Abstract
Chronic lymphocytic leukemia (CLL) development and progression are thought to be driven by unknown antigens/autoantigens through the B cell receptor (BCR) and environmental signals for survival and expansion including toll-like receptor (TLR) ligands. CD180/RP105, a membrane-associated orphan receptor of the TLR family, induces normal B cell activation and proliferation and is expressed by approximately 60% of CLL samples. Half of these respond to ligation with anti-CD180 antibody by increased activation/phosphorylation of protein kinases associated with BCR signaling. Hence CLL cells expressing both CD180 and the BCR could receive signals via both receptors. Here we investigated cross-talk between BCR and CD180-mediated signaling on CLL cell survival and apoptosis. Our data indicate that ligation of CD180 on responsive CLL cells leads to activation of either prosurvival Bruton tyrosine kinase (BTK)/phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT-mediated, or proapoptotic p38 mitogen-activated protein kinase (p38MAPK)-mediated signaling pathways, while selective immunoglobulin M (sIgM) ligation predominantly engages the BTK/PI3K/AKT pathway. Furthermore, pretreatment of CLL cells with anti-CD180 redirects IgM-mediated signaling from the prosurvival BTK/PI3K/AKT toward the proapoptotic p38MAPK pathway. Thus preengaging CD180 could prevent further prosurvival signaling mediated via the BCR and, instead, induce CLL cell apoptosis, opening the door to therapeutic profiling and new strategies for the treatment of a substantial cohort of CLL patients.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is characterized by the clonal expansion of CD5+CD19+CD23+ cells in peripheral lymphoid organs, tissues and bone marrow (1,2). The disease has a variable clinical course, progression and survival rate. It is proposed that CLL cell growth, survival and expansion are driven by unknown antigens/autoantigens through the B-cell antigen receptor (BCR), and supported by microenvironmental signals (3) including the toll-like receptors (TLRs), in particular CD180/RP105 (4,5) and TLR9 (6–10). CD180/RP105 is a membrane-associated orphan receptor that drives normal human and mouse B-cell activation and proliferation (11–14). Anti-CD180 mono-clonal antibody (mAb) induces upregulation of MHC class II, CD40 and CD80/CD86 on human and mouse B cells (4,11,15) and differentiation and rapid secretion of immunoglobulin G (IgG) in vivo (16).
We have shown previously that approximately 60% of CLL samples express CD180. Half of these responded to ligation with anti-CD180 mAb by activation and proliferation, and were termed responders (R-CLL) (4,5). We further demonstrated that CD180 ligation led to a strong upregulation of phosphorylated zeta-chain-associated protein kinase 70 (ZAP-70)/Syk, p38 mitogen-activated protein kinase (p38MAPK), extracellular-signal-regulated kinase (ERK) and, particularly, AKT protein kinase in normal B cells and R-CLL cells (5). Since phosphorylation of AKT has been associated with prosurvival signaling pathways in CLL previously (17,18) we have examined the relationship between AKT phosphorylation and CLL survival/apoptosis following CD180 ligation.
The BCR plays an important role in the maintenance and survival of CLL cells (19–23) and IgM-mediated prosurvival signaling is associated with activation of AKT, ERK and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (24). Therefore CLL samples expressing CD180 and BCR could receive both antigen-mediated and environmental signals, possibly via overlapping signaling pathways. BCR and CD180-mediated responses have not been correlated in CLL previously. Here we investigate cross-talk between BCR and CD180 pathways and how CD180 ligation impinges on BCR-driven CLL cell signaling and survival.
MATERIALS AND METHODS
Patients
Heparinized peripheral blood was collected with informed consent from 60 patients with CLL (47 to 89 years of age, median age 67.9 years) following ethical approval from the University College London Hospitals (UCLH, 08/H0714/6). Fifty three patients were at Binet stage A with white blood cell (WBC) count of 14.0–100.2 × 109/L, three at stage B (WBC count of 27.6–76.6 × 109/L) and four at stage C (WBC count of 12.3–81.0 × 109/L). From this cohort, 28 patients have been identified as IGHV mutated (M)-CLL and 19 patients as IGHV unmutated (U)-CLL. Patients were untreated or had not received treatment for 6 months prior to the study. Fifteen age-matched (50 to 78 years of age, median age 63.5 years) healthy volunteers served as controls.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs) and Purified CD19+ cells
PBMCs were isolated in Histopaque-1077 gradient (Sigma-Aldrich, Dorset, UK), and cell concentration was adjusted as required in RPMI-1640 medium supplemented with 10% fetal bovine serum (both Sigma-Aldrich). Control CD19+ B cells were enriched from PBMCs using an EasySep Human B Cell Enrichment Kit (Stemcell Technologies, Vancouver, BC, Canada). The level of purification was routinely >95%.
Cell Phenotyping
Fc-receptors on PBMCs were blocked with purified human immunoglobulins (Sigma-Aldrich) and the cells were immunophenotyped using unconjugated primary mAbs: IgG1 isotype control, anti-CD180 (clone G28-8, IgG1), anti-IgM (BD Biosciences, Oxford, UK), anti-CD79b, anti-CD38 (both: Fitzgerald, North Acton, MA) and anti-IgD (Sigma-Aldrich). The cells were stained with FITC-conjugated rabbit anti-mouse F(ab)2 (Dako, Ely, UK), blocked with mouse serum (Dako), treated with PE-Cy5 anti-CD19 mAb and fixed. The results were analyzed by flow cytometry (CyAn, Beckman Coulter, High Wycombe, UK) and expressed as percentages of positive cells (4,5).
Cell Stimulation
PBMCs or purified CD19+ cells were incubated with anti-CD180 mAb for 10 to 20 min or with goat anti-human IgM F(ab)2 (Southern Biotech, Birmingham, AL, USA) for 10 min at a final concentration 20 μg/mL at 37°C and 5% CO2. Optimal stimulation time (20 min) for anti-CD180 mAb was established previously (data not shown). Unstimulated cultures were used as negative controls. In separate experiments, cells were sequentially incubated with anti-CD180 for 10 to 20 min followed by anti-IgM for 10 min or vice versa.
Prior to the stimulation with anti-CD180, ten CLL samples were pretreated at 37°C for 2 h with specific inhibitors of AKT (Akti-1/2, Sigma-Aldrich, 20 μmol/L) or p38MAPK (SB2035804, Sigma-Aldrich, 20 μmol/L).
Assessment of Phosphorylation of Intracellular Protein Kinases
Flow cytometry
Following stimulation, cells were stained with PE-Cy5 conjugated anti-CD19 mAb, washed, fixed and permeabilized with Fix&Perm Kit (ADG [An Der Grub Bio Research GmbH] [Nordic-MUbio, Susteren, the Netherlands]). Cells were further stained with either of the following anti-human Abs: anti-phospho(p)-ZAP-70/Syk-Alexa Fluor-647 (BD Phosflow [BD, Franklin Lakes, NJ, USA]), anti-p-AKT-Alexa Fluor-488, anti-p-p38MAPK-Alexa Fluor-488 (Cell Signaling Technology, Danvers, MA, USA), anti-p-ERK-Alexa Fluor-488 (BD Biosciences [BD]), anti-p-BTK-Alexa Fluor-647 (BD Phosflow) and incubated at room temperature for 30 min. Cells were analyzed by flow cytometry, and the results expressed as percentages of positive cells compared with unstimulated cells.
Immunoblotting
Proteins were extracted from stimulated and unstimulated cells (10 × 106/mL) and applied to gel electrophoresis as described previously (25). Rainbow Molecular Weight Markers (3.5–260 kDa, Invitrogen [Thermo Fisher Scientific Inc., Waltham, MA, USA]) and the following primary antibodies were used against: AKT, p-AKT(Ser473), ERK(p44/42), p-ERK(Thr202/Tyr204), p38MAPK, p-p38MAPK(Thr180/Tyr182), PI3K(p85), p-PI3Kp85(tyr458)/p55(tyr199), Mcl-1 and Bcl-xL, (all Cell Signaling Technology). B-cell lymphoma 2 (Bcl-2; Sigma-Aldrich) was used as a loading control (25). Bands were visualized using appropriate secondary antibodies (Dako) and enhanced chemiluminescence system (Amersham [GE Healthcare, Little Chalfont, UK]). Relative optical density normalized to Bcl-2 was measured.
Apoptosis
Stimulated and unstimulated cells were stained with anti-human CD19 mAb conjugated to PE-Cy5, loaded with 0.2 μmol/L of DiOC6 (3,3′-dihexyloxacabocyanine iodide; Molecular Probes [Thermo Fisher Scientific]) and incubated at 37°C for 20 min, washed and analyzed by flow cytometry. Apoptosis was measured as the percentages of DiOC6dim cells in the CD19+ subset as described previously (5).
Statistical Analysis
Mann-Whitney nonparametric U test and paired t test were applied where appropriate.
RESULTS
Ligation of CD180 on CLL Cells from Most Patients Leads to an Alternative Phosphorylation of Either AKT or p38MAPK
We have established that CD180+CLL samples can be subdivided into responders (R-CLL) and nonresponders (NR-CLL), based on upregulation of CD86 and Ki67 following ligation of CD180 (4). Treatment of R-CLL cells, but not NR-CLL cells, with anti-CD180 mAb led to an increase in phosphorylation of AKT(Ser473) (5). Here, using a larger cohort of patients, we recategorized CLL samples into AKT signalers (AKT-S) and AKT nonsignalers (AKT-NS) based on their ability to increase phosphorylation of AKT above the basal levels following CD180 ligation (Figures 1A, C, D). It was important to determine other signaling molecules involved in these two categories of cells.
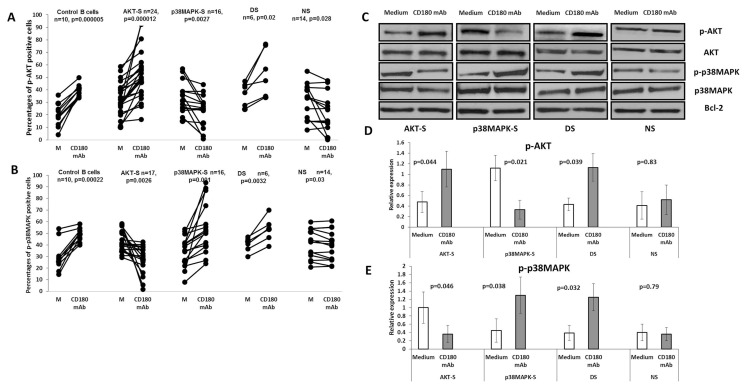
Figure 1.
Phosphorylation of AKT and p38MAPK protein kinases in AKT-S, p38MAPK-S, double signaler (DS) and nonsignaler (NS) categories of CLL samples following stimulation with anti-CD180 mAb. (A and B) CLL cells and control B cells were incubated with anti-CD180 mAb for 20 min (CD180 mAb) or left unstimulated in medium (Medium), washed, stained with anti-CD19 mAb, fixed, permeabilized and stained with anti-p-AKT(Ser473) (A) or with p-anti-p38MAPK (B) mAbs as described in the Materials and Methods, analyzed by flow cytometry and expressed as percentages of positive cells. P values were calculated using the paired t test. (C) Representative immunoblots with the levels of total AKT, p-AKT(Ser473), total p38MAPK and p-p38MAPK in CLL samples following stimulation with anti-CD180 mAb and in unstimulated cultures (Medium) as described in the Materials and Methods. Bcl-2 was used as a loading control. (D and E) Relative optical density, normalized to Bcl-2, for p-AKT and p-p38MAPK immunoblots respectively, n = 6.
Stimulation with anti-CD180 of AKT-S CLL samples showed a significant down-regulation of p-p38MAPK basal expression (Figure 1B) compared with AKT-NS CLL samples that showed a significant increase in p-p38MAPK measured by flow cytometry (see Figure 1B) and confirmed by immunoblotting (Figures 1C, E). We categorize this group as p38MAPK signalers (p38MAPK-S). These data are consistent with a hypothesis that elevation of AKT-P or p-p38MAPK indicates two possible alternative pathways following CD180 ligation.
One explanation for this dichotomy could be related to the surface density of the CD180. However, no significant differences in CD180, CD38 or CD79b expression were observed between AKT-S and p38MAPK-S (data not shown).
Only six CLL samples responded to CD180 ligation by increasing levels of both p-AKT and p-p38MAPK, however, all control B cells did (Figures 1A–E) and were marked as double-signalers (DS). By contrast, the remaining CLL samples down-regulated percentages of both p-AKT– and p-p38MAPK–expressing cells following CD180 ligation (Figures 1A, B) and were categorized as NS.
We have therefore identified CLL B cells with four patterns of CD180-mediated signaling: AKT-S, p38MAPK-S, NS and a minor subset of AKT/p38MAPK DS. No significant differences in phosphorylation of ERK were detected between the four categories of cells.
CD180-Induced AKT-Mediated Signaling in CLL Cells Involves BTK and PI3K and Leads to the Survival of CLL Cells, While the p38MAPK Pathway Favors Apoptosis
Since AKT (26) and associated phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and Bruton tyrosine kinase (BTK) pathways (23,27) have been shown to be important in the survival of CLL cells mediated through the BCR (18,28–31), we next determined whether CD180-mediated survival of CLL cells involved these pathways.
CD180 ligation of AKT-S CLL samples induced upregulation of p-BTK (Figure 2A) and p-PI3K, accompanied by an increased expression of Bcl-xL and Mcl-1 (Figures 2B, C) and substantial survival from apoptosis (Figure 2D). Control B cells also exhibited increased survival, following CD180 ligation (5) (see Figure 2D).
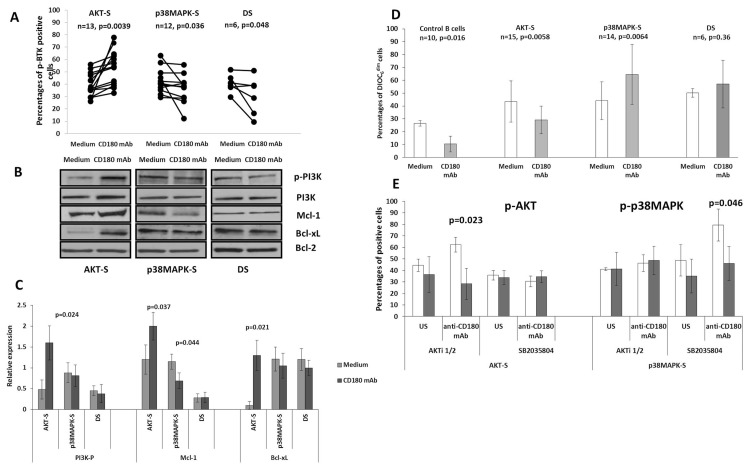
Figure 2.
Anti-CD180 mediated phosphorylation of BTK and PI3K, expression of Mcl-1, Bcl-xL and apoptosis in AKT-S, p38MAPK-S and DS CLL cells. (A) Percentages of cells expressing p-BTK in AKT-S, p38MAPK-S and DS categories of CLL samples. Cells were incubated with anti-CD180 mAb for 20 min (CD180 mAb) or left unstimulated (Medium), washed, stained with anti-CD19 mAb, fixed, permeabilized and stained with anti-p-BTK mAb as described in the Materials and Methods and analyzed by flow cytometry. P values were calculated using paired t test. (B) Representative immunoblots show the levels of total PI3K, p-PI3K, Bcl-xL and Mcl-1 in AKT-S, p38MAPK-S and DS categories of CLL samples following stimulation with anti-CD180 mAb (CD180 mAb) or in unstimulated cultures (Medium) as described in the Materials and Methods. Bcl-2 was used as loading control. (C) Relative optical density, normalized to Bcl-2, for p-PI3K, Mcl-1 and Bcl-xL immunoblots, n = 6. (D) The percentages of DiOC6dim (apoptotic) cells in control B cells, AKT-S, p38MAPK-S and DS CLL samples measured following stimulation with anti-CD180 mAb for 24 h, compared with unstimulated cultures (Medium), as described in the Materials and Methods. The values are mean ± SD, p values were calculated using a nonparametrical U test. (E) Percentages of cells expressing p-AKT and p-p38MAPK in unstimulated (US) and stimulated with anti-CD180 mAb AKT-S and p38MAPK-S CLL samples untreated (white bars) or pretreated (gray bars) with specific inhibitors AKTi1/2 or SB2035804. P values between the treated and untreated with inhibitors cells were calculated using the paired t test.
By contrast, treatment of p38MAPK-S CLL cells with anti-CD180 mAb resulted in a significant decrease in the percentages of p-BTK-expressing cells below the basal level (see Figure 2A) with no appreciable changes in p-PI3K (Figures 2B, C). Most importantly, there was a significant (if heterogeneous) increase in DiOC6dim apoptotic p38MAPK-S cells after CD180 ligation (see Figure 2D) accompanied by a decrease in Mcl-1, but not Bcl-xL (Figures 2B, C).
In the small cohort of DS samples, there were no CD180-mediated changes in either the cell survival or the levels of Bcl-xL and Mcl-1 (Figures 2B–D), despite a decrease in p-BTK (see Figure 2A).
In a pilot experiment, we used specific inhibitors of p-AKT and p-p38MAPK to confirm involvement of these protein kinases in CD180-mediated signaling. As expected, pretreatment of AKT-S CLL cells with AKT inhibitor Akti1/2 resulted in a significant decrease in the number of p-AKT cells, while no effect was seen for p38MAPK-S cells (Figure 2E). Likewise, p38MAPK inhibitor SB2035804 suppressed CD180-stimulated levels of p-p38MAPK in p38MAPK-S, but not in AKT-S cells.
Thus, our data suggest that CD180-mediated intracellular signaling in CD180+CLL cells can engage two major pathways: one being prosurvival, operating via BTK/PI3K/AKT, and the other being proapoptotic and favoring the p38MAPK pathway.
sIgM-Induced Activation Favors the Prosurvival Signaling Pathway in AKT-S CLL Cells, but Not a Proapoptotic Pathway in p38MAPK-S CLL Cells
Since AKT also plays a central role in BCR-mediated prosurvival signaling in CLL cells (23,32,33), it was important to compare the signaling patterns of sIgM with those mediated by CD180 in the major categories of CLL cells defined above. Double positive CD180+sIgM+ CLL samples were selected for this study.
Our data showed striking similarities between CD180 and sIgM-mediated signaling in the AKT-S category of cells. Treatment of AKT-S CLL samples with anti-IgM induced significant phosphorylation above the basal levels of AKT (Figure 3A), BTK (Figure 3C) and PI3K (Figures 3D, E), downregulation of the basal levels of p-p38MAPK (Figure 3B) accompanied by an increase in Mcl-1 and Bcl-xL (Figures 3D, E) and protection from apoptosis (Figure 3F). Whilst anti-CD180 induced activation of the circuit BTK/PI3K/AKT in all AKT-S CLL samples studied (Figures 1, 2), the effect of anti-IgM was more heterogeneous: 4/20 AKT-S samples responded instead by downregulation of p-AKT (see Figure 3A) and 2/15 AKT-S samples by phosphorylation of p38MAPK (see Figure 3B). However, overall, we can conclude that responses of 70% of AKT-S CLL samples to anti-CD180 and anti-IgM were similar and led to the recruitment of BTK/PI3K/AKT, downregulation of p-p38MAPK and protection from apoptosis.
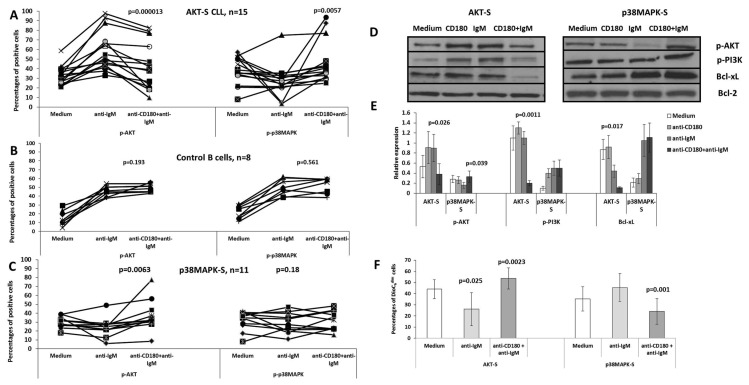
Figure 3.
Anti-IgM mediated phosphorylation of AKT, p38MAPK, BTK and PI3K, expression of Mcl-1, Bcl-xL and apoptosis in AKT-S and p38MAPK-S CLL cells. (A, B, C) Control B cells or AKT-S and p38MAPK-S CLL samples were incubated with anti-IgM F(ab)2 for 10 min (anti-IgM) or left unstimulated in medium (Medium), washed, stained with anti-CD19 mAb, fixed, permeabilized and stained with anti-p-AKT, anti-p-p38MAPK and anti-p-BTK mAbs as described in the Materials and Methods. The results were analyzed by flow cytometry and expressed as percentages of positive cells. P values were calculated using the paired t test. (D) Representative immunoblots show the levels of total PI3K, p-PI3K, Mcl-1 and Bcl-xL in AKT-S and p38MAPK-S CLL samples in unstimulated cultures (Medium) and following stimulation with anti-IgM F(ab)2 as described in the Materials and Methods. Bcl-2 was used as a loading control. (E) Relative optical density, normalized to Bcl-2, for p-PI3K, Mcl-1 and Bcl-xL immunoblots, n = 5. (F) The percentages of DiOC6dim (apoptotic) cells in control B cells, AKT-S, p38MAPK-S and DS CLL samples measured following stimulation with anti-IgM F(ab)2 for 24 h, compared with unstimulated cultures (Medium), as described in the Materials and Methods. The values are mean ± SD, p values were calculated using a nonparametrical U test.
By contrast, in only 3/13 cases (23%) did p38MAPK-S samples respond to anti-IgM by increasing phosphorylation of p38MAPK (see Figure 3B), while expression of p-BTK and Mcl-1 were decreased and no changes were detected in the levels of p-PI3K and Bcl-xL (Figures 3C–E). Our data indicate that (a) phosphorylation of p38MAPK following ligation of sIgM is a less frequent event, as compared with the stimulation with anti-CD180, (b) phosphorylation of p38MAPK is crucial for the induction of apoptosis that has not been observed in p38MAPK signalers in response to anti-IgM (Figure 3F) in contrast to anti-CD180 (see Figure 2D).
Thus, our data suggest that both CD180 or sIgM ligation on CLL cells results in activation of a prosurvival signaling pathway operating via BTK/PI3K/AKT. However, whereas anti-CD180 mAb can activate an alternative proapoptotic pathway mediated via p38MAPK, anti-sIgM alone rarely leads to the activation of p38MAPK.
Pretreatment of CLL Cells with Anti-CD180 mAb Rewires sIgM-Mediated Intracellular Signaling from Prosurvival BTK/PI3K/AKT to a Proapoptotic p38MAPK Pathway
Since Yamashita et al. (34) showed that pretreatment of murine B cells with anti-CD180/RP105 led to apoptosis following ligation of the BCR, we next tested whether preengagement of CD180 on CLL cells would affect signaling of CLL cells through the BCR. In all 15 AKT-S CLL samples, pretreatment with anti-CD180 mAb, followed by anti-IgM F(ab)2, led to a significant decrease in cells expressing p-AKT (Figures 4A, D, E), albeit not to baseline level, and a decrease in p-PI3K (Figures 4D, E) compared with anti-IgM alone. Simultaneously, we observed a decrease in percentages of cells expressing p-BTK from 44.3 ± 11.4% (anti-IgM alone) to 32.6 ± 13.5% (anti-CD180 + anti-IgM, n = 11, p = 0.02) with baseline levels of 38.3 ± 6.5%. Expression of Bcl-xL also was decreased appreciably (Figures 4D, E).
Figure 4.
Modulation of sIgM-mediated signaling by sensitization with anti-CD180 mAb. (A–C) Control B cells or CD180+sIgM+CLL samples were treated with anti-IgM F(ab)2 alone or pretreated with anti-CD180 followed with anti-IgM F(ab)2 as described in the Materials and Methods. Unstimulated cultures (Medium) were used as controls. The cells were stained with anti-CD19, fixed, permeabilized and stained with antibodies to p-AKT and p-p38MAPK. P values were calculated using the paired t test. (D) Representative immunoblots of p-AKT, p-PI3K, p-p38MAPK, Mcl-1 and Bc-xL following sequential ligation of CD180 followed by sIgM. Bcl-2 was used as a loading control. The bands were assessed and visualized as described in the Materials and Methods. (E) Relative optical density, normalized to Bcl-2, for p-AKT, p-PI3K, p-p38MAPK, Mcl-1 and Bcl-xL immunoblots, n = 5. (F) The percentages of DiOC6dim (apoptotic) cells in unstimulated CLL cells (Medium) or stimulated with anti-IgM F(ab)2 alone or first with anti-CD180 followed by anti-IgM F(ab)2 for 24 h, stained with anti-CD19 mAb, loaded with DiOC6 and analyzed by flow cytometry. The values represent mean ± SD, p value calculated using the nonparametrical U test.
Perhaps most relevant is that the reduction in BTK/PI3K/AKT signaling in 14 out of 15 of these samples was accompanied by a significant increase in the percentages of cells expressing p-p38MAPK following sequential ligation of CD180 and IgM (Figure 4A), as well as by a significant increase in apoptotic cells compared with the engagement of IgM alone (p = 0.025) and above baseline apoptosis (p = 0.026, Figure 4F). Therefore sensitization of AKT-S CLL cells with anti-CD180 leads to rewiring of anti-IgM-mediated signaling from the prosurvival BTK/PI3K/AKT to the proapoptotic p38MAPK pathway. Interestingly, the modulation appears to be a feature of CLL cells since no similar changes were observed in normal B cells (Figure 4B).
Differences in the optimal stimulation time for anti-CD180 and anti-IgM antibodies did not affect the redirection phenomenon described in this study. The percentages of cells expressing p-AKT following stimulation with anti-IgM for 30 min were somewhat lower than those after 10 min (49.3 ± 8.9% versus 59.4 ± 11.0%, p = 0.09). However, this did not affect an outcome of prestimulation with anti-CD180 where the numbers of p-AKT cells further dropped down to 26.4 ± 13.5%, p = 0.028. The percentages of p-p38MAPK expressing cells did not differ in the range of 10 to 30 min stimulation with anti-IgM (data not shown).
The results in Figure 5 demonstrate that sensitization with anti-IgM antibodies leads to a downregulation of CD180-mediated expression of p-AKT to basal levels in all 12 AKT-S CLL samples, accompanied by a significant increase in the expression of p-p38MAPK, compared with anti-CD180 alone (Figure 5A). In addition, pretreatment with anti-IgM led to downregulation of CD180-mediated levels of p-PI3K and Bcl-xL (Figures 5D, E) and percentages of cells expressing p-BTK from 45.0 ± 15.8% (anti-CD180) down to 35.8.4 ± 11.3% (anti-IgM + anti-CD180, n = 11, p = 0.019) with the baseline levels of 38.6 ± 7.1%. By contrast, pretreatment of control B cells with anti-IgM had an additive effect with anti-CD180 mAb on p-AKT, while no changes in the levels of p-p38MAPK were observed (Figure 5B). We conclude that there is cross-talk between the BCR and CD180 in the AKT-S CLL samples, leading to substantial rewiring of intracellular signaling from BTK/PI3K/AKT to p38MAPK.
Figure 5.
Modulation of CD180-mediated signaling by sensitization with anti-IgM F(ab)2. (A–C) Control B cells or CD180+sIgM+CLL samples were treated with anti-CD180 mAb or first with anti-IgM F(ab)2 followed by anti-CD180 as described in the Materials and Methods. Unstimulated cultures (Medium) were used as controls. The cells were stained with anti-CD19, fixed, permeabilized and stained with antibodies to p-AKT-P and p-p38MAPK. P values were calculated using the paired t test. (D) Representative immunoblots of p-AKT, p-PI3K, p-p38MAPK, Mcl-1 and Bcl-xL following sequential ligation of sIgM followed by CD180. Bcl-2 was used as a loading control. The bands were assessed and visualized as described in the Materials and Methods. (E) Relative optical density, normalized to Bcl-2, for p-AKT, p-PI3K, p-p38MAPK, Mcl-1 and Bcl-xL immunoblots, n = 5.
Interestingly, pretreatment of all eleven p38MAPK-S samples with anti-CD180 enhanced anti-IgM mediated levels of p-AKT (Figure 4C), as well as p-PI3K and Bcl-xL (Figures 4D, E), and increased their survival (see Figure 4F). Likewise, pretreatment of p38MAPK-S CLL cells with anti-IgM augmented the CD180-mediated percentages of p-AKT expressing cells in 8/10 samples (Figure 5C), as well as Bcl-xL, but not p-PI3K (Figures 5D, E). Thus, in contrast to AKT-S cells, sensitization of p38MAPK-S cells with either anti-CD180 or anti-IgM often leads to additive phosphorylation of AKT by both antibodies. We therefore conclude that in AKT-S CLL samples where ligation of either sIgM or CD180 leads to a substantial activation of the prosurvival BTK/PI3K/AKT pathway, preengagement of the other receptor redirected intracellular signaling toward the proapoptotic p38MAPK pathway, whereas in p38MAPK-S CLL samples similar presensitization favors the BTK/PI3K/AKT pathway.
DISCUSSION
CD180 Ligation on B-CLL Cells Leads to Alternative Phosphorylation of Either AKT or p38MAPK
We have identified four patterns of CD180-mediated signaling in CLL cells: AKT-signalers (AKT-S); p38MAPK- signalers (p38MAPK-S); nonsignalers (NS); and a minor subset of double AKT/p38MAPK signalers (DS). Of the 60 CD180+CLL samples, 24 responded to CD180 ligation by a significant upregulation of p-AKT (AKT-S) (Figures 1A, C, D) which is essential for the BCR-mediated growth and survival of B lymphocytes (35) and survival of CLL cells (23,36,37). Of the remaining 36 samples 16 responded by increased phosphorylation of p38MAPK (p38MAPK-S) (Figures 1B, C, E) and only in a small cohort of 6 CLL samples did CD180 ligation result in activation of both p-AKT and p-p38MAPK (DS) (Figure 1). Application of specific inhibitors of AKT and p38MAPK signaling pathways (see Figure 2E) confirmed that, in many of the CLL samples, activation of AKT and p38MAPK pathways is exclusive. This appears to be a feature of CLL cells, and not of normal B cells that responded to CD180 ligation as double AKT/p38MAPK signalers (5) (Figures 1A, B).
There was a significant drop in p-p38MAPK basal levels in the AKT-S cells following CD180 ligation (Figures 1B, C, E) and in the basal levels of p-AKT in p38MAPK-S cells (Figures 1A, C, D). Decrease in the BCR-stimulated signal intensity of p38MAPK has been reported previously (24), but not linked with AKT activation. Our data suggest a regulatory influence of one pathway on another.
The fact that 14 CD180+CLL samples failed to activate either of the two pathways (NS, Figure 1) suggests that an alternative pathway could be being used, or that they are totally refractive to ligation of CD180. Of note, CD180 nonsignaler CLL samples remained unresponsive to the ligation of sIgM that could indicate their anergic status (38).
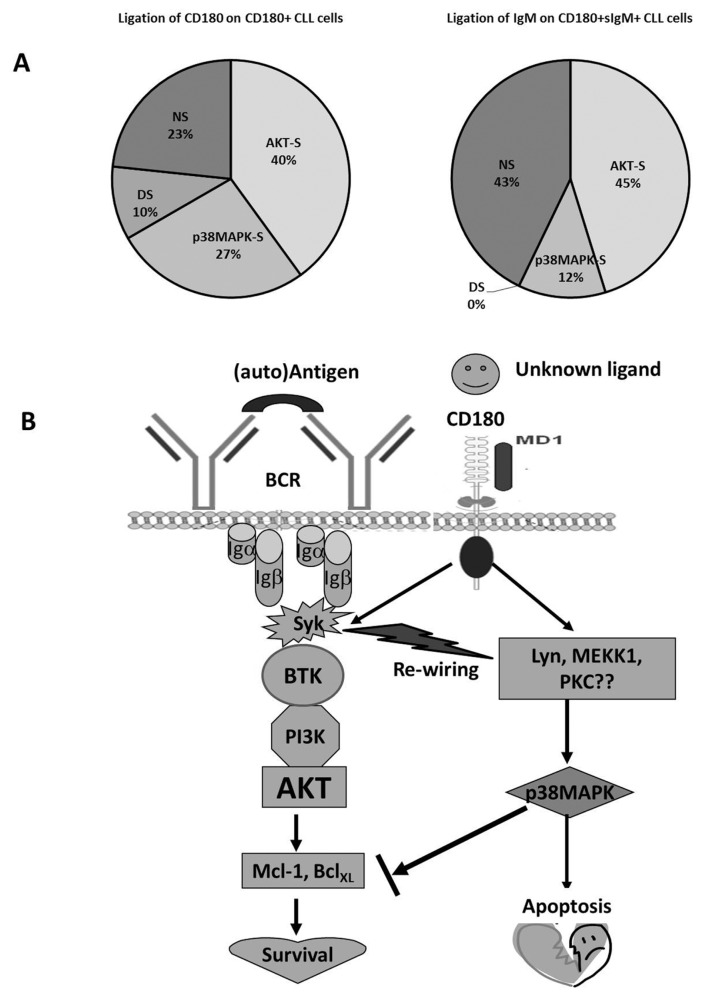
The distribution of CD180-mediated signaling categories of CLL cells that we have identified is shown in Figure 6A. In addition, more U-CLL samples (71%) were CD180+sIgM+ than CD180+sIgMneg/low as demonstrated by us previously (4) attributing U-CLL samples mostly to the AKT-S and NS signaling categories. However, in our hands, M-CLL samples were evenly distributed between AKT-S, p38MAPK-S and NS categories. Interestingly, all six DS CLL samples belonged to M-CLL group, which is associated with increased anergy (38).
Figure 6.
Proportion of CLL samples exhibiting four different patterns of signaling and hypothetical scheme of a cross-talk between CD180 and BCR signaling pathways. (A) treatment with anti-CD180 mAb of CD180+ CLL samples or treatment with anti-IgM F(ab)2 of CD180+sIgM+ CLL samples. AKT-S, AKT-signalers; p38MAPK-S, p38MAPK signalers; DS, double AKT/p38MAPK signalers; NS, nonsignalers; (B) CD180-mediated pathway can operate via both prosurvival BTK/PI3K/AKT or proapoptotic p38MAPK pathway, while sIgM-mediated signaling mostly operates through BTK/PI3K/AKT. Cross-talk between the two receptors redirects signaling pathway from BTK/PI3K/AKT to p38MAPK. Hypothetical precursors of p38MAPK activation are suggested.
There were no phenotypic differences between the four signaling groups, apart from sIgD, that was highly expressed by p38MAPK-S compared with AKT-S cells: 83.5% ± 15.1% versus 48.5% ± 33.0%, p = 0.0055, n = 10. The relevance of this is currently unclear, but suggests that sIgD could be involved in the p38MAPK-pathway mediated through CD180, which we are currently investigating.
CD180-Mediated AKT-Signaling Pathway in CLL Involves Activation of BTK and PI3K and is Prosurvival, While p38MAPK Activation Favors Apoptosis
BTK, a signaling element of the BCR pathway (39), has been shown to be important for the survival of CLL cells (40) as is PI3K (19,28–31,33,36,37,41–48). In murine B cells, engagement of CD180/RP105 leads to the activation of PI3K (49,50) followed by the recruitment of AKT (51). Our data indicate that CD180 engagement of AKT-S CLL samples involves the recruitment of the prosurvival kinases BTK and PI3K, while suppressing the p38MAPK pathway and leading to the reduction in apoptosis.
However, BTK and PI3K are not recruited in the p38MAPK-S CLL samples, resulting in the increased apoptosis (Figure 2). The role of p38MAPK-mediated signaling in CLL is so far unclear. Activation of p38MAPK has been associated with proliferation of various cells in response to CpG-ODN (52–56). In CLL, p38MAPK-signaling is involved in the regulation of cell survival (57–59), establishment of the tolerant status (60) and apoptosis (61). Our data strongly suggests that CD180-mediated activation of p38MAPK is associated with apoptosis of CLL cells. Thus, stimulation of intracellular signaling via CD180 in the p38MAPK-S category of CLL cells could potentially serve as a new profiling tool, particularly of those patients who do not respond effectively to BTK inhibitors (39,40).
CD180 ligation appears to have a pleiotropic effect on the regulation of apoptosis in CLL cells. In our hands, CD180 ligation enhanced the activation of the BTK/PI3K/AKT signaling circuit in AKT-S, but not in p38MAPK-S or NR-CLL samples (Figure 2). Hypothesizing that CLL cells would have received various microenvironmental stimuli, in vivo, including those through CD180, our findings suggest that therapeutic application of the BTK inhibitors might be limited to the AKT-S CLL cells.
sIgM Ligation Favors Prosurvival BTK/PI3K/AKT Signaling Pathways in AKT-S CLL Cells
Since our working hypothesis is that, like the BCR, CD180 is an important receptor that interacts with microenvironmental ligands, we assessed sIgM-mediated signaling in the categories of CLL cells defined through their responses to CD180. Our data strongly suggest that the prosurvival BTK/PI3K/AKT pathway in AKT-S CLL cells is activated following the ligation of either receptor, CD180 (Figure 2) or sIgM (Figure 3), leading to the upregulation of antiapoptotic molecules (see Figures 3D, E) and significant survival of CLL cells (Figure 3F). However, unlike in CD180, we failed to detect appreciable activation of p38MAPK through the engagement of BCR in the p38MAPK-S or DS CLL samples. This might suggest that the lack of BTK phosphorylation abrogates both arms of BCR signaling (via AKT and via p38MAPK, [62–65]), while leaving CD180-mediated p38MAPK activation intact.
Our data indicate, that, differently from BCR, CD180 can signal downstream to p38MAPK, bypassing BTK, and this pathway favors apoptosis over survival. Although there is a substantial overlap by CD180 and sIgM in activation of a prosurvival signaling circuit BTK/PI3K/AKT, ligation of CD180 can activate an alternative proapoptotic pathway mediated via p38MAPK. This prompted us to study how sequential ligation of CD180 and BCR would impact signal transduction through the AKT and p38MAPK pathways.
Pretreatment of CLL Cells with Anti-CD180 Antibodies Rewires the sIgM Signaling Pathway from Prosurvival to Proapoptotic
Treatment with anti-CD180 mAb of murine B cells sensitized them toward anti-IgM-induced apoptosis (34), while combining CD180 and BCR signaling, in vivo, induced rapid proliferation and antigen-specific antibody responses in mice (66). Our data indicate that pretreatment of AKT-S CLL cells with anti-CD180 mAb substantially diminished the prosurvival BTK/PI3K/AKT signaling pathway induced through subsequent ligation of sIgM and redirected it toward the p38MAPK pathway (Figures 4A, D, E) and, most importantly, apoptosis (see Figure 4F). Similar results were obtained when the sequence of the ligation of the two receptors was reversed (Figure 5). Since CLL cells are believed to express BCR in proliferation centers (67) and we have shown in pilot experiments that CD180 is expressed in bone marrow and lymph nodes of CLL patients (data not shown), it seems plausible that CD180+sIgM+ CLL cells could receive simultaneous signals through both receptors in vivo. We hypothesize that CD180-mediated activation of CLL cells through a putative endogenous ligand might mimic continuous BCR-mediated signaling postulated recently (68). The resulting cross-talk between CD180 and BCR in AKT-S CLL samples, could rewire intracellular signaling in favor of the proapoptotic p38MAPK pathway. Such modulation of signaling could influence blood cell counts, long-term disease progression and could provide a basis for therapy. Future experiments will address the significance of these pathways in relation to prognosis and clinical criteria. A hypothetical scheme for cross-talk between CD180 and the BCR is shown in Figure 6B. Why there appears to be no cross-talk of this kind between BCR and CD180 in control B cells (Figures 4B, 5B), is unclear at present, but indicates that this modulation is unique to CLL, facilitating possible therapeutic interventions.
That a universal approach to CD180 treatment of CLL patients might be inappropriate is clear from the data on p38MAPK-S CLL samples. Where sIgM ligation alone (Figures 4C, D) or CD180 alone (Figures 5C, D) resulted in poor activation of PI3K/AKT pathway, preengagement of CD180 or sIgM respectively led to the opposite effect, that is, potentiation of the prosurvival signal.
By using stimulatory antibodies we attempted to mimic, in vivo, interactions of CD180+sIgM+ CLL cells with the putative microenvironmental ligand CD180 and sIgM (auto)antigen(s) that would define their cellular fate. We have shown previously that CD180neg CLL cells were poor responders to anti-CD40 and IL-4 (5) and failed to upregulate p-AKT orp-p38MAPK in response to anti-IgM (data not shown). Thus, it appears that CD180 represents an essential component of the CLL signaling machinery through its interaction with and modulation of sIgM-mediated responses.
CONCLUSION
In conclusion, we hypothesize that interaction between CD180 and sIgM leads to convergence of certain key signaling pathways. Whereas the prosurvival pathway appears to be operating through the BTK/PI3K/AKT circuit, the proapoptotic pathway is activated via p38MAPK, perhaps leading to inhibition of BTK/PI3K/AKT. The elements of the proapoptotic pathway upstream to p38MAPK have yet to be identified.
Our data suggest that, in a substantial number of CLL samples, by preengaging CD180, we could prevent further prosurvival signaling mediated via sIgM and, instead, induce CLL cell apoptosis, which opens the door to new strategies for the treatment of a large cohort of CLL patients.
ACKNOWLEDGMENTS
The research was funded by the University of Westminster, London, UK. We are grateful to the CLL patients who consented to participate in this project. The research was supported in part by RO1 grant CA 081554 from the NIH National Cancer Institute to N Chiorazzi.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Porakishvili N, et al. (2015) Rewiring of sIgM-mediated intracellular signaling through the CD180 toll-like receptor. Mol. Med. 21:46–57.
REFERENCES
- 1.Ghia P, Ferreri AM, Caligaris-Cappio F. Chronic lymphocytic leukemia. Crit Rev Oncol Hemato. 2007;64:234–46. doi: 10.1016/j.critrevonc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu Rev Immunol. 2003;21:841–94. doi: 10.1146/annurev.immunol.21.120601.141018. [DOI] [PubMed] [Google Scholar]
- 3.Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Int Med. 2008;264:549–62. doi: 10.1111/j.1365-2796.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 4.Porakishvili N, et al. Differential expression of CD180 and IgM by B-cell chronic lymphocytic leukaemia cells using mutated and unmutated immunoglobulin VH genes. Br J Haematol. 2005;131:313–9. doi: 10.1111/j.1365-2141.2005.05775.x. [DOI] [PubMed] [Google Scholar]
- 5.Porakishvili N, et al. CD180 functions in activation, survival and cycling of B chronic lymphocytic leukaemia cells. Br J Haematol. 2011;153:486–98. doi: 10.1111/j.1365-2141.2011.08605.x. [DOI] [PubMed] [Google Scholar]
- 6.Decker T, et al. Immunostimulatory CpG-oligonucleotides induce functional high affinity IL-2 receptors on B-CLL cells: costimulation with IL-2 results in a highly immunogenic phenotype. Exp Hematol. 2000;28:558–68. doi: 10.1016/s0301-472x(00)00144-2. [DOI] [PubMed] [Google Scholar]
- 7.Jahrsdörfer B, et al. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J Leukoc Biol. 2001;69:81–8. [PubMed] [Google Scholar]
- 8.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 9.Tromp JM, et al. Dichotomy in NF-kappaB signaling and chemoresistance in immunoglobulin variable heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9 triggering. Oncogene. 2010;29:5071–82. doi: 10.1038/onc.2010.248. [DOI] [PubMed] [Google Scholar]
- 10.Muzio M, Fonte E, Caligaris-Cappio F. Toll-like receptors in chronic lymphocytic leukemia. Mediterr J Hematol Infect Dis. 2012;4:e2012055. doi: 10.4084/MJHID.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valentine MA, Clark EA, Shu GL, Norris NA, Ledbetter JA. Antibody to a novel 95-kDa surface glycoprotein on human B cells induces calcium mobilization and B cell activation. J Immunol. 1988;140:4071–8. [PubMed] [Google Scholar]
- 12.Miyake K, Yamashita Y, Ogata M, Sudo T, Kimoto M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol. 1995;154:3333–40. [PubMed] [Google Scholar]
- 13.Otipoby KL, Nagai Y, Shu GL, Miyake K, Clark EA. CD180 (RP105/Bgp95) Workshop Report. In: Mason DY, editor. Leukocyte Typing VII White Cell Differentiation Antigens. Oxford University Press; 2002. pp. 120–3. [Google Scholar]
- 14.Ogata H, et al. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–9. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura Y, et al. RP105 is associated with MD-1 and transmits an activation signal in human B cells. Blood. 1998;92:2815–22. [PubMed] [Google Scholar]
- 16.Chaplin JW, Kasahara S, Clark EA, Ledbetter JA. Anti-CD180 (RP105) activates B cells to rapidly produce polyclonal Ig via a T cell and MyD88-independent pathway. J Immunol. 2011;187:4199–209. doi: 10.4049/jimmunol.1100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo PG, et al. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia. 2007;21:110–20. doi: 10.1038/sj.leu.2404417. [DOI] [PubMed] [Google Scholar]
- 18.De Frias M, et al. Akt inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Haematologica. 2009;94:1698–707. doi: 10.3324/haematol.2008.004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernal A, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 20.Allsup DJ, et al. B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia. Cancer Res. 2005;65:7328–37. doi: 10.1158/0008-5472.CAN-03-1563. [DOI] [PubMed] [Google Scholar]
- 21.Burger JA, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurse-like cell cocultures and after BCR stimulation. Blood. 2009;113:3050–8. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroga MP, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–37. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Packham G, Stevenson F. The role of the B-cell receptor in the pathogenesis of chronic lymphocytic leukaemia. Semin Cancer Biol. 2010;20:391–9. doi: 10.1016/j.semcancer.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Petlickovski A, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105:4820–7. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 25.Steele AJ, et al. The JAK3-selective inhibitor PF-956980 reverses the resistance to cyto-toxic agents induced by interleukin-4 treatment of chronic lymphocytic leukemia cells: potential for reversal of cytoprotection by the microenvironment. Blood. 2010;116:4569–77. doi: 10.1182/blood-2009-09-245811. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang J, et al. Akt is activated in chronic lymphocytic leukemia cells and delivers a prosurvival signal: the therapeutic potential of Akt inhibition. Haematologica. 2010;95:110–8. doi: 10.3324/haematol.2009.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman SE, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barragan M, et al. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99:2969–76. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- 29.Barragan M, Campas C, Bellosillo B, Gil J. Protein kinases in the regulation of apoptosis in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44:1865–70. doi: 10.1080/1042819031000110964. [DOI] [PubMed] [Google Scholar]
- 30.Barragan M, et al. Regulation of Akt/PKB by phosphatidylinositol 3-kinase-dependent and -independent pathways in B-cell chronic lymphocytic leukemia cells: role of protein kinase Cb. J Leukoc Biol. 2006;80:1473–9. doi: 10.1189/jlb.0106041. [DOI] [PubMed] [Google Scholar]
- 31.Nedellec S, et al. B cell response to surface IgM cross-linking identifies different prognostic groups of B chronic lymphocytic leukemia patients. J Immunol. 2005;174:3749–56. doi: 10.4049/jimmunol.174.6.3749. [DOI] [PubMed] [Google Scholar]
- 32.Wickremasinghe RG, Prentice AG, Steele AJ. Aberrantly activated anti-apoptotic signaling mechanisms in chronic lymphocytic leukaemia cells: clues to the identification of novel therapeutic targets. Br J Haematol. 2011;153:545–56. doi: 10.1111/j.1365-2141.2011.08676.x. [DOI] [PubMed] [Google Scholar]
- 33.Scuppoli MT, Pizzolo G. Signaling pathways activated by the B cell receptor in chronic lymphocytic leukemia. Expert Rev Hematol. 2012;5:341–8. doi: 10.1586/ehm.12.21. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita Y, et al. Activation mediated by RP105 but not CD40 makes normal B cells susceptible to anti-IgM-induced apoptosis: a role for Fc receptor colligation. J Exp Med. 1996;184:113–20. doi: 10.1084/jem.184.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodland RT, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–60. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–82. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Longo PG, et al. The Akt/Mcl-1pathway plays a prominent role in mediating anti-apoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–55. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 38.Packham G, et al. The outcome of B-cell receptor signaling in chronic lymphocytic leukemia: proliferation or anergy. Haematologica. 2014;99:1138–48. doi: 10.3324/haematol.2013.098384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger JA, et al. The Bruton’s tyrosine kinase inhibitor, PCI-32765, is well tolerated and demonstrates promising clinical activity in chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): an update on ongoing phase 1 studies. Blood. 2010;116:32a. [Google Scholar]
- 40.Ponader S, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efremov DG, Gobessi S, Longo PG. Signaling pathways activated by antigen-receptor engagement in chronic lymphocytic leukemia B-cells. Autoimmun Rev. 2007;7:102–8. doi: 10.1016/j.autrev.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signaling: a distinctive role for the p110d isoform of PI3K. Trends Immunol. 2007;28:80–7. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman SE, et al. Phosphatidylinositol 3-kinase-d inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuni S, et al. A sustained activation of PI3K/NFkappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 46.Ringshausen I, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase CD. Blood. 2002;100:3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 47.Plate JM. PI3-kinase regulates survival of chronic lymphocytic leukemia B-cells by preventing caspase8 activation. Leuk Lymphoma. 2004;45:1519–29. doi: 10.1080/10428190410001683642. [DOI] [PubMed] [Google Scholar]
- 48.Lannutti BJ, et al. CAL-101, a p110 {delta} selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yazawa N, et al. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood. 2003;102:1374–80. doi: 10.1182/blood-2002-11-3573. [DOI] [PubMed] [Google Scholar]
- 50.Chan VW, et al. The molecular mechanism of B cell activation by toll-like receptor protein RP-105. J Exp Med. 1998;188:93–101. doi: 10.1084/jem.188.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hebeis B, Vigorito E, Kovesdi D, Turner M. Vav proteins are required for B lymphocyte responses to LPS. Blood. 2005;106:635–40. doi: 10.1182/blood-2004-10-3919. [DOI] [PubMed] [Google Scholar]
- 52.Takeshita F, et al. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol. 2004;16:17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad-Nejad P, et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–68. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 54.Park Y, Lee SW, Sung YC. Cutting edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-30-OH kinase pathway. J Immunol. 2002;168:5–8. doi: 10.4049/jimmunol.168.1.5. [DOI] [PubMed] [Google Scholar]
- 55.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–6. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Yi AK, Yoon JG, Krieg AM. Convergence of CpG DNA- and BCR mediated signals at the c-Jun N-terminal kinase and NF-kappaB activation pathways: regulation by mitogen-activated protein kinases. Int Immunol. 2003;15:577–91. doi: 10.1093/intimm/dxg058. [DOI] [PubMed] [Google Scholar]
- 57.Grumont RJ, et al. B lymphocytes differentially use the Rel and nuclear factor kappaB1 (NF-kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J Exp Med. 1998;187:663–74. doi: 10.1084/jem.187.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piatelli MJ, et al. Phosphatidylinositol 3-kinase-dependent mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 and NF-kappa B signaling pathways are required for B cell antigen receptor-mediated cyclin D2 induction in mature B cells. J Immunol. 2004;172:2753–62. doi: 10.4049/jimmunol.172.5.2753. [DOI] [PubMed] [Google Scholar]
- 59.Furman RR, Asgary Z, Mascarenhas JO, Liou HC, Schattner EJ. Modulation of NF-kappa B activity and apoptosis in chronic lymphocytic leukemia B cells. J Immunol. 2000;164:2200–6. doi: 10.4049/jimmunol.164.4.2200. [DOI] [PubMed] [Google Scholar]
- 60.Ntoufa S, et al. Distinct innate immunity pathways to activation and tolerance in subgroups of chronic lymphocytic leukemia with distinct immunoglobulin receptors. Mol Med. 2012;18:1281–91. doi: 10.2119/molmed.2011.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Negro R, et al. Overexpression of the auto-immunity-associated phosphatase PTPN22 promotes survival of antigen-stimulated CLL cells by selectively activating AKT. Blood. 2012;119:6278–87. doi: 10.1182/blood-2012-01-403162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fukuda M, Kojima T, Kabayama H, Mikoshiba K. Mutation of the pleckstrin homology domain of Bruton’s tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J Biol Chem. 1996;271:30303–6. doi: 10.1074/jbc.271.48.30303. [DOI] [PubMed] [Google Scholar]
- 63.Khan WN, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–99. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 64.Bajpai UD, Zhang K, Teutsch M, Sen R, Wortis HH. Bruton’s tyrosine kinase links the B cell receptor to nuclear factor kappaB activation. J Exp Med. 2000;191:1735–44. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton’s tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–54. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaplin JW, Chappell CP, Clark EA. Targeting antigens to CD180 rapidly induces antigen-specific IgG, affinity maturation and immunologic memory. J Exp Med. 2013;210:2135–46. doi: 10.1084/jem.20130188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrarini M, Totero D, Colombo M, Cutrona G. Facts and speculations on the onset and clonal expansion of chronic lymphocytic leukemia cells. Int Trends Immunity. 2014;2:1–10. [Google Scholar]
- 68.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukaemia. Trends Immunol. 2013;34:592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]