Abstract
Voltage-gated sodium channels (Nav) are essential for initiation and propagation of action potentials. Previous in vitro studies reported that exposure to the Nav toxins veratridine and α scorpion toxin cause persistent downregulation of Nav mRNA in vitro. However the mechanism of this downregulation is not well characterized. Here, we report that the type-II pyrethroid deltamethrin, which has a similar mechanism as these toxins, elicited an approximate 25% reduction in Nav 1.2 and Nav 1.3 mRNA in SK-N-AS cells. Deltamethrin-induced decreases of Nav mRNA were blocked with the Nav antagonist tetrodotoxin, demonstrating a primary role for interaction with Nav. Pre-treatment with the intracellular calcium chelator BAPTA-AM and the calpain inhibitor PD-150606 also prevented these decreases, identifying a role for intracellular calcium and calpain activation. Because alterations in Nav expression and function can result in neurotoxicity, additional studies are warranted to determine whether or not such effects occur in vivo.
Keywords: Pyrethroid, Deltamethrin, Sodium Channel, Intracellular Calcium, Calpain, Tetrodotoxin
INTRODUCTION
Pyrethroids are potent neurotoxic insecticides that account for approximately 25% of the total annual insecticide use in the world [1–3]. The primary mechanism by which pyrethroids exert their neurotoxic effects in insects involves delaying the inactivation of voltage-gated sodium channels (Nav) [4]. At sufficient doses, delayed inactivation increases the probability of repeated action potential firing, increasing neuronal excitability, and ultimately leading to convulsions and death [5]. In vitro mechanistic studies have reported that concentrations of the pyrethroid insecticide deltamethrin over 1 μM result in apoptosis that is dependent on interaction of deltamethrin with the Nav, which causes intracellular calcium overload and activation of the endoplasmic reticulum (ER) stress pathway [6]. However, reports on the effects of lower doses of deltamethrin on neuronal cells are scarce.
Nav are integral membrane proteins principally composed of a large (~260 kDa) α subunit that forms a central ion-conducting pore and one or more smaller auxiliary β subunits that are important modifiers of channel-gating kinetics, cell-surface expression, and cell-cell interactions [7]. The mammalian genome contains 10 sodium channel α subunit isoforms and four β subunit isoforms, yielding multiple subunit combinations, some of which are differentially sensitive to the effects of pyrethroids [8]. In an oocyte expression system, deltamethrin exhibited a pronounced effect on sodium currents through Nav1.3 [9] and Nav1.6 channels [10]. This may be particularly important to the potential developmental neurotoxicity of deltamethrin, as Nav1.3 channel complexes are highly expressed in the developing rodent brain [11] and the Nav1.6 isoform is abundantly expressed in the nodes of Ranvier, dendrites, and synapses [12].
In previous in vitro studies, compounds such as veratridine and scorpion toxin, that also delay the inactivation of Nav, produced Nav mRNA downregulation [13]. However, the mechanism of this effect remains to be established. Here, we report that exposure to the pyrethroid deltamethrin results in down-regulation of Nav 1.2 and Nav 1.3 mRNA expression in vitro. This downregulation of Nav expression was prevented by the Nav antagonist tetrodotoxin (TTX), depletion of intracellular calcium by BAPTA-AM, and the calpain inhibitor PD150606 prevents Nav mRNA downregulation. Taken in concert, these data identify downregulation of Nav mRNA as a consequence of pyrethroid pesticide exposure and establish a signaling cascade that regulates mRNA expression of Nav.
MATERIALS AND METHODS
Chemicals
Deltamethrin was purchased from ChemService (99.5% purity and lot 418–66B; West Chester, PA). TTX, BAPTA-AM, and protease inhibitor cocktail were obtained from Sigma-Aldrich (St. Louis, MO). The calpain inhibitor PD 150606 was obtained from Alexis Biochemicals (San Diego, CA). Cell culture media and reagents were obtained from Mediatech (Herndon, VA). All other reagents were purchased from Sigma-Aldrich unless otherwise noted.
Cell Culture
SK-N-AS neuroblastoma cells were obtained from American Type Culture Collection (Manassas, VA) and grown in minimal essential media supplemented with 10% heat-inactivated fetal bovine serum, sodium pyruvate (5 mM), nonessential amino acids (5 mM), and penicillin/streptomycin (50 IU/50 μg/mL). Cells were maintained at 37°C with 5% CO2. SK-N-AS cells were chosen as an in a vitro model because we have determined that they express Nav 1.2 and 1.3 isoforms (data not shown). Previous studies from our laboratory have reported TTX-sensitive sodium influx in response to deltamethrin in SK-N-AS cells [6].
In Vitro Exposures
Cells were seeded at 3.0 × 105 per well in a six-well plate and treated 24 h after plating. A concentration–effect curve was generated to determine the optimal concentration of deltamethrin for Nav mRNA down-regulation. Concentrations used were 100 pM, 1 nM, 10 nM,100nM,and 1 μM.A stock solution of deltamethrin (10 mM) was prepared in absolute ethanol (EtOH), and dilutions were made in cell culture medium such that the final concentration of EtOH was less than 0.5% for all experiments. This concentration of EtOH had no effect on the parameters measured in these experiments (data not shown).
To determine the mechanism of Nav mRNA down-regulation, cells were pretreated with TTX (1 μM in H2O; Sigma), the intracellular calcium chelator BAPTA-AM (5 μM in DMSO; Sigma), or the calpain inhibitor PD 150606 (1 μM in DMSO; Alexis Biochemicals) 20 min prior to treatment with 100 nM deltamethrin for 24 h. Cells were lysed and harvested 24 h after treatment for RNA extraction using the Qiagen RNeasy extraction kit according to the manufacturer’s instructions. cDNA construction and quantitative real-time polymerase chain reaction (qPCR) was performed as described below. Human primers are presented in Supplementary Table 1 in the Supporting Information. All cell culture experiments were repeated at least three times using separate frozen stocks of cells.
Quantitative Real-Time Polymerase Chain Reaction
qPCR was performed as described previously [14]. Briefly total RNA was isolated using Qiagen RNeasy mini kits and RNA (1.0 μg) was reverse-transcribed using Superscript II (Invitrogen, Carlsbad, CA). qPCR reactions were performed in duplicate using an ABI 7900HT and SYBR Green (Applied Biosystems, Carlsbad, CA) detection. β-actin was used as the normalizing gene, and data were calculated using the 2ΔΔCt method as described previously [14]. Primers were designed using the Primer Blast program (NCBI) and are given in Supplementary Table 1 in the Supporting Information.
Statistical Analysis
All statistical analyses were performed on normalized raw data. For cell culture experiments, assays were repeated a minimum of three times on different days and with different vials of frozen stocks of cells. On individual days, experiments were performed in duplicate or triplicate and average to form a single experimental unit. Normalized values, determined using the 2ΔΔCt method, were analyzed by ANOVA, and Dunnett’s post hoc tests were performed to compare treatments to control. Variability across control plates was included so that the assumptions underlying an ANOVA were met. For experiments with antagonists, 2-way ANOVA followed by the Student-Neuman Keuls post hoc test was performed. Statistical significance was determined at level of p ≤ 0.05. Data are presented as mean ± SEM.
RESULTS
Deltamethrin Downregulates Nav Expression In Vitro
To characterize the effects of deltamethrin on the expression of mRNAs encoding Nav1.2 and Nav1.3, SK-N-AS cells were exposed to deltamethrin for 24 h and a concentration–effect curve was generated using doses ranging from 1 nM to 1 μM. These isoforms were chosen because they represent the predominant isoforms present in the brain during development. The highest concentration of deltamethrin (1 μM) was well below that which is known to cause cytotoxicity in SK-N-AS cells (10 μM [6]). Exposure to deltamethrin (10 nM to 1 μM) for 24 h produced concentration–dependent reductions of Nav1.2 (10%–37%) and Nav1.3 (13%–26%) mRNA expression (Figs. (Figs 1A and 1B).
FIGURE 1.
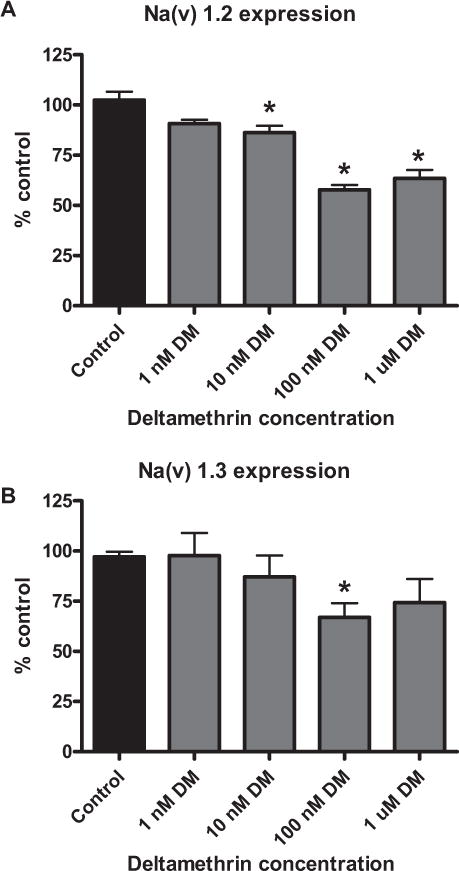
Concentration response for Nav downregulation in SK-N-AS cells. Cells were exposed to deltamethrin for 24 h and mRNA expression determined. (A) Nav 1.2 mRNA expression. (B) Nav 1.3 mRNA expression. Data represent 2ΔΔCt analysis of qPCR data, normalized to control. Significance determined by one-way ANOVA followed by Dunnett’s post hoc test * = p < 0.05 compared to control; n = 3–4. Error bars represent ± SEM
Tetrodotoxin Prevents Deltamethrin-Induced Downregulation of Nav Expression
Based on the robust effects of 100 nM deltamethrin on the downregulation of Nav1.2 and Nav1.3 mRNA expression observed in SK-N-AS cells following 24 h of exposure, this concentration was chosen for subsequent mechanistic studies. We first sought to test the requirement of interaction with the Nav by replicating our observations with the Nav agonist veratridine. Exposure of SK-N-AS cells to veratridine (5 ?M) for 24 h resulted in a 22% and 28% reduction in Nav1.2 and Nav1.3 mRNA expression, respectively, which was similar in magnitude to that from deltamethrin exposure (Figs. (figs 2A and 2B). Furthermore, pre-treatment with the sodium channel antagonist TTX completely abolished the effects of deltamethrin, suggesting that the down-regulation of Nav by deltamethrin involved a direct interaction with sodium channels.
FIGURE 2.
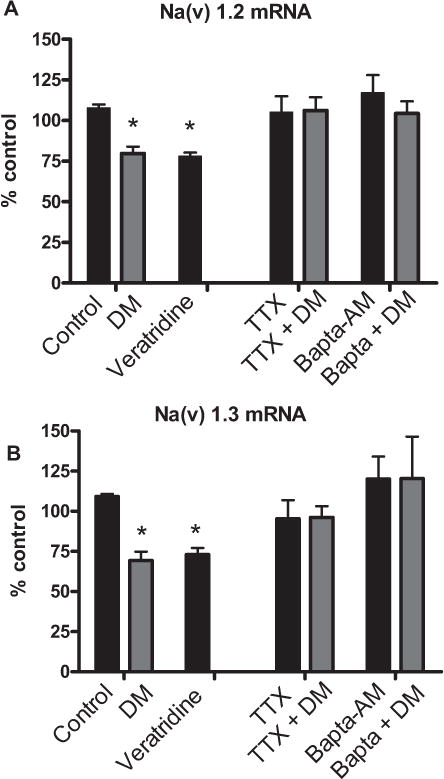
Downregulation Nav mRNA in SK-N-AS cells by deltamethrin is prevented by the Nav antagonist TTX and the intracellular calcium chelator BAPTA-AM. (A) Nav 1.2 mRNA relative expression in response to pretreatment of cells with the Nav antagonist TTX and depletion of intracellular calcium. (B) Nav 1.3 mRNA expression in response to pretreatment of cells with the Nav inhibitor TTX and depletion of intracellular calcium. Data represent 2ΔΔCt analysis of qPCR data, normalized to control. Significance determined by 2-way ANOVA with Student-Neuman Keul’s post hoc test. * = p < 0.05 compared to control; n = 3–4. Error bars represent ±SEM
Calpain Activation Is Associated with Decreased Nav Expression In Vitro
Previously, we reported that higher concentrations (>1 μM) of deltamethrin resulted in a secondary rise of intracellular calcium and activation of calpain, following interaction with Nav [6]. Elevation of intracellular calcium and activation of calpain by a calcium ionophore has been demonstrated to downregulate Nav mRNA expression in adrenal chromaffin cells [15]. Therefore, we sought to determine the role of calcium and calpain on the expression of Nav following exposure to deltamethrin. Pre-treatment of cells with either the intracellular calcium chelator BAPTA-AM (5 μM) (Figs. 2A and 2B) or the calpain inhibitor PD 150606 (1 μM) completely abolished the effects of deltamethrin on Nav mRNA expression (Figs. 3A and 3B).
FIGURE 3.
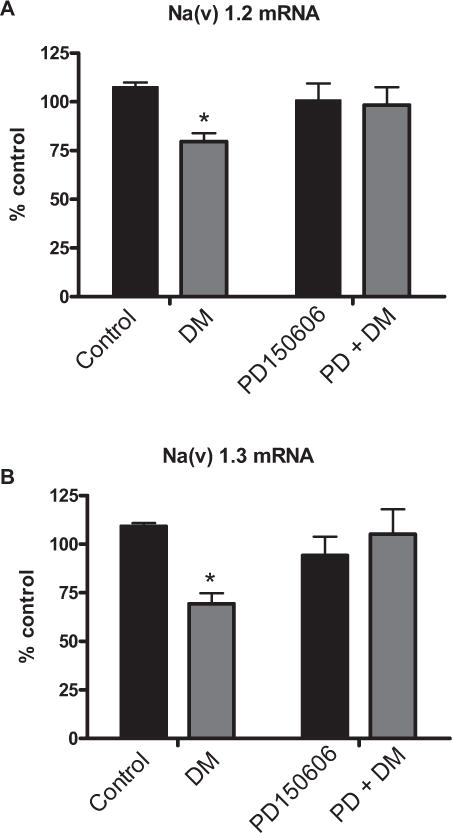
Calpain inhibition prevents deltamethrin-induced downregulation of Nav mRNA. Pretreatment of cells with the calpain inhibitor PD150606 prevents deltamethrin-induced downregulation of (A) Nav 1.2 mRNA and (B) Nav 1.3 mRNA. Data represent 2ΔΔCt analysis of qPCR data, normalized to control. Significance determined by 2-way ANOVA with Student-Neuman Keul’s post hoc test. * = p < 0.05 compared to control; n = 3–4. Error bars represent ±SEM
DISCUSSION
Here, we report that deltamethrin exposure caused a significant reduction of Nav α subunit mRNAs in SK-N-AS neuroblastoma cells, similar to studies that found α-scorpion toxin and veratridine downregulated Nav mRNA in cultured fetal neurons. From a mechanistic standpoint, downregulation of Nav mRNA was prevented by TTX pretreatment, demonstrating the requirement of interaction with the Nav. It is also important to note that interaction of deltamethrin with the Nav was required for this downregulation, as exposure to the general depolarizing agent KCl was unable to produce this effect (data not shown). Deltamethrin-induced downregulation of sodium channel mRNA was also be prevented by the calcium chelator BAPTA-AM, which was previously shown to be partially effective in inhibiting the rapid, short-term down-regulation of Nav by scorpion toxin at the protein [16], but not at the mRNA level [13]. Although, mRNA downregulation is the most likely cause of the reductions we measured, it is also possible that posttran-scriptional changes, such as mRNA degradation, could play a role. Taken together, these results indicate that the downregulation of Nav mRNA by deltamethrin is primarily Na+ dependent and secondarily Ca2+ dependent.
Based on our finding of the Na+ and Ca2+ dependency of Nav mRNA downregulation, we next focused on the potential role of calpain. Calpain, a nonprotea-somal calcium-dependent protease, has recently been shown to cleave Nav protein in rat brain homogenates [17] and cultured neurons. Here, pretreatment with the calpain inhibitor PD 150606 prevented Nav mRNA downregulation in cells exposed to deltamethrin. To our knowledge, this is the first time calpain activation has been shown to be a requirement for Nav mRNA downregulation. Although the exact mechanism for this effect is not clear, the proteolytic activity of calpain is involved in the transduction of neuronal depolarization into changes in gene expression through generation or degradation of active transcription factors [18–21]. Indeed, calpain has been shown to cleave the immediate early genes c-Fos and c-Jun in vitro leaving the DNA-binding domain intact [22, 23]. This binding domain accumulates in the cell allowing for transcriptional repression of target genes [22, 23]. Calpain is also able to cleave transcription factors in such a way as to preserve their DNA-binding domains but remove their transcriptional ability, which would further block transcription of target genes [22, 23]. This mechanism is similar to that which has been observed for other transmembrane proteins such as voltage-gated calcium channels [20, 21] and some metabotropic glutamate receptors [24]. Additionally, protein kinase C, which can regulate Nav mRNA expression, has been shown to be cleaved by calpain, resulting in translocation of a regulatory fragment to the nucleus where it regulates gene transcription [25]. Further studies are required to identify which of these mechanisms, or others, are responsible for the effects observed here.
In summary, this study demonstrates that deltamethrin downregulates Nav mRNA through its interaction with the Nav and subsequent increase in intracellular calcium and activation of calpain. Because alterations in Nav expression and function can result in significant alteration of neurotransmission [26–30], additional studies are warranted to determine whether these effects are observed in vivo identify and what physiological consequences may arise following such changes.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health (R01ES015991 and P30ES005022) and a graduate fellowship to JPM from Bristol Myers Squibb. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or Bristol Myers Squibb.
Footnotes
Supporting Information is available in the online issue at www.wileyonlinelibrary.com
SUPPORTING INFORMATION
References
- 1.Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu Rev Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A, Whyatt RM. Characterization of residential pest control products used in inner city communities in New York City. J Expo Sci Environ Epidemiol. 2011;21(3):291–301. doi: 10.1038/jes.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan MK. Children’s exposures to pyrethroid insecticides at home: a review of data collected in published exposure measurement studies conducted in the United States. Int J Environ Res Public Health. 2012;9(8):2964–2985. doi: 10.3390/ijerph9082964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narahashi T. Mode of action of pyrethroids. Bull World Health Organ. 1971;44(1–3):337–345. [PMC free article] [PubMed] [Google Scholar]
- 5.Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol. 1996;79(1):1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 6.Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci. 2011;122(2):512–525. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isom LL. Sodium channel beta subunits: anything but auxiliary. Neuroscientist. 2001;7(1):42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- 8.Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol. 2012;86(2):165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to alpha-cyano containing pyrethroids. Toxicol Appl Pharmacol. 2008;231(3):273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Na(v)1.6 sodium channels. Toxicol Appl Pharmacol. 2010;247(3):229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albrieux M, Platel JC, Dupuis A, Villaz M, Moody WJ. Early expression of sodium channel transcripts and sodium current by cajal-retzius cells in the preplate of the embryonic mouse neocortex. J Neurosci. 2004;24(7):1719–1725. doi: 10.1523/JNEUROSCI.3548-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97(10):5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lara A, Dargent B, Julien F, Alcaraz G, Tricaud N, Couraud F, Jover E. Channel activators reduce the expression of sodium channel alpha-subunit mRNA in developing neurons. Brain Res Mol Brain Res. 1996;37(1–2):116–124. doi: 10.1016/0169-328x(95)00286-2. [DOI] [PubMed] [Google Scholar]
- 14.Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a gender-specific manner. Neurotoxicology. 2008;29(1):855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiraishi S, Shibuya I, Uezono Y, Yokoo H, Toyohira Y, Yamamoto R, Yanagita T, Kobayashi H, Wada A. Heterogeneous increases of cytoplasmic calcium: distinct effects on down-regulation of cell surface sodium channels and sodium channel subunit mRNA levels. Br J Pharmacol. 2001;132(7):1455–1466. doi: 10.1038/sj.bjp.0703960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dargent B, Jullien F, Couraud F. Internalization of voltage-dependent sodium channels in fetal rat brain neurons: a study of the regulation of endocytosis. J Neurochem. 1995;65(1):407–413. doi: 10.1046/j.1471-4159.1995.65010407.x. [DOI] [PubMed] [Google Scholar]
- 17.von Reyn CR, Spaethling JM, Mesfin MN, Ma M, Neumar RW, Smith DH, Siman R, Meaney DF. Calpain mediates proteolysis of the voltage-gated sodium channel alpha-subunit. J Neurosci. 2009;29(33):10350–10356. doi: 10.1523/JNEUROSCI.2339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munarriz E, Bano D, Sayan AE, Rossi M, Melino G, Nicotera P. Calpain cleavage regulates the protein stability of p73. Biochem Biophys Res Commun. 2005;333(3):954–960. doi: 10.1016/j.bbrc.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 19.Tremper-Wells B, Vallano ML. Nuclear calpain regulates Ca2+-dependent signaling via proteolysis of nuclear Ca2+/calmodulin-dependent protein kinase type IV in cultured neurons. J Biol Chem. 2005;280(3):2165–2175. doi: 10.1074/jbc.M410591200. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127(3):591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104(12):1373–1381. doi: 10.1161/CIRCRESAHA.108.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirai S, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991;287(1–2):57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- 23.Watt F, Molloy PL. Specific cleavage of transcription factors by the thiol protease, m-calpain. Nucleic Acids Res. 1993;21(22):5092–5100. doi: 10.1093/nar/21.22.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch DR, Gleichman AJ. Picking up the pieces: the roles of functional remnants of calpain-mediated proteolysis. Neuron. 2007;53(3):317–319. doi: 10.1016/j.neuron.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Matkovich SJ, Duan X, Diwan A, Kang MY, Dorn GW., 2nd Receptor-independent protein kinase C alpha (PKCalpha) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J Biol Chem. 2011;286(30):26943–26951. doi: 10.1074/jbc.M111.234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellerkmann RK, Remy S, Chen J, Sochivko D, Elger CE, Urban BW, Becker A, Beck H. Molecular and functional changes in voltage-dependent Na(+) channels following pilocarpine-induced status epilepticus in rat dentate granule cells. Neuroscience. 2003;119(2):323–333. doi: 10.1016/s0306-4522(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 27.Gu XQ, Haddad GG. Decreased neuronal excitability in hippocampal neurons of mice exposed to cyclic hypoxia. J Appl Physiol. 2001;91(3):1245–1250. doi: 10.1152/jappl.2001.91.3.1245. [DOI] [PubMed] [Google Scholar]
- 28.Kullmann DM, Waxman SG. Neurological channelopathies: new insights into disease mechanisms and ion channel function. J Physiol. 2010;588(Pt 11):1823–1827. doi: 10.1113/jphysiol.2010.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanWart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking nav1.6 sodium channels. J Neurosci. 2006;26(27):7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waxman SG. Channel, neuronal and clinical function in sodium channelopathies: from genotype to phenotype. Nat Neurosci. 2007;10(4):405–409. doi: 10.1038/nn1857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


