustained growth suppressive effect against human colon carcinoma SW480 xenograft by targeting multiple signaling molecules. Pharmaceutical research. 2010;27(10):2085–2097. doi: 10.1007/s11095-010-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derry MM, Raina K, Balaiya V, et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer prevention research (Philadelphia, Pa) 2013;6(7):625–633. doi: 10.1158/1940-6207.CAPR-13-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 Suppl):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Dormoy V, Danilin S, Lindner V, et al. The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth. Molecular cancer. 2009;8:123. doi: 10.1186/1476-4598-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer research. 2006;66(14):7041–7049. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 34.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nature immunology. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 35.Chang F, Steelman LS, Lee JT, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 36.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer treatment reviews. 2004;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Gu M, Dhanalakshmi S, Mohan S, Singh RP, Agarwal R. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005;26(8):1404–1413. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- 38.Gu M, Dhanalakshmi S, Singh RP, Agarwal R. Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(5):1344–1349. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- 39.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67(7):3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 40.Bigelow RL, Jen EY, Delehedde M, Chari NS, McDonnell TJ. Sonic hedgehog induces epidermal growth factor dependent matrix infiltration in HaCaT keratinocytes. J Invest Dermatol. 2005;124(2):457–465. doi: 10.1111/j.0022-202X.2004.23590.x. [DOI] [PubMed] [Google Scholar]
- 41.So PL, Wang GY, Wang K, et al. PI3K-AKT signaling is a downstream effector of retinoid prevention of murine basal cell carcinogenesis. Cancer Prev Res (Phila) 2014;7(4):407–417. doi: 10.1158/1940-6207.CAPR-13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tewari-Singh N, Jain AK, Inturi S, Agarwal C, White CW, Agarwal R. Silibinin attenuates sulfur mustard analog-induced skin injury by targeting multiple pathways connecting oxidative stress and inflammation. PLoS ONE. 2012;7(9):e46149. doi: 10.1371/journal.pone.0046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kavitha CV, Deep G, Gangar SC, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits prostate cancer cells- and RANKL-induced osteoclastogenesis by targeting NFATc1, NF-kappaB, and AP-1 activation in RAW264.7 cells. Mol Carcinog. 2014;53(3):169–180. doi: 10.1002/mc.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9(2):240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 46.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 47.Urabe A, Nakayama J, Taniguchi S, Kuroki R, Hori Y. Expression of the fos oncogene in basal cell carcinoma. J Dermatol Sci. 1994;8(1):50–53. doi: 10.1016/0923-1811(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 48.Bonifas JM, Pennypacker S, Chuang PT, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116(5):739–742. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 49.Rubin AI, Chen EH, Ratner D. Basal-Cell Carcinoma. New England Journal of Medicine. 2005;353(21):2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 50.Hetal TB, Patel, Sneha, Thakkar A review on techniques for oral bioavailability enhancement of drugs. International Journal of Pharmaceutical Sciences Review & Resear. 2010;4(3):203–223. [Google Scholar]
- 51.Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (Feed Studies) National Toxicology Program technical report series. 2011;(565):1–177. [PubMed] [Google Scholar]
- 52.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytotherapy research: PTR. 2010;24(10):1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]