Abstract
Doxorubicin (DOX) is a widely used, potent chemotherapeutic agent; however, its clinical application is limited because of its dose-dependent cardiotoxicity. DOX’s cardiotoxicity involves increased oxidative/nitrative stress, impaired mitochondrial function in cardiomyocytes/endothelial cells and cell death. Cannabidiol (CBD) is a nonpsychotropic constituent of marijuana, which is well tolerated in humans, with antioxidant, antiinflammatory and recently discovered antitumor properties. We aimed to explore the effects of CBD in a well-established mouse model of DOX-induced cardiomyopathy. DOX-induced cardiomyopathy was characterized by increased myocardial injury (elevated serum creatine kinase and lactate dehydrogenase levels), myocardial oxidative and nitrative stress (decreased total glutathione content and glutathione peroxidase 1 activity, increased lipid peroxidation, 3-nitrotyrosine formation and expression of inducible nitric oxide synthase mRNA), myocardial cell death (apoptotic and poly[ADP]-ribose polymerase 1 [PARP]-dependent) and cardiac dysfunction (decline in ejection fraction and left ventricular fractional shortening). DOX also impaired myocardial mitochondrial biogenesis (decreased mitochondrial copy number, mRNA expression of peroxisome proliferator-activated receptor γ coactivator 1-alpha, peroxisome proliferator-activated receptor alpha, estrogen-related receptor alpha), reduced mitochondrial function (attenuated complex I and II activities) and decreased myocardial expression of uncoupling protein 2 and 3 and medium-chain acyl-CoA dehydrogenase mRNA. Treatment with CBD markedly improved DOX-induced cardiac dysfunction, oxidative/nitrative stress and cell death. CBD also enhanced the DOX-induced impaired cardiac mitochondrial function and biogenesis. These data suggest that CBD may represent a novel cardioprotective strategy against DOX-induced cardiotoxicity, and the above-described effects on mitochondrial function and biogenesis may contribute to its beneficial properties described in numerous other models of tissue injury.
INTRODUCTION
Doxorubicin (DOX) is one of the most commonly used broad-spectrum chemotherapeutic drugs; however, its clinical application is limited because of its dose-dependent cardiotoxicity, which may lead to the development of irreversible cardiomyopathy and/or heart failure (1). The mechanism of DOX’s cardiotoxicity is complex and may involve oxidative (2,3), nitrosative and nitrative stress (4,5), mitochondrial dysfunction/toxicity (1,6–8), dysregulation of various metabolic (9) and lipid signaling pathways (10–12), activation of various stress kinases and cell death mechanisms (both apoptotic and necrotic) (13), triggering of secondary inflammation and remodeling (14), eventually culminating in cardiac dysfunction and heart failure (1,15).
Cannabidiol (CBD) is the most abundant nonpsychoactive constituent of marijuana (Cannabis Sativa), which was considered initially to be biologically inactive (16,17). It has a negligible effect on conventional cannabinoid 1 and 2 receptors (CB1/2) in vivo (16,17) and is very safe in humans (18). A pioneering study by J Axelrod and D Wink in 1998 (19) demonstrated that CBD was a potent neuroprotective antioxidant. They found that it was more protective against glutamate-induced neurotoxicity than either ascorbate or α-tocopherol, suggesting that it has potential therapeutic utility in neurodegenerative disorders associated with oxidative stress (19). Later, large numbers of preclinical studies also confirmed potent antioxidant and antiinflammatory effects of CBD in preclinical models of colitis, ischemic reperfusion injury, various neurodegenerative and cardiovascular disorders, diabetes and diabetic complications (20), among others (17,21). An oromucosal spray containing 50% CBD (Sativex) is approved in the United Kingdom, Canada and various other European countries to alleviate pain and spasticity associated with multiple sclerosis (22), and CBD recently received orphan drug approval by FDA for the treatment of refractory childhood epilepsy.
In this study, we aimed to explore the effects of CBD in a well-established, clinically relevant mouse model of DOX-induced cardiomyopathy (4,5,23), particularly focusing on oxidative and nitrative stress and mitochondrial dysfunction/biogenesis. Our results may also provide a novel mechanistic insight on the protective effects of CBD in various models of tissue injury and a promising tool for the prevention of devastating cardiovascular complications of DOX chemotherapy.
MATERIALS AND METHODS
Animals/Drugs
All protocols involving the use of animals were approved by the Institutional Animal Care and Use Committees and were performed in line with the Guidelines for the Care and Use of Laboratory Animals adopted by the National Institutes of Health (NIH) (24). Male C57BL/6J mice weighing 22–30 g were acutely administered with single high dose (20 mg/kg) of DOX (Sigma-Aldrich, St. Louis, MO, USA) intraperitoneally (IP) freshly dissolved in physiological saline. CBD obtained from Tocris (Ellisville, MO, USA) or isolated as described earlier (25) was dissolved in vehicle solution (one drop of Tween-80 in 3 mL 2.5% DMSO in saline). Mice were treated with CBD 10 mg/kg IP, or vehicle started 1.5 h before the DOX injection and once every day. Mice were subjected to hemodynamic measurements at the end of study (5 d). Hearts were excised and snap frozen in liquid nitrogen for biochemical measurements or fixed for histological evaluation as described previously (10,11).
Hemodynamic Measurements in Mice
Echocardiography was performed by using the VisualSonics Vevo0770 system (VisualSonics Inc., Toronto, ON, Canada), which is equipped with a 30 MHz mechanical scan probe which can obtain high resolution 2-dimensional images. Animals were anesthetized with isoflurane (2%) inhalation anesthesia. B mode images were obtained in the plane containing aortic and mitral valves. M mode images were obtained from the parasternal short-axis view at the level of papillary muscles. LV end-diastolic diameters, ejection fraction and fractional shortening were calculated by using Vevo Analysis software as described previously. The investigators performing echocardiography were blinded to the treatment status.
Serum Creatine Kinase (CK) and Lactate Dehydrogenase (LDH) Levels
The blood samples were collected and the serum was removed immediately for CK and LDH measurements using a clinical chemistry analyzer system (VetTest 8008, IDEXX Laboratories, Westbrook, ME, USA).
Myocardial 3-Nitrotyrosine (NT) Determination
Myocardial 3-nitrotyrosine content, a marker of nitrative stress (26), was determined by nitrotyrosine enzyme-linked immunosorbent assay (ELISA) according to the protocol supplied with the kit (Cell Biolabs, San Diego, CA, USA) as previously described (11,12).
Determination of Myocardial Caspase 3/7 and Poly(ADP-Ribose) Polymerase (PARP) Activities
Caspase 3/7 activity in the heart homogenates was determined using the fluorimetric-based Apo-ONE homogenous assay kit (Promega, Madison, WI, USA) as described (11,12). Myocardial PARP activity was assayed using a colorimetric kit according to manufacturer’s protocol (Trevigen, Gaithersburg, MD, USA) as described (11,12).
Myocardial DNA Fragmentation ELISA
The quantitative determinations of cytoplasmic histone-associated-DNA-fragmentation (mono and oligonucleosomes) due to in vivo cell death were measured using ELISA kit (Roche Diagnostics GmbH, Indianapolis, IN, USA) (12).
Determination of Myocardial Glutathione (GSH) Content
Myocardial GSH content was determined using kits from Trevigen according to the manufacturer’s protocols, as previously reported (12).
Histological Examination of Heart Sections
Heart samples were fixed in 4% buffered formalin. After embedding and cutting 5 μm slices, all sections were stained with nitrotyrosine (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s protocol and/or as described (20). Nitrotyrosine-stained sections were counterstained with hematoxylin. The specific staining was visualized and images were acquired using BX-41 microscope and U-TV1 × 2 camera (Olympus, Tokyo, Japan) with 200 × fold magnification.
Real-Time PCR Analyses of mRNA
Total RNA was isolated from heart homogenate using TRIzol reagents (Invitrogen [Thermo Fisher Scientific Inc., Waltham, MA, USA]) according to manufacturer’s instructions. The isolated RNA was treated with RNase-free DNaseI (Ambion [Thermo Fisher Scientific]) to remove traces of genomic DNA contamination. Each RNA sample (1 μg) was mixed with 2 μL of 5× Genomic DNA Elimination Buffer to remove any residual genomic DNA as supplied with the kit and made up to a final volume of 10 μL. RT cocktail (prepared according to the manufacturer’s instruction) was added to RNA containing Genomic DNA Elimination Mixture and incubated at 42° C for 15 min, followed by heating at 95° C for 5 min. Each 20 μL of cDNA Synthesis Reaction received an addition of 91 μL of sterile water. Real-time PCR was performed using Oxidative Stress and Anti-oxidant Defense RT Profiler PCR array or specific primer sets (SAB Bioscience, Frederick, MD, USA). Relative quantification was calculated using the comparative CT method. The normalizer used for each cDNA sample was housekeeping gene β-actin as described (11,12,20,27).
PARP Activity
Myocardial PARP activity was assayed using a colorimetric kit according to the manufacturer’s protocol (Trevigen) as described (12).
Myocardial 4-Hydroxy-2-Nonenal (4-HNE) Content
Lipid peroxides are unstable indicators of oxidative stress in cells that decompose to form more complex and reactive compounds such as 4-HNE, which has been shown to be capable of binding to proteins and forming stable HNE adducts. HNE in myocardial tissues was determined using a kit (Cell Biolabs). In brief, BSA or Myocardial tissue extracts were adsorbed onto a 96-well plate for overnight at 4° C. HNE adducts present in the sample or standard were probed with anti-HNE antibody, followed by an HRP-conjugated secondary antibody. The HNE protein adduct content in an unknown sample was determined by comparing with a standard curve (12).
Myocardial Glutathione Peroxidase Assay
Glutathione peroxidase enzyme activity was assayed using a SpectraMax spectrophotometer (Trevigen) according to the manufacturer’s instructions.
Determination of Myocardial Mitochondrial Complex Activity
Microplate assay kits were obtained from MitoSciences (Eugene, OR, USA) and used to determine the activity of mitochondrial complex I, complex II and complex IV according to the manufacturer’s instructions. The complex enzymes were immunocaptured in the microplate and activities were determined in Spectramax M3 (Molecular Devices [Thermo Fisher Scientific]). Complex activities were expressed as percentage activity compared with the heart samples of the vehicle-treated mice similar to that described previously for liver (27).
Mitochondrial DNA Content
Mitochondrial DNA (mtDNA) copy/content determination was performed as described previously (27).
Analyses of Data
Results are expressed as means ± SEM. Statistical significance among groups was determined by paired student t test or ANOVA followed by Tukey post hoc test for multiple comparisons using GraphPad Prism 6 software (San Diego, CA, USA). Probability values of P < 0.05 were considered significant.
RESULTS
CBD Attenuates DOX-Induced Cardiac Injury and Cardiac Dysfunction
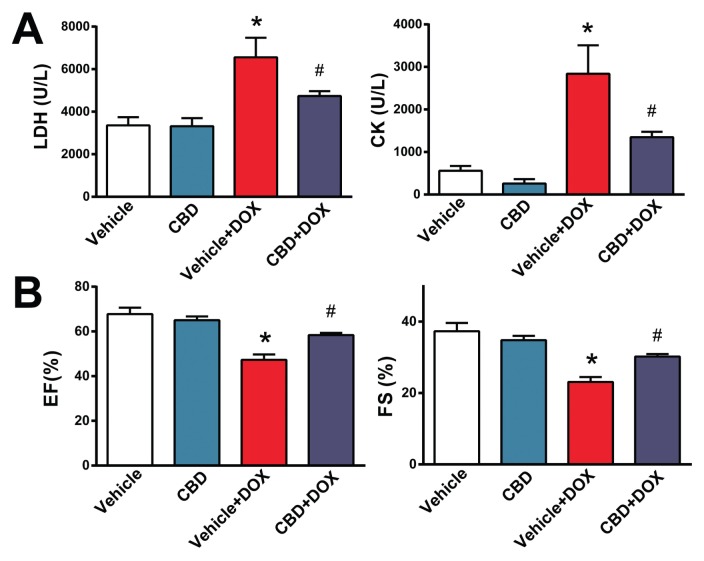
First we investigated if CBD attenuates the DOX-induced increased serum LDH and CK levels (markers of tissue/cardiac injury) and cardiac dysfunction (evaluated by measurement of ejection fraction [EF] and fractional shortening [FS] using echocardiography). DOX-markedly increased serum levels of LDH and CK (Figure 1A) and reduced EF and FS (Figures 1B, C). The DOX-induced tissue injury and cardiac dysfunction were markedly attenuated by CBD treatment (Figures 1A, B).
Figure 1.
CBD treatment attenuates markers of DOX-induced cardiac injury and dysfunction. The effect of DOX and CBD treatment on (A) serum LDH and CK levels and on (B) EF and FS. *P < 0.05 versus vehicle group; #P < 0.05 versus DOX group; n = 5–9/group
CBD Attenuates DOX-Induced Myocardial Oxidative Stress
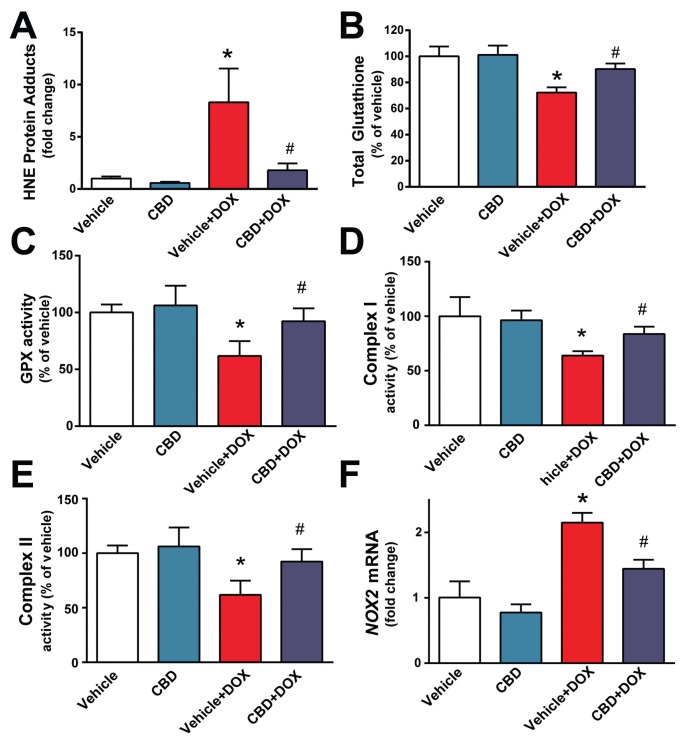
Next we explored the effects of CBD on myocardial oxidative stress. DOX induced marked increase in myocardial HNE content (Figure 2A), reduced total glutathione content (Figure 2B), attenuated glutathione peroxidase (Figure 2C) and mitochondrial complex I and II (but not IV) activities (Figures 2D, E) (the unchanged complex IV activity is not shown). DOX also induced a marked increase in the myocardial expression of reactive oxygen species generation NADPH oxidase isoform NOX2 (Figure 2F). All markers of DOX-induced oxidative stress were attenuated by CBD treatment (see Figures 2A–F).
Figure 2.
CBD treatment attenuates DOX-induced myocardial oxidative stress and mitochondrial dysfunction. The effect of DOX and CBD treatment on myocardial (A) HNE adducts, (B) total glutathione content, (C) GPX activity, (D,E) mitochondrial complex I and II activities and (F) NOX2 mRNA expression. *P < 0.05 versus vehicle group; #P < 0.05 versus DOX group; n = 4–6/group.
CBD Attenuates DOX-Induced Myocardial Nitrative Stress
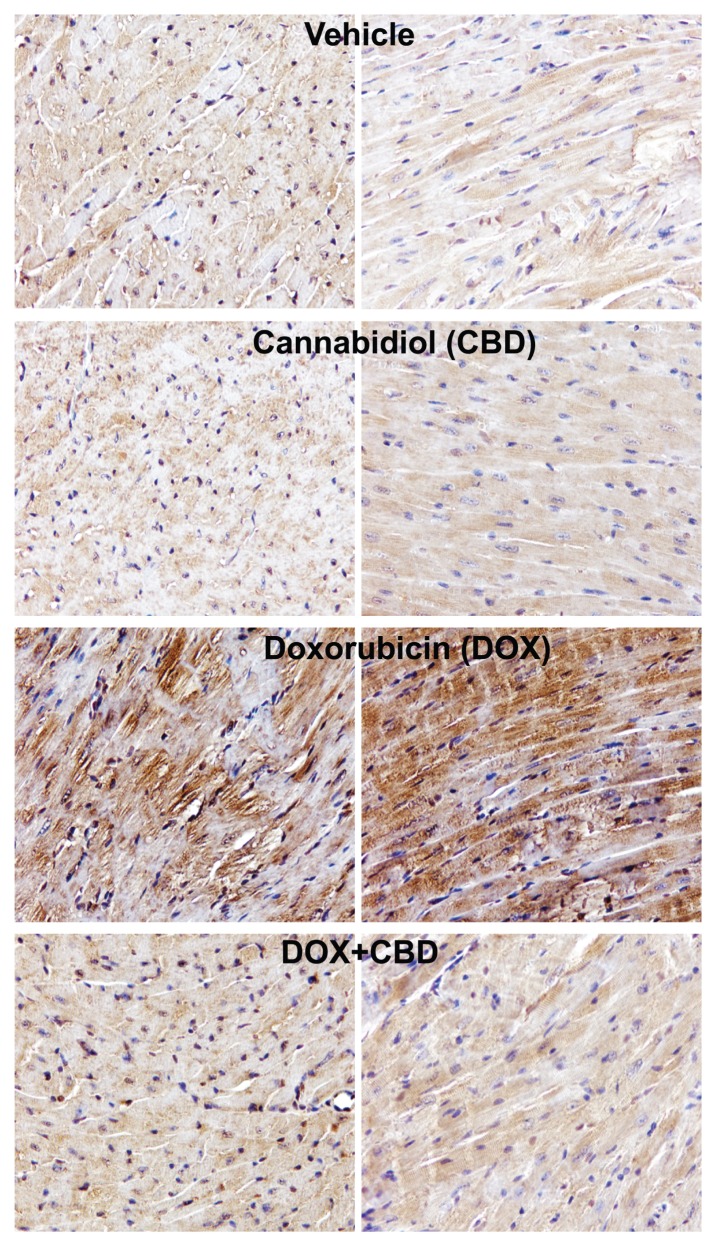
We also explored effects of CBD on DOX-induced increased nitrative stress. DOX markedly increased myocardial inducible nitric oxide synthase (iNOS) expression (Figure 3A) and 3-nitrotyrosine formation (Figures 3B, 4). CBD largely attenuated the DOX-induced increased iNOS expression and nitrotyrosine formation (Figures 3, 4).
Figure 3.
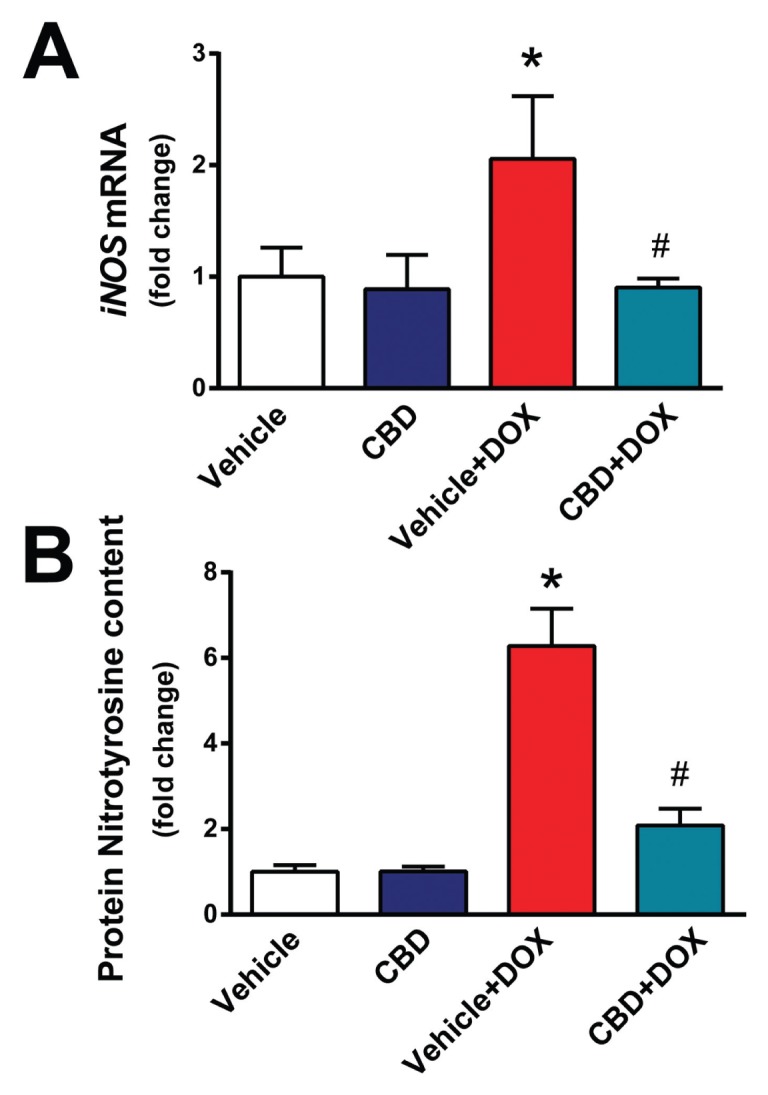
CBD treatment attenuates DOX-induced myocardial iNOS expression and 3-nitrotyrosine accumulation. The effect of DOX and CBD treatment on myocardial (A) iNOS mRNA and (B) protein 3-nitrotyrosine content measured by ELISA *P < 0.05 versus vehicle group; #P < 0.05 versus DOX group; n = 4–6/group.
Figure 4.
CBD treatment attenuates DOX-induced myocardial 3-nitrotyrosine staining. Representative formalin-fixed myocardial tissue sections from indicated groups immunostained for 3-nitrotyrosine (brown staining) (200× magnification).
CBD Enhances DOX-Induced Impaired Mitochondrial Biogenesis
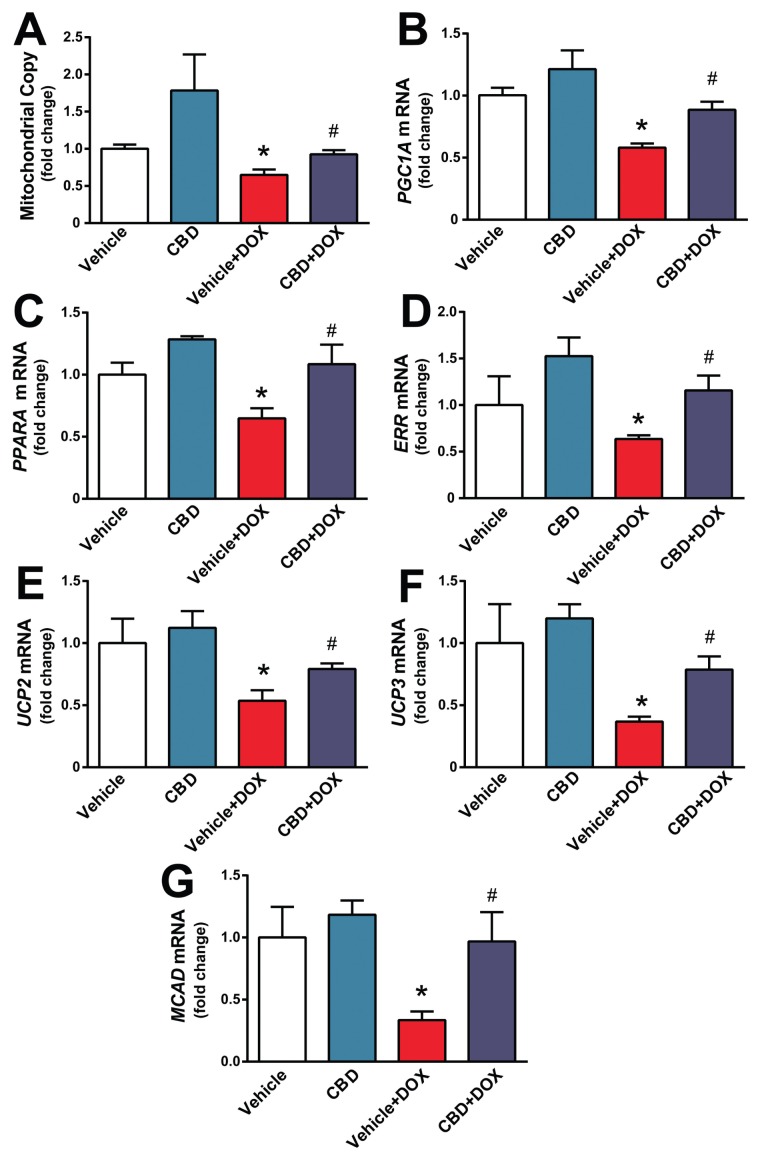
Since DOX-induced cardiotoxicity is known to be associated with mitochondrial injury and dysfunction (see also Figure 2), we further explored the effects of CBD on mitochondrial copy number and various markers of mitochondrial biogenesis. DOX decreased the myocardial mitochondrial copy number and attenuated expression of mRNA of various markers of mitochondrial biogenesis such as peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1A), peroxisome proliferator-activated receptor α (PPARA), estrogen-related receptor α (ERRA), uncoupling protein 2 and 3 (UCP2, UCP3) and medium-chain acyl-CoA dehydrogenase (MCAD) (Figures 5 A–G). CBD largely prevented the above-mentioned changes (see Figures 5A–G), suggesting that it enhanced mitochondrial biogenesis in damaged hearts.
Figure 5.
CBD attenuates DOX-induced impaired myocardial mitochondrial biogenesis. The effect of DOX and CBD treatment on (A) mitochondrial copy number and expression of (B) PGC1A, (C) PPARA, (D) ERRA, (E,F) UCP2 and UCP3 and (G) MCAD mRNA levels. Pre-treatment with CBD improves DOX-induced decreased expression of mitochondrial biogenesis genes. *P < 0.05 versus vehicle group; #P < 0.05 versus DOX group; n = 4–6/group.
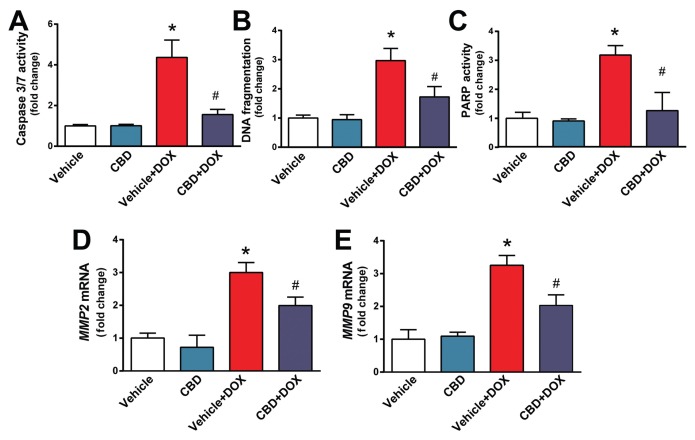
CBD Attenuates DOX-Induced Myocardial Cell Death and Matrix Metalloproteinases 2 and 9 (MMP2 and MMP9) Activation
Cell death of cardiomyocytes and endothelial cells, as well as activation of proteolytic MMP enzymes, is a consequence of increased oxidative and nitrative stress and mitochondrial injury. We found that DOX-induced marked increases in myocardial apoptotic and PARP-dependent cell death in the myocardium and in mRNA expression of MMP2 and MMP9, which all were largely attenuated by CBD treatment (Figures 6A–E).
Figure 6.
CBD treatment attenuates DOX-induced myocardial cell death and MMP2 and MMP9 mRNA expression. Effect of DOX and CBD treatment on myocardial (A) caspase 3/7 activity, (B) chromatin fragmentation and (C) PARP activation. (D,E) Expression of MMP2 and MMP9 mRNA. *P < 0.05 versus vehicle group; #P < 0.05 versus DOX group; n = 4–6/group.
CBD Attenuates DOX-Induced Myocardial Inflammation
Myocardial injury is known to trigger secondary proinflammatory response. We found that DOX increased myocardial expression of mRNAs of tumor necrosis factor-α (TNFA), interleukin-1β (IL1B) and monocyte chemoattractant protein-1 (MCP-1) (Figures 7A–C). These changes were attenuated by CBD treatment (see Figures 7A–C).
Figure 7.
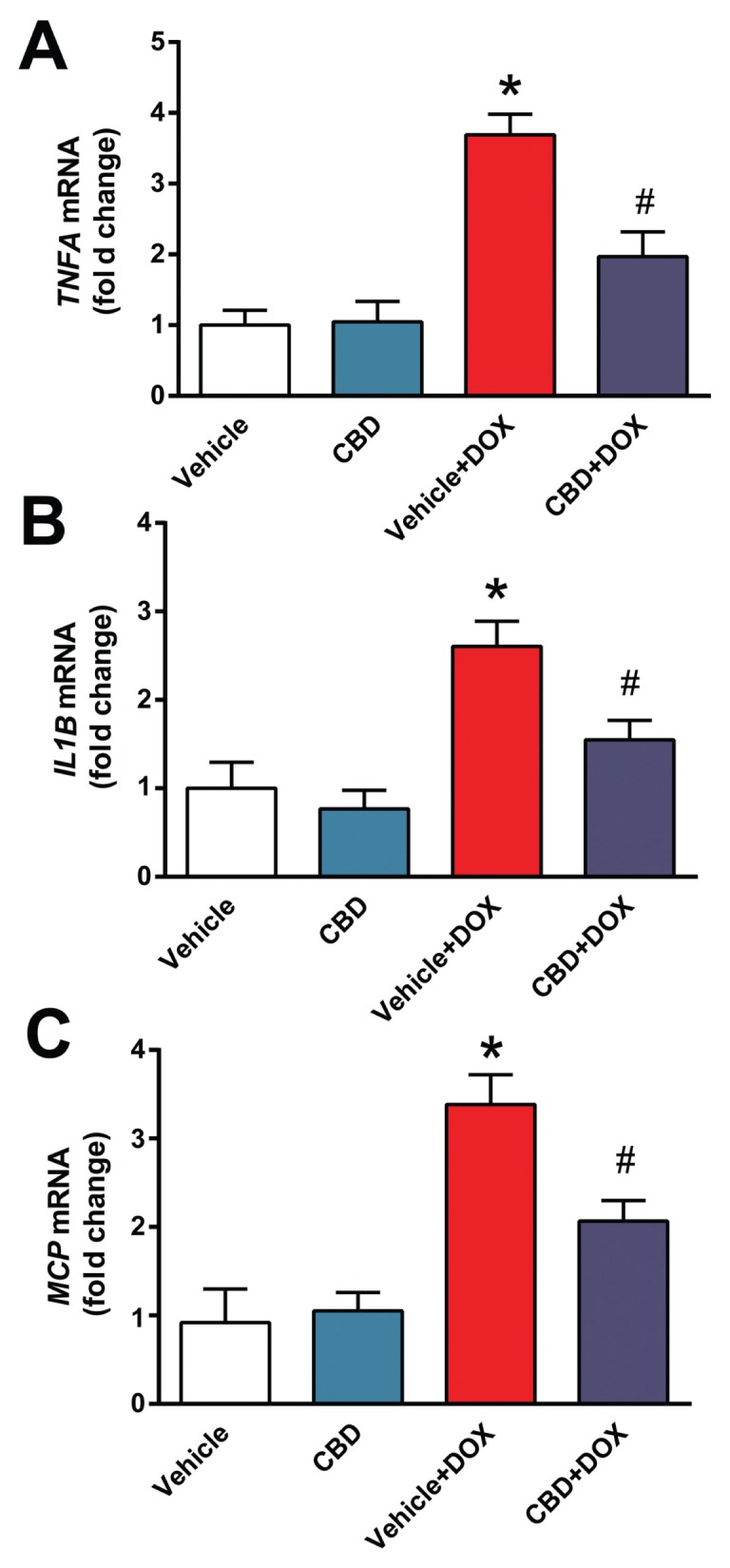
CBD attenuates DOX-induced proinflammatory response in the heart. Real-time PCR shows significant increase of (A) TNFA, (B) IL1B, (C) MCP-1 mRNA level. Pretreatment with CBD significantly attenuates DOX-induced increased proinflammatory chemokine levels. *P < 0.05 versus vehicle group; #P < 0.05 versus DOX group; n = 4–6/group.
DISCUSSION
DOX-induced cardiomyopathy/heart failure is a serious complication of chemotherapy with very limited therapeutic options, mostly including supportive treatment or heart transplantation (1). The currently available preventive strategies are not satisfactory and there is an urgent need for development of novel approaches to prevent this devastating complication of DOX chemotherapy. CBD is a nonpsychoactive constituent of marijuana with potent antioxidant and antiinflammatory effects in preclinical disease models which are independent from classical G-protein coupled cannabinoid 1 and 2 receptors (16,17).
Herein, we show that CBD exerts protective effects against DOX-induced cardiotoxicity and cardiac dysfunction by (i) attenuating oxidative and nitrative stress, (ii) improving mitochondrial function, (iii) enhancing mitochondrial biogenesis, (iv) decreasing cell death and expression of MMPs and (v) decreasing myocardial inflammation.
Consistent with numerous previous reports (1–8) we found that DOX treatment markedly increased oxidative stress and impaired antioxidant defense in the heart. DOX also decreased myocardial mitochondrial complex I and II activities, as well as the activity of the glutathione peroxidase (GPX). This is consistent with studies suggesting a key role for mitochondrial dysfunction and reactive oxygen species generation in triggering the deleterious cascade of the DOX-induced cardiotoxicity (4,5,28,29). In addition to mitochondrial, NADPH oxidase-dependent ROS generation (particularly NOX2-dependent) also may contribute to DOX-induced cardiotoxicity (4,30). We also found that DOX increased myocardial expression of iNOS and enhanced 3-nitrotyrosine (3-NT) formation. 3-NT is a marker of peroxynitrite formation, or, more broadly, of nitrative stress (26). Peroxynitrite formed from the diffusion limited reaction of superoxide anion and NO recently was implicated as the key downstream effector of promoting DOX-induced cardiotoxicity (26). Peroxynitrite may induce lipid peroxidation, nitration of key contractile proteins and/or sarco/endoplasmic reticulum Ca2+ pump, trigger activation of mitogen-activated protein kinases promoting apoptotic cell death and impair mitochondrial function favoring increased reactive oxygen species generation, eventually culminating in cardiac dysfunction (26). In addition, peroxynitrite also can diffuse to the nucleus, inducing DNA breaks and consequent over-activation of the nuclear enzyme PARP-1, which, in turn, leads to energetic crisis and cell necrosis (26). Indeed, both neutralization of peroxynitrite by peroxynitrite decomposition catalysts or inhibition of iNOS or PARP is protective against DOX-induced cardiotoxicity in murine models of DOX-induced cardiomyopathies. (4,5,13). DOX via increased generation of reactive oxygen and nitrogen species also leads to activation of MMP enzymes (31), which in turn promotes pathological remodeling (4,14,32,33).
CBD attenuated DOX-induced myocardial lipid peroxidation and the decline in antioxidant glutathione level and in the activity of glutathione peroxidase, which could be the consequence of attenuated formation or enhanced inactivation of reactive oxygen and nitrogen species. CBD also decreased the DOX-induced increased iNOS expression in the myocardium, most likely contributing to the attenuation of DOX-induced myocardial nitrotyrosine formation as a consequence of decreased NO availability for peroxynitrite formation from NO and superoxide anion. In agreement with this, CBD also attenuated nuclear factor-κB (NF-κB) and/or iNOS expression in a model of liver ischemia-reperfusion injury (34), cisplatin-induced nephropathy (35), and in diabetic cardiomyopathy (20,36). In these models, CBD also attenuated oxidative stress by attenuating mitochondrial dysfunction and reactive oxygen species generation or by decreasing the expression of various reactive oxygen species generating NADPH oxidase isoforms (20,35,36). CBD also attenuated the DOX-induced decreased myocardial mitochondrial complex I activity, in agreement with its effect in a liver ischemia-reperfusion injury model (34). Furthermore, CBD also reduced the DOX-induced decline in myocardial mitochondrial copy number and enhanced mitochondrial biogenesis in injured hearts. Consistent with previous studies, DOX also induced both apoptotic and PARP-dependent cell death in hearts (5,12,23,37), which could be largely attenuated by CBD. CBD also attenuated the DOX-induced increased myocardial inflammation. These results are in agreement with the attenuation of proinflammatory response by CBD in diabetic hearts (20) or in human cardiomyocytes (20) or coronary artery endothelial cells (36) exposed to high glucose, likewise with beneficial effects of CBD in rodent models of myocardial infarction, primary diabetes and diabetic complications (17,38–40).
So far, over 50 metabolites of CBD were identified in the urine, showing considerable variations between different species (including humans), however their biological activity was not evaluated in detail (41,42). Substantial evidence from in vitro or in vivo studies suggests that CBD by itself exerts potent antiinflammatory and antioxidant effects, independently from cannabinoid receptors (for example in liver ischemia/reperfusion injury model CBD exerted comparable antiinflammatory effects in both cannabinoid 2 receptor knockout mice and their wild-type littermates). Nevertheless, the potential protective effect of certain CBD metabolites in vivo cannot be excluded either.
Another issue which deserves some discussion is whether the above-mentioned antioxidant and antiinflammatory effects of CBD would interfere with the antitumor activity of DOX. The answer is that it is not likely to do so. For example, mitochondrial-targeted antioxidants appear to have synergistic effects with various chemotherapeutic agents in tumor cell killing (43). Activation of MMPs may be involved in tumor progression and metastasis formation, which is inhibited by CBD, likewise the NF-κB signaling. Furthermore, CBD by itself has been reported to have antitumor effects in a large variety of cancer cell lines, as well as in some explanted tumor models (44–46), which would rather predict a synergistic effect with antineoplastic drugs. In fact, Insys Therapeutics just received FDA orphan drug designation for CBD as a potential treatment for glioblastoma multiforme in humans.
CONCLUSION
In summary, our results indicate that CBD may represent a novel promising approach for the prevention of DOX-induced cardiomyopathy/heart failure by attenuating oxidative/nitrative stress, mitochondrial dysfunction, cell death and inflammation, and by promoting mitochondrial biogenesis. These results, coupled with the excellent safety of CBD in humans, its protective effects against cisplatin-induced nephropathy, its recently reported antineoplastic properties in various malignancies and its orphan drug approval for glioblastoma multiforme and childhood epilepsy, are particularly encouraging from a therapeutic point of view.
ACKNOWLEDGMENTS
This study was supported by the Intramural Research Program of National Institutes of Health/NIAAA. P Pacher is grateful to George Kunos, Scientific Director of NIAAA, for continuous support, and dedicates this study to collaborator and friend, Itai Bab. The study also was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (to P Pacher).
Footnotes
Online address: http://www.molmed.org
DISCLOSURES
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Hao E et al. (2015) Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 21:38–45.
REFERENCES
- 1.Carvalho FS, et al. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34:106–35. doi: 10.1002/med.21280. [DOI] [PubMed] [Google Scholar]
- 2.Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res. 1988;48:4766–9. [PubMed] [Google Scholar]
- 3.Sterba M, et al. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal. 2013;18:899–929. doi: 10.1089/ars.2012.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacher P, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay P, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–83. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–74. [PubMed] [Google Scholar]
- 7.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa Y, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617–30. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J Mol Cell Cardiol. 2006;41:389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay P, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–36. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhopadhyay P, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–84. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay P, et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011;50:179–95. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacher P, et al. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–7. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 14.Bai P, et al. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol Rep. 2004;11:505–8. [PubMed] [Google Scholar]
- 15.Azuma J, et al. Adriamycin cardiotoxicity: possible pathogenic mechanisms. J Mol Cell Cardiol. 1981;13:381–397. doi: 10.1016/0022-2828(81)90281-9. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–27. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Cunha JM, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 19.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–73. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajesh M, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–25. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley CP, Hind WH, O’Sullivan SE. Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol. 2013;75:313–22. doi: 10.1111/j.1365-2125.2012.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J. 2013;280:1918–43. doi: 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay P, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–36. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press; 2011. [Google Scholar]
- 25.Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–24. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- 26.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay P, et al. Poly (ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology. 2014;59:1998–2009. doi: 10.1002/hep.26763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–7. [PubMed] [Google Scholar]
- 29.Chandran K, et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardio-protection by Mito-Q. Biophys J. 2009;96:1388–98. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 2010;70:9287–97. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–10. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kizaki K, et al. Enhanced gene expression of myocardial matrix metalloproteinases 2 and 9 after acute treatment with doxorubicin in mice. Pharmacol Res. 2006;53:341–6. doi: 10.1016/j.phrs.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Spallarossa P, et al. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res. 2006;69:736–45. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay P, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–81. doi: 10.1016/j.freeradbiomed.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan H, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–14. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajesh M, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293:H610–9. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pacher P, et al. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med. 2006;17:369–75. [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss L, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–51. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- 39.Weiss L, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54:244–9. doi: 10.1016/j.neuropharm.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvath B, Mukhopadhyay P, Hasko G, Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am J Pathol. 2012;180:432–42. doi: 10.1016/j.ajpath.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin BR, Harvey DJ, Paton WD. Bio-transformation of cannabidiol in mice. Identification of new acid metabolites. Drug Metab Dispos. 1977;5:259–67. [PubMed] [Google Scholar]
- 42.Harvey DJ, Samara E, Mechoulam R. Comparative metabolism of cannabidiol in dog, rat and man. Pharmacol Biochem Behav. 1991;40:523–32. doi: 10.1016/0091-3057(91)90358-9. [DOI] [PubMed] [Google Scholar]
- 43.Mukhopadhyay P, et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med. 2012;52:497–506. doi: 10.1016/j.freeradbiomed.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solinas M, et al. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PloS One. 2013;8:e76918. doi: 10.1371/journal.pone.0076918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacher P. Towards the use of non-psychoactive cannabinoids for prostate cancer. Br J Pharmacol. 2013;168:76–8. doi: 10.1111/j.1476-5381.2012.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303–12. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]