Abstract
Background
It is well established that chronic ethanol (EtOH) consumption is associated with increased incidence and disease severity of respiratory infections. Our recent work demonstrates this increase in disease severity to influenza virus (IAV) infections is due, in part, to a failure to mount a robust IAV-specific CD8 T cell response along with a specific impairment in the ability of these T cells to produce IFNγ. However, the full extent of the lesion in the effector CD8 T cell compartment during chronic EtOH consumption remains unknown.
Methods
Utilizing the Meadows-Cook murine model of chronic alcohol consumption, mice received EtOH in their drinking water for 8 or 12 weeks. Mice were challenged intranasally with IAV and the activation and effector functions of IAV specific CD8 T cells were determined in both the lung-draining lymph nodes and lungs.
Results
Our results confirm the defect in interferon γ (IFNγ) production, however, the ability of IAV-specific T cells to produce tumor necrosis factor α (TNFα) and interleukin-2 (IL-2) in EtOH-consuming mice remains unaltered while interferon γ (IFNγ) production. In contrast, EtOH consumption significantly reduces the ability of CD8 T cells to degranulate and kill IAV-specific targets. Finally, our findings suggest the lesion begins during the initial activation of CD8 T cells, as we observe early defects in proliferation in the lung-draining lymph nodes (dLN) of IAV-infected, EtOH-consuming mice.
Conclusions
These findings highlight the previously unrecognized depth of the lesion in the IAV-specific CD8 T cell response during chronic EtOH consumption. Given the important role CD8 T cell immunity plays in control of IAV, these findings may aid in the development of vaccination and/or therapeutic strategies to reverse these defects in the CD8 T cell response and reduce serious disease outcomes associated with IAV infections in alcoholics.
Keywords: Influenza Virus, CD8 T cells, lung, lymph node, Chronic Ethanol Consumption
Introduction
Chronic alcohol abuse by humans is associated with increased incidence and severity of pulmonary bacterial and viral infections (Happel and Nelson, 2005, Nelson and Kolls, 2002, Zhang et al., 2002, Cook, 1998). While studies have begun to elucidate the mechanisms leading to this increased pulmonary disease severity in alcoholics, much remains unknown (Happel and Nelson, 2005, Legge and Waldschmidt, 2014, Meyerholz et al., 2008, Joshi and Guidot, 2007, Nelson et al., 1991, Zhang et al., 2002, Brown et al., 2004, Downs et al., 2013, Sisson, 2007, Bird and Kovacs, 2008). Therefore, it has been of continued interest to determine the effect of chronic alcohol consumption on the pulmonary adaptive immune response. Previously, our group has demonstrated increased morbidity and mortality along with an increase in viral titers following infection with influenza A virus (IAV) in a murine model of chronic ethanol (EtOH) consumption (Meyerholz et al., 2008, Langlois et al., 2010). This defect in viral clearance and increased disease is correlated with a decrease in the number of total and IAV-specific CD8 T cells in mice chronically consuming EtOH. Further, the IAV-specific CD8 T cells in EtOH-consuming mice also exhibit decreased interferon γ (IFNγ) production (Meyerholz et al., 2008).
The development of a cytotoxic CD8 T cell response is required to clear an acute IAV infection (Bender et al., 1992, Doherty et al., 1997). In addition to producing pro-inflammatory cytokines [e.g. IFNγ, tumor necrosis factor α (TNFα), and interleukin-2 (IL-2)], CD8 T cells can also kill virally infected cells via perforin/granzymes, tumor necrosis factor receptor apoptosis-inducing ligand- (TRAIL-), and/or Fas-mediated apoptosis (Brincks et al., 2008, Topham et al., 1997, Doherty et al., 1997). However, what effect chronic EtOH exposure has on these functions remains unknown. Therefore, we determined the depth of the lesion in the effector response of CD8 T cells within EtOH-consuming mice during IAV infection.
Our results demonstrate that extension of our previous studies to 12 weeks of chronic EtOH consumption maintains the defect in IFNγ production, but surprisingly not TNFα or IL-2, by IAV-specific CD8 T cells. Given the known elevation in viral titers during IAV infection within EtOH-consuming mice (Langlois et al., 2010, Meyerholz et al., 2008), we also sought to determine whether any direct mechanisms of CD8 T cell killing were affected by chronic EtOH consumption. We observed a significant decrease in degranulation which correlates to a significant reduction in the ability of IAV-specific CD8 T cells from the lungs of IAV-infected, EtOH-exposed mice to kill IAV-peptide pulsed targets in vitro.
As the defects in the IAV-specific CD8 T cell population were observed at the peak of the immune response, we also sought to identify when this lesion in the IAV-specific CD8 T cell response first appears. It has been demonstrated that IAV-specific CD8 T cells first acquire effector functions in draining lymph nodes (dLN) (Kim and Braciale, 2009, Legge and Braciale, 2003). Further, the acquisition of IFNγ production has been linked to cell division, with only activated CD8 T cells that have undergone 4 or more rounds of division expressing IFNγ (Lawrence and Braciale, 2004). It has also been documented that IAV-specific CD8 T cells must interact with pulmonary dendritic cells (DCs) within the lungs following activation in order to avoid apoptosis and continue to proliferate and develop a robust T cell response (McGill and Legge, 2009, McGill et al., 2008). This indicates that signals in the dLN and/or lungs could be contributing to the lesion within the IAV-specific CD8 T cell response of mice chronically consuming EtOH. Our results suggest that the lesion in the effector response begins within the dLN, as we observed a significant defect in proliferation and IFNγ production by IAV-specific CD8 T cells within the dLN of EtOH-consuming mice. Together, these findings highlight the previously unrecognized depth of the lesion in the IAV-specific CD8 T cell response within mice chronically consuming EtOH. Targeting to reverse these specific defects in proliferation, degranulation, cytotoxicity, and IFNγ production may aid in treating increased disease severity and delayed viral clearance during IAV infection of chronic alcoholics.
Materials and Methods
Mice
Six- to eight-week-old wild type (WT) C57Bl/6 and BALB/c mice were obtained from The National Cancer Institute (Frederick, MD). Clone 4 (CL-4) CD90.1+ mice containing T cell receptor (TCR) transgenic T cells specific for the HA533 epitope of A/PR/8/34 were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed and maintained in the specific pathogen-free animal care facility at the University of Iowa (Iowa City, IA). All experiments were performed in accordance with regulatory standards and guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Iowa. Unless noted otherwise, all experiments herein were performed with C57Bl/6 mice.
EtOH Administration
Mice were administered EtOH using the Meadows-Cook model of administration (Cook et al., 2007, Cook et al., 2004, Gurung et al., 2009, Meyerholz et al., 2008, Ness et al., 2008, Song et al., 2002, Parlet and Schlueter, 2013). After 1 week of acclimation, mice were separated into two groups of equal size. One group was transitioned to pharmaceutical grade EtOH by receiving the following in drinking water: 10% for 2 days, 15% for 5 days, and 20% for 12 wks. BALB/c mice received the following: 10% for 2 days, 15% for 5 days, and 18% for 8 wks. These EtOH solutions were the only source of water available for the mice in the EtOH group. The control animals were given the same double-distilled water used for the EtOH solutions. Mice were given access to the appropriate source of drinking fluid and laboratory chow ad libitum throughout the treatment period. With this ethanol administration protocol, mice were visibly inebriated in the early morning following nocturnal drinking, and blood alcohol levels were as high as 400 mg/dL, with much lower levels later in the day (Song et al., 2002). Mice remained on this EtOH protocol throughout the duration of experiments.
Influenza A virus infection
Influenza A virus strain A/PR/8/34 was prepared from stocks as described previously (Legge and Braciale, 2003). Mice were anesthetized with isoflurane and infected intranasally with 1.1×103 tissue culture infectious units of virus.
MHC I Tetramers
MHC class I tetramers specific for influenza nucleocapsid protein (NP)366-374 (H-2D(b)/ASNENMETM) and influenza acidic polymerase protein (PA)224-233 (H-2D(b)/SSLENFRAYV) were obtained from the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility (Atlanta, GA).
Surface Staining
Single-cell suspensions of lungs were prepared by pressing them through wire mesh screens. 1×106 cells/well were placed in a 96-well plate and blocked with 2 μl rat serum in FACS buffer for 30 min at 4°C.
Following blocking, cells were incubated with FACS buffer containing rat anti-mouse CD8α monoclonal antibody (mAb) (53-6.7), rat anti-mouse CD3ε mAb (145-2C11), hamster anti-mouse CD95L mAb (MFL3), rat anti-mouse TRAIL mAb (N2B2), mouse anti-human/mouse granzyme B mAb (GB11), and NP366 and PA224 tetramers for 30 min at 4°C. Cells were then fixed in FACS Lysis Buffer (BD) per manufacturer’s instructions and resuspended in PBS. Data was acquired on a BD FACSCanto II and analyzed with FlowJo software (TreeStar, Inc.).
In Vitro Intracellular Cytokine and CD107a Assay
Intracellular cytokine staining was performed as previously described (Hemann et al., 2013). Briefly, 5×105 cells from lung single-cell suspensions were incubated for 6 h with NP366 or PA224 peptides in complete media containing recombinant human IL-2 (Novartis) and brefeldin A (Sigma). For CD107a degranulation, this incubation was performed in the absence of brefeldin A. Anti-mouse CD107a mAb (1D4B) was present during peptide incubation in order to measure degranulation (Brincks et al., 2008). Following incubation, cells were surface stained with rat anti-mouse CD8αmAb (53-6.7). Following fixation, cells were permeablized by incubation for 30 min at 4°C in FACS Buffer containing 0.5% saponin (ACROS) and subsequently stained with rat anti-mouse IFNγ (XMG1.2), rat anti-mouse TNFα (MP6-XT22), and rat anti-mouse IL-2 (JES6-5H4) for 30 min at 4°C in FACS Buffer containing 0.5% saponin. Data was acquired on a BD FACSCanto II and analyzed with FlowJo software (TreeStar, Inc.).
In Vitro Cytotoxicity Assay
In vitro cytotoxicity assay was performed as previously described (Brincks et al., 2011, Legge and Braciale, 2005). On day 8 following IAV infection, lungs were harvested from water- and EtOH-consuming mice without perfusion or prior BAL wash, homogenized, and CD8 T cells from total lung homogenate were MACS purified (>95% purity). A portion of the purified T cells was then stained with anti-CD8α and anti-CD11a mAbs. The percentage of CD11a+ CD8αlo T cells was used to calculate the number of antigen experienced (IAV-specific) effectors (Rai et al., 2009). For target cells, naïve C57Bl/6 splenocytes were labeled with 2μM PKH. One half was subsequently labeled with 0.5μM CFSE, and the other half was labeled with 3μM CFSE. The CFSElo cells were pulsed with 10μM OVA257 peptide as a control while the CFSEhi cells were pulsed with 10 μM NP366 and PA224 peptide for 1 h at 37°C. The effector CD8 T cells were mixed with the peptide-pulsed target cells at a 10:1 and 25:1 E:T ratio and cultured for 8 h in complete media. Following incubation, samples were run on the flow cytometer to determine cell populations present. The percent of CFSEhi and CFSElo cells were adjusted based on the adjustment of the target only wells to 50:50. The percent specific killing was then calculated: ([adjusted CFSEhi cell #/adjusted CFSElo cell #] × 100).
In Vivo Intracellular Cytokine Assay
5×106 naïve, CFSE-labeled, CL-4 CD90.1 cells (H-2d) from the spleens of EtOH-or water-consuming mice were transferred intravenously into equivalent (EtOH- or water-consuming) BALB/c hosts (H-2d). Mice were infected with a 0.1 LD50 of A/PR/8/34. On day 4 post infection (p.i.), mice were administered 500 μg monensin intraperitoneally as previously described (Hufford et al., 2011, Liu and Whitton, 2005). 6 hours following treatment, mice were sacrificed, dLN were harvested and single-cell suspensions prepared. Cells were stained extracellularly with mouse anti-rat/mouse CD90.1 (OX-7). Following fixation, cells were permeablized by incubation for 30 min at 4°C in FACS Buffer containing 0.5% saponin (ACROS) and subsequently stained with rat anti-mouse IFNγ (XMG1.2), rat anti-mouse TNFα (MP6-XT22), rat anti-mouse IL-2 (JES6-5H4) and mouse anti-human/mouse granzyme B (GB11) for 30 min at 4°C in FACS Buffer containing 0.5% saponin. Data was acquired on a BD FACSCanto II and analyzed with FlowJo software (TreeStar, Inc.). Percent divided and proliferation index were determined using proliferation measures within the FlowJo software.
Statistical Analysis
Data was compiled in graphical format using Prism software (Graphpad Software, San Diego, CA). Error bars represent the SEM. Statistical significance was determined using unpaired, two-tailed Student’s t tests.
Results
Dysregulation of cytokine production by CD8 T cells in chronic EtOH consumers during IAV challenge is limited to IFNγ
Mice chronically consuming EtOH have been demonstrated to be more susceptible to IAV infections (Meyerholz et al., 2008). One of the mechanisms of this increased susceptibility in murine models of chronic EtOH consumption is due to reduced IFNγ production by IAV-specific CD8 T cells along with a reduction in the total IAV- specific CD8 T cell population detected by major histocompatibility complex (MHC) class I-viral peptide tetramers (Meyerholz et al., 2008). Similar to our previous studies analyzing IAV-specific CD8 T cell responses in mice consuming EtOH for four to eight weeks, C57Bl/6 mice chronically consuming 20% EtOH for twelve weeks prior to IAV infection display a significant reduction in the number of IAV-specific CD8 T cells as determined by tetramer staining (Fig. 1A). However, this decrease in the total numbers of IAV-specific CD8 T cells is due to the documented decrease in the total CD8 T cell population as the frequency of tetramer+ CD8 T cells remains equal between water- and EtOH-consuming mice during IAV infection [(Meyerholz et al., 2008) and Fig. 1A]. In contrast, mice chronically consuming 20% EtOH for twelve weeks have a significant reduction in both the frequency and number of IFNγ-producing, IAV-specific CD8 T cells within the lungs on day 8 p.i. (Fig. 1B). This supplemental lesion within the frequency of IAV-specific CD8 T cells that can produce IFNγ in EtOH-exposed mice demonstrates that in addition to the EtOH-induced reduction in global CD8 T cell immunity, EtOH exposure results in the selective inhibition of IFNγ production by those effector IAV-specific T cells that do develop. Interestingly, this specific defect in IFNγ production by IAV-specific CD8 T cells is rescued when the T cells are stimulated ex vivo with PMA and ionomycin [data not shown and (Meyerholz et al., 2008)], indicating this defect in IFNγ production is specific to a signaling event upstream of Ca2+ flux and PKCθ activation.
Figure 1. Chronic EtOH consumption leads to a significant reduction the number and IFNγ effector ability of IAV-specific CD8 T cells following IAV infection.
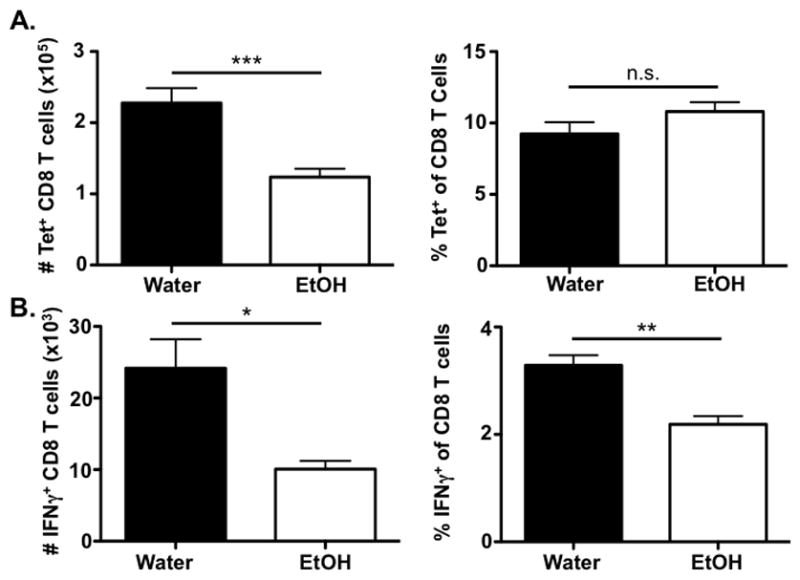
C57Bl/6 EtOH- and water-consuming mice were challenged with a sublethal dose of IAV. A) On day 8 p.i. lungs were harvested and PA224 and NP366 tetramer positive CD3ε+/CD8α+ T cells were identified by flow cytometry. B) On day 8 p.i. lungs were harvested and stimulated with PA224 and NP366 for 6 h. Following stimulation, CD8α+ T cells producing IFNγ were identified by flow cytometry. Data are representative of two independent experiments. n=7–8 mice/group. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001
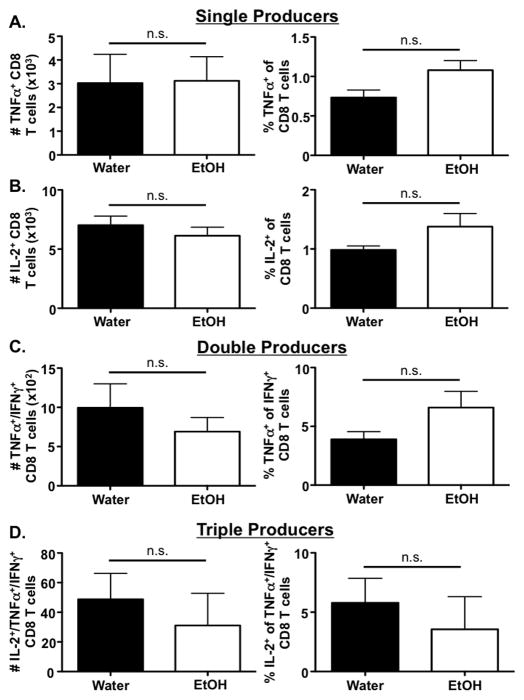
IAV-specific effector CD8 T cells have been shown to produce IFNγ, TNFα and IL-2 synchronously during IAV infection (La Gruta et al., 2004). While TNFα aids in inhibition of viral replication and enhancement of cytolytic activity (Wortzman et al., 2013), IL-2 has been implicated in the activation and expansion of IAV-specific CD8 T cells (Malek, 2008). Given the severe defect in IFNγ production by CD8 T cells during IAV infection in mice chronically consuming EtOH, we next investigated whether the ability of the IAV-specific CD8 T cell population to produce TNFα and/or IL-2 was also altered by EtOH exposure. Surprisingly, the ability of IFNγ+ IAV-specific CD8 T cells to produce TNFα (Fig. 2A) or IL-2 (Fig. 2B) during IAV challenge remains unaltered during chronic EtOH consumption. Further, co-production of either TNFα (Fig. 2C) or TNFα and IL-2 (Fig. 2D) in addition to IFNγ during IAV challenge remains unaltered by chronic EtOH consumption. However, it should be noted that these polyfunctional CD8 T cells represent a very small fraction of the total IAV-specific CD8 T cell population in both water and EtOH-consuming mice (Fig. 1 & Fig. 2). Together, these results demonstrate the EtOH-induced defect in IAV-specific CD8 T cell cytokine production appears to be limited to the IFNγ compartment.
Figure 2. Chronic EtOH consumption does not alter the numbers or frequency of IL-2 and TNFα single-producing or polyfunctional CD8 T cells (IFNγ/TNFα and IFNγ/TNFα/IL-2).
C57Bl/6 EtOH- and water-consuming mice were challenged with a sublethal dose of IAV. On day 8 p.i., the lungs were harvested and stimulated with PA224 and NP366 for 6 h. Following stimulation, the frequency and numbers of CD8α+ T cells producing A) TNFα, and B) IL-2, C) TNFα+/IFNγ+, or D) IL-2+/TNFα+/IFNγ+ were enumerated via flow cytometry. Data are representative of two independent experiments. n=7–8 mice/group.
Chronic EtOH consumption does not reduce FasL and TRAIL expression by IAV-specific CD8 T cells
CD8 T cells utilize multiple mechanisms to control IAV infection within the lungs. In addition to producing inflammatory cytokines, CD8 T cells can induce lysis of virally infected cells by FasL-, and/or TRAIL-dependent mechanisms (Brincks et al., 2008, Doherty et al., 1997, Topham et al., 1997). Therefore, we sought to determine the surface levels of these molecules on IAV-specific CD8 T cells in order to begin to understand whether any of these mechanisms could potentially be involved in the increased susceptibility to IAV infection observed in EtOH-consuming mice (Meyerholz et al., 2008, Langlois et al., 2010). We detected no difference in the frequency of IAV-specific CD8 T cells expressing FasL or the mean fluorescence intensity (MFI) of FasL on IAV-specific CD8 T cells between water and EtOH-consuming mice (Fig. 3A & 3B, left column). Consistent with the EtOH-induced decrease in the total CD8 T cell numbers (Fig. 1), we did detect a significant reduction in the total numbers of IAV-specific CD8 T cells expressing FasL (Fig. 3C, left column). These results indicate that while the total number of FasL+ IAV-specific CD8 T cells is impaired by chronic EtOH consumption, the amount of FasL present on a per cell basis within the IAV-specific CD8 T cell population remains equivalent. We observed a similar trend when analyzing TRAIL expression of IAV-specific CD8 T cells within the lungs of water- and EtOH-consuming mice. While the total number of TRAIL+ IAV-specific CD8 T cells is significantly diminished in EtOH-consuming mice (Fig. 3C, right column), the frequency of TRAIL+ cells within the IAV-specific CD8 T cell population along with TRAIL MFI per cell remained unaltered by chronic EtOH exposure (Fig. 3A & 3B, right column). Together, these results indicate that while the EtOH-induced reduction in total numbers of FasL+ and TRAIL+ IAV-specific CD8 T cells likely contribute to an overall reduced CD8 T cell mediated cytotoxicity of IAV-infected epithelial cells, it appears unlikely that there is a specific, intrinsic defect in FasL- and/or TRAIL-mediated cytotoxicity by IAV-specific CD8 T cells (Meyerholz et al., 2008).
Figure 3. Chronic EtOH consumption leads to a significant reduction in the total number of IAV-specific CD8 T cells in the lung following IAV infection, but does not alter the frequency or MFI of IAV-specific CD8 T cells expressing FasL or TRAIL.
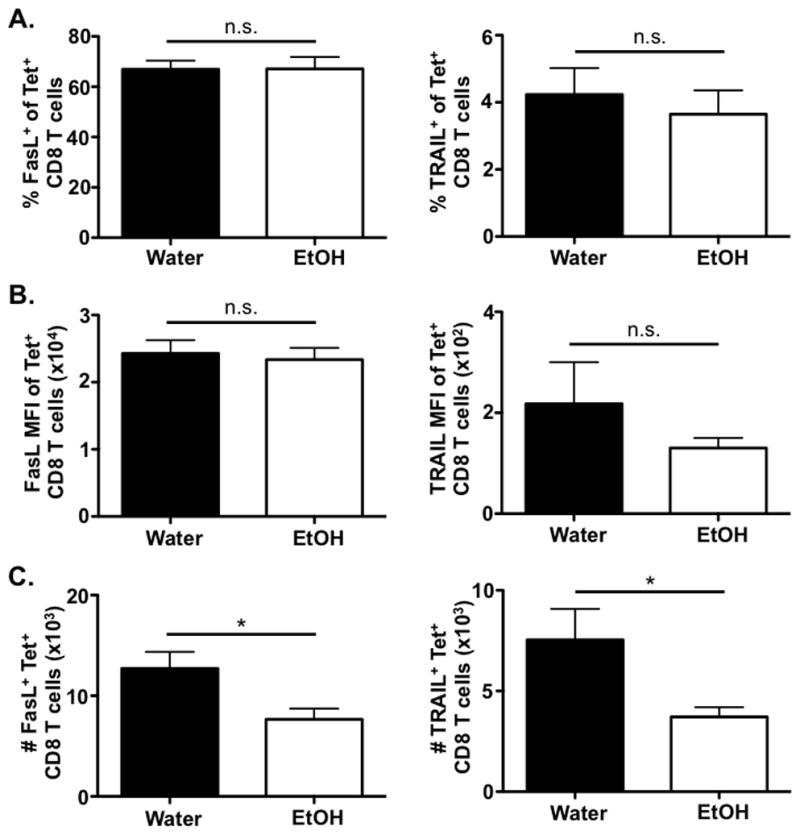
C57Bl/6 EtOH- and water-consuming mice were challenged with a sublethal dose of IAV. On day 8 p.i., the lungs of mice were harvested and PA224 and NP366 tetramer+/CD3ε+/CD8α+ cells were examined for FasL and TRAIL expression via flow cytometry. The A) percent, B) MFI, and C) number of FasL+ and TRAIL+ IAV-specific CD8 T cells were quantified. Data are representative of two independent experiments. n=7–9 mice/group. * indicates p<0.05
Chronic EtOH decreases IAV-specific CD8 T cell degranulation, limiting their cytotoxic ability
In addition to their use of surface expressed death-inducing ligands to mediate cytoxicity, CD8 T cells can release perforin and granzymes to induce the lysis of target cells. Therefore, we next determined whether EtOH exposure altered granzyme B expression within IAV-specific CD8 T cells. As shown in Figure 4, we detected no differences in the frequency, MFI or numbers of granzyme B+ IAV-specific CD8 T cells within the lungs on day 8 p.i. between water- and EtOH-consuming mice (Fig. 4A–4C), indicating chronic EtOH consumption does not alter the granzyme B+ IAV-specific CD8 T cell population during IAV challenge.
Figure 4. Chronic EtOH consumption does not alter granzyme B expression by IAV-specific CD8 T cell, but EtOH exposure reduces the ability of IAV-specific CD8 T cells to degranulate.
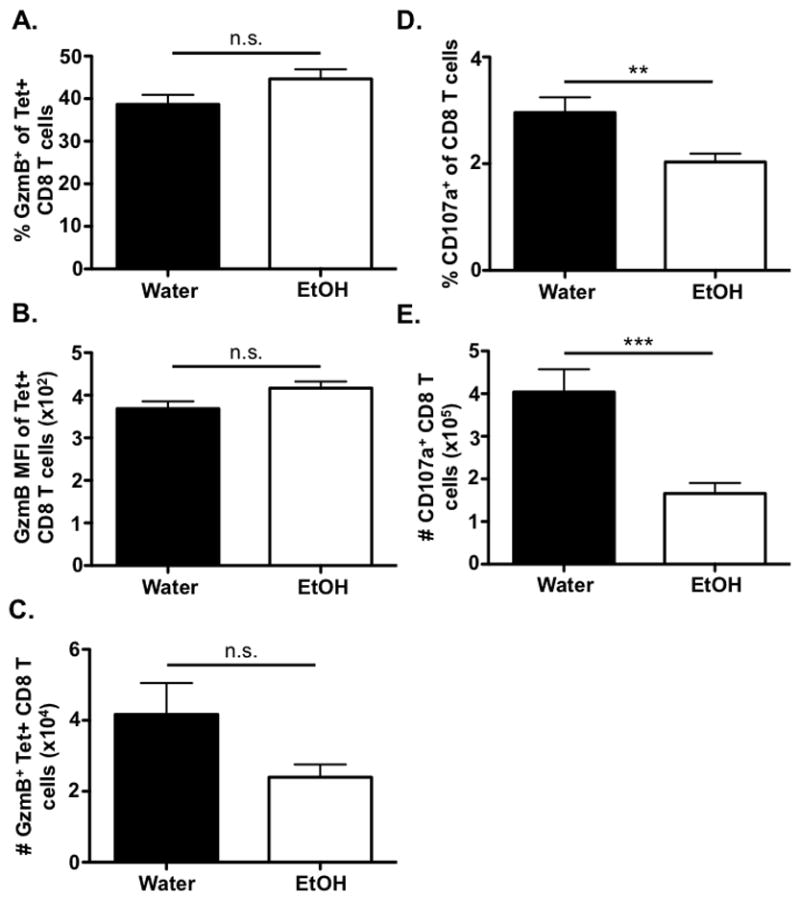
C57Bl/6 EtOH- and water-consuming mice were challenged with a sublethal dose of IAV. On day 8 p.i., the lungs of mice were harvested and PA224 and NP366 tetramer+/CD3ε+/CD8α+ cells were examined for granzyme B expression via flow cytometry. The A) percent, B) MFI, and C) number of granzymeB+ IAV-specific CD8 T cells were quantified. D & E) On day 8 p.i., the lungs were harvested and stimulated with PA224 and NP366 peptides in the presence of anti-CD107a mAb for 6 h. The D) frequency and E) numbers of CD3ε+/CD8α+ cells positive for CD107a were identified by flow cytometry. Data are representative of two independent experiments. n=7–9 mice/group. ** indicates p<0.01, *** indicates p<0.001
Although we detected no difference in the granzyme B expression of IAV-specific CD8 T cells in EtOH-consuming mice during IAV challenge, it is possible that an EtOH-induced defect in cytotoxic T lymphocyte (CTL) killing via the perforin/granzyme pathway could still be present due to altered ability of IAV-specific CD8 T cells to degranulate and thereby release granzyme B. When we monitored the ability of water- and EtOH-exposed CD8 T cells to degranulate (measured by surface CD107a expression), we observed a significant decrease in both the frequency and number of CD107a+ CD8 T cells in EtOH-consuming mice compared to water controls (Fig. 4D & 4E).
The observed EtOH-induced reduction in degranulation suggests IAV-specific CD8 T cells from the lungs of EtOH exposed mice are likely defective in their ability to kill IAV-infected cells. A defect in killing by IAV-specific CD8 T cells from EtOH-exposed mice is consistent with the observed increase in IAV virus titers in EtOH- compared to control water-consuming mice (Meyerholz et al., 2008, Langlois et al., 2010). In order to determine the cytotoxic effector ability of IAV-specific CD8 T cells, we utilized an in vitro cytotoxicity assay. In agreement with the observed EtOH-induced reduction in degranulation, purified IAV-specific CD8 T cells from the lungs of EtOH-consuming mice are significantly reduced in their ability to lyse IAV-peptide pulsed targets relative to control OVA-peptide pulsed targets at a 10:1 and 25:1 E:T ratio (Fig. 5). This data indicates IAV-specific CD8 T cells from the lungs of EtOH-consuming mice on day 8 p.i. are defective in their ability to kill at a per cell level compared to their counterparts from water-drinking controls. This defect in killing may be responsible for the higher viral titer observed within the lungs of mice chronically consuming EtOH during IAV infection (Meyerholz et al., 2008, Langlois et al., 2010).
Figure 5. IAV-specific CD8 T cells from chronic ETOH mice are defective in their ability to kill IAV peptide-pulsed targets.
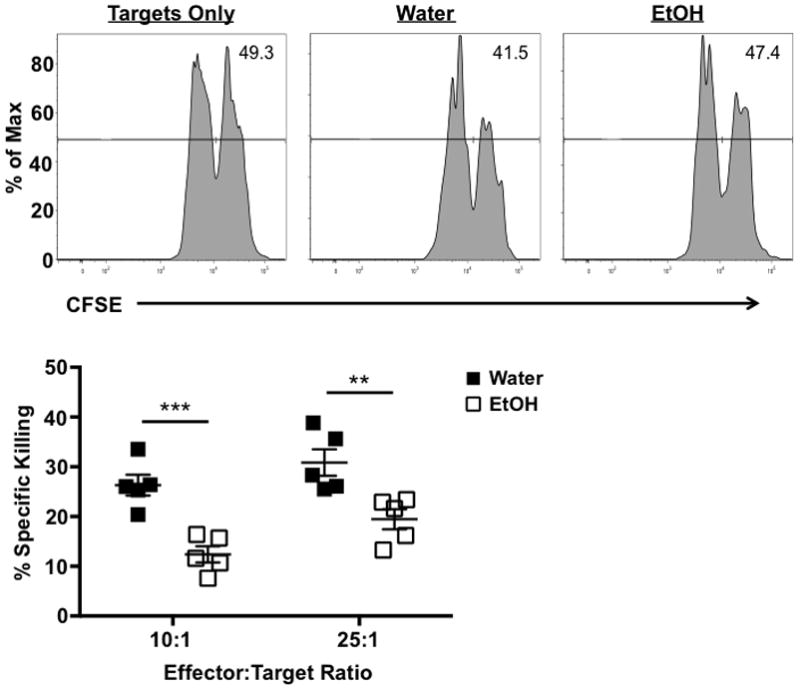
C57Bl/6 EtOH- and water-consuming mice were challenged with a sublethal dose of IAV. On day 8 p.i., the lungs of mice were harvested, homogenized, and CD8α+ cells were isolated by MACS. The effector T cells were then co-cultured with a 1:1 mixture of CFSEhi/IAV peptide-pulsed and CFSElo/OVA peptide-pulsed targets at either a 10:1 or 25:1 E:T ratio for 8 h. The percent of specific killing was determined by flow cytometry following incubation. n=5 mice/group. ** indicates p<0.01, *** indicates p<0.001
The EtOH induced lesion within the IAV-specific CD8 T cell response begins in the dLN during the T cells’ initial activation
The acquisition, expression, and regulation of effector functions by CD8 T cells is linked to both their initial activation in the dLN as well as subsequent programming at peripheral sites. Initially, the production of both IFNγ and granzyme B by CD8 T cells is tied to proliferation, beginning after 4–6 rounds of division following activation in the dLN (Lawrence and Braciale, 2004, Jenkins et al., 2008). Further, our own work, as well as that of others, has documented that IAV-specific CD8 T cells must interact with pulmonary DCs within the lungs following activation in order to avoid apoptosis and continue to proliferate and develop a robust T cell response (McGill and Legge, 2009, McGill et al., 2008). Therefore, regulation/signals in the dLN and/or lungs could be contributing the EtOH-induced lesion within IAV-specific CD8 T cell response. In order to determine whether the EtOH-induced decrease in IFNγ+ IAV-specific CD8 T cells begins in the dLN and correlates with a reduction in division of activated IAV-specific CD8 T cells, we adoptively transferred CFSE-labeled, naïve, CD90.1+ CL-4 cells from water- or EtOH-consuming donors into matched CD90.2+ congenically disparate water- or EtOH-consuming BALB/c hosts. Twenty-four hours later we infected mice, and on day 4 p.i. mice were treated with monensin intraperitoneally in order to block release of intracellular materials. Compared to CL-4 T cells in the water controls, the donor EtOH CL-4 T cells in the dLN of chronic EtOH-consuming hosts exhibit reduced proliferation in response to IAV infection (Fig. 6A), even though they were adoptively transferred at equal numbers to that of water T cells in order to correct for the reduced numbers present in EtOH-exposed mice (Fig. 1). This overall reduced proliferation appears to not be due to a defect in the initial triggering of the naive T cells, as the percent of the initial population that has divided is equal (Fig. 6A-middle panel), but is instead due to reduced overall proliferation of EtOH-exposed CL-4 T cells compared to CL-4s from water-drinking mice measured by proliferation index (Fig. 6A-right panel). Further, this reduced proliferation is not due to an increased death of dividing cells, as measured by Annexin V positivity, in the EtOH-consuming mice (data not shown).
Figure 6. Chronic EtOH consumption leads to an early defect in proliferation and IFNγ production in lung-draining lymph nodes during IAV infection.
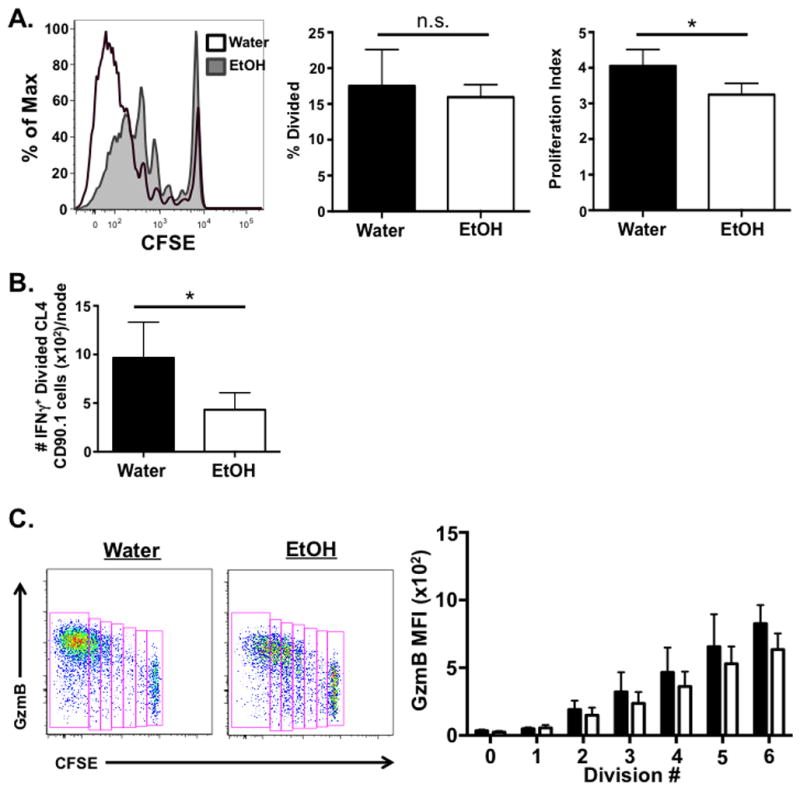
5×106 EtOH- or water-exposed, CFSE-labeled, donor CL-4 CD90.1+ cells were adoptively transferred i.v. to EtOH- and water-consuming CD90.2+ BALB/c hosts, respectively. Mice were challenged with a sublethal dose of IAV 24 h later. On day 4 p.i. mice were administered 500 μg monensin i.p.. Six h following treatment, dLN were harvested and stained for analysis by flow cytometry. A) Percent divided and proliferation index was determined within the CL-4 Thy1.1+ population. B) The number of IFNγ+, divided CL-4 Thy1.1 cell population was determined. C) The MFI of granzyme B expression in dividing CL-4 T cells was determined. Data are representative of two independent experiments. n=6–9 mice/group. * indicates p<0.05
Given the link between proliferation and the ability to make cytokines (Lawrence and Braciale, 2004, Jenkins et al., 2008), we next determined whether this decreased division number within the IAV-specific CD8 T cells in EtOH-consuming mice correlated with a reduction in the number of cytokine+ IAV-specific CD8 T cells that develop in the dLN. Consistent with the reduced division of IAV-specific CD8 T cells, we observed a significant decrease in the number of IFNγ+ IAV-specific CD8 T cells within the dLN of EtOH-consuming mice compared to water controls (Fig. 6B). Although trending toward a reduction, there was no statistically significant reduction in the frequency of dividing, IFNγ CD8 T cells in EtOH-consuming mice (data not shown). Further, we detected few TNFα– or IL-2- producing, IAV-specific CD8 T cells among the dividing T cells of either group (data not shown), suggesting that these cytokines may be upregulated in IAV-specific CD8 T cells later during infection or within the lung. Together these data suggest that the EtOH-induced defect in the number, and potentially IFNγ effector ability, of IAV-specific T cells likely begins in dLN.
Given the correlation between division of CD8 T cells and upregulation of granzymes (Jenkins et al., 2008), we also assessed granzyme B production by IAV-specific CD8 T cells within dLN on day 4 p.i.. Similar to pulmonary CD8 T cells analyzed for granzyme B expression ex vivo (Fig. 4), our results show there is no significant difference in the MFI of granzyme B based upon cell division number between water- and EtOH-consuming mice (Fig. 6C). Together, these results demonstrate the defect in the CD8 T cell response of mice chronically consuming EtOH is apparent as early as day 4 p.i. within dLN following IAV infection, suggesting EtOH-induced intrinsic defects in the CD8 T cells and/or defects in CD8 T cell priming during chronic EtOH exposure.
Discussion
Previous work has demonstrated that mice chronically consuming EtOH exhibit enhanced morbidity, mortality, viral titers and pulmonary pathology following IAV challenge (Meyerholz et al., 2008). This increase in overall IAV-induced disease in EtOH-consuming mice is accompanied by a severe defect in the total number of IAV-specific CD8 T cells, as well as their ability to produce IFNγ. In this report we demonstrate that the lesion in the IAV-specific CD8 T cell response of mice chronically consuming EtOH is more severe than previously appreciated. In addition to the defects in effector functionality previously observed, we detected a significant reduction in the total number of TRAIL+ and FasL+ IAV-specific CD8 T cells on day 8 p.i. (Fig. 3). However, this defect in TRAIL and FasL cells appears not to be intrinsic to IAV-specific CD8 T cells of mice chronically consuming EtOH, as the per cell expression of these effector molecules is equivalent, but rather due to the documented reduction in the total CD8 T cell numbers within the lungs of EtOH-exposed mice (Meyerholz et al., 2008). In contrast to FasL and TRAIL, we observed a significant reduction in the ability of EtOH CD8 T cells to degranulate when stimulated ex vivo with IAV peptides (Fig. 4). This reduction in degranulation correlates with the defect in the ability of IAV-specific CD8 T cells from the lungs of EtOH-consuming mice to kill IAV-pulsed targets in vitro (Fig. 5) and likely contributes to the increased viral titers observed in IAV-infected chronic EtOH-consuming mice (Langlois et al., 2010, Meyerholz et al., 2008). Further, this result may not be unique to IAV infection, but rather a more global defect due to EtOH consumption as CD8 T cells from EtOH mice challenged with Listeria monocytogenes are defective in their ability to degranulate following ex vivo Listeria peptide stimulation (Prajwal Gurung and Robert Cook, unpublished data).
Surprisingly, however, our findings suggest the defect in cytokine production is limited to IFNγ, as IAV-specific CD8 T cells in EtOH-consuming mice have no defect in total TNFα or IL-2 production (Fig. 2A & 2B). Further, the small fraction of polyfunctional IAV-specific CD8 T cells present within the lungs during IAV infection remains unaltered in mice chronically consuming EtOH (Fig. 2C & 2D). The mechanism controlling the selective loss of one effector cytokine by IAV-specific CD8 T cells (i.e. loss of IFNγ but not TNFα and/or IL-2) in chronic EtOH-consuming mice remains unknown. However, it is likely that other defects within mice chronically consuming EtOH (such as IL-2 expression by CD4 T cells and defects within APCs) contribute to inappropriate and/or insufficient priming of IAV-specific CD8 T cells within dLN. Changes in antigen presenting cell populations have been well documented to occur during EtOH exposure (Fan et al., 2011, Ness et al., 2008, Parlet and Schlueter, 2013, Edsen-Moore et al., 2008, Legge and Waldschmidt, 2014, Gurung et al., 2009, Buttari et al., 2008, Happel and Nelson, 2005, Joshi and Guidot, 2007, Szabo and Mandrekar, 2009, Zhang et al., 2002, Eken et al., 2011, Joshi et al., 2005, Lau et al., 2007, Mehta and Guidot, 2012, Rendon et al., 2012, Siggins et al., 2009, Szabo and Mandrekar, 2008). While dendritic cell functionality during IAV challenge of mice chronically consuming EtOH has not been completely investigated, the migration of dermal DCs, cutaneous DCs and Langerhans cells is delayed following FITC sensitization (Ness et al., 2008, Parlet and Schlueter, 2013). Following IAV infection, DCs from mice consuming EtOH are severely inhibited in migrating from the lungs to the dLN (Legge and Waldschmidt, 2014). If DCs are not able to migrate efficiently to the lung-draining lymph nodes following IAV challenge, an appropriate CD8 T cell response will not be primed to clear the infection (Meyerholz et al., 2008). In addition to their required presence in the dLN for T cell activation, the maturation and activation state of DC is known to have a profound connection to T cell tolerance/activation/effector function fate decisions. In this regard, DCs from alcoholic humans have decreased CD86 expression and a reduced ability to prime T cells following LPS stimulation compared to DCs from healthy individuals (Buttari et al., 2008). Data from our group also suggests DCs from the lungs of mice chronically consuming EtOH are defective in their ability to upregulate the costimulatory molecules CD40 and CD80 (Legge and Waldschmidt, 2014). Overall, these data suggest DCs from mice chronically consuming EtOH may not be able to appropriately activate IAV-specific CD8 T cells, even if they make it to dLN, consistent with our finding of hindered proliferation of IAV-specific CD8 T cells within dLN of chronic EtOH-exposed mice (Fig 6). In the future it will be important to determine the contribution of these chronic EtOH-induced DC defects to the overall defects observed in the activation of naïve, IAV-specific CD8 T cells and their subsequent effector functions.
In addition to these extrinsic effects that may contribute to altered T cell functions in chronic EtOH-consuming mice, another possible explanation for the changes observed may be due to T cell intrinsic effects, such as signaling changes within CD8 T cells. It has been demonstrated that chronic EtOH exposure reduces TCR signaling in primary human CD4 T cells (Ghare et al., 2011). Reduced TCR signaling would affect signaling upstream of PKCθ and calcium flux. This would correlate with data from our laboratory demonstrating reduced IFNγ production by CD8 T cells stimulated with IAV peptides while these same cells produced IFNγ appropriately when stimulated with PMA/ionomycin [data not shown and (Meyerholz et al., 2008)].
In summary, our findings detail a large, multifactorial lesion within the IAV-specific CD8 T cells likely contributes significantly to the increased severity of disease and viral titers observed during chronic EtOH exposure. This defect includes a reduction of the total number of IAV-specific CD8 T cells, but also a significant decrease in IFNγ production of IAV-specific CD8 T cells, but not TNFα, IL-2 or polyfunctional (IFNγ/TNFα and IFNγ/TNFα/IL-2 producers) cytokine producers during IAV challenge. This EtOH-induced defect in IFNγ is apparent early in the dLN and is accompanied by a significant reduction in proliferation following IAV infection. Finally, our findings importantly suggest the EtOH induced lesion in CD8 T cell effector immunity is not limited to IFNγ, as we observed a significant reduction in the ability of CD8 T cells from EtOH-consuming mice to degranulate and kill IAV targets compared to water controls. Perhaps targeting these early defects in proliferation and/or cytotoxicity of CD8 T cells in the development of IAV vaccination strategies and during IAV infections could aid in limiting the increased incidence and severity of respiratory disease in alcoholics.
Acknowledgments
We thank Drs. Robert Cook, Annette Schlueter, Steven Varga, and Thomas Waldschmidt for their critical evaluation of this manuscript.
This work was supported by NIH awards R01 AA018671 (to K.L.L.), R01 AA019438 (to Thomas Waldschmidt), R21 AA021205 (to K.L.L.), T32 AI007533 (to E.A.H.), T32 AI007485 (to E.A.H.), and the University of Iowa Department of Pathology.
References
- Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. The Journal of experimental medicine. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. Journal of leukocyte biology. 2008;84:607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincks EL, Gurung P, Langlois RA, Hemann EA, Legge KL, Griffith TS. The magnitude of the T cell response to a clinically significant dose of influenza virus is regulated by TRAIL. Journal of immunology. 2011;187:4581–4588. doi: 10.4049/jimmunol.1002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. Journal of immunology. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Buttari B, Profumo E, Mancinelli R, Cesta Incani U, Tosti ME, Attilia ML, Ceccanti M, Rigano R. Chronic and acute alcohol exposure prevents monocyte-derived dendritic cells from differentiating and maturing. International journal of immunopathology and pharmacology. 2008;21:929–939. doi: 10.1177/039463200802100417. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcoholism, clinical and experimental research. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcoholism, clinical and experimental research. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT, Zhu X, Coleman RA, Ballas ZK, Waldschmidt TJ, Ray NB, LaBrecque DR, Cook BL. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33:175–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunological reviews. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Downs CA, Trac D, Brewer EM, Brown LA, Helms MN. Chronic alcohol ingestion changes the landscape of the alveolar epithelium. BioMed research international. 2013;2013:470217. doi: 10.1155/2013/470217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsen-Moore MR, Fan J, Ness KJ, Marietta JR, Cook RT, Schlueter AJ. Effects of chronic ethanol feeding on murine dendritic cell numbers, turnover rate, and dendropoiesis. Alcoholism, clinical and experimental research. 2008;32:1309–1320. doi: 10.1111/j.1530-0277.2008.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken A, Ortiz V, Wands JR. Ethanol inhibits antigen presentation by dendritic cells. Clinical and vaccine immunology : CVI. 2011;18:1157–1166. doi: 10.1128/CVI.05029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Edsen-Moore MR, Turner LE, Cook RT, Legge KL, Waldschmidt TJ, Schlueter AJ. Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation. Alcoholism, clinical and experimental research. 2011;35:47–59. doi: 10.1111/j.1530-0277.2010.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghare S, Patil M, Hote P, Suttles J, McClain C, Barve S, Joshi-Barve S. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: potential mechanism of alcohol-induced immune suppression. Alcoholism, clinical and experimental research. 2011;35:1435–1444. doi: 10.1111/j.1530-0277.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. Journal of leukocyte biology. 2009;85:34–43. doi: 10.1189/jlb.0208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proceedings of the American Thoracic Society. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Hemann EA, Kang SM, Legge KL. Protective CD8 T Cell-Mediated Immunity against Influenza A Virus Infection following Influenza Virus-like Particle Vaccination. Journal of immunology. 2013;191:2486–2494. doi: 10.4049/jimmunol.1300954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. The Journal of experimental medicine. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MR, Mintern J, La Gruta NL, Kedzierska K, Doherty PC, Turner SJ. Cell cycle-related acquisition of cytotoxic mediators defines the progressive differentiation to effector status for virus-specific CD8+ T cells. Journal of immunology. 2008;181:3818–3822. doi: 10.4049/jimmunol.181.6.3818. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. Journal of immunology. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. American journal of physiology Lung cellular and molecular physiology. 2007;292:L813–823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PloS one. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. Journal of immunology. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- Langlois RA, Meyerholz DK, Coleman RA, Cook RT, Waldschmidt TJ, Legge KL. Oseltamivir treatment prevents the increased influenza virus disease severity and lethality occurring in chronic ethanol consuming mice. Alcoholism, clinical and experimental research. 2010;34:1425–1431. doi: 10.1111/j.1530-0277.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AH, Thomson AW, Colvin BL. Chronic ethanol exposure affects in vivo migration of hepatic dendritic cells to secondary lymphoid tissue. Human immunology. 2007;68:577–585. doi: 10.1016/j.humimm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. Journal of immunology. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Legge KL, Waldschmidt TJ. Chapter 10: Alcohol and the Adaptive Immune Response in the Airway: Dendritic Cell and Lymphocyte Impairments, in Alcohol Use Disorders and the Lung. In: GUIDOT DM, MEHTA AJ, editors. Alcohol Use Disorders and the Lung. Springer; New York: 2014. pp. 115–132. [Google Scholar]
- Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. Journal of immunology. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annual review of immunology. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- McGill J, Legge KL. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. Journal of immunology. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. The Journal of experimental medicine. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. The American journal of the medical sciences. 2012;343:244–247. doi: 10.1097/MAJ.0b013e31823ede77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. Journal of immunology. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Bagby G, Andresen J, Nakamura C, Shellito J, Summer W. The effects of ethanol, tumor necrosis factor, and granulocyte colony-stimulating factor on lung antibacterial defenses. Advances in experimental medicine and biology. 1991;288:245–253. doi: 10.1007/978-1-4684-5925-8_28. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nature reviews Immunology. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcoholism, clinical and experimental research. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlet CP, Schlueter AJ. Mechanisms by Which Chronic Ethanol Feeding Impairs the Migratory Capacity of Cutaneous Dendritic Cells. Alcoholism, clinical and experimental research. 2013;37:2098–2107. doi: 10.1111/acer.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. Journal of immunology. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon JL, Janda BA, Bianco ME, Choudhry MA. Ethanol exposure suppresses bone marrow-derived dendritic cell inflammatory responses independent of TLR4 expression. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2012;32:416–425. doi: 10.1089/jir.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Bagby GJ, Molina P, Dufour J, Nelson S, Zhang P. Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques. Alcoholism, clinical and experimental research. 2009;33:1524–1531. doi: 10.1111/j.1530-0277.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. Journal of leukocyte biology. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. Human monocytes, macrophages, and dendritic cells: alcohol treatment methods. Methods in molecular biology. 2008;447:113–124. doi: 10.1007/978-1-59745-242-7_9. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcoholism, clinical and experimental research. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. Journal of immunology. 1997;159:5197–5200. [PubMed] [Google Scholar]
- Wortzman ME, Lin GH, Watts TH. Intrinsic TNF/TNFR2 interactions fine-tune the CD8 T cell response to respiratory influenza virus infection in mice. PloS one. 2013;8:e68911. doi: 10.1371/journal.pone.0068911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S. Pulmonary host defenses and alcohol. Frontiers in bioscience : a journal and virtual library. 2002;7:d1314–1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]